Abstract
(1) Background: What is the effect of 16% and 40% concentration bleaching agents on dental structures in healthy patients compared to predialysis patients? (2) Methods: Forty teeth were included in the study (20 from healthy patients and 20 from predialysis patients). Each group was randomly divided into another two subgroups (n-10), depending on the bleaching agent concentration (16% and 40% gels). Color parameters were registered before and after the whitening process using a spectrophotometer. To determine enamel ultramicroscopic modifications, SEM and AFM analysis were performed before and after bleaching. (3) Results: An increasing trend was identified in the average values of ∆E and ∆L within the groups of predialysis teeth between teeth whitened with 40% concentration gel and those whitened with 16% concentration gel, while for the enamel samples from healthy patients the trend was reversed. The average values for roughness in the case of the two bleaching agents in healthy and predialysis teeth presented statistically significant differences (p < 0.05). (4) Conclusions: The effects of bleaching agents are less significant on teeth from predialysis compared to healthy patients. A direct link exists in terms of the clinical effect between the concentration of the whitening gel and color modifications.
1. Introduction
The use of different whitening techniques has attracted the interest of dental professionals, since these are relatively non-invasive and easy to perform. Contemporary bleaching systems are mainly based on hydrogen peroxide or one of its precursors, carbamide peroxide. These are often used in combination with an activating agent such as light or heat. Whitening agents can be applied to the external surface of the teeth (vital whitening) or inside the pulp chamber (non-vital whitening). Both techniques aim to whiten the chromogens inside the dental tissue, thus changing the tooth color [1].
There are several possible methods for monitoring color changes induced by teeth whitening treatments:
- -
- Clinical studies, by assessing the color parameters before and after applying a protocol to the patient;
- -
- Using extracted teeth; the advantage of this technique is that the results are not influenced by the patient’s eating habits, when multiple treatments are performed; the disadvantage is the impossibility of integral simulation of the oral environment;
- -
- Using processed enamel samples; the advantage is that it is possible to work on flat surfaces and use higher precision spectrophotometers.
The current literature states that most teeth can be whitened, but the treatment period required to achieve the desired result may vary [2].
Most of the data from color research studies in dentistry are based on the CIE L∗a∗b∗ system (CIE: Commission Internationale de l’Eclairage; International Commission on Illumination) and are reported by the corresponding symbols: L∗ (brightness), a∗ (green-red color coordinate), b∗ (blue-yellow color coordinate), C∗ (hue), h (saturation) and ΔEab (overall color difference).
For patients with chronic renal failure, predialysis, the appearance of the teeth is as important as for healthy patients. Oral manifestations of chronic kidney disease are represented by delays in the eruption of permanent teeth, enamel hypoplasia of temporary and permanent teeth with or without staining, narrowing or calcification of the pulp chamber of permanent teeth, and high or low prevalence of tooth decay [3].
Currently, there is only one study in the literature that investigated the effect of teeth whitening on hemodialyzed patients with chronic renal failure [4]. The researchers concluded that the negative effects of whitening gel with 16% carbamide peroxide on the dental substrate of uremic patients was less harmful and destructive compared to the effects on the teeth of healthy patients.
Based on these results obtained by Mahmoud et al. [4], the current study aims to investigate the effects of whitening treatment on the dental tissues of patients with chronic kidney disease, but in the stage of predialysis.
The null hypothesis tested is that the color parameters and microstructural changes of predialysis dental tissues should not differ from the corresponding values of healthy enamel.
This study was designed to compare the effect of teeth whitening on predialysis and healthy patients using two bleaching gels, with carbamide peroxide 16% and hydrogen peroxide 40%, simulating, respectively, at-home and in-office whitening. The assessment was carried out by spectrophotometry and by atomic force microscopy (AFM), and the observations were correlated with scanning electron microscopy (SEM) investigations.
2. Materials and Methods
2.1. Preparation of the Samples
Forty teeth were included in the current study, maxillary and mandibular teeth, extracted due to orthodontic or periodontal reasons, from patients aged between 18 and 30 years old [4,5]. Half of the teeth were collected from patients with chronic renal failure, predialysis, and half from healthy patients. Only caries-free teeth were included in the group, without coronary destruction and without pre-existing restorations. In order to meet the necessary hydration conditions after extraction, these teeth were preserved in distilled water after professional cleaning [6].
For better handling, the apical two-thirds of the teeth were embedded in self-curing acrylic resin (Duracryl Plus, Spofa Dental, Czech Republic, shade 0). After embedding, the crown and the cervical third of the root were impressed in a dental high consistency silicone impression material in order to obtain the casts necessary for manufacturing polyethylene caps. The latter were made by thermoforming, with the aim of achieving an accurate adaptation on the coronary surface. The caps had a double role: to allow registration of the color parameters by using the spectrophotometer before and after the whitening treatment, as well as to allow a more precise adaptation of the tip of the spectrophotometer on the dental surface. So, for each tooth, on the buccal and oral surface of the cap, perforations with a diameter equivalent to the diameter of the measuring tip of the spectrophotometer were made.
The two samples were each divided into two subsamples according to a whitening protocol: one for at-home whitening and one for in-office whitening, as follows:
Sample 1.1—10 extracted teeth from predialysis patients, whitened using Opalescence PF 16%;
Sample 1.2—10 extracted teeth from predialysis patients, whitened using Opalescence Boost 40%;
Sample 2.1—10 extracted teeth from healthy patients, whitened using Opalescence PF 16%;
Sample 2.2—10 extracted teeth from healthy patients, whitened using Opalescence Boost 40%.
