1. Introduction
The quinoline skeleton occurs in many natural products, including alkaloids. Some containing the quinoline ring natural products have been used as lead molecules for the development of novel biologically active compounds and drugs [
1,
2,
3,
4,
5]. Many modern drugs have been designed based on the quinoline scaffold. The quinoline scaffold has often been used for the design and synthesis of various synthetic compounds with pharmacological properties [
1,
2,
3,
4,
5,
6,
7,
8].
The quinoline ring is a structural part of many biologically active compounds. Quinoline derivatives exhibit a variety of biological activities including antibacterial, antivirus, anticancer, antifungal, antimalarial, cardiovascular, anticonvulsant, analgesic, antimycobacterial, anti-inflammatory, antihelminthic, antiprotozoal and antioxidant properties [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10]. Such antimalarial drugs as chloroquine, hydroxychloroquine, amodiaquine, and primaquine have been developed based on the quinoline scaffold [
1,
2,
3,
4,
5,
6,
7,
8].
Fluoroquinolone drugs are some of the most commonly used antibiotics in modern pharmacotherapy. These drugs are broad-spectrum bacteriocidals, which exhibit high activity against both Gram-negative and Gram-positive bacteria [
1,
2,
3,
4,
5,
6,
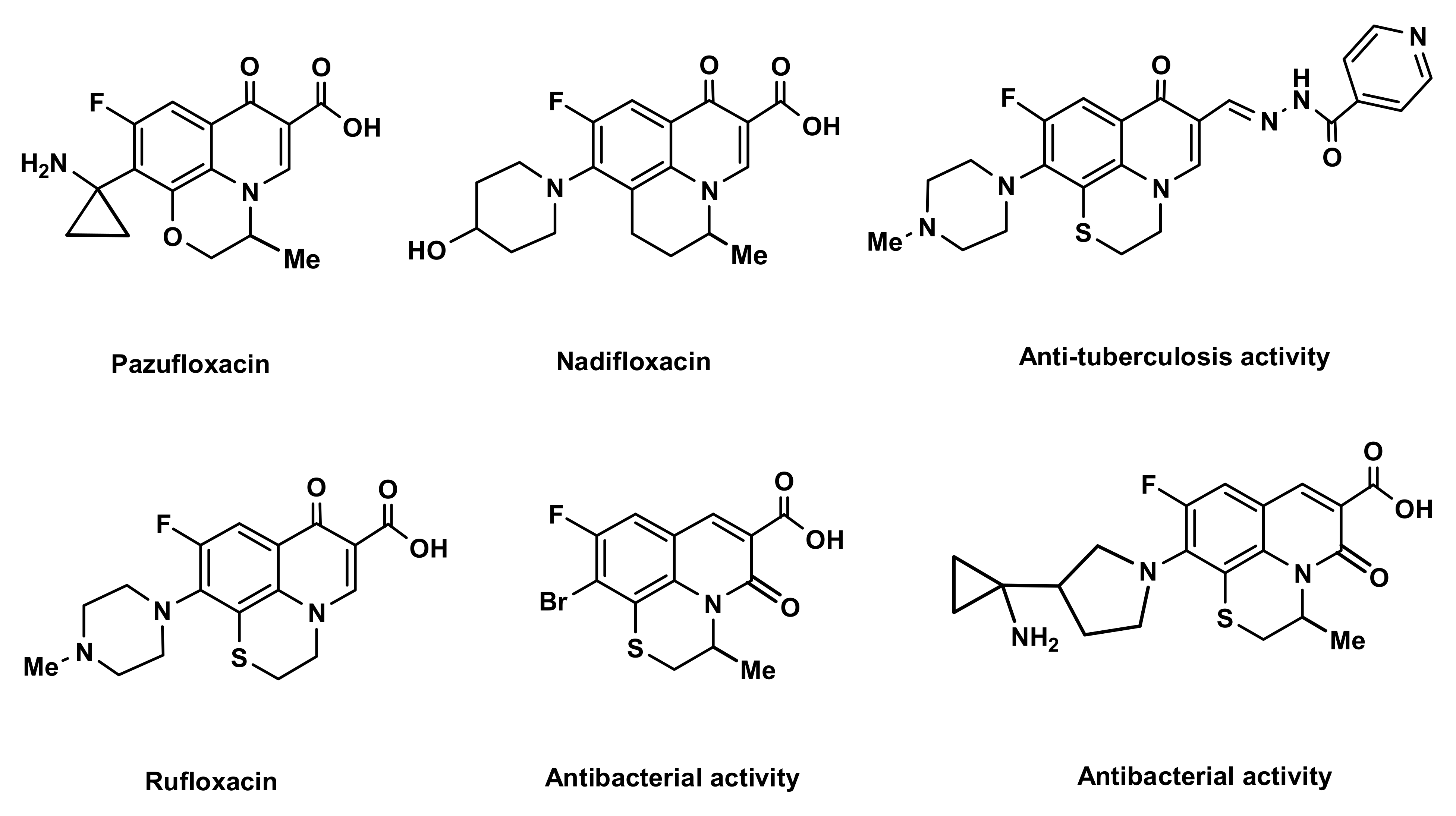
7]. A number of fluoroquinolone antibiotics have a tricyclic core structure (pazufloxacin, rufloxacin, nadifloxacin), in which the quinoline ring is condensed with six-membered cycles (2,3-dihydro-1,4-thiazine, 2,3-dihydro-1,4-oxazine,
Figure 1).
