Doxycycline and Minocycline Act as Positive Allosteric Modulators of the PAC1 Receptor and Induce Plasminogen Activators in RT4 Schwann Cells
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. RT4 Schwann Cell Line
2.2. ELISA tPA and uPA Activity
2.3. Cell Viability Assay
2.4. Zymography of tPA and uPA Activity
2.5. Immunocytochemistry
2.6. Western Blot
2.7. Statistical Analysis
3. Results
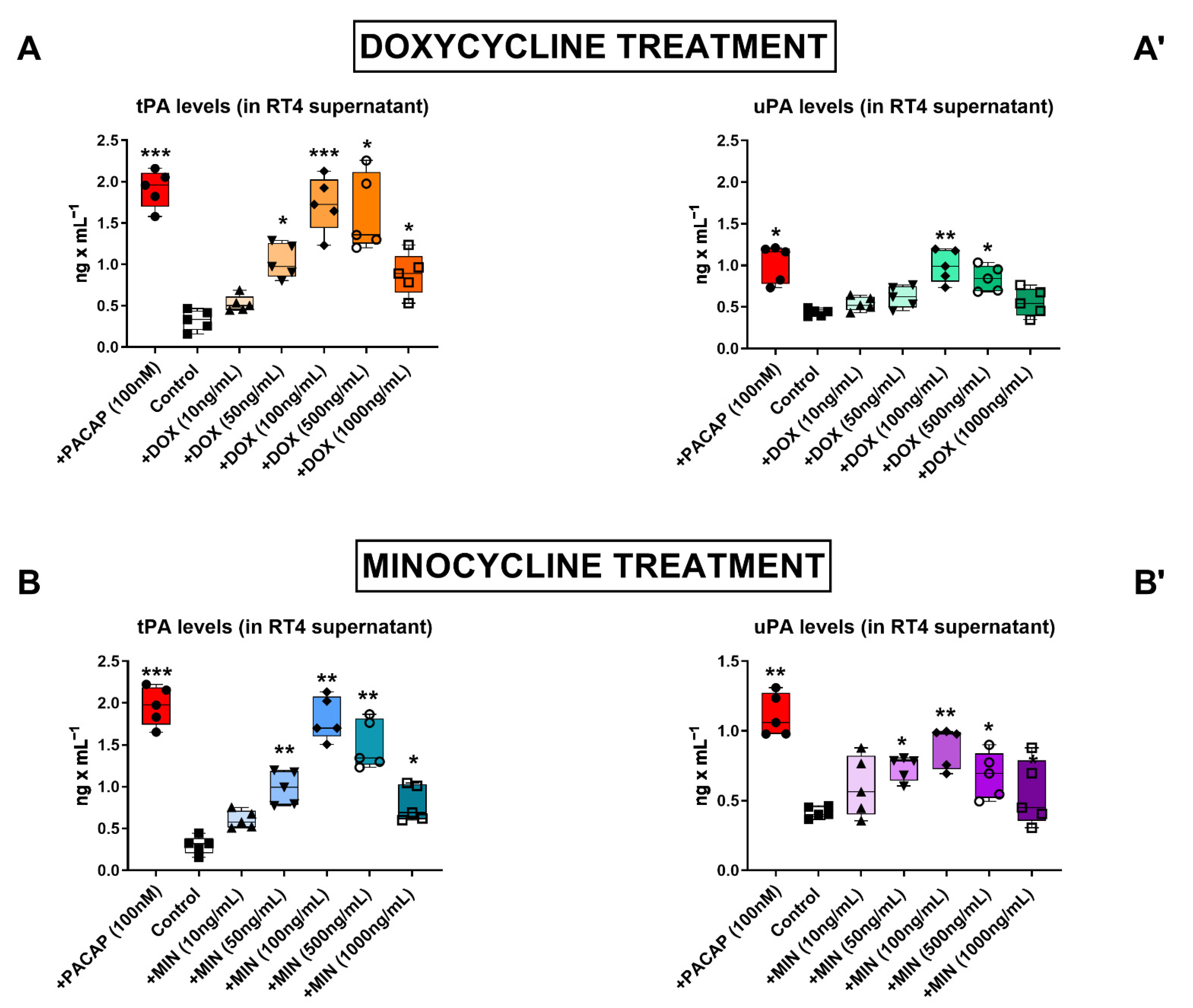
3.1. Dose–Response Effects of Doxycycline and Minocycline on tPA and uPA Secretion in RT4 Cells
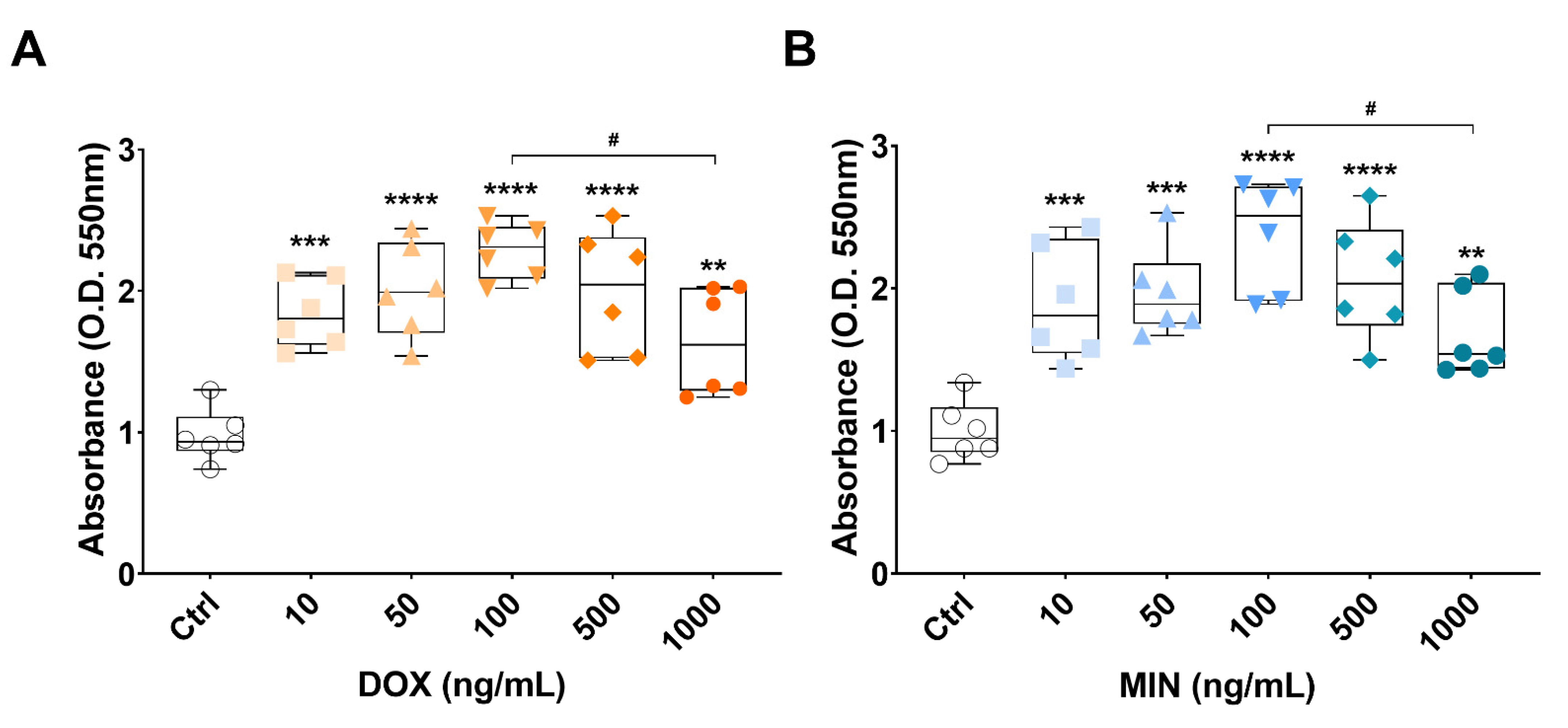
3.2. Effects of Doxycycline or Minocycline Treatment on RT4 Cell Viability
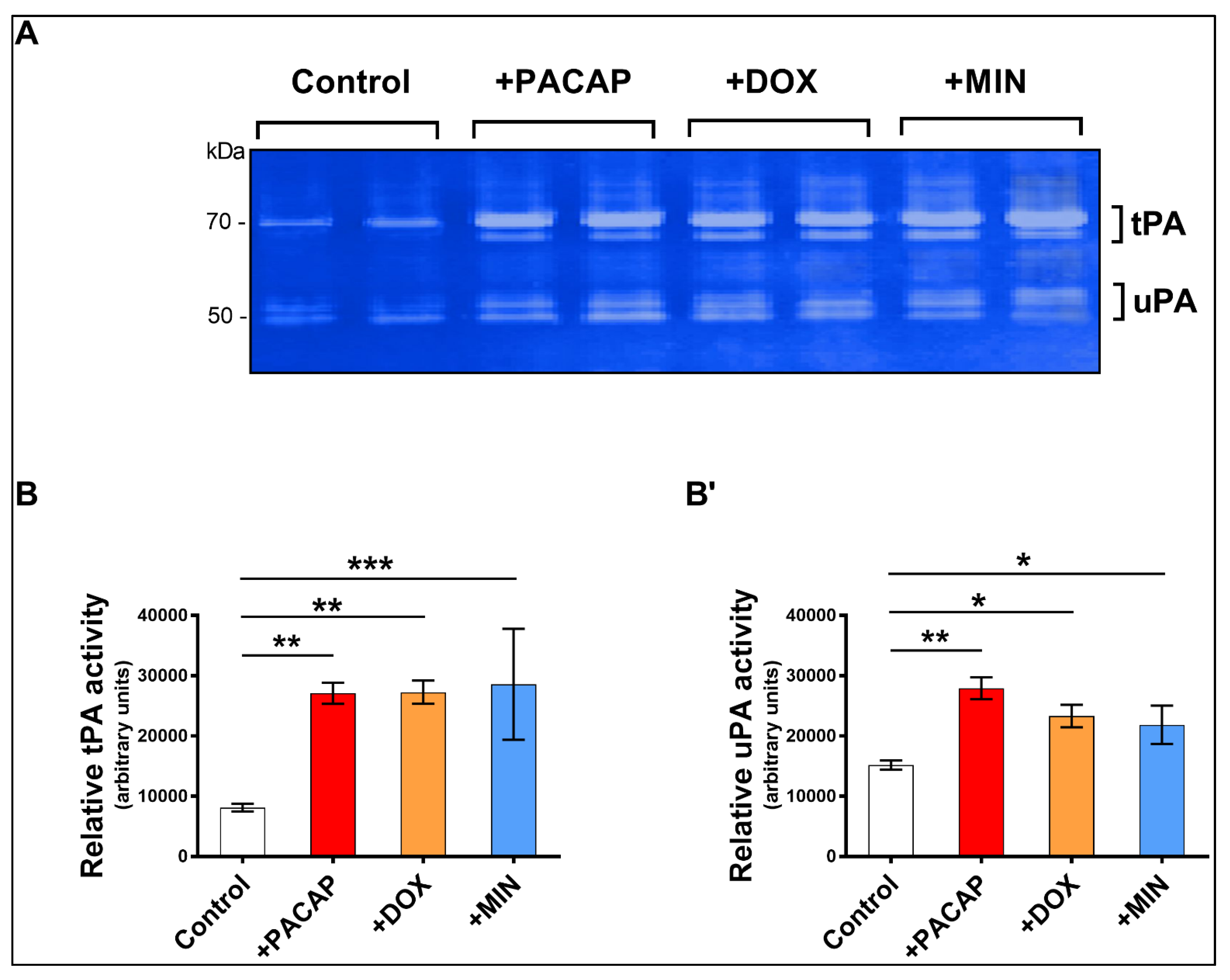
3.3. Enzymatic Activity of tPA and uPA in RT4 Cells Exposed to Doxycycline or Minocycline
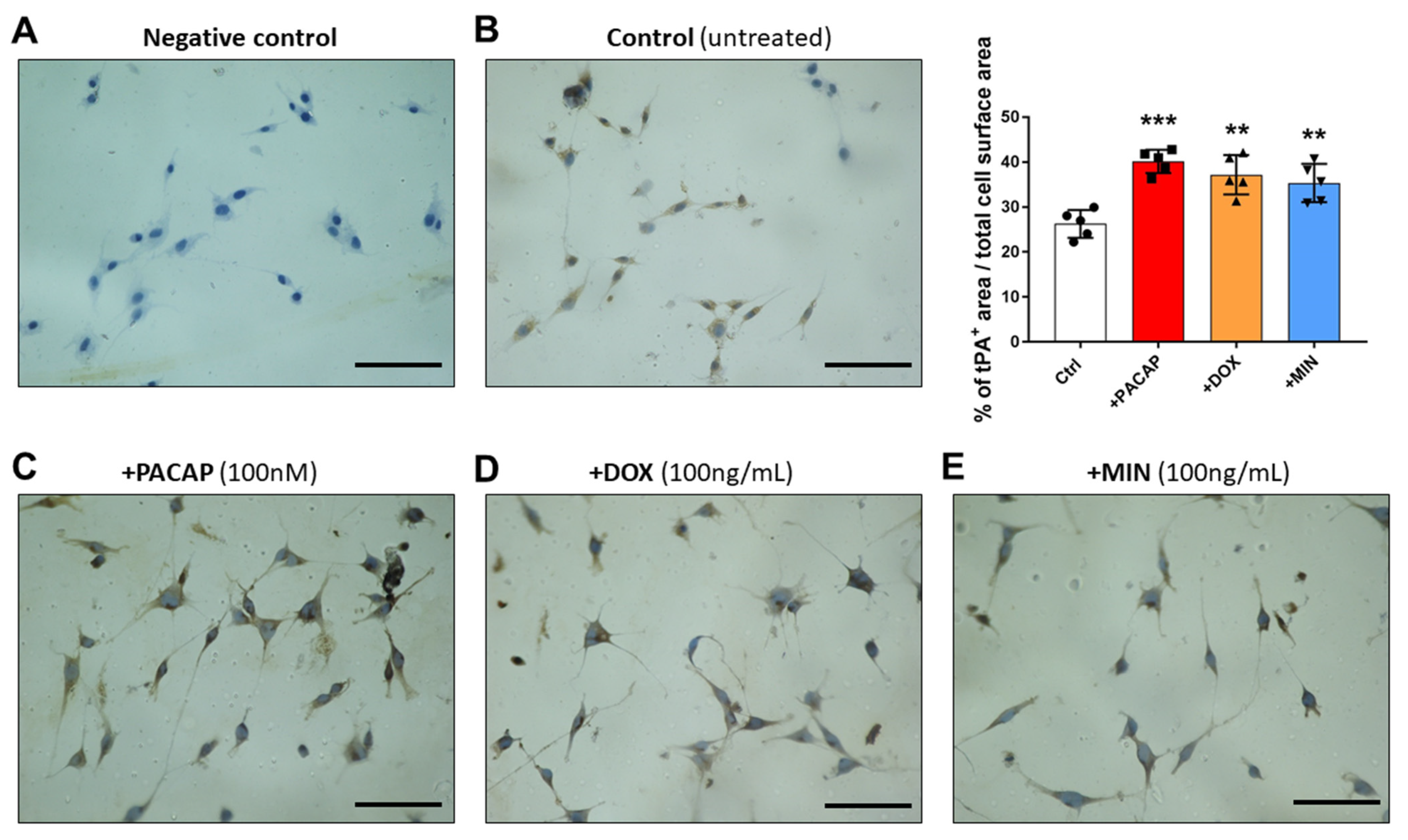
3.4. tPA-like Immunoreactivity in RT4 Cells Exposed to PACAP, Doxycycline or Minocycline
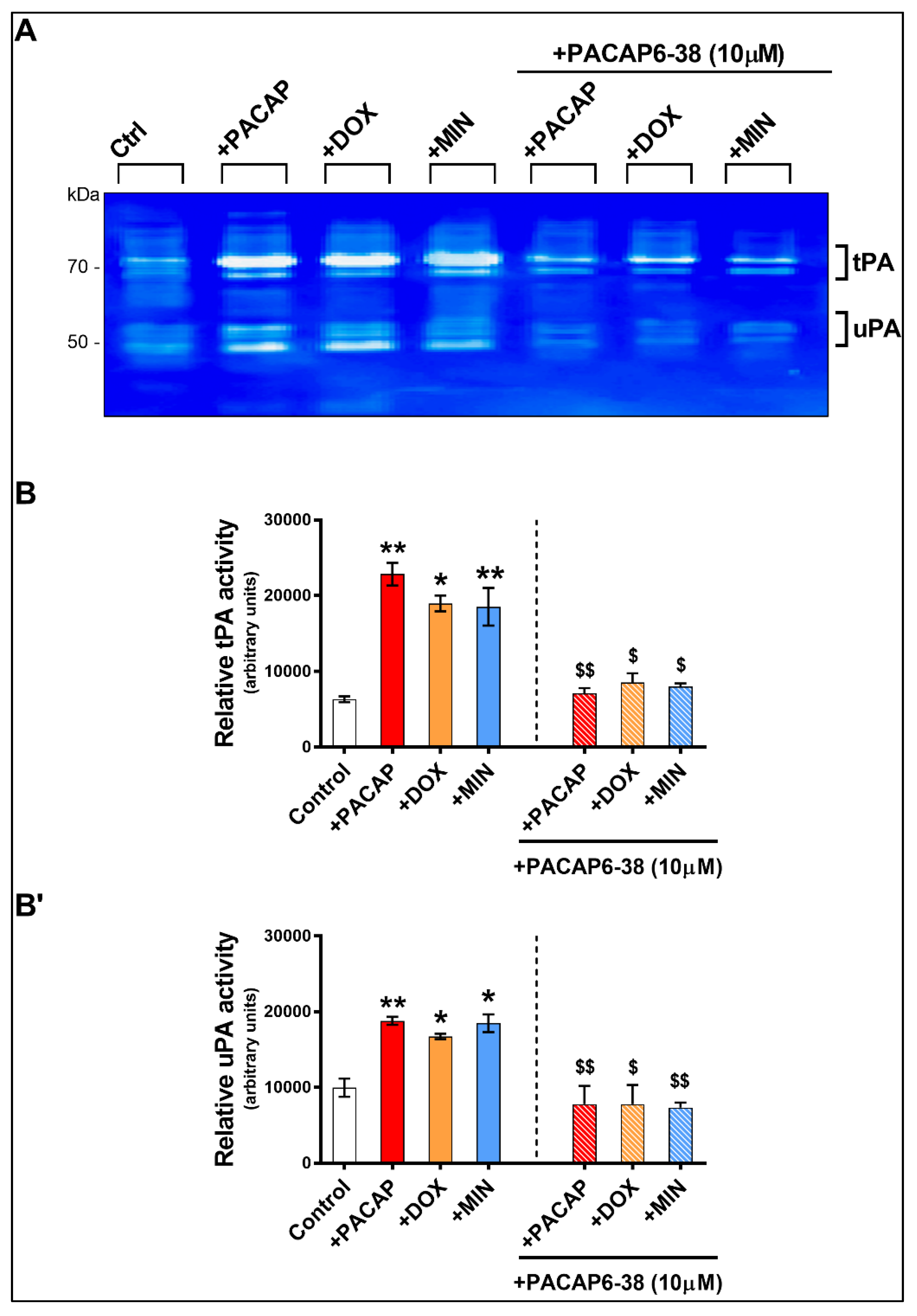
3.5. Pre-Treatment with PACAP6-38 Blocks Doxycycline- and Minocycline-Induced tPA Activity
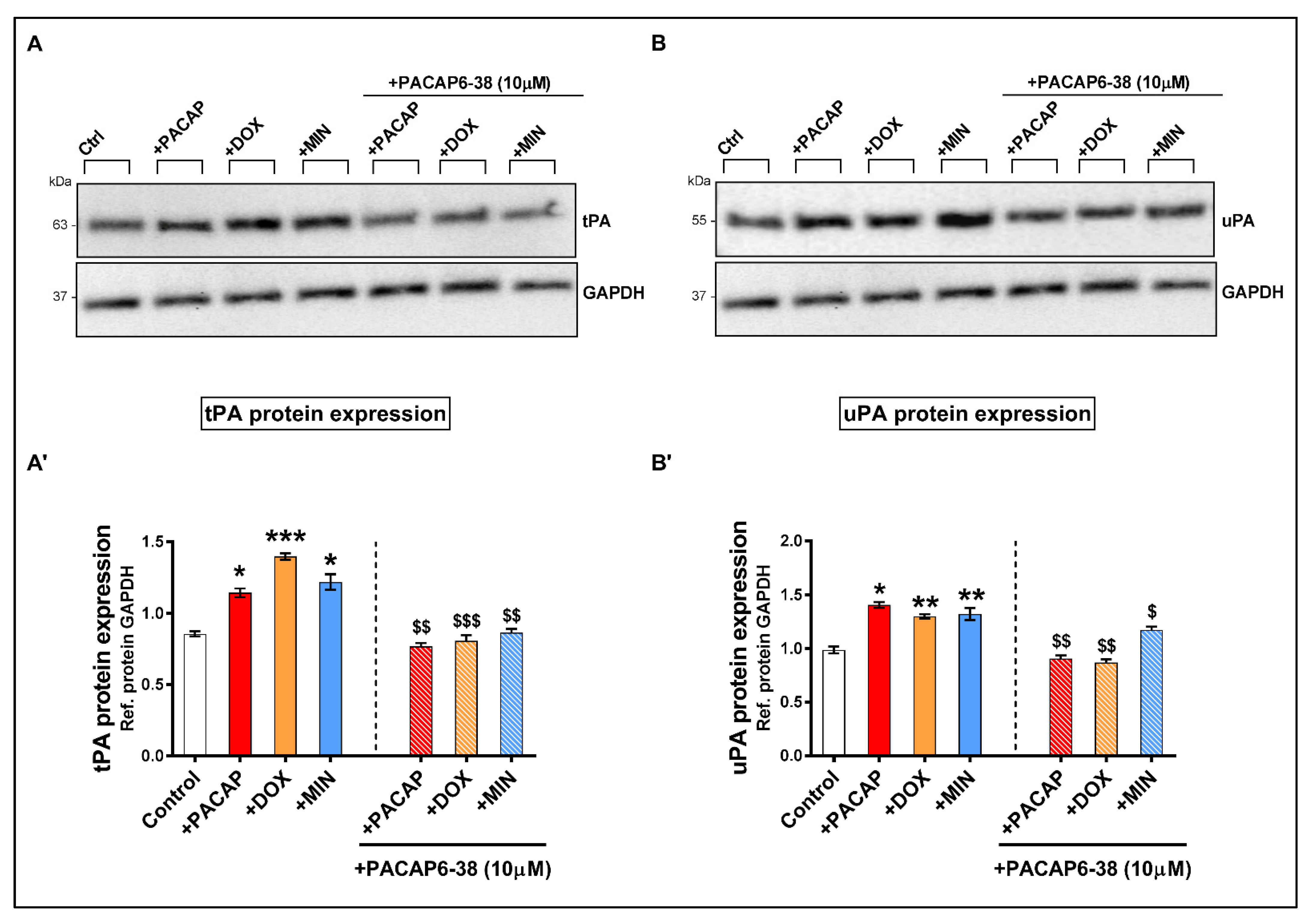
3.6. Pre-Treatment with PACAP6-38 Blocks Doxycycline- and Minocycline-Induced tPA and uPA Protein Expression Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Experientia 2020, 77, 3977–3989. [Google Scholar] [CrossRef] [Green Version]
- Stierli, S.; Imperatore, V.; Lloyd, A.C. Schwann cell plasticity-roles in tissue homeostasis, regeneration, and disease. Glia 2019, 67, 2203–2215. [Google Scholar] [CrossRef]