2.2. Teeth Whitening Treatment
The whitening protocol aimed to simulate at-home and in-office whitening treatments. Two commercial materials were used for this: one based on carbamide peroxide, Opalescence® PF 16% (Ultradent Products, USA)—used for at-home treatment—and one based on hydrogen peroxide, Opalescence® Boost PF 40% (Ultradent Products, USA)—used for in-office treatment. The protocol lasted for 14 days, with a continuous application of whitening material for 5 h per day for Opalescence® PF 16% and 2 sessions (3 applications of 20 min each, initially, and the same one week later) for Opalescence® Boost PF 40%, on the buccal surfaces of the extracted teeth. After each step, the teeth were washed under warm water for 60 s and then kept in artificial saliva. The artificial saliva used in this study contained 1.5 mmol/L Ca, 0.9 mmol/L P, 150 mmol/L KCl, 0.1 mol/L Tris buffer, and pH 7.0 [7].
2.3. Spectrophotometric Assessment
To measure the color parameters, the Vita Easyshade spectrophotometer (VITA Zahnfabrik, Bad Säckingen, Germany) was used. This consists of three main elements: a light source, a guide that directs the light from the source to an object and receives the light reflected by the object, and a spectrometer that measures the intensity of the received light according to its wavelength. A major advantage of this device is its ability to determine tooth color regardless of lighting conditions, thanks to its own light source.
The measurements were performed before and after the whitening protocol and used to determine the color parameters. They were performed on the buccal and oral surfaces of each tooth and for each area four measurements were made. The tip of the spectrophotometer was applied perpendicularly and mesio-distally centered, 1 mm above the enamel–cement junction.
The mean of the measurements was used to calculate the color difference according to the formula CIELab [8]:
where: L1* (Brightness), a1* (chromatic parameter in the red-green axis), b1* (chromatic parameter in the yellow-blue axis) represent the chromatic parameters on the buccal surface before whitening protocol and L2*, a2*, b2* represent the chromatic parameters on the buccal surface after the whitening protocol. The mean values of brightness differences (ΔL*), color parameters (Δa*, Δb*) and color differences (ΔE*) for each sample were statistically analyzed.
2.4. Atomic Force Microscopy (AFM) Analysis
The AFM was performed on a Ntegra Spectra commercial microscope (NT-MDT, Moscow, Russia), at room temperature, in intermittent contact (semicontact), with a rectangular silicon cantilever, nominal elasticity constant k = 40 N/m, resonant frequency—240–440 kHz, peak radius < 10 nm. After acquisition, the images were processed using Nova v1.1.0.1837 (NT-MDT, Moscow, Russia) software.
For the roughness analysis, the statistical parameters calculated for the image were used, on which a “fit lines” correction was applied, through which a second-degree polynomial was found and extracted for each scan line. As the image processing history is important for the validity of the roughness comparison of two or more surfaces—the leveling procedure applied to each image, directly affecting the roughness values, as well as the image size for which they are calculated—all AFM data were processed identically.
2.5. Scanning Electron Microscopy (SEM) Analysis
An INSPECT S microscope (FEI Company, Hillsboro, OR, USA) was used to observe the morphology of dental surfaces before and after the whitening treatment. The samples were examined using a gold-rich substrate—QUANTA 133 (FEI Company, Hillsboro, OR, USA). For each tooth, the investigations were performed on two surfaces: the buccal surface treated with the whitening gel and the oral surface, considered as a control. For each surface, different magnifications were used for image capture.
2.6. Statistical Analysis
Prior to any statistical assessment, the adequate number of samples to be included in the study was calculated by performing Power analysis, using G3*Power calculation software version 3.1.9.6 (Erdfelder, Faul, and Buchner, 1996) [4,9]. It was observed, from the results of similar studies, that the expected mean value of ∆E would be about 5 and ∆L about 3, with corresponding standard deviations of about 0.1 and 0.3, respectively [4,10]. By using these data, we had an estimated effect size that we used to calculate the minimum number of samples necessary for analysis of variance. All data were collected and statistically analyzed using PASW Statistics 18 (SBAS, Hong Kong). ANOVA and t test were used and p values < 0.05 were considered statistically significant. The Scheffe PostHoc was used to identify the pairs between which the differences of the mean values of roughness are statistically significant.
3. Results
An increasing trend was identified in the average values of ∆E and ∆L within the groups of predialysis teeth between the samples whitened with 40% concentration gel and those whitened with 16% concentration gel. Regarding the groups of teeth from healthy patients, the trend is reversed (Table 1).

Table 1.
Mean values, variance and statistical significance in ∆E and ∆L values between groups.
The statistical analysis showed no significant clinical chromatic modification before and after whitening in the case of the teeth from predialysis patients bleached with 40% gel (ΔE < 3), while in the teeth from healthy patients and bleached with 40% gel, perceptible color changes were identified (ΔE > 3.5). When comparing the effects of different concentrations of bleaching gels, significant changes have been observed between the two materials (p < 0.05).
The highest variation in color change before and after bleaching was observed in teeth from healthy patients bleached with 40% gel, while the lowest difference was identified in teeth from predialysis patients bleached with the same material.
