Production of Arthrospira (Spirulina) platensis Enriched in β-Glucans through Phosphorus Limitation
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Cultivation Conditions
2.2. Analytical Methods
2.2.1. Biomass Biochemical Composition
2.2.2. Glucans Determination
2.2.3. High Pressure Liquid Chromatography for Monosaccharides Profile Determination
2.2.4. Total Dietary Fibers
2.2.5. Fourier-Transform Infrared Spectroscopy (FTIR) Spectra
3. Results and Discussion
3.1. Biomass Production and Proximate Biochemical Composition
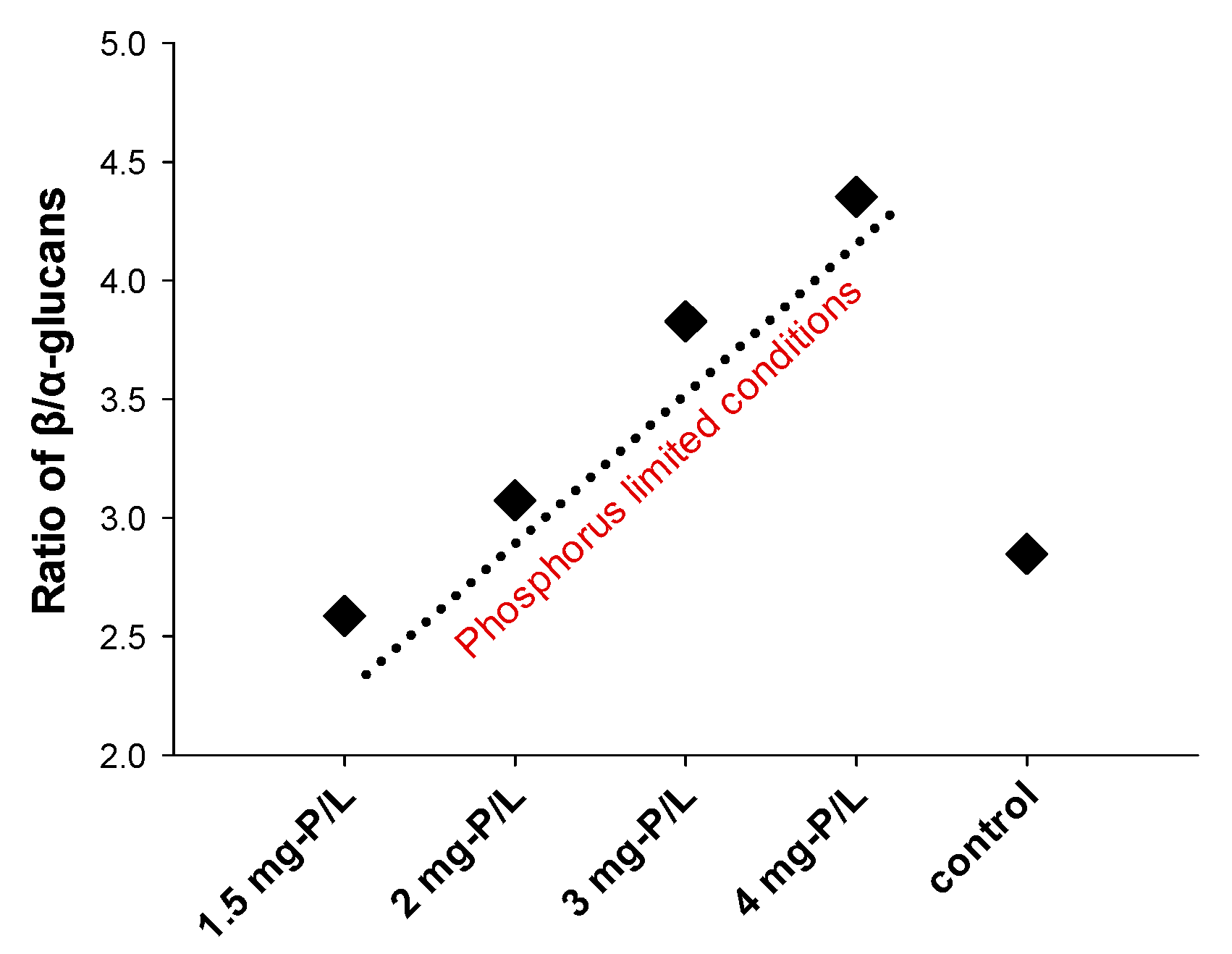
3.2. Content of Glucans
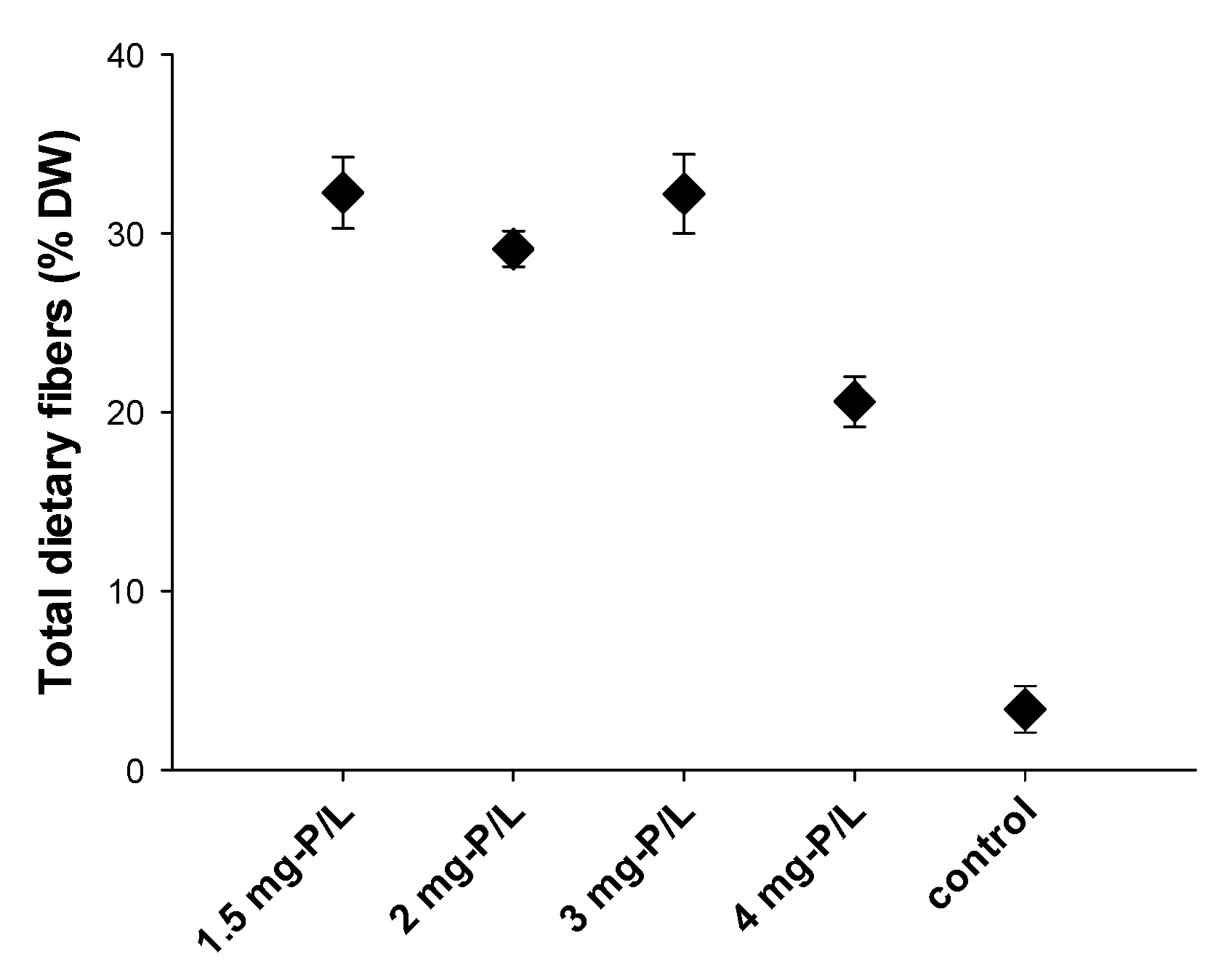
3.3. Total Dietary Fiber
3.4. Monosaccharide Profile of A. platensis
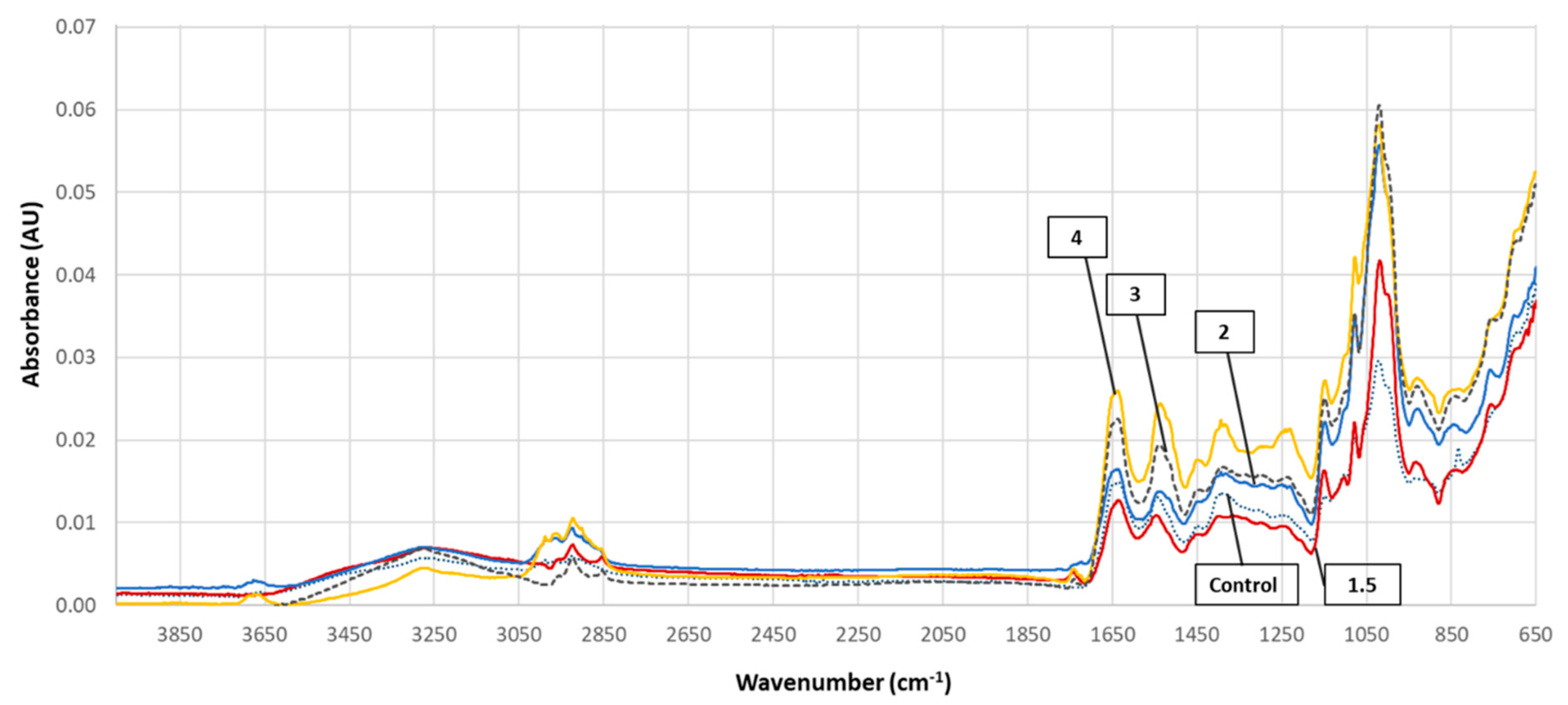
3.5. FTIR Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ghosh, S.; Dubinsky, Z.; Verdelho, V.; Iluz, D. Unconventional high-value products from microalgae: A review. Bioresour. Technol. 2021, 329, 124895. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Goswami, S. Microalgae–A green multi-product biorefinery for future industrial prospects. Biocatal. Agric. Biotechnol. 2020, 25, 101580. [Google Scholar] [CrossRef]
- Barkallah, M.; Ben Atitallah, A.; Hentati, F.; Dammak, M.; Hadrich, B.; Fendri, I.; Ayadi, M.A.; Michaud, P.; Abdelkafi, S. Effect of Spirulina platensis biomass with high polysaccharides content on quality attributes of common Carp (Cyprinus carpio) and Common Barbel (Barbus barbus) fish burgers. Appl. Sci. 2019, 9, 2197. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, P.Q.M.; Moraes, L.; Cardoso, L.G.; Druzian, J.I.; Morais, M.G.; Nunes, I.L.; Costa, J.A.V. Spirulina sp. LEB 18 cultivation in seawater and reduced nutrients: Bioprocess strategy for increasing carbohydrates in biomass. Bioresour. Technol. 2020, 316, 123883. [Google Scholar] [CrossRef]