- Siconolfi, L.B.; Seeds, N.W. Induction of the Plasminogen Activator System Accompanies Peripheral Nerve Regeneration after Sciatic Nerve Crush. J. Neurosci. 2001, 21, 4336–4347. [Google Scholar] [CrossRef] [Green Version]
- Akassoglou, K.; Kombrinck, K.W.; Degen, J.L.; Strickland, S. Tissue plasminogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury. J. Cell Biol. 2000, 149, 1157–1166. [Google Scholar] [CrossRef]
- Musumeci, G.; Leggio, G.M.; Marzagalli, R.; Al-Badri, G.; Drago, F.; Castorina, A. Identification of Dysregulated microRNA Networks in Schwann Cell-Like Cultures Exposed to Immune Challenge: Potential Crosstalk with the Protective VIP/PACAP Neuropeptide System. Int. J. Mol. Sci. 2018, 19, 981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodley, P.K.; Min, Q.; Li, Y.; Mulvey, N.F.; Parkinson, D.B.; Dun, X.-P. Distinct VIP and PACAP Functions in the Distal Nerve Stump During Peripheral Nerve Regeneration. Front. Neurosci. 2019, 13, 1326. [Google Scholar] [CrossRef] [Green Version]
- Castorina, A.; Vogiatzis, M.; Kang, J.W.; Keay, K.A. PACAP and VIP expression in the periaqueductal grey of the rat following sciatic nerve constriction injury. Neuropeptides 2018, 74, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Giunta, S.; Castorina, A.; Marzagalli, R.; Szychlinska, M.A.; Pichler, K.; Mobasheri, A.; Musumeci, G. Ameliorative Effects of PACAP against Cartilage Degeneration. Morphological, Immunohistochemical and Biochemical Evidence from in Vivo and in Vitro Models of Rat Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 5922–5944. [Google Scholar] [CrossRef] [Green Version]
