Abstract
Environmental microplastics are gaining interest due to their ubiquity and the threat they pose to environmental and human health. Critical studies have revealed the abundance of microplastics in nature, while others have tested the impacts of these small plastics on organismal health in the laboratory. Yet, there is often a mismatch between these two areas of research, resulting in major discrepancies and an inability to interpret certain findings. Here, we focus on several main lines of inquiry. First, even though the majority of environmental microplastics are plastic microfibers from textiles, laboratory studies still largely use spherical microbeads. There are also inconsistencies between the measurements of microplastics in the environment as compared to the concentrations that tend to be used in experimental studies. Likewise, the period of exposure occurring in experimental studies and in the environment are vastly different. Lastly, although experimental studies often focus on a particular subset of toxic chemicals present on microplastics, textile microfibers carry other dyes and chemicals that are understudied. They also cause types of physical damage not associated with microspheres. This review will analyze the literature pertaining to these mismatches, focusing on aquatic organisms and model systems, and seek to inform a path forward for this burgeoning area of research.
1. Introduction
Ever since the discovery of microplastics (MPs) in the ocean, there have been large numbers of laboratory studies on their effects. Many studies have been conducted on aquatic organisms, many of which consume these pollutants, and some of which are eaten by humans. Other experimental studies have been on mammalian surrogates (rats or mice) or tissue cultures to provide evidence for potential impacts of these ubiquitous pollutants on humans. For both types of studies, questions have arisen about the lack of realism. For example, Burns and Boxall (2018) noted that the levels detected in the environment are orders of magnitude lower than those used in studies of effects on biochemistry, reproduction, growth, feeding, and inflammation in aquatic organisms [1]. Similarly, Bucci et al. (2020) who performed a meta-analysis of the literature, concluded that there was a mismatch between field studies and the laboratory studies, which tend to use different plastic shapes (fibers being the most common in the field; spheres in the lab), virgin MPs in the lab as compared to aged MPs with biofilm in the field, and different sizes of MPs [2]. Fibers have been found to be the most common MP type in aquatic ecosystems [3]. These fibers come predominantly from washing of synthetic textiles, but also from cigarette butts [4]. The degree of effect detected is a response to concentration, shape, particle size, and polymer type. Comparing concentrations used in most lab exposures to the concentrations and sizes of MPs in the environment, Bucci et al. (2020) noted that 17% of the concentrations used in lab studies have been seen in the environment, and that 80% of the particle sizes used in experimental studies are below the sizes found in most environmental sampling [2].
Kogel et al. (2020) in a general review found that toxicity of plastic particles depends on particle size, concentration, particle condition, exposure time, polymer type, and shape [5]. Other factors, relating to the experimental subject included species, sex, developmental stage, and food availability. Effects that have been found in experimental studies were on energy metabolism, feeding, growth, activity, metabolism, physiological stress, pathology, immune system, intestinal damage, development, hormonal regulation, and cell death. Photosynthetic disruption was reported in phytoplankton.
In this review, we examine effects of MPs on aquatic animals, and on mammalian surrogates and cell cultures, representing risks to human health. We view these studies in terms of what type of MP was chosen for experimentation: shape (e.g., spheres in experiments, fibers in the environment) and size, concentrations used, length of time of exposure, and coating (e.g., chemical additives, biofilms, “virgin”). Critically, in some cases we show that the dearth of environmentally relevant data emphasizes what needs to be done, rather than what we already know.
2. Shape (Spheres vs. Fibers)
2.1. Aquatic Effects
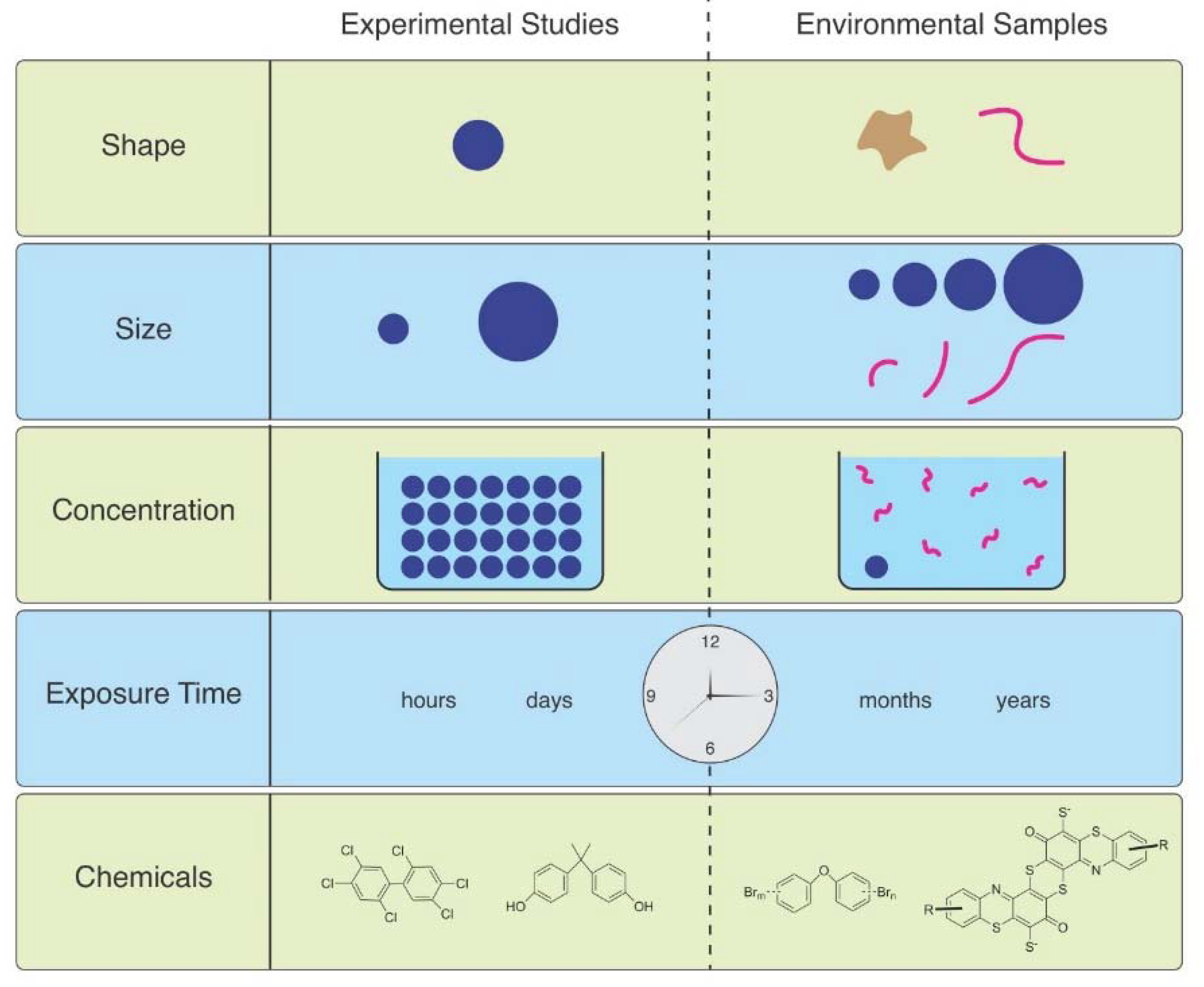
As Ward et al. (2019) point out, microspheres are available to be purchased with uniform size and shape, with added fluorescence or dyes allowing them to be visualized in tissues [6]. Therefore, they are useful for studies of ingestion and egestion in many species. However useful they are, they are not representative of MPs in the environment, and are therefore not realistic (Figure 1). Despite not being so easily obtained, other researchers have found ways to study effects of microfibers, which are the predominant shape of MPs in the water, air, and soil. They can be acquired from ropes, lint in dryers, textile samples, microfilaments used to make synthetic textiles, and other methods.
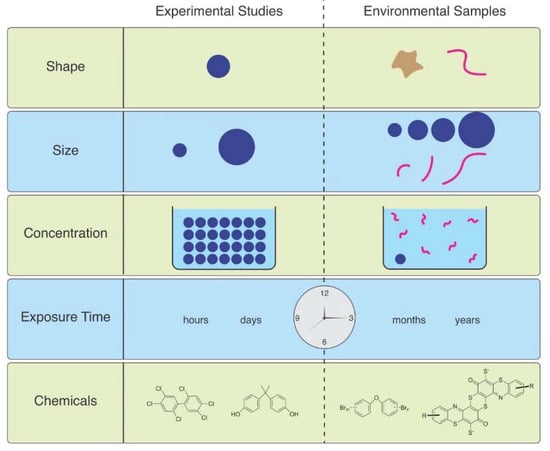
Figure 1.