- Tourang, M.; Baghdadi, M.; Torang, A.; Sarkhosh, S. Optimization of carbohydrate productivity of Spirulina microalgae as a potential feedstock for bioethanol production. Int. J. Environ. Sci. Technol. 2019, 16, 1303–1318. [Google Scholar] [CrossRef]
- Chentir, I.; Hamdi, M.; Doumandji, A.; HadjSadok, A.; Ouada, H.B.; Nasri, M.; Jridi, M. Enhancement of extracellular polymeric substances (EPS) production in Spirulina (Arthrospira sp.) by two-step cultivation process and partial characterization of their polysaccharidic moiety. Int. J. Biol. Macromol. 2017, 105, 1412–1420. [Google Scholar] [CrossRef]
- Ravindran, R.; Rajauria, G. Chapter 5-Carbohydrates derived from microalgae in the food industry. In Cultured Microalgae for the Food Industry; Lafarga, T., Acién, G., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 127–146. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. β-glucan metabolic and immunomodulatory properties and potential for clinical application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef]
- Bai, J.; Ren, Y.; Li, Y.; Fan, M.; Qian, H.; Wang, L.; Wu, G.; Zhang, H.; Qi, X.; Xu, M.; et al. Physiological functionalities and mechanisms of β-glucans. Trends Food Sci. Technol. 2019, 88, 57–66. [Google Scholar] [CrossRef]
- Rahar, S.; Swami, G.; Nagpal, N.; Nagpal, M.A.; Singh, G.S. Preparation, characterization, and biological properties of β-glucans. J. Adv. Pharm. Technol. Res. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Vogler, B.W.; Brannum, J.; Chung, J.W.; Seger, M.; Posewitz, M.C. Characterization of the Nannochloropsis gaditana storage carbohydrate: A 1,3-beta glucan with limited 1,6-branching. Algal Res. 2018, 36, 152–158. [Google Scholar] [CrossRef]