- Waschek, J. VIP and PACAP: Neuropeptide modulators of CNS inflammation, injury, and repair. Br. J. Pharmacol. 2013, 169, 512–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moody, T.W.; Ito, T.; Osefo, N.; Jensen, R.T. VIP and PACAP: Recent insights into their functions/roles in physiology and disease from molecular and genetic studies. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Van, C.; Condro, M.C.; Lov, K.; Zhu, R.; Ricaflanca, P.; Ko, H.H.; Diep, A.L.; Hoang, A.Q.; Pisegna, J.; Rohrer, H.; et al. PACAP/PAC1 Regulation of Inflammation via Catecholaminergic Neurons in a Model of Multiple Sclerosis. J. Mol. Neurosci. 2018, 68, 439–451. [Google Scholar] [CrossRef]
- Schaler, A.W.; Runyan, A.M.; Clelland, C.L.; Sydney, E.J.; Fowler, S.L.; Figueroa, H.Y.; Shioda, S.; Santa-Maria, I.; Duff, K.E.; Myeku, N. PAC1 receptor–mediated clearance of tau in postsynaptic compartments attenuates tau pathology in mouse brain. Sci. Transl. Med. 2021, 13, eaba7394. [Google Scholar] [CrossRef]
- Reglodi, D.; Vaczy, A.; Beltrán, A.E.R.; MaassenVanDenBrink, A. Protective effects of PACAP in ischemia. J. Headache Pain 2018, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Edvinsson, L.; Tajti, J.; Szalardy, L.; Vécsei, L. PACAP and its role in primary headaches. J. Headache Pain 2018, 19, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.; De Molliens, M.P.; Schneebeli, S.T.; Brewer, M.; Song, G.; Chatenet, D.; Braas, K.M.; May, V.; Li, J. Targeting the PAC1 Receptor for