The mismatch between MPs used in laboratory studies and those in the environment.
Microfiber (MF) uptake by Asian clams (Corbicula fluminea) varied by polymer type and size. The uptake of fibers was greater in those exposed to greater concentrations than lower concentrations, and clams were more likely to take up polyester fibers of smaller size [7]. Horn et al. (2019) used polypropylene rope as a source of microfibers, the concentrations of which were based on concentrations found in the beach from where the experimental subjects, the mole crab, Emerita analoga, were collected [8]. Adult crabs exposed to these microfibers had increased mortality and impaired embryo development. Larval midges, Chironomus riparius, were exposed to PET MFs, and effects including survival, time till emergence, growth, head capsule length, and general stress response, were examined in 28-day sediment chronic toxicity tests [9]. They used artificial sediments spiked with MFs at concentrations of 500, 5000, and 50,000 particles/kg sediment dry weight. The lowest concentration was comparable to 500 particles/kg sediment dry weight in Lake Ontario, Canada and 4900 particles/kg sediment dry weight as in the Rhine River. Larvae ingested the microfibers which were later found in the adults. However, no significant effects were seen on time until emergence, head capsule lengths, weight, or HSP 70 (stress response) compared to control organisms. Alnajar et al. (2021) conducted a seven-day exposure of the mussel Mytilus galloprovinciallis to MFs at 56–180 mg L−1 (far higher than environmental levels) and observed a reduction in mean clearance rate, abnormality in gills and digestive gland, and an increase in DNA damage [10]. They felt that these effects were due to a combination of the fibrous material itself and chemicals mobilized from the polymers into seawater or the digestive tract, the latter being consistent with an increase in trace elements (e.g., zinc) in the exposure medium with increasing lint concentration. Lobster (Homarus americanus) larvae were exposed to 0, 1, 10 and 25 MF mL−1 [11]. Only the highest concentration decreased early larval survival, and the timing or rate of molting was not altered. All larval and post-larval stages accumulated MFs under the carapace, and ingestion increased with larval stage and with MF concentration; oxygen consumption rates were reduced in later larval stages exposed to high concentrations.
Some studies have compared responses to and effects of fibers vs. spheres. Ward et al. (2019) indicated that mussels reject most of the fibers and spheres they ingest, but a much smaller percentage of spheres were rejected than polyester fibers (ca. 65–260 μm long × 16 μm wide) [6]. The amphipod Hyalella azteca had slower egestion of fibers than microspheres, but eventually both showed complete egestion [12]. MFs had greater toxicity than microbeads however, possibly because of slower gut passage. In a 28-day feeding experiment, Blarer and Burkhart-Holm (2016) studied effects of fibers and spheres on the feeding rate, assimilation efficiency and change in wet weight of the amphipod Gammarus fossarum [13]. While both types were ingested and egested, only the fibers impaired the health of the animals. Lower concentrations of MF had more severe effects on the amphipod G. fossarum than higher concentrations of MP particles. Frydkjær et al. (2017) reported that elevated concentrations of PE particles decreased the mobility of Daphnia magna, while irregular shaped fragments (10–75 µm) had greater effects than beads (10–106 µm) [14]. D. magna egested regular-shaped PE faster than irregular ones, indicating that “spiky” particles are retained longer and have more severe effects. Polyethylene terephthalate granular particles (p-PET, approximately 150 μm diam) and fibers (f-PET, approximately 3–5 mm L and 20 μm diam) were compared for effects on development of zebrafish (Danio rerio) embryos and their joint effects with cadmium [15]. Both types of MP accelerated blood flow and heart rate and inhibited hatching. Both forms decreased the toxicity of Cd. The detoxification effect of f-PET was greater than that of p-PET. Mendrik et al. (2021) found that MP fibers, but not spheres, reduced photosynthesis of algal symbionts of Acropora sp. corals, with a 41% decrease in photochemical efficiency after 12 days [16]. Grass shrimp (Palaemonetes pugio) were exposed to either sediment, polyethylene spheres, polypropylene fragments, tire fragments, polyester fibers, or clean-water for 96 h at a nominal concentration of 50,000 particles/L before a bacterial challenge with V. campbellii [17]. Mortality was not observed in any of the exposures, and survival following the bacterial challenge was similar among shrimp exposed to particle-free water, sediment, polypropylene fragments, polyethylene spheres, tire fragments, and polyester fibers. The grass shrimp cleared most of the ingested particles and all of the ventilated particles within 48 h.
2.2. Human Effects
In humans, relevant MP shapes will depend on exposure source (i.e., air, water, food), as well as how particles degrade over time in the environment and in the body. One of the earliest signs of MPs in human tissues came in 1998, when plastic microfibers were identified in biopsies of human lung tissue [18]. This study indicated two things: humans are exposed to MPs from the environment, and these MPs become embedded in our tissue. This study also importantly brought to our attention the relevance of plastic microfibers, as compared to more commonly studied spherical MPs. A more recent study built upon this work, showing that a combination of plastic particles and fibers were present in 13 of 20 human lung tissue samples examined [19].
Although inhaling airborne MPs is a major route of exposure, MPs also enter through ingestion and can become embedded in other organs. In a screening of 11 human colon samples, 96.1% of embedded particles were plastic filaments or fibers [20]. Furthermore, as was widely publicized, MPs were recently identified in human placentas, with a total of 12 irregularly shaped MP fragments being found in 4 placenta samples [21].
Interestingly, in a characterization of excreted MPs in human stool samples, mostly fragments and films were identified [22]. As few plastic microfibers were found in human stool samples and fibers are a major class of environmental MPs, one can infer that plastic microfibers that enter our bodies are becoming embedded in our tissues. This conclusion is in line with the aforementioned data.
Through experimental studies, it is known that ingested MP spheres (5 µm) hamper metabolism and gut microbial function. Furthermore, these spheres accumulate in the gut [23]. Spherical MPs have also been used in new in vitro models and organoid cultures of the human intestines. Similar to mammalian models, these systems show varied effects, ranging from no significant cytotoxicity to high levels of cytotoxicity and a strong immune response [24]. The advantage of these in vitro approaches is that they have the potential to more closely resemble how humans might respond to MP exposure. However, the lack of available evidence surrounding how both mammalian models and in vitro systems respond to plastic microfibers as compared to spherical MPs leaves much to be desired.
A more recent studied began to explore this question, studying how MPs of varied shape relates to cytotoxicity in human cell lines. They found that MPs with rigid, sharp, or irregular edges appeared to be more harmful than those with smooth, round edges [25]. Future studies should find which shapes of MPs are most prevalent in various tissues, and then test the impacts of MPs with those same shapes in mammalian models or in vitro cultures for the corresponding cell types.
3. Size
3.1. Aquatic Effects
Particle size is a critical factor in uptake and egestion. For small organisms such as plankton, particles larger than their mouth opening cannot be ingested. However, using only particles small enough to be eaten is also unrealistic, because it maximizes organism exposure [26]. One might expect that after ingestion, smaller particles would be better able to penetrate through tissues and cause greater toxicity, but that is not always the case. Roch et al. (2021) found that rainbow trout evacuated 50% of particles in 12 h for 42.7 µm particles and 4 h for 1086 µm particles (which is less than the time for evacuating food) [27]. In contrast, the differences between sizes for evacuation by common carp were smaller: 7 h for 42.7 µm particles and 4.6 h for 1086 µm particles. They concluded that it is likely that large particles in rainbow trout must be actively transported out of the stomach, since they had shorter evacuation times than food, while in carp, evacuation rates of all particle sizes were in the same range as food, suggesting a passive process.
In Daphnia, accumulation of fragments was higher than that of beads, and inhibition of feeding and growth depended on the size of MP fragments [28]. Survival of D. magna exposed to small- and large-sized fragments (17 vs. 34 μm) was significantly lower than that of daphnids exposed to MP beads. Small fragments significantly reduced feeding, and body retention time in the digestive system.