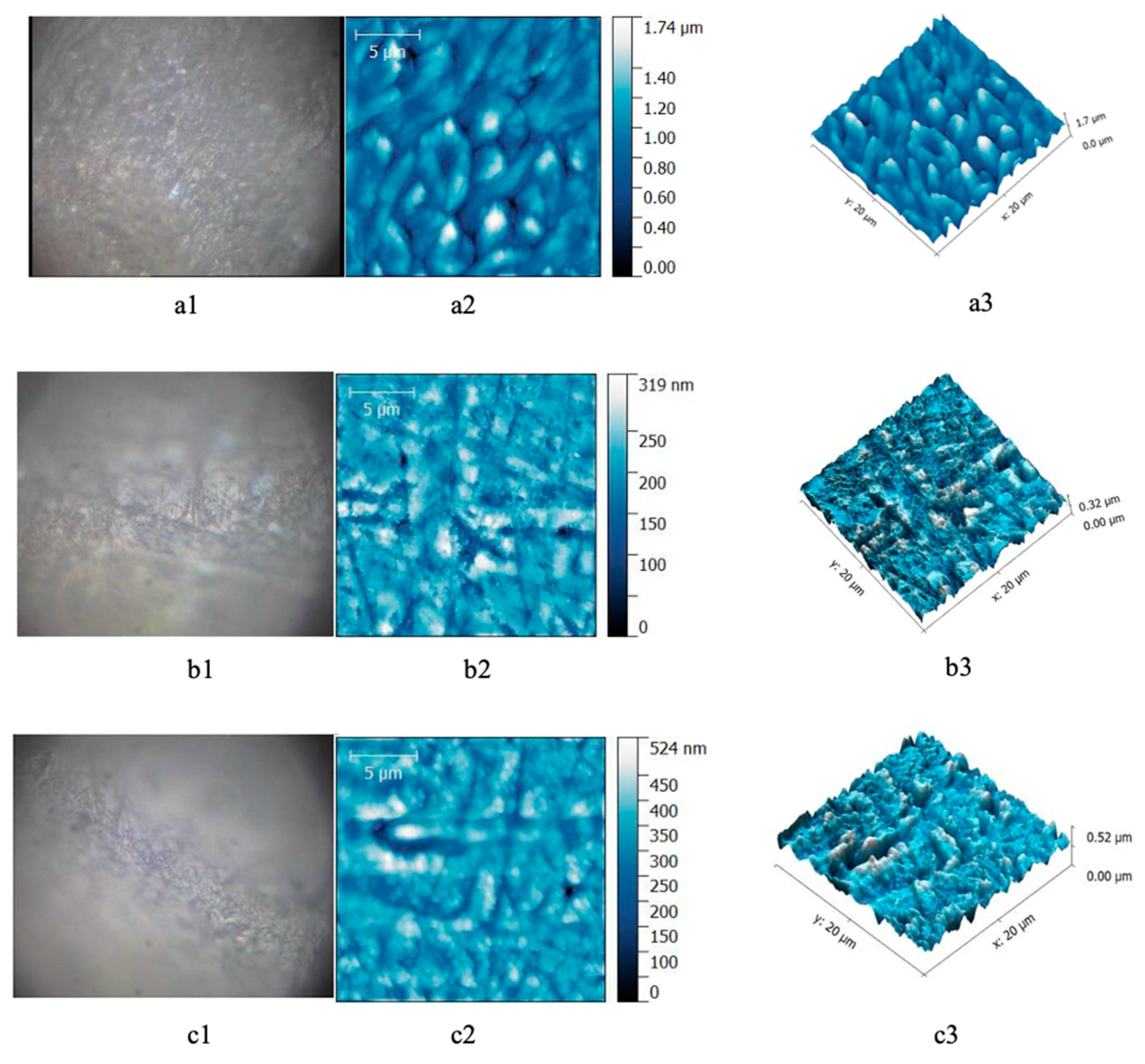
AFM investigation allowed the analysis of surface modifications before and after bleaching in every group. The images obtained from healthy teeth before bleaching showed the enamel surface with homogenous aspect. After bleaching with 40% gel, the enamel presented heterogenic surface with microporosity in depth (Figure 1).
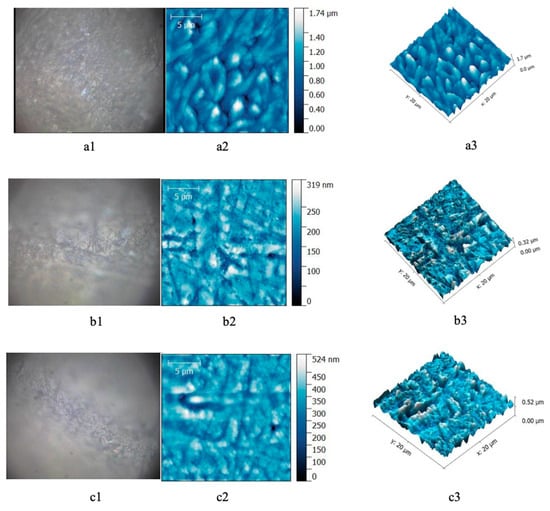
Figure 1.
Enamel samples form healthy patients. (a) Before bleaching; (b) after 16% gel bleaching; (c) after 40% gel bleaching. (a1,b1,c1) Optical image; (a2,b2,c2) AFM bidimensional image; (a3,b3,c3) AFM tridimensional image. Scanning parameters: scanned area—20 × 20 μm2, 556 × 556 pts, scan frequency—1 Hz, FB 1, scan speed—97.85 μm/s.