- Sadovskaya, I.; Souissi, A.; Souissi, S.; Grard, T.; Lencel, P.; Greene, C.M.; Duin, S.; Dmitrenok, P.S.; Chizhov, A.O.; Shashkov, A.S.; et al. Chemical structure and biological activity of a highly branched (1→3,1→6)-β-d-glucan from Isochrysis galbana. Carbohydr. Polym. 2014, 111, 139–148. [Google Scholar] [CrossRef]
- Schulze, C.; Wetzel, M.; Reinhardt, J.; Schmidt, M.; Felten, L.; Mundt, S. Screening of microalgae for primary metabolites including β-glucans and the influence of nitrate starvation and irradiance on β-glucan production. J. Appl. Phycol. 2016, 28, 2719–2725. [Google Scholar] [CrossRef]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina-From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Kurd, F.; Samavati, V. Water soluble polysaccharides from Spirulina platensis: Extraction and in vitro anti-cancer activity. Int. J. Biol. Macromol. 2015, 74, 498–506. [Google Scholar] [CrossRef]
- Wu, X.; Li, R.; Zhao, Y.; Liu, Y. Separation of polysaccharides from Spirulina platensis by HSCCC with ethanol-ammonium sulfate ATPS and their antioxidant activities. Carbohydr. Polym. 2017, 173, 465–472. [Google Scholar] [CrossRef]
- Lee, J.-B.; Srisomporn, P.; Hayashi, K.; Tanaka, T.; Sankawa, U.; Hayashi, T. Effects of structural modification of calcium spirulan, a sulfated polysaccharide from Spirulina platensis, on antiviral activity. Chem. Pharm. Bull. 2001, 49, 108–110. [Google Scholar] [CrossRef] [Green Version]
- Luo, A.; Feng, J.; Hu, B.; Lv, J.; Chen, C.Y.O.; Xie, S. Polysaccharides in Spirulina platensis improve antioxidant capacity of Chinese-style sausage. J. Food Sci. 2017, 82, 2591–2597. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, C.; Sun, Y.; Chen, W.; Tan, H.; Cao, X.; Xue, S.; Yin, H. Production and structural characterization of a new type of polysaccharide from nitrogen-limited Arthrospira platensis cultivated in outdoor industrial-scale open raceway ponds. Biotechnol. Biofuels 2019, 12, 131. [Google Scholar] [CrossRef]