In the marine medaka (Oryzias melastigma), body weight, adipocyte size and liver lipid contents were significantly increased in fish exposed to large (200 μm) PS-MPs, while fish exposed to smaller (2 and 10 μm) MPs had liver injury, specifically fibrosis and inflammation [29]. Since the larger particles did not enter the circulatory system, their impacts on intestinal microbes were investigated. Gut microbial diversity and composition were altered in fish exposed to PS-MPs, particularly the larger particles. However, when goldfish (Carassius auratus) were exposed to two sizes (0.25 and 8 μm) of polystyrene MPs at different environmentally relevant concentrations, enzyme changes and histological lesions were more severe in those that were exposed to the smaller sized MPs [30].
Bour et al., 2018 exposed the benthic bivalves Ennucula tenuis and Abra nitida to polyethylene MPs at 1, 10, and 25 mg/kg of sediment for four weeks [31]. Three sizes (4–6; 20–25 and 125–500 um) were used. No effects were seen on survival, condition index or burrowing behavior. However, A. nitida showed a significant decrease of protein in those exposed to the largest particles, at all concentrations. No changes were seen in protein, carbohydrate or lipid in E. tenuis, but total energy decreased in a dose-related manner in ones exposed to the largest size particles.
In a rare (and welcome) paper comparing effects of polyethylene terephthalate MFs of different lengths (50 and 100 μm), mussels (Mytilus galloprovincialis) were exposed to environmental (0.5 μg/L) and high (100 mg/L) MF concentrations for four days [32]. Short MFs accumulated in the lower intestinal organs, but long MFs were only observed in the upper intestinal organs. Both sized MFs affected necrosis, DNA damage, reactive oxygen species, nitric oxide, and acetylcholinesterase. Fiber length-dependent effects occurred at environmental concentrations for DNA damage (long MFs) and AChE activity (short MFs).
3.2. Human Effects
Due to the availability of various sized MP spheres, there is more known about how particle size impacts mammalian models and human cells. In human lung tissue samples, MP particles were found to be smaller than 5.5 µm, whereas plastic microfibers were found to range from 8.12 to 16.8 µm [19]. In human colon samples, MPs were surprisingly larger, with an average size of 1.1 mm and a range of 0.8 to 1.6 mm [20]. This is particularly relevant because it suggests that different tissues might be exposed to sizes of MPs that vary greatly. Perhaps those that are airborne and inhaled are generally smaller than those ingested. These considerations should be incorporated into future studies.
Keeping this point in mind, human colon cell lines (Caco-2 cells) were exposed to 0.1 µm and 5 µm MP spheres. Although both sizes disrupted mitochondrial membrane potential, 5 µm particles displayed a stronger effect. However, both sizes inhibited ATP-binding cassette transporter activity, but in this context, 0.1 µm MPs were more disruptive [33].
The size-dependent impacts of MPs were found in another study in mice. Pregnant mice were exposed to either 0.5 µm or 5.0 µm polystyrene MP. Their offspring were examined for metabolic disorders, and it was found that those coming from mothers exposed to 5.0 µm MPs had more severe metabolic defects, including altered metabolites and hepatic gene expression [34]. Similarly, in a different study larger MPs (10 µm) were shown to more strongly damage testis tissue architecture, and decrease viability than smaller MPs (0.5 µm), although all three size were able to enter cells in vitro [35]. Interestingly, in a human vascular endothelial cell line (HUVECs), size-dependent impacts of MPs were also observed. However, in this case, smaller MPs (0.5 µm) were shown to have a greater impact on cell viability in an autophagy-dependent manner, as compared to larger MPs (5.0 µm) [36].
Furthermore, a study where mice were fed with MPs found that smaller MPs (5 µm) were more likely to translocate to the gut and kidney after ingestion. Larger MPs (20 µm) accumulated in the liver [37].
As these selected references show, there is a clear size dependent impact of MPs. Yet, in a recent report, no effects were observed at any MP size tested—1 µm, 4 µm, 10 µm—despite all three having some level of cellular uptake [38]. The discrepancies between these results, and between the sizes of MPs observed in various tissues compared to those used in experimental studies requires further investigation. Furthermore, there are a dearth of studies investigating how plastic microfibers of various sizes interact with cells and tissues in mammalian models and human cells. This is critical, as it appears that fibers are one of the more common classes of MP that become embedded in tissues.
4. Concentration
4.1. Aquatic Effects
MP concentrations in various marine environments ranges from approximately 1 mg/L–1 ng/L, generally <20 MPs/ m3 in water [39] but most lab exposures use far higher concentrations. Lenz et al. (2016) state that experimental exposures are two to seven orders-of-magnitude higher than concentrations in the environment [39]. Horton et al. (2017) reviewed particle counts in marine and freshwater surface water and found them to be extremely variable [40]. MP concentrations in marine surface waters are reported from 0.0005 particles L−1 to 16 particles L−1 with intermediate concentrations. Freshwater surface samples have concentrations around the lower end of marine surface concentrations, in general. According to Bucci et al. (2020) only 17% of concentrations used in laboratory experiments have been found in nature, and 80% of particle sizes used in experiments fall below the size range seen in the majority of environmental sampling [2]. In their review article, Thomas et al. (2021) found 14 papers that found exposure with no or minor effects [41]. Some of these used low concentrations, but some used high concentrations. In contrast they found 81 papers that reported adverse effects, most of which used unrealistically high concentrations (Figure 1).
However, current MP sampling is likely to underestimate concentrations in the water [42]. Using different mesh sizes for collection results in greatly different counts. MPs collected with a 100 μm net were 10-times greater than samples collected with a 500 μm net. Covernton et al. (2019), comparing samples collected with a standard plankton net (300 μm mesh) verses a bulk sample filtered through an 8 μm filter, found 8.5 times as many in the filtered sample, including a much greater proportion of microfibers [43]. This effect is important when comparing surface water samples, since differences in sampling methods lead to a lack of comparability. Sediment concentrations tend to be higher than in water in UK river sediments, which was also observed in coastal sediments in Slovenia [44,45]. High concentrations (thousands of particles kg−1 of dry sediment) were found in river sediments in Germany, similar to the 2000–8000 particles kg−1 reported in coastal sediments in Canada [46,47].
Experiments do not always use unreasonably high concentrations, and there are many studies that find deleterious effects at ranges close to environmental concentrations. Lobster (Homarus americanus) larvae were exposed to MFs at 0, 1, 10 and 25 MF mL−1 [11]. Only the highest MF concentration decreased early larval survival. MFs did not affect the timing or rate of molting. Mussels (Mytilus edulis) were fed a diet of plankton with MF concentrations up to 30 MF mL−1 and filtration rates were greatly reduced [48]. Horn et al. (2020) used environmentally relevant concentrations of MFs (1 mm long 0.1 mm diameter) to expose mole crabs, Emerita analoga [8]. The site from which crabs were collected had an average of 15 pieces/100 mL of sand, water had 3–7 MF/L water. They found that crabs ingested MFs and showed deleterious effects on survival, reproduction, and embryo development. Mohsen et al. (2021) performed 60-day exposures of sea cucumbers, Apostichopus japonicus to MFs [49]. Their food was mixed with MFs at 0.6 and 1.2 MFs g−1, based on environmentally relevant concentrations. In the third treatment, MFs were mixed in the diet at 10 MF g−1 to simulate the worst-case scenario. Exposures did not significantly affect growth or fecal production rate. However, acid phosphatase and alkaline phosphatase activity were altered, and total antioxidant capacity was reduced in juveniles and adults. Hermit crabs (Pagurus bernhardus) living in suboptimal shells were exposed to polyethylene particles at 25 particles L−1 [50], which is lower than most exposure studies. After five days, crabs showed impaired shell selection. They were less likely to contact or enter optimal shells and took longer to contact and enter an optimal shell compared to controls. Thus, MPs impair information-gathering and processing, which are essential survival behaviors.
There are also studies that find minimal or no effects from exposure to high concentrations. Two amphipods, Echinogammarus marinus and Gammarus pulex, showed no impacts after exposure to 100,000 MP/L and 4,000,000 MP/L [51,52]. Likewise, no effects were seen in mussels Mytilus galloprovincialis exposed to 10 and 1000 MP mL−1, nor the oyster Ostrea edulis exposed for 60 days to 80 μg L−1 [53,54]. The lugworm (Arenicola marina) was exposed to up to 100 g of MP per liter of sediment; reduced feeding was the only effect seen during the experiment [55].