Neurological and Metabolic Disorders. Curr. Top. Med. Chem. 2019, 19, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Lutfy, K.; Shankar, G. Emerging evidence for the role of pituitary adenylate cyclase-activating peptide in neuropsychiatric disorders. Prog. Mol. Biol. Transl. Sci. 2019, 167, 143–157. [Google Scholar] [CrossRef]
- Yang, R.; Jiang, X.; Ji, R.; Meng, L.; Liu, F.; Chen, X.; Xin, Y. Therapeutic potential of PACAP for neurodegenerative diseases. Cell. Mol. Biol. Lett. 2015, 20, 265–278. [Google Scholar] [CrossRef]
- Reglodi, D.; Tamas, A.; Jungling, A.; Vaczy, A.; Rivnyak, A.; Fulop, B.; Szabo, E.; Lubics, A.; Atlasz, T. Protective effects of pituitary adenylate cyclase activating polypeptide against neurotoxic agents. NeuroToxicology 2018, 66, 185–194. [Google Scholar] [CrossRef]
- Zhou, X.; Rodriguez, W.I.; Casillas, R.A.; Ma, V.; Tam, J.; Hu, Z.; Lelievre, V.; Chao, A.; Waschek, J.A. Axotomy-induced changes in pituitary adenylate cyclase activating polypeptide (PACAP) and PACAP receptor gene expression in the adult rat facial motor nucleus. J. Neurosci Res. 1999, 15, 953–961. [Google Scholar] [CrossRef]
- Armstrong, B.; Abad, C.; Chhith, S.; Cheung-Lau, G.; Hajji, O.; Nobuta, H.; Waschek, J. Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience 2008, 151, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Castorina, A.; Tiralongo, A.; Giunta, S.; Carnazza, M.L.; Rasi, G.; D’Agata, V. PACAP and VIP prevent apoptosis in schwannoma cells. Brain Res. 2008, 1241, 29–35. [Google Scholar] [CrossRef]