A variety of biologically active compounds are based on scaffolds in the form of a combination of nitrogen and sulfur heterocycles [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20]. Pazufloxacin, rufloxacin and nadifloxacin antibiotics have the quinoline core structure condensed with six-membered heterocycles (
Figure 1) [
17,
18,
19]. The well-known antibiotics penicillin and cephalosporin are examples of drugs containing fused nitrogen and sulfur heterocycles.
The [1,4]thiazino[2,3,4-
ij]quinolin-4-ium scaffold can be considered as being a result of annulation of the quinoline core structure with the thiazine heterocycle. The [1,4]thiazino[2,3,4-
ij]quinolin-4-ium derivatives exhibit a broad spectrum of biological activities [
18,
19,
20,
21,
22,
23], including anticancer [
21], antibacterial [
22] and anti-tuberculosis [
23] properties. The commonly used fluoroquinolone antibiotic rufloxacin belongs structurally to this class of compounds (
Figure 1).
Figure 1.
Known biologically active compounds structurally related to the 2
H,3
H-[1,4]thiazino[2,3,4-
ij]quinolin-4-ium scaffold, which have the quinoline ring condensed with six-membered cycles (fluoroquinolone antibiotics [
17,
18,
19], compounds with antibacterial [
22] and anti-tuberculosis [
23] activity).
Figure 1.
Known biologically active compounds structurally related to the 2
H,3
H-[1,4]thiazino[2,3,4-
ij]quinolin-4-ium scaffold, which have the quinoline ring condensed with six-membered cycles (fluoroquinolone antibiotics [
17,
18,
19], compounds with antibacterial [
22] and anti-tuberculosis [
23] activity).
In the last decade, we have developed efficient regioselective approaches to novel heterocyclic and condensed organochalcogen compounds by means of cyclization and annulation reactions based on chalcogen-containing reagents and unsaturated compounds [
24,
25,
26,
27,
28,
29,
30,
31,
32,
33]. Recently, we carried out the annulation reactions of 8-quinolinesulfenyl halides with vinyl heteroatom compounds and cycloalkenes to obtain novel [1,4]thiazino[2,3,4-
ij]quinolin-4-ium derivatives in high yields [
32,
33]. For example, the annulation reactions with divinyl sulfide and divinyl selenide proceeded with the addition of the sulfur atom of 8-quinolinesulfenyl electrophile at the β-position of the vinylchalcogenyl group (the Markovnikov direction), affording 3-(vinylsulfanyl)- and 3-(vinylselanyl)-2
H,3
H-[1,4]thiazino[2,3,4-
ij]quinolin-4-ium chlorides in 94% and 50% yields, respectively. However, in the case of tetravinyl silane, the attachment of the sulfur atom occurred at the
α-carbon atom of the vinylsilyl group (the anti-Markovnikov direction), leading to 2-(trivinylsilyl)-2
H,3
H-[1,4]thiazino[2,3,4-
ij]quinolin-4-ium chloride in a 98% yield [
32].