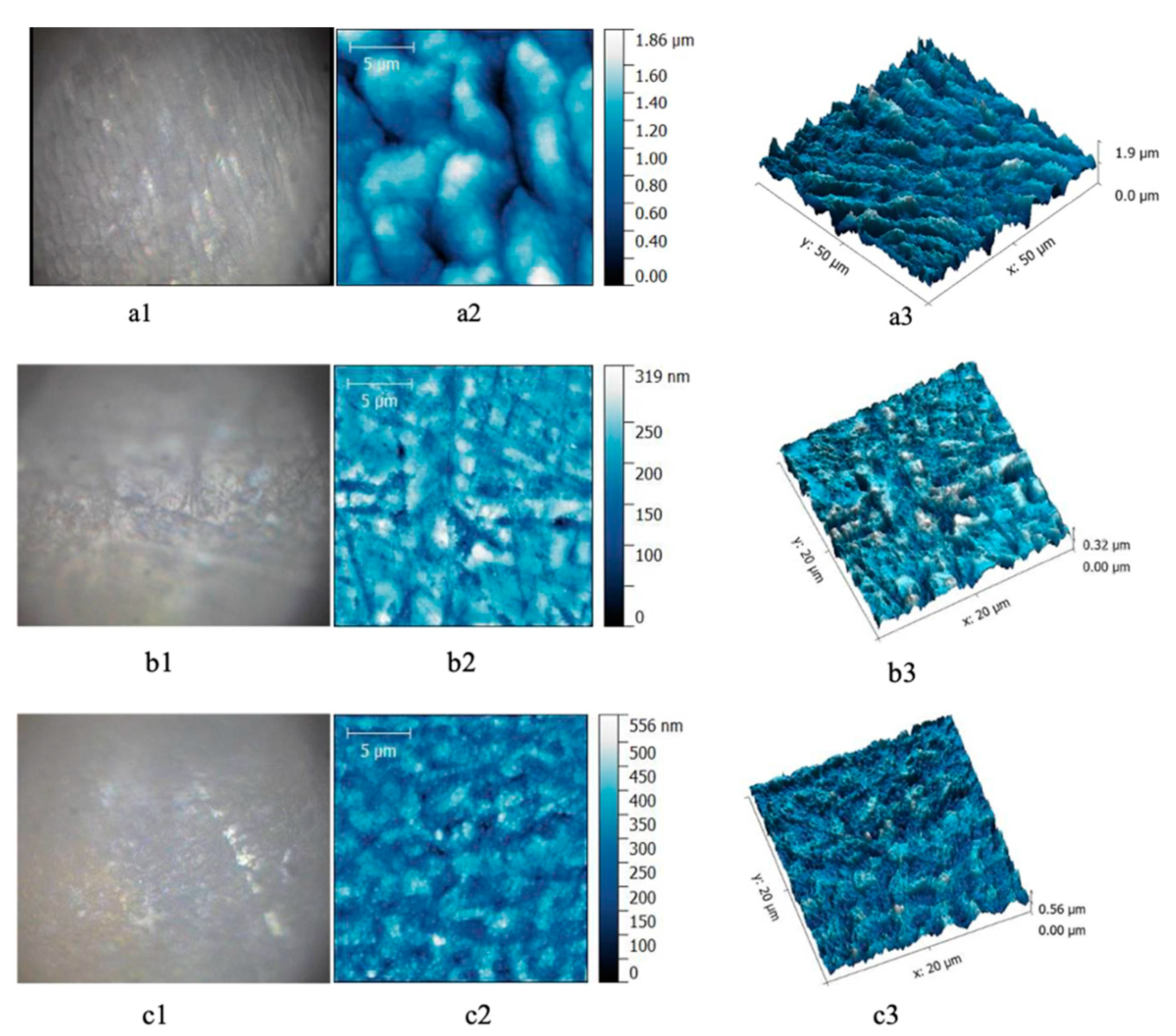
In the case of enamel samples of teeth from the predialysis patients, the micromorphology AFM images show small enamel rods, with light surface depressions, more superficial, and longitudinal channels between the enamel rods.
After 40% gel bleaching, in the enamel of teeth from the predialysis patients a fairly uniform structure was observed, without significant depressions (Figure 2).

Figure 2.
Enamel samples from predialysis patients. (a) Before bleaching; (b) after 16% gel bleaching; (c) after 40% gel bleaching. (a1,b1,c1) Optical image; (a2,b2,c2) AFM bidimensional image; (a3,b3,c3) AFM tridimensional image. Scanning parameters: scanned area for—20 × 20 μm2, 556 × 556 pts, scan frequency—1 Hz, FB 1, scan speed—97.85 μm/s.
The results recorded after the Ra (Sa) arithmetic mean roughness test, shown in Table 2, indicate values between 28 and 256 nm. The results of the ANOVA test for enamel roughness before and after bleaching reveal that between the average values of the two bleaching materials tested on healthy and predialysis teeth, there are statistically significant differences (p < 0.05). The Scheffe PostHoc test indicates the pairs between which the differences of the mean values are statistically significant.