- Lai, Y.H.; Puspanadan, S.; Lee, C.K. Nutritional optimization of Arthrospira platensis for starch and total carbohydrates production. Biotechnol. Prog. 2019, 35, e2798. [Google Scholar] [CrossRef]
- Chentir, I.; Doumandji, A.; Ammar, J.; Zili, F.; Jridi, M.; Markou, G.; Ouada, H.B. Induced change in Arthrospira sp.(Spirulina) intracellular and extracellular metabolites using multifactor stress combination approach. J. Appl. Phycol. 2018, 30, 1563–1574. [Google Scholar] [CrossRef]
- Markou, G. Alteration of the biomass composition of Arthrospira (Spirulina) platensis under various amounts of limited phosphorus. Bioresour. Technol. 2012, 116, 533–535. [Google Scholar] [CrossRef]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol. 2012, 96, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Xia, J.; Levert, J.M. Fractionation and characterization of polysaccharides from cyanobacterium Spirulina (Arthrospira) maxima in nitrogen-limited batch culture. J. Cent. South Univ. of Technol. 2002, 9, 81–86. [Google Scholar] [CrossRef]
- Sekharam, K.M.; Venkataraman, L.; Salimath, P. Structural studies of a glucan isolated from blue-green alga Spirulina platensis. Food Chem. 1989, 31, 85–91. [Google Scholar] [CrossRef]
- Kochert, G. Carbohydrate determination by phenol-sulfuric acid method. In Handbook of Phycological Methods. Physiological and Biochemical Methods; Hellebust, J.A., Craige, J.S., Eds.; Cambridge University Press: London, UK, 1978; pp. 95–97. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Izard, J.; Limberger, R.J. Rapid screening method for quantitation of bacterial cell lipids from whole cells. J. Microbiol. Methods 2003, 55, 411–418. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Ravishankar, G.A.; Ambati, R.R. Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef]