The reactions of 8-quinolinesulfenyl halides (chloride and bromide) with cycloalkenes (cyclopentene, cyclohexene, and cyclooctene), depending on the nature of the halogen and the reaction conditions, gave products of electrophilic addition or annulation products in high yields [
33]. The reactions of 8-quinolinesulfenyl chloride with cycloalkenes afforded 8-[(2-chlorocycloalkyl)sulfanyl]quinolines in quantitative yields, while condensed tetracyclic compounds were synthesized in 90–100% yields from 8-quinolinesulfenyl bromide and cycloalkenes. Thus, the reaction of 8-quinolinesulfenyl chloride with cyclopentene at room temperature in methylene chloride gave 8-[(2-chlorocyclopentyl)sulfanyl]quinoline in a quantitative yield, whereas condensed tetracyclic compound, 8,9,10,10a-tetrahydro-7a
H-cyclopenta[5,6][1,4]thiazino[2,3,4-
ij]quinolin-11-ium bromide (
1), was obtained in a 98% yield by the annulation reaction of 8-quinolinesulfenyl bromide with cyclopentene in chloroform under reflux (
Scheme 1) [
33].
Although some synthetic methods for the preparation of [1,4]thiazino[2,3,4-
ij]quinolin-4-ium derivatives have been developed [
32,
33,
34,
35,
36,
37,
38,
39,
40], the annulation reactions of 8-quinolinesulfenyl halides with natural products such as eugenol, isoeugenol, methyleugenol, anethole as well as with 1
H-indene, styrene derivatives (4-methylstyrene and α-methylstyrene) and simple alkenes (1-hexene and 1-heptene) have not been described in the literature.
The development of efficient selective methods for the preparation of new [1,4]thiazino[2,3,4-ij]quinolin-4-ium derivatives with promising biological activity is an urgent task. The aim of this research is the development of the regioselective synthesis of new derivatives of [1,4]thiazino[2,3,4-ij]quinolin-4-ium based on annulation reactions of 8-quinolinesulfenyl halides with natural products (eugenol, isoeugenol, methyleugenol, trans-anethole) as well as with 1-hexene, 1-heptene, 1H-indene and styrene derivatives (4-methylstyrene and α-methylstyrene) and the evaluation of their antimicrobial activity.
2. Results and Discussion
The starting compounds, 8-quinolinesulfenyl chloride
3 and bromide
4, were generated in situ from di(8-quinolinyl) disulfide (
2) in methylene chloride or chloroform and used without isolation in further reactions with alkenes and natural products (eugenol derivatives,
trans-anethole) (
Scheme 2).
Taking into account the known data on the reactions of 8-quinolinesulfenyl halides with cycloalkenes [
33], which produced electrophilic addition products of sulfenyl chloride
3 and annulation products in the case of sulfenyl bromide
4 (
Scheme 1), it could be assumed that the reactions of 8-quinolinesulfenyl halides with 1-alkenes would proceed similarly. However, the reactions of sulfenyl chloride
3 with 1-alkenes gave a complex mixture of compounds including electrophilic addition products under the same conditions as indicated in
Scheme 1. Nevertheless, the reactions of sulfenyl bromide
4 with 1-alkenes at room temperature in methylene chloride led to annulation products
5 and
6 in 85% and 81% yields, respectively (
Scheme 3).
Compounds 5 and 6 are water-soluble light-yellow powders with a melting point of about 120 °C.
The naturally occurring products eugenol (4-allyl-2-methoxyphenol) and isoeugenol (2-methoxy-4-(1-propenyl)phenol) were involved in the annulation reactions with 8-quinolinesulfenyl chloride
3. The reaction of sulfenyl chloride
3 with isoeugenol smoothly proceeded at room temperature in chloroform, giving compound
7 in a 90% yield (
Scheme 4).
The reaction of sulfenyl chloride
3 with eugenol under the same conditions as the synthesis of compound
7 was very sluggish (40% yield of the annulation product). However, carrying out the reaction of sulfenyl chloride
3 with eugenol under reflux in chloroform for 7 h allowed us to obtain annulation product
8 in a 75% yield (
Scheme 4).
The reaction of sulfenyl chloride 3 with eugenol includes electrophilic addition of the sulfur atom of sulfenyl electrophile to the α-carbon atom of the vinyl group (the anti-Markovnikov direction), while the opposite regiochemistry is observed in the annulation reaction of sulfenyl chloride 3 with isoeugenol.
Another naturally occurring compound,
trans-anethole (1-methoxy-4-(
E-1-propenyl)benzene), appears to be very reactive in annulation reactions. The reaction of sulfenyl chloride
3 with
trans-anethole was carried out at room temperature in methylene chloride, affording quinolinium chloride
9 in a quantitative yield (
Scheme 5).