Table 2.
Mean values and standard deviation for roughness.
Low roughness values were identified in the enamel from predialysis patients treated with Opalescence 40%, closely followed by the roughness in the enamel of healthy patients treated with the same gel. In this study, there were no statistically significant differences within the same test group, i.e., teeth from healthy patients and teeth from predialysis patients for Opalescence gel with a concentration of 16%. The ANOVA results indicate higher tooth enamel roughness in healthy patients before and after whitening treatment, but the enamel substrate in either predialysis or healthy patients reveals a significant effect on the interaction of the enamel with the whitening gel (p = 0.000).
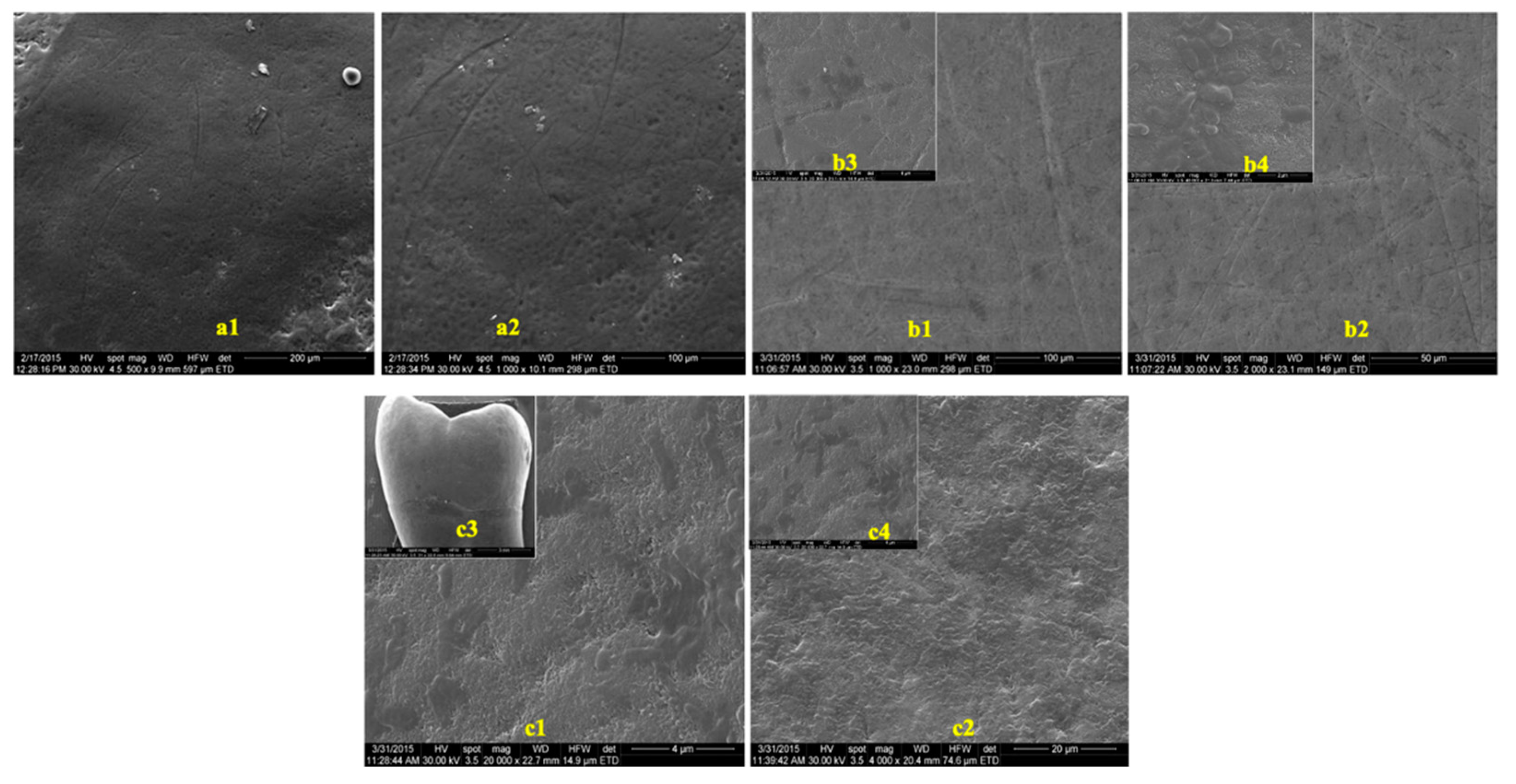
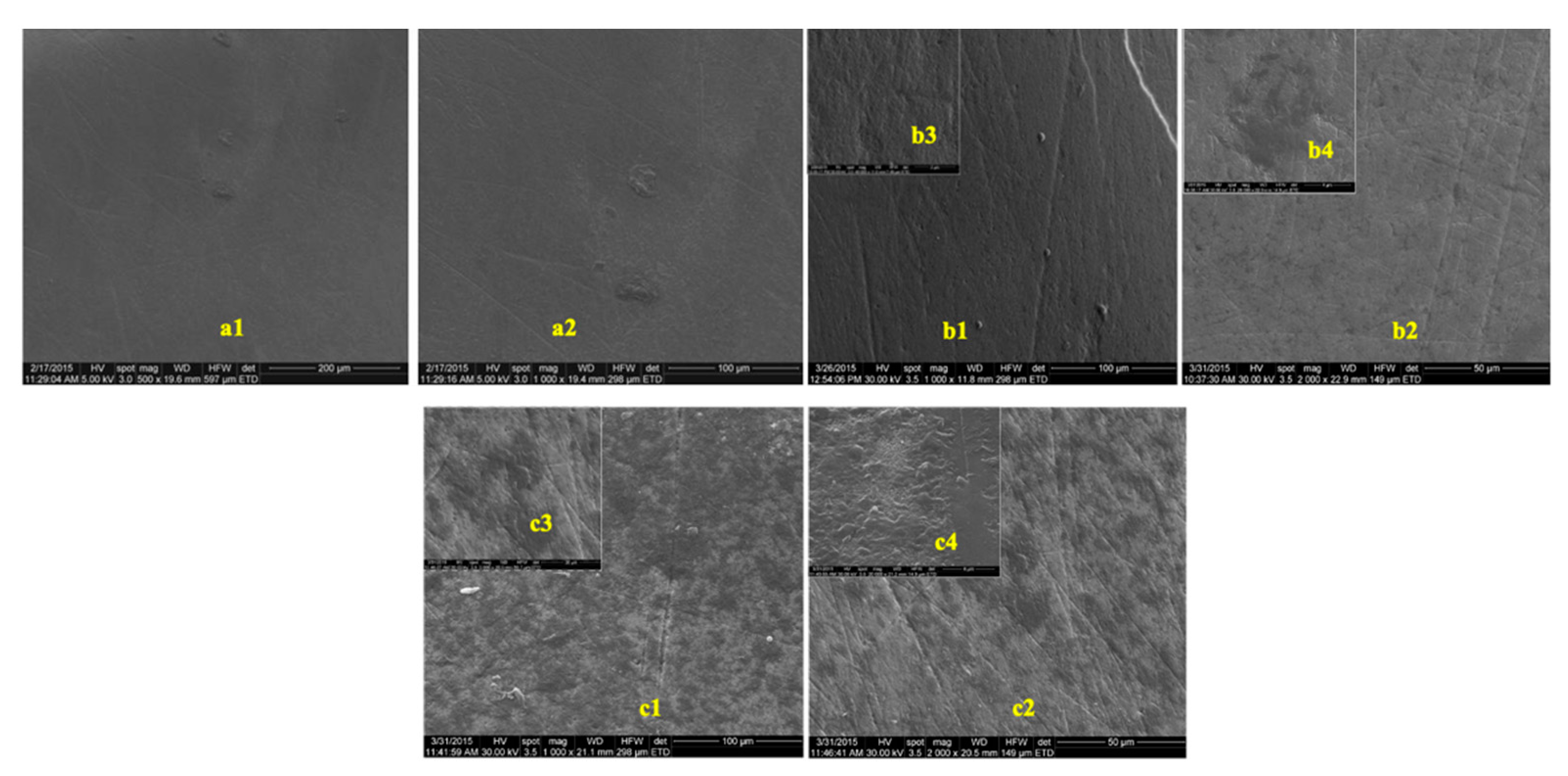
The images obtained to evaluate the possible effects of tooth whitening on tooth enamel by SEM, on two groups of teeth, extracted from predialysis patients and from healthy patients, are presented in Figure 3 and Figure 4. The images were recorded before and after the whitening treatment with the two commercial whitening gels at several scanning magnifications.