- Bertocchi, C.; Navarini, L.; Cesàro, A.; Anastasio, M. Polysaccharides from cyanobacteria. Carbohydr. Polym. 1990, 12, 127–153. [Google Scholar] [CrossRef]
- De Philippis, R.; Sili, C.; Vincenzini, M. Glycogen and poly-β-hydroxybutyrate synthesis in Spirulina maxima. Microbiology 1992, 138, 1623–1628. [Google Scholar] [CrossRef] [Green Version]
- Aikawa, S.; Izumi, Y.; Matsuda, F.; Hasunuma, T.; Chang, J.S.; Kondo, A. Synergistic enhancement of glycogen production in Arthrospira platensis by optimization of light intensity and nitrate supply. Bioresour. Technol. 2012, 108, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Kremmyda, A.; MacNaughtan, W.; Arapoglou, D.; Eliopoulos, C.; Metafa, M.; Harding, S.E.; Israilides, C. The detection, purity and structural properties of partially soluble mushroom and cereal β-D-glucans: A solid-state NMR study. Carbohydr. Polym. 2021, 266, 118103. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Anti-infectious and Anti-tumor Activities of β-glucans. Anticancer Res. 2020, 40, 3139–3145. [Google Scholar] [CrossRef]
- Food and Drug Administration; HHS. Food Labeling: Health Claims; Soluble Dietary Fiber from Certain Foods and Coronary Heart Disease. Federal Register 2003, 70, 44207–44209. [Google Scholar]
- Sharoba, A.M. Nutritional value of spirulina and its use in the preparation of some complementary baby food formulas. J. Food Dairy Sci. 2014, 5, 517–538. [Google Scholar] [CrossRef]
- Seghiri, R.; Kharbach, M.; Essamri, A. Functional composition, nutritional properties, and biological activities of Moroccan Spirulina microalga. J. Food Qual. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- FoodData Central Search Results. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170495/nutrients (accessed on 30 August 2021).