The reaction of sulfenyl chloride
3 with methyl eugenol (4-allyl-1,2-dimethoxybenzene) seems to proceed more slowly than with eugenol. Under the same conditions as the synthesis of product
9, the reaction of sulfenyl chloride
3 with methyl eugenol afforded the annulation product in a 57% yield. However, after refluxing the mixture of sulfenyl chloride
3 with methyl eugenol in chloroform for 3 h, annulation product
10 was obtained in a 98% yield (
Scheme 5).
The double bond in trans-anethole and isoeugenol occurs in conjugation with the benzene ring, and these compounds demonstrate higher activity in the annulation reactions compared to eugenol and methyl eugenol bearing the allyl fragment without conjugation of the double bond.
Such substrates as styrene, 4-methylstyrene and α-methylstyrene and 1H-indene also have the double bond, which is in conjugation with the benzene ring. We assumed that these substrates may be active in the annulation and carried out the reactions of sulfenyl chloride 3 with them.
The reactions of quinoline sulfenyl chloride
3 with styrene proceeded smoothly in methylene chloride at room temperature for 24 h to give quinolinium chloride
11 in a 97% yield (
Scheme 6).
Under the same conditions, the reactions of sulfenyl chloride
3 with 1
H-indene afforded the condensed five-membered product in only a 43% yield. Refluxing the reaction mixture resulted in the formation of a small amount of by-product along with the target compound. However, when the reaction time was increased to 65 h at room temperature, pure annulation product
12 was obtained in an 80% yield (
Scheme 6).
The reaction of sulfenyl chloride
3 with 4-methylstyrene was carried out at room temperature for 24 h in methylene chloride affording compound
13 in quantitative yield (
Scheme 7). Under the same conditions as the synthesis of product
13, the reaction of sulfenyl chloride
3 with α-methylstyrene gave annulation product
14 in an 87% yield (
Scheme 7).
The reaction of sulfenyl chloride 3 with α-methylstyrene seems to proceed more slowly than with 4-methylstyrene and styrene. The methyl substituent at position 4 of the benzene ring has little influence on the yield of the product, and compounds 11 and 13 (derived from both styrene and 4-methylstyrene) were obtained in 97% and quantitative yields, while the introduction of the methyl substituent to the α-position of the double bond affects on the annulation reaction and slightly decreases the the annulation product yield to 87% under the same conditions.
All the studied substrates can be schematically divided into two groups: terminal alkenes including allyl arenes and styrene derivatives, which contain the double bond in conjugation with the benzene ring. In general, the latter group of compounds demonstrates the higher activity in the annulation reactions compared to the terminal alkenes (
Scheme 8).
The annulation reactions with the terminal alkenes, whose double bond is not in conjugation with the benzene ring, proceed with the attachment of the sulfur atom of sulfenyl halides
3,
4 at the α-position of the double bond (the anti-Markovnikov direction). In the case of styrene derivatives, the addition of the sulfur atom occurs at the terminal carbon atom of the double bond (the Markovnikov direction). Possible intermediates
A and
B, which correspond to two directions of these reactions, can be considered for the explanation of these trends (
Scheme 8).
We assume that the reactions with terminal alkenes proceed via three-membered thiiranium intermediates
A. It is known that the electrophilic addition reactions of sulfenyl chlorides [
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54] with linear 1-alkene mainly give anti-Markovnikov products [
41,
42,
43], and thiiranium cations are regarded as intermediates of these reactions [
42,
43,
44,
45]). Nucleophilic attack of the nitrogen atom of the quinoline ring occurs at the unsubstituted carbon atom of thiiranium intermediates
A due to the steric factor, which determines the anti-Markovnikov direction of the reactions (
Scheme 8).
It is assumed that linear carbocations
B are involved as intermediates in the reactions with styrene derivatives. In this case, linear carbocations
B are energetically favorable due to their stabilization by the benzene ring that provides the Markovnikov direction of the reactions (
Scheme 8). It is known that electrophilic addition of sulfenyl chlorides to styrene leads to Markovnikov products [
52,
53].
The antibacterial activity of the synthesized compounds was evaluated. The minimal inhibitory concentration (MIC) was determined using the broth standard microdilution method [
55].
The obtained results are presented in
Table 1. Compounds
1,
5–
14 were tested in vitro for antimicrobial activity against strains of the Gram-positive bacteria
Bacillus subtilis B-406 and
Enterococcus durans B-603 (which are similar in properties and taxonomic affiliation to bacteria
Staphylococcus aureus) and the Gram-negative bacteria
Escherichia coli B-1238 (the bacterial strains were taken from the All-Russian Collection of Microorganisms).