Cunningham and Sigwart’s (2019) meta-analysis points to three issues that need improvement in future work: use of extremely high dosages, incompatible units, and the lack of good controls [56]. They found that 5% of laboratory exposures did not use any control treatment at all, and 82% used “dramatically elevated” concentrations far greater than environmental concentrations. Only 23 studies tested biological impacts from experimental exposures at environmentally realistic levels. The studies they considered to use “high” concentrations used a range of multiple orders of magnitude, to extreme levels with no environmental relevance. They consider 100 MPs/L to be “high” compared to environmental levels.
4.2. Human Effects
Although it is likely to have major impacts on human health, the concentration of MPs used during experimental studies is not often considered, and is sometimes critiqued. Evidence regarding the concentration of MPs in human tissues does exist in some cases, albeit in a nascent form. In a screening of human colon samples, 28.1 (±15.4) particles/gram of tissue were found [20]. Yet, as with size and shape, the concentration of MPs in human tissue appears to vary in an organ-dependent manner. In human lung tissue, recent research estimated 0.56 MP particles/gram of lung tissue (equaling about 470 particles in an average set of lungs). The authors indicate that this suggests most particles inhaled are retained in airways [19], an observation in line with previous characterizations.
Many experimental studies focusing on MPs in the context of human health either use a single concentration of MPs or use a range that is not environmentally relevant. One recent report studying human cells (HUVECs) used a range of MPs at 0–100 µg/mL [36]. Based on previous findings, these authors argued that this would be a good estimate for the actual concentration of MPs in human blood (~99.4 µg/mL). They suggest that the actual concentration might be higher if there are low turnover rates. Indeed, the evidence mentioned here indicates that only a fraction of MPs that enter our body are excreted—with some likely remaining in the blood or getting embedded in other organs [37]. For 0.5 µm sized particles, regardless of concentration cell viability decreases drastically at 72 h after of exposure. For 1 µm sized particles, at 72 h cell viability is impacted in a cell concentration-dependent manner, with 100 µg/mL having the strongest effect. Regardless of concentration, 5 µm particles did not significantly alter cell viability [36]. Although the authors proposed that MPs induce autophagy-dependent cell death and negatively interact with cell-surface receptors, the reasoning for the differences observed between 0.5 µm and 1.0 µm remain unclear.
In another study using human lung epithelial cells (BEAS-2B), two concentrations of polystyrene MPs were used. At low doses (10 µg/µL), cytotoxicity, an inflammatory response, and barrier dysfunction were observed. High doses (1000 µg/µL) exacerbated the same effects, but also reduced alpha1-antitrypsin levels, increasing the risk for COPD [57].
These results elicit a more thorough characterization of the concentrations of MPs human cells are exposed to in different tissues. First it is necessary to recognize that there will be concentrations that persist over prolonged periods (those MPs embedded in tissues), and more transient concentrations (MPs that move through the body). These two categories might be regional or tissue-specific, depending on how MPs translocate and are circulated in different organs. Once this improved understanding is reached, similar concentrations should be used in experimental studies to properly model the prolonged and transient scenarios.
5. Duration of Exposure
5.1. Aquatic Effects
The duration of exposure is an important factor that determines the magnitude of effects that will be produced. However, the vast majority of exposure studies in the laboratory are short-term (days) (Figure 1), which might act as a counterbalance to the general use of excessively high concentrations. However, short-term exposures to high concentrations are not equivalent to long-term chronic exposures to lower, more realistic concentrations. The Horn et al. (2019) study of effects of MFs on reproduction in mole crabs is a welcome exception [8]. Not only did they use MFs at environmental levels, but they exposed the animals for 71 days, which is time for two reproductive cycles in the species. A recent paper examined in situ effects of high concentrations (80 g m2) of irregularly shaped mixed MPs on freshwater benthic communities, with an exposure of 100 days [58]. However, no effects on abundance, biomass, species richness, Shannon or Simpson measures of community diversity were found.
5.2. Human Effects
Similar to the lack of studies described in aquatic systems for long-term exposure, there are a dearth of studies describing how long-term exposure to MPs impacts human cells or surrogates. With evidence that MPs can become embedded in tissue, studying the long-term presence of MPs and their cytotoxic impacts over time are of high importance.
In addition, the length of exposure and the length of the experiment can also influence how far MPs travel in the body, and their translocation within an organ or tissue. As we have shown here that MPs do indeed become embedded in cells and tissues, it is necessary to factor timing into an experiment in order to properly mimic the in vivo dynamics.
6. Leached Chemicals
6.1. Aquatic Effects
Plastics contain chemical additives such as phthalates and BPA (bisphenol a) that can leach out and cause toxicity [59]. In addition, contaminants, such as PCBs (polychlorinated biphenyls), metals, and pesticides, adsorb to plastic [60]. Many studies investigating the impacts of MPs use fresh (“virgin”) plastic particles, but after weathering in the environment, particles acquire attached chemicals and are potentially more toxic than virgin particles. The use of pristine particles in many experimental studies could lead to an underestimation of the toxicological impacts. MPs could be considered “vectors” for moving contaminants into animals and up the food chain [61,62]. The leaching of plastic additives as well as adsorbed chemicals makes MPs a “cocktail” of toxic contaminants [63].
Similar to other studies, most of the studies on chemical toxicity of MPs have used microbeads, not MFs, while studies comparing the two have suggested that MFs are more detrimental to organisms than microbeads (see earlier section), which could lead to further underestimation of MP impacts. A study of MFs from cigarette butts emphasized the concern about attached chemicals from tobacco as a source of ecotoxicity [4].
MFs are rarely studied for the toxicity of associated chemicals, even though dyes and hundreds of other chemicals used in the manufacture of textiles (e.g., antimicrobials, fire retardants, stain resistance chemicals) are toxic [64,65] (Figure 1).
A key question is how tightly bound the chemicals are to the plastic versus how available they are to biota. The degree of availability depends on factors including the polymer, the chemical, and the surrounding environment. After ingestion, additives may leach and adsorbed chemicals may desorb [66,67,68]. Since ingestion is the most often studied type of exposure, it is important to study what proportion of the plastic additives and attached contaminants can be “pulled off” in the digestive system of different animals during the period of time that the MPs are going through the gut; this is likely to vary with the chemical, the type of MP (as seen in [69]), and the complexity, length, and chemistry of the gut (some invertebrates have alkaline stomachs rather than acidic stomachs similar to vertebrates). Bakir et al. (2014) investigated the potential for polyvinylchloride and polyethylene MPs to sorb and desorb various organic chemicals including 14C-DDT, 14C-phenanthrene,14C-perfluorooctanoic acid and 14C-di-2-ethylhexyl phthalate [70]. Desorption was measured in seawater and under simulated gut conditions. Desorption was faster with gut surfactant and increased more in conditions simulating warm blooded organisms. Chua et al. (2014) exposed amphipods (Allorchestes compressa) to MPs spiked with polybrominated diphenyl ethers (PBDEs) and found the amphipods assimilated the PBDEs [71].
Studies have found evidence for transfer of contaminants from MPs into organisms. Japanese medaka (Oryzias latipes) were fed diets for ten weeks with 500, 1000, or 2000 μg/g 10 μm fluorescent spherical polystyrene MPs during maturation from juveniles to reproductive adults [72]. No changes in mortality, behavior, or growth were seen, and MPs were egested after 3–4 days. Nevertheless, females had dose-dependent decreases in number of eggs, and histological analysis showed changes in spleen and kidney. However, microscopic examination, histologic sections, and scanning electron microscopy found no MPs in any internal organs. Since MPs did not penetrate into tissues, toxic effects were attributed to the leaching of chemicals from the particles. Hu et al. (2020) found abnormalities in MF-exposed medaka (Oryzias latipes), including various pathologies in the gills [73]. They acknowledged that changes they observed could have been caused by mechanical damage or leached chemicals, or both. They mentioned that some textile dyes, namely benzothiazoles (Avagyan et al., 2015), induced gill alterations in fish that resemble those they observed from mf exposure [74]. Similarly, Rochman et al. (2014) exposed adult medaka (Oryzias latipes) to polyethylene MPs and found changes in estrogen receptor-mediated gene expression and histopathology of the testes, implying altered endocrine function [75]. These effects resemble those caused by the plastic additives di-(2-ethylhexyl)-phthalate (DEHP) and its metabolite mono(2-ethylhexyl)-phthalate (MEHP) in the medaka [76].