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Fuller, S.; Beck, E.; Salman, H.; Tapsell, L. New horizons for the study of dietary fiber and health: A review. Plant Foods Hum. Nutr. 2016, 71, 1–12. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Pistorius, A.M.; DeGrip, W.J.; Egorova-Zachernyuk, T.A. Monitoring of biomass composition from microbiological sources by means of FT-IR spectroscopy. Biotechnol. Bioeng. 2009, 103, 123–129. [Google Scholar] [CrossRef]
- Giordano, M.; Kansiz, M.; Heraud, P.; Beardall, J.; Wood, B.; McNaughton, D. Fourier transform infrared spectroscopy as a novel tool to investigate changes in intracellular macromolecular pools in the marine microalga Chaetoceros muellerii (Bacillariophyceae). J. Phycol. 2001, 37, 271–279. [Google Scholar] [CrossRef]
- Murdock, J.N.; Wetzel, D.L. FT-IR microspectroscopy enhances biological and ecological analysis of algae. Appl. Spectrosc. Rev. 2009, 44, 335–361. [Google Scholar] [CrossRef]
- Grace, C.E.E.; Lakshmi, P.K.; Meenakshi, S.; Vaidyanathan, S.; Srisudha, S.; Mary, M.B. Biomolecular transitions and lipid accumulation in green microalgae monitored by FTIR and Raman analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117382. [Google Scholar] [CrossRef]
- Nicolai, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Mayers, J.J.; Flynn, K.J.; Shields, R.J. Rapid determination of bulk microalgal biochemical composition by Fourier-Transform Infrared spectroscopy. Bioresour. Technol. 2013, 148, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bataller, B.G.; Capareda, S.C. A rapid and non-destructive method for quantifying biomolecules in Spirulina platensis via Fourier transform infrared–Attenuated total reflectance spectroscopy. Algal Res. 2018, 32, 341–352. [Google Scholar] [CrossRef]
- Stehfest, K.; Toepel, J.; Wilhelm, C. The application of micro-FTIR spectroscopy to analyze nutrient stress-related changes in biomass composition of phytoplankton algae. Plant Physiol. Biochem. 2005, 43, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yao, C.; Xue, S.; Yang, H. Application of Fourier transform infrared (FT-IR) spectroscopy in determination of microalgal compositions. Bioresour. Technol. 2014, 151, 347–354. [Google Scholar] [CrossRef]


| P-Concentration (mg-P/L) | Total Proteins (%DW) | Total Carbohydrates (%DW) | Total Lipids (%DW) |