- Castorina, A.; Waschek, J.A.; Marzagalli, R.; Cardile, V.; Drago, F. PACAP Interacts with PAC1 Receptors to Induce Tissue Plasminogen Activator (tPA) Expression and Activity in Schwann Cell-Like Cultures. PLoS ONE 2015, 10, e0117799. [Google Scholar] [CrossRef]
- Song, S.; Wang, L.; Li, J.; Huang, X.; Yu, R. The allosteric modulation effects of doxycycline, minocycline, and their derivatives on the neuropeptide re-ceptor PAC1-R. Acta Biochim Biophys Sin. 2019, 51, 627–637. [Google Scholar] [CrossRef]
- Khanna, D.; Kalra, S. Minocycline and doxycycline: More Than Antibiotics. Curr. Mol. Pharmacol. 2021, 14, 1. [Google Scholar] [CrossRef]
- Santa-Cecília, F.V.; Leite, C.A.; Del-Bel, E.; Raisman-Vozari, R. The Neuroprotective Effect of Doxycycline on Neurodegenerative Diseases. Neurotox. Res. 2019, 35, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Festoff, B.W.; Ameenuddin, S.; Arnold, P.M.; Wong, A.; Santacruz, K.S.; Citron, B.A. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J. Neurochem. 2006, 97, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Adembri, C.; Selmi, V.; Vitali, L.; Tani, A.; Margheri, M.; Loriga, B.; Carlucci, M.; Nosi, D.; Formigli, L.; De Gaudio, A.R. Minocycline But Not Tigecycline Is Neuroprotective and Reduces the Neuroinflammatory Response Induced by the Superimposition of Sepsis Upon Traumatic Brain Injury. Crit. Care Med. 2014, 42, e570–e582. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Gebara, E.; Toni, N. Doxycycline increases neurogenesis and reduces microglia in the adult hippocampus. Front. Neurosci. 2013, 7, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-S.; Suh, Y.-H. Minocycline and neurodegenerative diseases. Behav. Brain Res. 2009, 196, 168–179. [Google Scholar] [CrossRef]