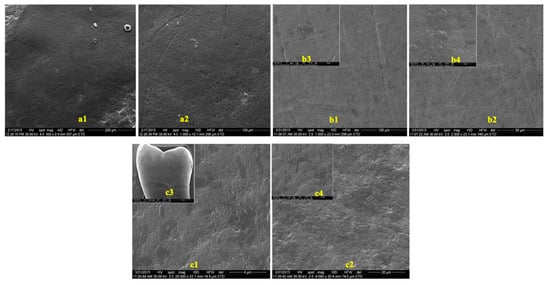
Figure 3.
SEM images of enamel samples from predialysis patients: (a) before bleaching; (b) after 16% whitening; (c) after 40% whitening. (a1) Magnification—500×; (a2) magnification—1000×; (b1,c1) magnification—1000×; (b2,c2) magnification—2000×; (b3) magnification—40,000×; (b4,c4) magnification—20,000×; c3: magnification—5000×.

Figure 4.
SEM images of enamel samples from healthy patients: (a) before bleaching; (b) after 16% whitening; (c) after 40% whitening. (a1) Magnification—500×; (a2) magnification—1000×; (b1) magnification—1000×; (b2,c2) magnification—2000×; (b3,c1,c4) magnification—20,000×; (b4,c4) magnification—40,000×; (c3) magnification—30×.
In the presented images, areas characterized by deep channels, generating a much more variable appearance of the enamel surface, suggestive of an increase in enamel porosity, can be observed (Figure 4(c1),(c2)). No difference was observed between the samples from healthy or predialysis patients, treated with whitening gel of 16% concentration. In the dental enamel samples from the predialysis patients after bleaching with 40% gel (Figure 4(c4)), important areas of dissolution were observed that involved either the center or the periphery of the enamel prisms, generating characteristic engraving patterns.
4. Discussion
The effect of whitening agents on the enamel surface is a controversial issue and the results of experiments are influenced by a multitude of variables. The use of in vitro studies allows valuable information to be obtained regarding the safety of whitening products, their effects on dental tissues, the differences between the concentrations used or between different action times.
In this study, a cyclic model was used, with periods of teeth whitening followed by periods of remineralization in artificial saliva, in order to simulate the physiological conditions present during teeth whitening. The quantities of whitening gels chosen were sufficient to completely cover the tooth surface subject to whitening. Additionally, to simulate human saliva, the artificial saliva in which the teeth were kept contained electrolytes of sodium, potassium, calcium and magnesium.
Spectrophotometers are extremely sensitive devices that can detect even minimal color changes that are not clinically detectable. For this reason, spectrophotometric analysis was used in the current study to determine and assess tooth color change, using the CIE parameters L*a*b* and ΔE, as well as the closest shades.
A precise time interval for clinically noticeable color change differences between bleached and unbleached enamel surfaces has not been determined yet. The time required for the discoloration to be clinically observed depends on many factors, including the layer thickness, the nature of the gels and their concentration, and the quality and quantity of the seal [11,12,13]. The differences between the results of previous and current studies could be attributed to the methodologies used. In the present study, the effect of differentiated discoloration between enamel surfaces from different patients treated with whitening gels of different concentrations was determined by in vitro means using the most standardized protocols and using a spectrophotometer that allows a detailed, pointed and synchronized analysis of two identical images made at different spatial moments, while previous studies in the literature use spectrophotometers that do not allow this synchronization. Considering the results of the present study, ∆E variations are higher than the general average variations of ∆L in the case of the Opalescence gel with a concentration of 40%, but smaller for Opalescence 16%. Using a combination of in-office and at-home whitening techniques leads to the most effective result in the shortest time. Teeth whitening after in-office whitening treatments can be further maintained and enhanced by using at-home whitening treatments.
The AFM analysis after the cyclical period of 14 days of whitening treatment with two commercial products on the healthy patients’ teeth demonstrated the presence of some microporosities on the enamel surface. These observations are consistent with those presented by Hegedüs et al. [14], who concluded that whitening products are capable of altering the surface of the enamel. The hypothesis that peroxide bleaching agents affect the organic component of the enamel is mentioned. Additionally, peroxides can affect not only the surface but also the internal structure of the enamel, because they have a low molecular weight that allows them to penetrate this dental layer [15,16].
On the other hand, analyzing the AFM images of tooth enamel extracted from patients with renal insufficiency, predialysis, a lower roughness was noticed, which indicates a resistance to demineralization. An explanation can be given in terms of changes in calcium and phosphorus metabolism caused by chronic kidney disease. Phosphate retention can cause an increase in its level in saliva and thus, along with the composition of saliva (often modified) and its alkaline pH, tooth enamel is more resistant to acid demineralization as well, as has previously been demonstrated [17]. In this way, it is normal for the changes found in the enamel after whitening to be less obvious compared to those present in the tooth enamel of healthy patients.
From the literature, it can be noticed that bleaching treatments induce changes in surface roughness and, therefore, influence the formation and retention of sub- and supragingival bacterial plaque. Therefore, the adhesion of Streptococcus mutans to the enamel is increased [18]. The same is true of Streptococcus sobrinus, but, surprisingly, not of Actinomyces viscosus [19]. Such an undesirable effect may have some implications for the development of a future carious lesions. However, there are currently no clinical reports available on the possible cariogenicity of whitening treatments.
Therefore, the majority of bleaching agents induce mild to moderate changes in the enamel surface, with a decrease in its microhardness. However, these microlesions are less intense than those resulting from phosphoric acid etching, but may interfere with the adhesive properties of restorative materials. After a while, the porosity gradually decreases. Attempts to reduce mineral loss and microdefects on the enamel surface were performed with fluoride-containing products [20,21].