|---|---|---|---|
| 1.5 | 22.45 ± 3.55 | 65.25 ± 3.88 | 5.56 ± 1.03 |
| 2 | 23.09 ± 1.57 | 65.01 ± 3.24 | 6.68 ± 1.16 |
| 3 | 25.88 ± 1.99 | 51.06 ± 3.24 | 7.98 ± 1.66 |
| 4 | 33.02 ± 2.91 | 33.42 ± 2.67 | 7.38 ± 1.98 |
| 89 (Control) | 59.03 ± 3.61 | 23.04 ± 1.95 | 8.58 ± 1.43 |
| P-Concentration (mg-P/L) | Total Glucans (α + β) (%DW) | 1.3:1.6-β-Glucans (%DW) | 1.3:1.4-β-Glucans (%DW) | α-Glucans (%DW) |
|---|---|---|---|---|
| 1.5 | 42.96 | 30.98 ± 1.56 | Not detectable | 11.98 ± 1.55 |
| 2 | 44.56 | 33.62 ± 0.63 | Not detectable | 10.94 ± 0.84 |
| 3 | 35.17 | 27.88 ± 0.16 | Not detectable | 7.29 ± 0.18 |
| 4 | 24.97 | 20.30 ± 1.43 | Not detectable | 4.66 ± 0.07 |
| 89 (Control) | 18.54 | 13.72 ± 0.40 | Not detectable | 4.82 ± 0.57 |
| P-Concentration (mg-P/L) | Glucose (%DW) | Glucose (Ratio to Total Sugars, %) | Galactose (%DW) | Galactose (Ratio to Total Sugars, %) | Mannose (%DW) | Mannose (Ratio to Total Sugars, %) |
|---|---|---|---|---|---|---|
| 1.5 | 53.2 ± 0.50 | 97.3 | 0.6 ± 0.09 | 1.1 | 0.9 ± 0.01 | 1.6 |
| 2 | 55.3 ± 0.23 | 97.5 | 0.5 ±0.01 | 0.9 | 0.9 ± 0.01 | 1.6 |
| 3 | 43.9 ± 7.67 | 96.6 | 0.7 ± 0.08 | 1.6 | 0.8 ± 0.13 | 1.8 |
| 4 | 28.9 ± 0.38 | 94.9 | 0.9 ± 0.1 | 3.1 | 0.6 ± 0.07 | 2.0 |
| 89 (Control) | 24.6 ± 0.01 | 95.3 | 0.7 ± 0.02 | 2.8 | 0.5 ± 0.04 | 2.8 |
| Frequency (cm−1) | Assignments | Reference |
|---|---|---|
| 3630–3040 (3280) a | N-H Stretch/protein (overtone of amide II band) | [45,48] |
| 3040–2800 (2860,2930, num b) | C–H symmetric stretch/CH2, CH3 lipid acyl chains, CH stretch/aminoacids or carbohydrates | [45,46,47,48] |
| 1775–1720 (1745) | C=O symmetric stretch/lipids, fatty acids | [45,48] |
| 1590–1715 (1650) | CO stretch, CN stretch, NH bend/amide I band (proteins), C=C stretch/olefinic and aromatic compounds | [45,47] |
| 1490–1590 (1540) | NH bend, CN stretch, CH stretch/amide II band (proteins) | [45,47,48] |
| 1490–1430 (1450) | CH3 and CH2 assymetric bend/proteins or lipids | [47,48,49] |
| 1430–1350 (1400) | CH3 and CH2 Symmetric bend/proteins or lipids, COO- symmetric stretch/acids | [47,49] |
| 1280–1185 (1240) | P = O asymmetric stretch/nucleic acids (DNA and RNA) or phosphorylated proteins, C-O-S/sulpholipids | [45,47,49] |
| 1185–1135 (1150) 1135–1095 (1105) | C-O-C stretch and O-H bend/carbohydrates or lipids | [45,46,47,49] |