- Yu, R.; Zheng, L.; Cui, Y.; Zhang, H.; Ye, H. Doxycycline exerted neuroprotective activity by enhancing the activation of neuropeptide GPCR PAC1. Neuropharmacology 2015, 103, 1–15. [Google Scholar] [CrossRef]
- Musumeci, G.; Magro, G.G.; Cardile, V.; Coco, M.; Marzagalli, R.; Castrogiovanni, P.; Imbesi, R.; Graziano, A.C.E.; Barone, F.; Di Rosa, M.; et al. Characterization of matrix metalloproteinase-2 and -9, ADAM-10 and N-cadherin expression in human glioblastoma multiforme. Cell Tissue Res. 2015, 362, 45–60. [Google Scholar] [CrossRef]
- Hai, M.; Muja, N.; DeVries, G.H.; Quarles, R.H.; Patel, P.I. Comparative analysis of Schwann cell lines as model systems for myelin gene transcription studies. J. Neurosci. Res. 2002, 69, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Glenn, T.D.; Talbot, W.S. Signals regulating myelination in peripheral nerves and the Schwann cell response to injury. Curr. Opin. Neurobiol. 2013, 23, 1041–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castorina, A.; Scuderi, S.; D’Amico, A.G.; Drago, F.; D’Agata, V. PACAP and VIP increase the expression of myelin-related proteins in rat schwannoma cells: Involvement of PAC1/VPAC2 receptor-mediated activation of PI3K/Akt signaling pathways. Exp. Cell Res. 2014, 322, 108–121. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.; Musumeci, G.; Reglodi, D.; D’Agata, V. Effects of Pacap on Schwann Cells: Focus on Nerve Injury. Int. J. Mol. Sci. 2020, 21, 8233. [Google Scholar] [CrossRef] [PubMed]