MP studies on the lugworm, Arenicola marina included transfer of PCBs (polychlorinated biphenyls) from polystyrene (PS) MPs [77]. Effects on survival, activity, and bodyweight, and the transfer of PCBs were assessed. The concentration of MPs in the spiked sediment was directly related to the uptake of MPs and to weight loss. Reduced feeding was observed at a dose of 7.4% dry weight. There were statistically significant effects on bioaccumulation and fitness, but the magnitude of effects was small. However, in similar studies, polyethelene MPs (PE) did not act as a measurable vector of PCBs [77]. Browne et al. (2013) exposed lugworms (Arenicola marina) to sand with 5% PVC MP that was spiked with the pollutants nonylphenol and phenanthrene and additive chemicals triclosan and PBDE-47 [69]. The pollutants and the additives were found to be transferred into gut tissues and to cause some biological effects. However, sand transferred greater amounts of the pollutants to the worms than did MPs. Uptake of nonylphenol from MPs or sand reduced the ability to remove pathogenic bacteria. Uptake of Triclosan from PVC reduced the worms’ ability to burrow and engineer sediments and caused mortality (both by >55%) while PVC alone made the lugworms more susceptible to oxidative stress.
In contrast, some studies have found that chemicals bind so tightly to MPs that the overall bioavailability is reduced and that there is less transfer of toxic chemicals from ingested plastics than from sediments, indicating that plastic is a less important source of contaminants to animals [78,79,80]. Herring (Clupea harengus) larvae were tube-fed a dose of up to 200 MP spheres spiked with 14C-labelled PCB-153 while control larvae were tube-fed an isotonic solution without MPs [81]. Most larvae evacuated all or almost all MPs by 24 h and tracer levels in exposed fish were not significantly different from control larvae. There was no significant transfer observed of PCB from MPs into larvae which had gut-transit times of <24 h. Koelmans et al. (2014) assessed the potential of leaching of nonylphenol (NP) and bisphenol A (BPA) in the digestive system of the lugworm, Arenicola marina and cod, Gadus morhua [82]. They used a biodynamic model to estimate the relative contribution of plastic ingestion to total exposure to chemicals in the ingested plastic. They concluded that MP ingestion by the lugworm or cod is probably not a relevant exposure pathway for either chemical for either animal.
Several studies have compared the toxicity of fresh (virgin) MPs with that of “weathered” MPs that have been in the environment and have had time to adsorb contaminants from the surrounding water, or with virgin MPs that have been spiked with chemicals. Virgin MPs are assumed to have no attached toxicants, while weathered ones would be expected to have accumulated them from the environment. Intertidal snails (Littorina littorea) were exposed to leachates from both virgin and beached pellets [59]. While virgin pellets impaired vigilance and antipredator behavior slightly, beached pellets severely inhibited these behaviors. These results indicate that effects from MP leachates may have serious consequences for animals that rely on chemosensory cues to escape predation. In a study of early life stages, marine medaka Oryzias melastigma embryos and larvae were exposed to suspended MPs spiked with benzo(a)pyrene (MP-BaP), perfluorooctanesulfonic acid (MP-PFOS), or benzophenone-3 (MP-BP3) for 12 days [83]. The MPs agglomerated on the surface of the egg chorion (outer membrane) but did not penetrate it. While embryos exposed to virgin MPs showed no effects, those exposed to MPs with PFOS had decreased survival and did not hatch. Larvae exposed to MPs spiked with BaP or with BP3 showed developmental anomalies, reduced growth, and abnormal behavior. Thus, compared to similar water concentrations, BaP and PFOS spiked on MPs appeared to be more embryotoxic. These data suggest pollutant transfer by contact of MPs on the chorion of fish embryos. An effect of particle size was also seen, with smaller particles having more severe effects than larger ones. Bucci et al. (2021) exposed larval fathead minnows to pre-consumer MPs and environmental MPs and found environmental MPs caused a more drastic increase in length and weight and almost six times more deformities as the pre-consumer MPs [84].
Effects of low-density polyethylene (LDPE) MPs (11–13μm), with and without adsorbed contaminants (benzo[a]pyrene—BaP and perfluorooctane sulfonic acid—PFOS), were studied on the clam, Scrobicularia plana [85]. Environmentally relevant concentrations of contaminants were adsorbed onto MPs to evaluate their potential role as a vector for toxic chemicals after ingestion. S. plana were exposed at 1 mg L−1 for fourteen days and were sampled after three, seven, and fourteen days. BaP accumulation in tissues, enzymatic biomarkers, genotoxicity in the gills, digestive gland, and hemolymph, and neurotoxicity were evaluated. Results suggested that physical injury of gills by MPs could affect the biomarkers. The digestive gland was less affected by physical damage from virgin MPs than by MPs with adsorbed chemicals, indicating desorption and toxic effects on the biomarkers measured.
A 21 day exposure of the fish Sparus aurata juveniles to virgin and weathered MPs was performed [86]. Fish were analyzed for a suite of enzyme biomarkers and behavioral changes, specifically social interactions and feeding behavior. Results indicated cellular stress from virgin MPs, and greater stress from weathered MPs. Behavioral effects indicated that fish exposed to either type of MPs were significantly bolder during social interactions than controls.
The toxicity of leachates from beached MPs and virgin industrial MPs (PVC) to sea urchin embryos (Paracentrotus lividus) was investigated [87]. The weathered beached MPs, which contained polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs), produced severe, consistent, and specific developmental abnormalities in embryos and larvae. However, embryos exposed to virgin MP leachates without additives or environmental contaminants showed normal development, suggesting that the abnormalities observed from beached MPs are the result of exposure to either adsorbed contaminants or industrial additives.
Unlike other types of MPs, MFs from textiles have unique dyes and chemicals such as surface treatments, anti-microbials, brominated flame retardants that are used in the production of textiles, and which have been shown to be highly toxic [64,65,88]. Furthermore, chemical additives were detected in aqueous leachates of virgin and aged (photodegraded) MFs [89]. These have been greatly understudied.
6.2. Human Effects
Although many of the leached chemicals described above have been studied in the context of mammalian models, human cells, and human health, investigations of the dynamics of MPs and these chemical as MPs enter the body are less well known. When mice were fed MPs coated with phthalate esters (PAEs), this induced the accumulation of PAEs in the gut. As compared to virgin MPs, this resulted in inflammation, and altered intestinal gene expression [90]. Of all the PAEs tested, DEHP had the greatest adsorption capacity to MPs, and showed the strongest impacts on animal health [90]. In a different study, co-exposure to MPs and organophosphorus flame retardants worsened the impact of these chemicals, indicating that MPs act as more than just a vector for entry into the body [91]. These results aside, many more recent studies investigating how MPs impact mammalian models and human cells have used fresh, “virgin” MPs. Similar to aquatic organisms, this likely underestimates the effects observed after exposure to MPs.
MPs coated with chemical additives can degrade in the environment, or after entering the body. A recent study found that the associated release of chemical additives is not equivalent. By simulating the ingestion of MPs coated with bisphenols, researchers found that intestinal fluids intensified their release from PVC MPs. Interestingly, bisphenols released under intestinal conditions caused greater cytotoxicity to human MCF-7 cells as compared to bisphenols released from MPs in water [92]. These studies indicate that not only the type of chemical additive is important, but also the dynamics of the exposure.
6.3. The Role of Biofilms
6.3.1. Aquatic Effects
MPs in the aquatic environment, in addition to adsorbing chemicals from the water also acquire a community of microbes on their surface, the biofilm. Biofilm may play a crucial role in toxic chemical transport by MPs, and therefore, studies on MPs as a vector for toxic contaminants in aquatic habitats should include investigations on biofilm [93]. This is of ecotoxicological concern as it has been shown that biofilm is the reason that some animals are attracted to plastic as a source of food [94]. Microbes in the biofilm on marine MPs produced dimethyl sulfoxide (DMS), which acts as an attractant odor that increases ingestion of the plastic by plankton and attracts seabirds [94]. The biofilm itself has begun to be studied. Zhang et al. (2021) exposed polypropylene, polyethylene, and polylactic acid pellets, and glass particles for six weeks during different seasons in the Yellow Sea, China [95]. The total amount of biofilm that formed varied among seasons and locations; temperature of seawater was the most important factor. A diverse microbiome, primarily diatoms and bacteria formed on the MP surfaces with no preference for any particular polymer type.