| 1095–1070 (1080) 1070–950 (1015, 1005sh c) | C-O-H deformation, C-O-C stretch/polysaccharides, carbohydrates or lipids, P=O symmetric stretch/nucleic acids or phospholipids | [45,49] |
| 950–880 (940) | CH deformation/carbohydrates, P-O-P/polyphosphates | [45,48] |
| 880–825 (836-control samples only) | P-O assymetric stretch/lipids, CH deformation/carbohydrates, C-O-S/sulpholipids | [45] |
| 825–740 (760) | CH deformation/carbohydrates | [45] |
| 740–690 (690sh) | N-H bend/amide V band, CH2 rocking/lipids, CH2 rocking—NH deformation/polyglycines, O-CO/CO2/CO/C=O deformation/carboxylic acids or esters | [45] |
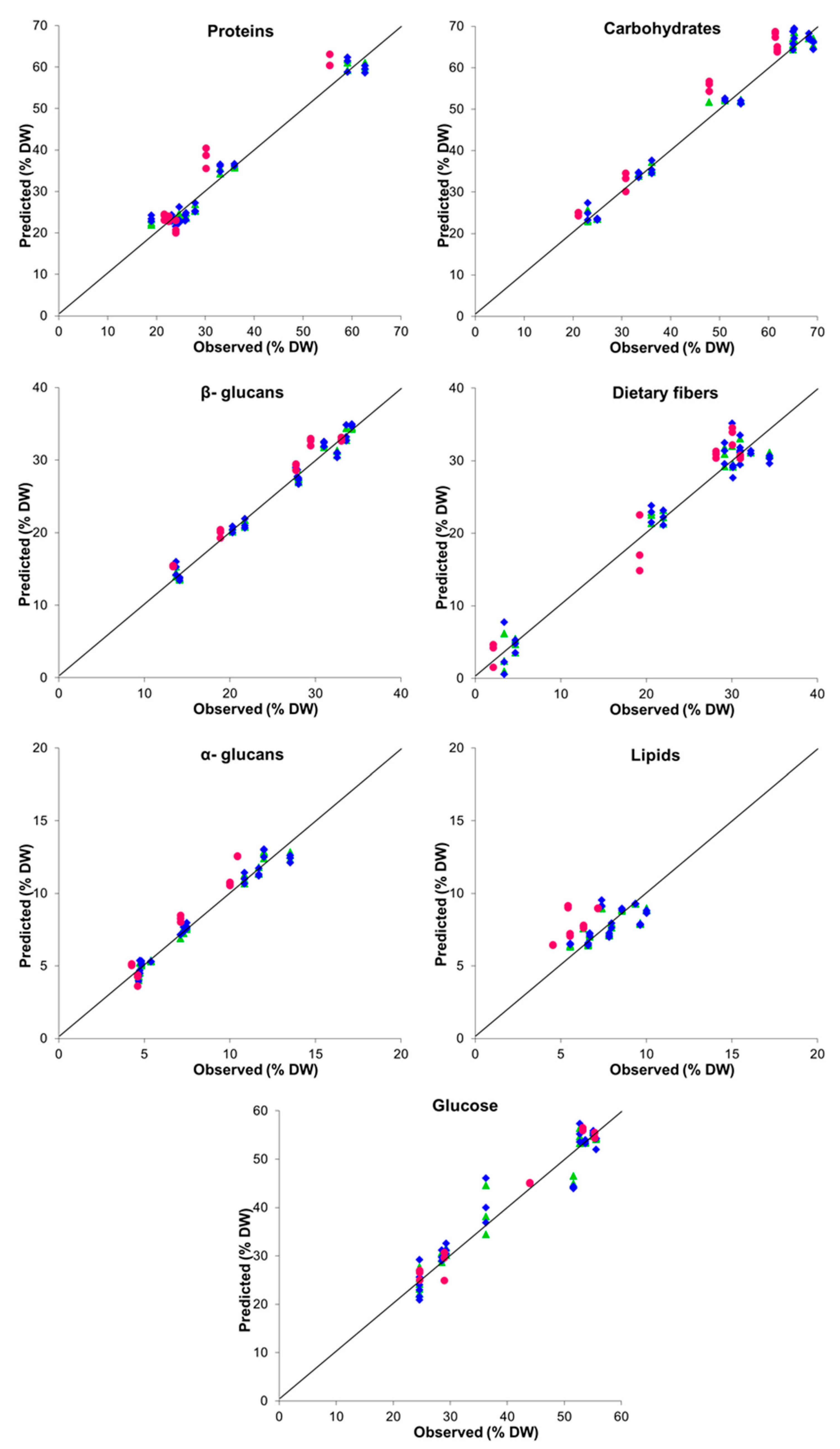
| Parameter | LV a | Performance Indices | |||||
|---|---|---|---|---|---|---|---|
| Internal Validation (LOOCV) | External Validation | ||||||
| R2 b | RMSE c | RPD d | R2 | RMSE | RPD | ||
| Proteins | 4 | 0.968 | 2.581 | 5.662 | 0.847 | 4.981 | 2.645 |
| Carbohydrates | 3 | 0.978 | 2.576 | 6.825 | 0.895 | 5.287 | 3.194 |
| Lipids | 2 | 0.440 | 1.054 | 1.360 | −5.449 | 2.255 | 0.408 |
| 1.3:1.6-β-glucans | 3 | 0.979 | 1.066 | 7.031 | 0.930 | 1.920 | 3.913 |
| α-glucans | 3 | 0.970 | 0.562 | 5.920 | 0.777 | 1.226 | 2.194 |
| Dietary fibers | 4 | 0.951 | 2.352 | 4.617 | 0.937 | 2.721 | 4.121 |
| Glucose | 4 | 0.921 | 3.630 | 3.623 | 0.973 | 2.053 | 6.286 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markou, G.; Eliopoulos, C.; Argyri, A.; Arapoglou, D. Production of Arthrospira (Spirulina) platensis Enriched in β-Glucans through Phosphorus Limitation. Appl. Sci. 2021, 11, 8121. https://doi.org/10.3390/app11178121
Markou G, Eliopoulos C, Argyri A, Arapoglou D. Production of Arthrospira (Spirulina) platensis Enriched in β-Glucans through Phosphorus Limitation. Applied Sciences. 2021; 11(17):8121. https://doi.org/10.3390/app11178121
Chicago/Turabian StyleMarkou, Giorgos, Christos Eliopoulos, Anthoula Argyri, and Dimitrios Arapoglou. 2021. "Production of Arthrospira (Spirulina) platensis Enriched in β-Glucans through Phosphorus Limitation" Applied Sciences 11, no. 17: 8121. https://doi.org/10.3390/app11178121
APA StyleMarkou, G., Eliopoulos, C., Argyri, A., & Arapoglou, D. (2021). Production of Arthrospira (Spirulina) platensis Enriched in β-Glucans through Phosphorus Limitation. Applied Sciences, 11(17), 8121. https://doi.org/10.3390/app11178121