The results of the AFM analysis in the present study are similar to those reported by Mahmoud et al. [4] on the micromorphology and roughness of the dental enamel of hemodialysed patients when 16% carbamide peroxide was used for teeth bleaching. They concluded that the effects of whitening are less harmful to the teeth of uremic patients.
According to SEM image analysis, the enamel surfaces of the teeth extracted from healthy patients show changes in morphology. The results are consistent with those obtained by Shannon et al. [22]. They evaluated the effects of three whitening agents with a concentration of 10% carbamide peroxide on the morphology of tooth enamel, also using SEM analysis. Based on their observations, they concluded that there are significant changes to the surface of the enamel after teeth whitening.
Previous studies indicated that bleaching products with concentrations of hydrogen peroxide of 30–35% cause superficial alterations and reduce the calcium–phosphorus ratio [23,24]. As hydrogen peroxide with a concentration of 40% is a strong oxidizing agent, it is only indicated for professional use in the dental office.
Carbamide peroxide is the most common product used for teeth whitening. When in contact with the outer surface of the enamel, it decomposes into water and oxygen which diffuses through the organic component of the enamel. This process causes the oxidation of organic pigments, located mostly in the dentin, resulting in a reduction in or even disappearance of dental staining [4].
Duschner et al. [25] studied the effects of whitening on healthy teeth and restorative treatments (dental amalgam, gold, ceramic, glass ionomer cement and composites), focusing on surface, hardness and morphology. Their results confirmed that, regardless of the whitening gel used, with hydrogen peroxide or carbamide peroxide, these systems do not produce changes in the surface morphology or microresistance of the restorative dental materials. These results support the clinical safety of commercial bleaching systems in the oral environment.
In addition, there are no clinical studies or case reports in the literature, macroscopic or clinical documents on visible damage due to the destruction of vital tissue after bleaching. Investigative studies on external whitening therapies are often applied and, usually, microresistance of structural defects of the enamel is tested. However, there are inconsistencies in the results of these studies. This could be due to the protocol differences of the studies, such as the use of dental substrate (human or bovine), microhardness testing (Knoop vs. Vickers hardness), storage conditions between whitening intervals (no remineralizing solution or artificial or human saliva), fluoridation measures (applied or unapplied) and the question of whether the studies were performed in vitro or in vivo.
In addition to the limitations offered by in vitro studies and the application of their results to clinical activity, the present study nevertheless presents some limitations that refer to the method of calculating color changes. In the current study, ∆E was calculated based on the CIELab formula, while the CIEDE2000 color difference formula can also be used. Further studies should also be performed to investigate long term dental tissue modifications and color stability. In addition to microscopic changes, from a clinical point of view, several side effects of dental bleaching have been reported such as rebound of stains, soft tissue irritation and, one of the most commonly reported, tooth sensitivity. Several studies indicated that using remineralizing agents following bleaching procedures could repair the microscopic side effects [26,27].
As can be seen from all the literature studies, the present study of the possible effects of whitening treatments on tooth enamel in patients with renal insufficiency, predialysis, compared to tooth enamel in healthy patients, studied using three different methods, is original in its approach and in the interpretation of the results.
5. Conclusions
The effects of bleaching agents on teeth from predialysis are less significant compared to healthy patients. A direct link exists in terms of the clinical effect of the concentration of the whitening gel and color modifications.
Author Contributions
Conceptualization, G.F.G., M.M., A.M.C. and B.D.; methodology, D.P. and O.E.A.; software, I.S.; validation, D.P., R.M.C. and L.G.; formal analysis, M.M.; investigation, R.M.C., D.P. and G.F.G.; resources, M.M. and B.D.; data curation, O.E.A.; writing—original draft preparation, G.F.G., A.M.C., R.M.C. and L.G.; writing—review and editing, M.M., A.M.C. and G.F.G.; visualization, R.M.C.; supervision, I.S.; project administration, G.F.G.; funding acquisition, G.F.G. and A.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sulieman, M.; Addy, M.; Macdonald, E.; Rees, J.S. A safety study in vitro for the effects of an in-office bleaching system on the integrity of enamel and dentine. J. Dent. 2004, 32, 581–590. [Google Scholar] [CrossRef]
- Dahl, J.E.; Pallesen, U. Tooth bleaching- A critical review of the biological aspects. Crit. Rev. Biol. Med. 2003, 14, 292–304. [Google Scholar] [CrossRef]
- Proctor, R.; Kumar, N.; Stein, A.; Moles, D.; Porter, S. Oral and dental aspects of chronic renal failure. J. Dent. Res. 2005, 84, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.H.; Elembaby, A.E.S.; Zaher, A.R.; Grawish, M.E.A.; Elsabaa, H.M.; El-Negoly, S.A.E.R.; Sobh, M.A.K. Effect of 16% carbamide peroxide bleaching gel on enamel and dentin surface micromorphology and roughness of uremic patients: An atomic force microscopic study. Eur. J. Dent. 2010, 4, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orilisi, G.; Tosco, V.; Monterubbianesi, R.; Notarstefano, V.; Özcan, M.; Putignano, A.; Orsini, G. ATR-FTIR, EDS and SEM evaluations of enamel structure after treatment with hydrogen peroxide bleaching agents loaded with nano-hydroxyapatite particles. PeerJ 2021, 29, e10606. [Google Scholar] [CrossRef] [PubMed]