- Baskozos, G.; Sandy-Hindmarch, O.; Clark, A.J.; Windsor, K.; Karlsson, P.; Weir, G.A.; McDermott, L.A.; Burchall, J.; Wiberg, A.; Furniss, D.; et al. Molecular and cellular correlates of human nerve regeneration: ADCYAP1/PACAP enhance nerve out-growth. Brain 2020, 143, 2009–2026. [Google Scholar] [CrossRef]
- Bourgault, S.; Vaudry, D.; Botia, B.; Couvineau, A.; Laburthe, M.; Vaudry, H.; Fournier, A. Novel stable PACAP analogs with potent activity towards the PAC1 receptor. Peptides 2008, 29, 919–932. [Google Scholar] [CrossRef]
- Zhu, L.; Tamvakopoulos, C.; Xie, D.; Dragovic, J.; Shen, X.; Fenyk-Melody, J.E.; Schmidt, K.; Bagchi, A.; Griffin, P.R.; Thornberry, N.A.; et al. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: In vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1–38). J. Biol. Chem. 2003, 278, 22418–22423. [Google Scholar] [CrossRef] [Green Version]
- Castorina, A.; Al-Badri, G.; Leggio, G.M.; Musumeci, G.; Marzagalli, R.; Drago, F. Tackling dipeptidyl peptidase IV in neurological disorders. Neural Regen. Res. 2018, 13, 26–34. [Google Scholar] [CrossRef]
- Bortolanza, M.; Nascimento, G.C.; Socias, S.B.; Ploper, D.; Chehín, R.N.; Raisman-Vozari, R.; Del-Bel, E. Tetracycline repurposing in neurodegeneration: Focus on Parkinson’s disease. J. Neural Transm. 2018, 125, 1403–1415. [Google Scholar] [CrossRef]