The community of microbes on MPs (the “plastisphere”) is the subject of intense study by microbiologists. A review of information about the plastisphere noted that among the taxa found are the dinoflagellate Pfiesteria which may be toxic [96,97]. Among the bacteria found are Vibrio, so it is possible that MPs could be vectors for pathogenic microorganisms [98]. Since the biofilm can concentrate toxin-producing microbes and disease microbes, it may be a vector for these hazards, as well as contaminants.
6.3.2. Human Health Effects
There are few relevant studies investigating the impacts of MP biofilms on humans. This will be a particularly relevant and fruitful area of study in the future, especially considering the epidemiological relevance of disease transmission.
7. Discussion
Numerous studies in aquatic organisms, mammalian model systems, and human cell lines have indicated that MPs pose a threat to biodiversity and human health. Historically, studies have been conducted at high concentrations using spherical MPs, some of which we have highlighted here. We have also indicated the need for studies that more closely mimic environmental conditions, namely the use of plastic microfibers, correct sized particles, and appropriate concentrations. In general, it appears that more studies in aquatic organisms have started to account for these parameters and use MPs that better represent those contaminating the environment.
Two other important considerations we have begun to explore here, in both aquatic organisms and in humans, are route of entry and dynamics once inside the body. If studying how MPs impact human lungs, it is necessary to have approximations for concentrations of airborne MPs. It is also necessary to understand how many MPs actually reach the lung tissue, and how long they stay there. It is possible, and likely, that a single tissue will not be exposed uniformly to MPs, with some regions having transient exposure to high levels, and other regions have long term exposure to lower levels. All of this must be considered and can likely be modeled.
Indeed, disparities between effects observed when particle shape, size, and concentration are varied leave much to be desired in the elucidation of how MPs might impact human health. The studies we have described here have formed a strong foundation for this field, and future inquiries will have increasing relevance to human health.
Author Contributions
Conceptualization, J.S.W. and K.H.P.; methodology and investigation, J.S.W. and K.H.P.; writing, J.S.W. and K.H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank Carl Arnold, a longtime advocate for the environment, who brought together this collaboration.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burns, E.E.; Boxall, A.B.A. Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 2018, 37, 2776–2796. [Google Scholar] [CrossRef] [Green Version]
- Bucci, K.; Tulio, M.; Rochman, C.M. What is known and unknown about the effects of plastic pollution: A meta-analysis and systematic review. Ecol. Appl. 2020, 30, e02044. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Belzagui, F.; Buscio, V.; Gutiérrez-Bouzán, C.; Vilaseca, M. Cigarette butts as a microfiber source with a microplastic level of concern. Sci. Total Environ. 2021, 762, 144165. [Google Scholar] [CrossRef] [PubMed]
- Kögel, T.; Bjorøy, Ø.; Toto, B.; Bienfait, A.; Sanden, M. Micro- and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 2020, 709, 136050. [Google Scholar] [CrossRef]
- Ward, J.E.; Rosa, M.; Shumway, S.E. Capture, ingestion, and egestion of microplastics by suspension-feeding bivalves: A 40-year history. Anthr. Coasts 2019, 2, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Su, L.; Cai, H.; Rochman, C.M.; Li, Q.; Kolandhasamy, P.; Peng, J.; Shi, H. The uptake of microfibers by freshwater Asian clams (Corbicula fluminea) varies based upon physicochemical properties. Chemosphere 2019, 221, 107–114. [Google Scholar] [CrossRef]
- Horn, D.A.; Granek, E.F.; Steele, C.L. Effects of environmentally relevant concentrations of microplastic fibers on Pacific mole crab (Emerita analoga) mortality and reproduction. Limnol. Oceanogr. Lett. 2020, 5, 74–83. [Google Scholar] [CrossRef] [Green Version]
- Setyorini, L.; Michler-Kozma, D.; Sures, B.; Gabel, F. Transfer and effects of PET microfibers in Chironomus riparius. Sci. Total Environ. 2021, 757, 143735. [Google Scholar] [CrossRef]
- Alnajar, N.; Jha, A.N.; Turner, A. Impacts of microplastic fibres on the marine mussel, Mytilus galloprovinciallis. Chemosphere 2021, 262, 128290. [Google Scholar] [CrossRef] [PubMed]
- Woods, M.N.; Hong, T.J.; Baughman, D.; Andrews, G.; Fields, D.M.; Matrai, P.A. Accumulation and effects of microplastic fibers in American lobster larvae (Homarus americanus). Mar. Pollut. Bull. 2020, 157, 111280. [Google Scholar] [CrossRef] [PubMed]
- Au, S.; TF, B.; Bridges, W.; Klaine, S. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 2015, 34, 2564–2572. [Google Scholar] [CrossRef]
- Blarer, P.; Burkhardt-Holm, P. Microplastics affect assimilation efficiency in the freshwater amphipod Gammarus fossarum. Environ. Sci. Pollut. Res. 2016, 23, 23522–23532. [Google Scholar] [CrossRef]
- Frydkjær, C.; Iversen, N.; Roslev, P. Ingestion and egestion of microplastics by the cladoceran daphnia magna: Effects of regular and irregular shaped plastic and sorbed phenanthrene. Bull. Environ. Contam. Toxicol. 2017, 99, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Feng, Y.; Duan, Z.; Duan, X.; Zhao, S.; Wang, Y.; Gong, Z.; Wang, L. Toxicities of microplastic fibers and granules on the development of zebrafish embryos and their combined effects with cadmium. Chemosphere 2021, 269, 128677. [Google Scholar] [CrossRef] [PubMed]
- Mendrik, F.M.; Henry, T.B.; Burdett, H.; Hackney, C.R.; Waller, C.; Parsons, D.R.; Hennige, S.J. Species-specific impact of microplastics on coral physiology. Environ. Pollut. 2021, 269, 116238. [Google Scholar] [CrossRef]
- Leads, R.R.; Burnett, K.G.; Weinstein, J.E. The effect of microplastic ingestion on survival of the grass shrimp Palaemonetes pugio (Holthuis, 1949) challenged with Vibrio campbellii. Environ. Toxicol. Chem. 2019, 38, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Pauly, J.L.; Stegmeier, S.J.; Allaart, H.A.; Cheney, R.T.; Zhang, P.J.; Mayer, A.G.; Streck, R.J. Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidemiol. Prev. Biomarkers 1998, 7, 419–428. [Google Scholar]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.S.; Anuar, S.T.; Azmi, A.A.; Khalik, W.M.A.W.M.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of microplastics in human colectomy specimens. JGH Open 2021, 5, 116–121. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool: A prospective case series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Fournier, E.; Etienne-Mesmin, L.; Grootaert, C.; Jelsbak, L.; Syberg, K.; Blanquet-Diot, S.; Mercier-Bonin, M. Microplastics in the human digestive environment: A focus on the potential and challenges facing in vitro gut model development. J. Hazard. Mater. 2021, 415, 125632. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Hwang, J.; Bang, J.; Han, S.; Kim, T.; Oh, Y.; Hwang, Y.; Choi, J.; Hong, J. In vitro toxicity from a physical perspective of polyethylene microplastics based on statistical curvature change analysis. Sci. Total Environ. 2021, 752, 142242. [Google Scholar] [CrossRef] [PubMed]
- Bour, A.; Hamann Sandgaard, M.; Syberg, K.; Palmqvist, A.; Carney Almroth, B. Comprehending the complexity of microplastic organismal exposures and effects, to improve testing frameworks. J. Hazard. Mater. 2021, 415, 125652. [Google Scholar] [CrossRef] [PubMed]
- Roch, S.; Ros, A.F.H.; Friedrich, C.; Brinker, A. Microplastic evacuation in fish is particle size-dependent. Freshw. Biol. 2021, 66, 926–935. [Google Scholar] [CrossRef]