- Chisnoiu, A.M.; Moldovan, M.; Sarosi, C.; Chisnoiu, R.M.; Rotaru, D.I.; Delean, A.G.; Pastrav, O.; Muntean, A.; Petean, I.; Tudoran, L.B.; et al. Marginal Adaptation Assessment for Two Composite Layering Techniques Using Dye Penetration, AFM, SEM and FTIR: An In-Vitro Comparative Study. Appl. Sci. 2021, 11, 5657. [Google Scholar] [CrossRef]
- Queiroz, C.S.; Hara, A.T.; Paes Leme, A.F.; Cury, J.A. pH cycling models to evaluate the effect of low fluoride dentifrice on enamel de- and remineralization. Braz. Dent. J. 2008, 19, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Domingues, G.H.D.; Oliveira, A.L.B.M.; Corona, S.A.M.; Vitti, R.P.; Scatolin, R.S. In vitro study on the color change of tooth enamel bleached with violet LED. J. Clin. Dent. Res. 2020, 17, 54–65. [Google Scholar] [CrossRef]
- Chisnoiu, R.M.; Moldovan, M.; Prodan, D.; Chisnoiu, A.M.; Hrab, D.; Delean, A.G.; Muntean, A.; Rotaru, D.I.; Pastrav, O.; Pastrav, M. In-Vitro Comparative Adhesion Evaluation of Bioceramic and Dual-Cure Resin Endodontic Sealers Using SEM, AFM, Push-Out and FTIR. Appl. Sci. 2021, 11, 4454. [Google Scholar] [CrossRef]
- Daley, T.D.; Wysocki, G.P.; Ulan, R.A. Dental changes in patients on chronic hemodialysis. Can. Dent. Res. 1982, 1, 17–21. [Google Scholar]
- Pini, N.I.P.; Piccelli, M.R.; Vieira-Junior, W.F.; Ferraz, L.N.; Aguiar, F.H.B.; Lima, D.A.N.L. In-office tooth bleaching with chitosan-enriched hydrogen peroxide gels: In vitro results. Clin. Oral. Investig. 2021, 6, 12. [Google Scholar]
- Vieira, I.; Vieira-Junior, W.F.; Pauli, M.C.; Theobaldo, J.D.; Aguiar, F.H.; Lima, D.A.; Leonardi, G.R. Effect of in-office bleaching gels with calcium or fluoride on color, roughness, and enamel microhardness. J. Clin. Exp. Dent. 2020, 12, e116–e122. [Google Scholar] [CrossRef]
- Kury, M.; Perches, C.; da Silva, D.P.; André, C.B.; Tabchoury, C.P.M.; Giannini, M.; Cavalli, V. Color change, diffusion of hydrogen peroxide, and enamel morphology after in-office bleaching with violet light or nonthermal atmospheric plasma: An in vitro study. J. Esthet. Restor. Dent. 2020, 32, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Hegedüs, C.; Bistey, T.; Flora-Nagy, E.; Keszthelyi, G.; Jenei, A. An atomic force microscopy study on the effect of bleaching agents on enamel surface. J. Dent. 1999, 27, 509–515. [Google Scholar] [CrossRef]
- Fernandes, B.M.; Tanaka, M.H.; De Oliveira, A.L.B.M.; Scatolin, R.S. Color stability of dental enamel bleached with violet LED associated with or without Low concentration peroxide gels. Photodiagnosis Photodyn Ther. 2021, 33, 102101. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Zanatta, R.F.; Silva, T.J.; Borges, A.B. Effect of calcium and fluoride addition to hydrogen peroxide bleaching gel on tooth diffusion, color, and microhardness. Oper. Dent. 2019, 44, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.H.; Ahmed, M.E.; Mahmoud, K.M.; Grawish, M.A.; Zaher, A.R. Effects of phosphoric acid concentration and etching duration on enamel and dentin tissues of uremic patients receiving hemodialysis: An AFM study. J. Adhes. Dent. 2012, 14, 215–221. [Google Scholar] [PubMed]
- Hosoya, N.; Honda, K.; Iino, F.; Arai, T. Changes in enamel surface roughness and adhesion of Streptococcus mutans to enamel after vital bleaching. J. Dent. 2003, 31, 543–548. [Google Scholar] [CrossRef]
- Goldberg, M.; Grootveld, M.; Lynch, E. Undesirable and adverse effects of tooth-whitening products: A review. Clin. Oral Investig. 2010, 14, 1–10. [Google Scholar] [CrossRef]
- Aushill, T.M.; Hellwig, E.; Schmidale, S.; Sculean, A.; Arweiler, N.B. Efficacity, side effects and patients’ acceptance of different bleaching techniques (OTC, in-office, at-home). Oper. Dent. 2005, 30, 156–163. [Google Scholar]
- Haywood, V.B.; Houck, V.M.; Heymann, H.O. Nightguard vital bleaching: Effects of various solutions on enamel surface texture and color. Quintessence Int. 1991, 22, 775–782. [Google Scholar]
- Shannon, H.; Spencer, P.; Gross, K.; Tira, D. Characterization of enamel exposed to 10% carbamide peroxide bleaching agents. Quintessence Int. 1993, 24, 39–44. [Google Scholar]
- Rotstein, I.; Danker, E.; Goldman, A.; Heling, I.; Stabholz, A.; Zalkind, M. Histochemical analysis of dental hard tissues following bleaching. J. Endod. 1996, 22, 23–26. [Google Scholar] [CrossRef]
- McGuckin, R.S.; Babin, J.F.; Meyer, B.J. Alterations in human enamel surface morphology following vital bleaching. J. Prosthet. Dent. 1992, 68, 754–760. [Google Scholar] [CrossRef]
- Duschner, H.; Götz, H.; White, D.J.; Kozak, K.M.; Zoladz, J.R. Effects of hydrogen peroxide bleaching strip gels on dental restorative materials in vitro: Surface microhardness and surface morphology. J. Clin. Dent. 2004, 15, 105–111. [Google Scholar] [PubMed]
- Scribante, A.; Poggio, C.; Gallo, S.; Riva, P.; Cuocci, A.; Carbone, M.; Arciola, C.R.; Colombo, M. In Vitro Re-Hardening of Bleached Enamel Using Mineralizing Pastes: Toward Preventing Bacterial Colonization. Materials 2020, 13, 818. [Google Scholar] [CrossRef] [Green Version]
- Tschoppe, P.; Zandim, D.L.; Sampaio, J.E.; Kielbassa, A.M. Saliva substitute in combination with high-concentrated fluoride toothpaste. Effects on demineralised dentin in vitro. J. Dent. 2010, 38, 207–213. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).