- Keilhoff, G.; Schild, L.; Fansa, H. Minocycline protects Schwann cells from ischemia-like injury and promotes axonal outgrowth in bioartificial nerve grafts lacking Wallerian degeneration. Exp. Neurol. 2008, 212, 189–200. [Google Scholar] [CrossRef]
- Keilhoff, G.; Langnaese, K.; Wolf, G.; Fansa, H. Inhibiting effect of minocycline on the regeneration of peripheral nerves. Dev. Neurobiol. 2007, 67, 1382–1395. [Google Scholar] [CrossRef]
- Sanchez-Brualla, I.; Calls-Cobos, A.; Udina, E. Minocycline Does Not Reduce the Regenerative Capacity of Peripheral Motor and Sensory Neurons after a Conditioning Injury in Mice. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2018, 301, 1638–1645. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas Broome, S.; Musumeci, G.; Castorina, A. Doxycycline and Minocycline Act as Positive Allosteric Modulators of the PAC1 Receptor and Induce Plasminogen Activators in RT4 Schwann Cells. Appl. Sci. 2021, 11, 7673. https://doi.org/10.3390/app11167673
Thomas Broome S, Musumeci G, Castorina A. Doxycycline and Minocycline Act as Positive Allosteric Modulators of the PAC1 Receptor and Induce Plasminogen Activators in RT4 Schwann Cells. Applied Sciences. 2021; 11(16):7673. https://doi.org/10.3390/app11167673
Chicago/Turabian StyleThomas Broome, Sarah, Giuseppe Musumeci, and Alessandro Castorina. 2021. "Doxycycline and Minocycline Act as Positive Allosteric Modulators of the PAC1 Receptor and Induce Plasminogen Activators in RT4 Schwann Cells" Applied Sciences 11, no. 16: 7673. https://doi.org/10.3390/app11167673