- An, D.; Na, J.; Song, J.; Jung, J. Size-dependent chronic toxicity of fragmented polyethylene microplastics to Daphnia magna. Chemosphere 2021, 271, 129591. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, K.; Ding, D.; Liu, J.; Lei, Z.; Chen, X.; Ye, G.; Zhang, J.; Shen, H.; Yan, C.; et al. Size-dependent adverse effects of microplastics on intestinal microbiota and metabolic homeostasis in the marine medaka (Oryzias melastigma). Environ. Int. 2021, 151, 106452. [Google Scholar] [CrossRef]
- Abarghouei, S.; Hedayati, A.; Raeisi, M.; Hadavand, B.S.; Rezaei, H.; Abed-Elmdoust, A. Size-dependent effects of microplastic on uptake, immune system, related gene expression and histopathology of goldfish (Carassius auratus). Chemosphere 2021, 276, 129977. [Google Scholar] [CrossRef]
- Bour, A.; Haarr, A.; Keiter, S.; Hylland, K. Environmentally relevant microplastic exposure affects sediment-dwelling bivalves. Environ. Pollut. 2018, 236, 652–660. [Google Scholar] [CrossRef]
- Choi, J.; Kim, K.; Hong, S.; Park, K.; Park, J. Impact of polyethylene terephthalate microfiber length on cellular responses in the Mediterranean mussel Mytilus galloprovincialis. Mar. Environ. Res. 2021, 168, 105320. [Google Scholar] [CrossRef]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, Y.; Wang, C.; Wang, X.; Zhou, J.; Shen, M.; Zhao, Y.; Fu, Z.; Jin, Y. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ. Pollut. 2019, 255, 113122. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Amarakoon, D.; Wei, C.; Choi, K.Y.; Smolensky, D.; Lee, S.-H. Adverse effect of polystyrene microplastics (PS-MPs) on tube formation and viability of human umbilical vein endothelial cells. Food Chem. Toxicol. 2021, 154, 112356. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.; et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Lenz, R.; Enders, K.; Nielsen, T.G. Microplastic exposure studies should be environmentally realistic. Proc. Natl. Acad. Sci. USA 2016, 113, E4121–E4122. [Google Scholar] [CrossRef] [Green Version]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.J.; Perono, G.; Tommasi, F.; Pagano, G.; Oral, R.; Burić, P.; Kovačić, I.; Toscanesi, M.; Trifuoggi, M.; Lyons, D.M. Resolving the effects of environmental micro- and nanoplastics exposure in biota: A knowledge gap analysis. Sci. Total Environ. 2021, 780, 146534. [Google Scholar] [CrossRef]
- Lindeque, P.K.; Cole, M.; Coppock, R.L.; Lewis, C.N.; Miller, R.Z.; Watts, A.J.R.; Wilson-McNeal, A.; Wright, S.L.; Galloway, T.S. Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Environ. Pollut. 2020, 265, 114721. [Google Scholar] [CrossRef]
- Covernton, G.A.; Pearce, C.M.; Gurney-Smith, H.J.; Chastain, S.G.; Ross, P.S.; Dower, J.F.; Dudas, S.E. Size and shape matter: A preliminary analysis of microplastic sampling technique in seawater studies with implications for ecological risk assessment. Sci. Total Environ. 2019, 667, 124–132. [Google Scholar] [CrossRef]
- Horton, A.A.; Svendsen, C.; Williams, R.J.; Spurgeon, D.J.; Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK—Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017, 114, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Laglbauer, B.J.L.; Franco-Santos, R.M.; Andreu-Cazenave, M.; Brunelli, L.; Papadatou, M.; Palatinus, A.; Grego, M.; Deprez, T. Macrodebris and microplastics from beaches in Slovenia. Mar. Pollut. Bull. 2014, 89, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.; Worch, E.; Knepper, T.P. Occurrence and spatial distribution of microplastics in river shore sediments of the rhine-main area in Germany. Environ. Sci. Technol. 2015, 49, 6070–6076. [Google Scholar] [CrossRef] [PubMed]
- Mathalon, A.; Hill, P. Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Mar. Pollut. Bull. 2014, 81, 69–79. [Google Scholar] [CrossRef]
- Woods, M.; Stack, M.; Fields, D.; Shaw, S.; Matrai, P. Microplastic fiber uptake, ingestion, and egestion rates in the blue mussel (Mytilus edulis). Mar. Pollut. Bull. 2018, 137, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.; Zhang, L.; Sun, L.; Lin, C.; Wang, Q.; Liu, S.; Sun, J.; Yang, H. Effect of chronic exposure to microplastic fibre ingestion in the sea cucumber Apostichopus japonicus. Ecotoxicol. Environ. Saf. 2021, 209, 111794. [Google Scholar] [CrossRef] [PubMed]
- Crump, A.; Mullens, C.; Bethell, E.J.; Cunningham, E.M.; Arnott, G. Microplastics disrupt hermit crab shell selection. Biol. Lett. 2020, 16, 20200030. [Google Scholar] [CrossRef] [PubMed]
- Bruck, S.; Ford, A.T. Chronic ingestion of polystyrene microparticles in low doses has no effect on food consumption and growth to the intertidal amphipod Echinogammarus marinus? Environ. Pollut. 2018, 233, 1125–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, A.; Scherer, C.; Brennholt, N.; Reifferscheid, G.; Wagner, M. PET microplastics do not negatively affect the survival, development, metabolism and feeding activity of the freshwater invertebrate Gammarus pulex. Environ. Pollut. 2018, 234, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Martins, M.; Sobral, P.; Costa, P.M.; Costa, M.H. An assessment of the ability to ingest and excrete microplastics by filter-feeders: A case study with the Mediterranean mussel. Environ. Pollut. 2019, 245, 600–606. [Google Scholar] [CrossRef]
- Green, D.S. Effects of microplastics on European flat oysters, Ostrea edulis and their associated benthic communities. Environ. Pollut. 2016, 216, 95–103. [Google Scholar] [CrossRef]
- Besseling, E.; Wegner, A.; Foekema, E.M.; Van den Heuvel-Greve, M.J.; Koelmans, A.A. Effects of Microplastic on Fitness and PCB Bioaccumulation by the Lugworm Arenicola marina (L.). Environ. Sci. Technol. 2012, 47, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, E.M.; Sigwart, J.D. Environmentally Accurate Microplastic Levels and Their Absence from Exposure Studies. Integr. Comp. Biol. 2019, 59, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.D.; Chen, C.W.; Chen, Y.C.; Chen, H.H.; Lee, J.S.; Lin, C.H. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater. 2020, 385, 121575. [Google Scholar] [CrossRef]
- Stanković, J.; Milošević, D.; Jovanović, B.; Savić-Zdravković, D.; Petrović, A.; Raković, M.; Stanković, N.; Stojković Piperac, M. In Situ Effects of a Microplastic Mixture on the Community Structure of Benthic Macroinvertebrates in a Freshwater Pond. Environ. Toxicol. Chem. 2021. [Google Scholar] [CrossRef]
- Seuront, L. Microplastic leachates impair behavioural vigilance and predator avoidance in a temperate intertidal gastropod. Biol. Lett. 2018, 14, 20180453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, N.; Rist, S.; Bodin, J.; Jensen, L.; Schmidt, S.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-Term Field Measurement of Sorption of Organic Contaminants to Five Types of Plastic Pellets: Implications for Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M. The Role of Plastic Debris as Another Source of Hazardous Chemicals in Lower-Trophic Level Organisms. Handb. Environ. Chem. 2016, 78, 281–295. [Google Scholar] [CrossRef]
- Rochman, C.M. The Complex Mixture, Fate and Toxicity of Chemicals Associated with Plastic Debris in the Marine Environment. Mar. Anthropog. Litter 2015, 117–140. [Google Scholar] [CrossRef] [Green Version]
- Athira, N.; Jaya, D.S. The Use of Fish Biomarkers for Assessing Textile Effluent Contamination of Aquatic Ecosystems: A Review. Nat. Environ. Pollut. Technol. 2018, 17, 25–34. [Google Scholar]
- Selvaraj, D.; Leena, R.; Kamal, D. Toxicological and histopathological impacts of textile dyeing industry effluent on a selected teleost fish Poecilia reticulata. Asian J. Pharmacol. Toxicol. 2015, 3, 26–30. [Google Scholar]
- Suhrhoff, T.; Scholz-Böttcher, B. Qualitative impact of salinity, UV radiation and turbulence on leaching of organic plastic additives from four common plastics—A lab experiment. Mar. Pollut. Bull. 2016, 102, 84–94. [Google Scholar] [CrossRef]
- Anbumani, S.; Kakkar, P. Ecotoxicological effects of microplastics on biota: A review. Environ. Sci. Pollut. Res. Int. 2018, 25, 14373–14396. [Google Scholar] [CrossRef]
- Turner, A.; Holmes, L.A.; Turner, A.; Holmes, L.A. Adsorption of trace metals by microplastic pellets in fresh water. Environ. Chem. 2015, 12, 600–610. [Google Scholar] [CrossRef]
- Browne, M.A.; Niven, S.J.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic Moves Pollutants and Additives to Worms, Reducing Functions Linked to Health and Biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef] [Green Version]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef]
- Chua, E.; Shimeta, J.; Nugegoda, D.; Morrison, P.; Clarke, B. Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes compressa. Environ. Sci. Technol. 2014, 48, 8127–8134. [Google Scholar] [CrossRef]
- Zhu, M.; Chernick, M.; Rittschof, D.; Hinton, D. Chronic dietary exposure to polystyrene microplastics in maturing Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2019, 220, 105396. [Google Scholar] [CrossRef]
- Hu, L.; Chernick, M.; Lewis, A.M.; Ferguson, P.L.; Hinton, D.E. Chronic microfiber exposure in adult Japanese medaka (Oryzias latipes). PLoS ONE 2020, 15, e0229962. [Google Scholar] [CrossRef] [Green Version]
- Avagyan, R.; Luongo, G.; Thorsén, G.; Östman, C. Benzothiazole, benzotriazole, and their derivates in clothing textiles—A potential source of environmental pollutants and human exposure. Environ. Sci. Pollut. Res. Int. 2015, 22, 5842–5849. [Google Scholar] [CrossRef]
- Rochman, C.; Kurobe, T.; Flores, I.; Teh, S. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef]
- Ye, T.; Kang, M.; Huang, Q.; Fang, C.; Chen, Y.; Shen, H.; Dong, S. Exposure to DEHP and MEHP from hatching to adulthood causes reproductive dysfunction and endocrine disruption in marine medaka (Oryzias melastigma). Aquat. Toxicol. 2014, 146, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Foekema, E.M.; Van den Heuvel-Greve, M.J.; Koelmans, A.A. The Effect of Microplastic on the Uptake of Chemicals by the Lugworm Arenicola marina (L.) under Environmentally Relevant Exposure Conditions. Environ. Sci. Technol. 2017, 51, 8795–8804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehse, S.; Kloas, W.; Zarfl, C. Microplastics Reduce Short-Term Effects of Environmental Contaminants. Part I: Effects of Bisphenol A on Freshwater Zooplankton Are Lower in Presence of Polyamide Particles. Int. J. Environ. Res. Public Health 2018, 15, 280. [Google Scholar] [CrossRef] [Green Version]
- Beckingham, B.; Ghosh, U. Differential bioavailability of polychlorinated biphenyls associated with environmental particles: Microplastic in comparison to wood, coal and biochar. Environ. Pollut. 2017, 220, 150–158. [Google Scholar] [CrossRef]
- Kleinteich, J.; Seidensticker, S.; Marggrander, N.; Zarfl, C. Microplastics Reduce Short-Term Effects of Environmental Contaminants. Part II: Polyethylene Particles Decrease the Effect of Polycyclic Aromatic Hydrocarbons on Microorganisms. Int. J. Environ. Res. Public Health 2018, 15, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norland, S.; Vorkamp, K.; Bogevik, A.S.; Koelmans, A.A.; Diepens, N.J.; Burgerhout, E.; Hansen, Ø.J.; Puvanendran, V.; Rønnestad, I. Assessing microplastic as a vector for chemical entry into fish larvae using a novel tube-feeding approach. Chemosphere 2021, 265, 129144. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Foekema, E.M. Leaching of plastic additives to marine organisms. Environ. Pollut. 2014, 187, 49–54. [Google Scholar] [CrossRef]
- Le Bihanic, F.; Clérandeau, C.; Cormier, B.; Crebassa, J.-C.; Keiter, S.H.; Beiras, R.; Morin, B.; Bégout, M.-L.; Cousin, X.; Cachot, J. Organic contaminants sorbed to microplastics affect marine medaka fish early life stages development. Mar. Pollut. Bull. 2020, 154, 111059. [Google Scholar]
- Bucci, K.; Bikker, J.; Stevack, K.; Watson-Leung, T.; Rochman, C. Impacts to Larval Fathead Minnows Vary between Preconsumer and Environmental Microplastics. Environ. Toxicol. Chem. 2021. [Google Scholar] [CrossRef]
- O’Donovan, S.; Mestre, N.C.; Abel, S.; Fonseca, T.G.; Carteny, C.C.; Cormier, B.; Keiter, S.H.; Bebianno, M.J. Ecotoxicological Effects of Chemical Contaminants Adsorbed to Microplastics in the Clam Scrobicularia plana. Front. Mar. Sci. 2018, 5, 143. [Google Scholar] [CrossRef] [Green Version]
- Rios-Fuster, B.; Arechavala-Lopez, P.; García-Marcos, K.; Alomar, C.; Compa, M.; Álvarez, E.; Julià, M.; Martí, A.; Sureda, A.; Deudero, S. Experimental evidence of physiological and behavioral effects of microplastic ingestion in Sparus aurata. Aquat. Toxicol. 2021, 231, 105737. [Google Scholar] [CrossRef] [PubMed]
- Rendell-Bhatti, F.; Paganos, P.; Pouch, A.; Mitchell, C.; D’Aniello, S.; Godley, B.J.; Pazdro, K.; Arnone, M.I.; Jimenez-Guri, E. Developmental toxicity of plastic leachates on the sea urchin Paracentrotus lividus. Environ. Pollut. 2021, 269, 115744. [Google Scholar] [CrossRef] [PubMed]
- Carney Almroth, B.; Cartine, J.; Jönander, C.; Karlsson, M.; Langlois, J.; Lindström, M.; Lundin, J.; Melander, N.; Pesqueda, A.; Rahmqvist, I.; et al. Assessing the effects of textile leachates in fish using multiple testing methods: From gene expression to behavior. Ecotoxicol. Environ. Saf. 2021, 207, 111523. [Google Scholar] [CrossRef]
- Sait, S.T.L.; Sørensen, L.; Kubowicz, S.; Vike-Jonas, K.; Gonzalez, S.V.; Asimakopoulos, A.G.; Booth, A.M. Microplastic fibres from synthetic textiles: Environmental degradation and additive chemical content. Environ. Pollut. 2021, 268, 115745. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, Z.; Shen, R.; Wang, M.; Huang, Y.; Ren, H.; Zhang, Y.; Lemos, B. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ. Int. 2020, 143, 105916. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Qiao, R.; Bonilla, M.M.; Yang, X.; Ren, H.; Lemos, B. Evidence that microplastics aggravate the toxicity of organophosphorus flame retardants in mice (Mus musculus). J. Hazard. Mater. 2018, 357, 348–354. [Google Scholar] [CrossRef]
- Wu, P.; Tang, Y.; Jin, H.; Song, Y.; Liu, Y.; Cai, Z. Consequential fate of bisphenol-attached PVC microplastics in water and simulated intestinal fluids. Environ. Sci. Ecotechnol. 2020, 2, 100027. [Google Scholar] [CrossRef]
- Mincer, T.J.; Zettler, E.R.; Amaral-Zettler, L.A. Biofilms on Plastic Debris and Their Influence on Marine Nutrient Cycling, Productivity, and Hazardous Chemical Mobility. Handb. Environ. Chem. 2016, 78, 221–233. [Google Scholar] [CrossRef]
- Savoca, M.S.; Tyson, C.W.; McGill, M.; Slager, C.J. Odours from marine plastic debris induce food search behaviours in a forage fish. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171000. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Liu, L.; Chen, L.; Teng, J.; Zhu, X.; Zhao, J.; Wang, Q. Spatial and seasonal variations in biofilm formation on microplastics in coastal waters. Sci. Total Environ. 2021, 770, 145303. [Google Scholar] [CrossRef] [PubMed]
- Amaral-Zettler, L.A.; Zettler, E.R.; Mincer, T.J. Ecology of the plastisphere. Nat. Rev. Microbiol. 2020, 18, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Kettner, M.T.; Oberbeckmann, S.; Labrenz, M.; Grossart, H.-P. The Eukaryotic Life on Microplastics in Brackish Ecosystems. Front. Microbiol. 2019, 10, 538. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Löder, M.G.J.; Labrenz, M.; Oberbeckmann, S.; Löder, M.G.J.; Labrenz, M. Marine microplastic-associated biofilms—A review. Environ. Chem. 2015, 12, 551–562. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).