As can be seen from the presented data (
Table 1), compound
1 is active against
Enterococcus durans, but has low activity against other microorganisms. Compounds
5 and
6 differ only by one group, CH
2, but the activity of these compounds varies considerably. Compound
5 with a shorter carbon chain shows low activity, while compound
6 is superior to antibiotic gentamicin against both the Gram-positive
Enterococcus durans and the Gram-negative
Escherichia coli (
Table 1).
The obtained results were compared with the activity of standard aminoglycoside antibiotic gentamicin, the minimal inhibitory concentrations of which are 25, 50 and 100 μg/mL against Enterococcus durans, Bacillus subtilis and Escherichia coli, respectively.
Having the same molecular formula, products
7 and
8 are isomeric compounds obtained by the reactions of sulfenyl chloride
3 with isoeugenol and eugenol, respectively. These compounds differ significantly (by about 10 times) in activity (
Table 1). The eugenol-derived product
8, as well as that obtained from methyl eugenol, compound
10, are highly active against bacteria
Enterococcus durans and
Bacillus subtilis and are superior in their activity compared the antibiotic gentamicin against these microorganisms.
The structurally related compounds
7 and
9 (obtained from methyl isoeugenol and
trans-anethole), which formally differ in one hydroxyl group, show activity against Gram-positive bacteria
Enterococcus durans and
Bacillus subtilis, but are inferior to gentamicin (
Table 1).
Comparison of the structurally related compounds
11, 13 and
14 reveals the higher activity of products
11 and
13 (obtained from styrene and 4-methylstyrene), which are superior to the activity of gentamicin against bacteria
Enterococcus durans. α-methylstyrene-derived product
14 shows lower activity (
Table 1).
The highest level of activity was shown by product 12 (obtained from 1H-indene), which significantly exceeds the activity of gentamicin and all the obtained compounds against the bacteria Enterococcus durans and is more than 15 times higher than the activity of this antibiotic against Bacillus subtilis.
The structural assignments of synthesized compounds were made using
1H- and
13C-NMR spectroscopy including
J-modulation
13C-NMR experiments and confirmed by elemental analysis (see more in
Supplementary Materials).
The groups SCH-CH2N+, SCH2-CHN+ and SCH-CHN+ provide characteristic signals in the 1H- and 13C-NMR spectra. Thus, the carbon atoms of these groups, bonded with one or two protons, reveal characteristic signals in the 13C-NMR spectra. For example, the CH2N+ group manifests itself at ~62 ppm, while signals of the CHN+ moiety are observed in the lowfield region of ~69–75 ppm in the 13C-NMR spectra of the obtained compounds. The regiochemistry of the products was determined based on the 1H- and 13C-NMR spectra, taking into account the number of protons bonded with the carbon atoms of these groups.
3. Experimental Section
3.1. General Information
1H (400.1 MHz) and 13C (100.6 MHz) NMR spectra were recorded on a Bruker DPX-400 spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany) in a 2–5% solution with D2O or DMSO-d6. 1H and 13C chemical shifts (δ) are reported in parts per million (ppm), relative to tetramethylsilane (external) or to the residual solvent peaks of D2O (δ = 4.79) and DMSO-d6 (δ = 2.50 and 39.52 ppm for 1H and 13C NMR, respectively). Elemental analysis was performed on a Thermo Scientific FLASH 2000 Organic Elemental Analyzer (Thermo Fisher Scientific Inc., Milan, Italy). Melting points were determined on a Kofler Hot-Stage Microscope PolyTherm A apparatus (Wagner & Munz GmbH, München, Germany). Absolute solvents were used in the reactions.
3.2. Synthesis of Products 5 and 6 from 1-Alkenes
2-Butyl-2H,3H-[1,4]thiazino[2,3,4-ij]quinolin-4-ium bromide (5). A solution of bromine (0.072 g, 0.45 mmol) in methylene chloride (10 mL) was added dropwise to a solution of di(8-quinolinyl) disulfide (0.144 g, 0.45 mmol) in methylene chloride (10 mL), and the mixture was stirred for 10 min at room temperature. A solution of hexene-1 (0.076 g, 0.9 mmol) in methylene chloride (10 mL) was added dropwise. The reaction mixture was stirred for 48 h at room temperature and left overnight. The mixture was filtered, and the solvent was removed by rotary evaporator. The residue was washed with cold hexane and dried in vacuum, giving the product (0.248 g, 85% yield) in the form of a yellow powder, mp 220–221 °C.
1H-NMR (400 MHz, DMSO-d6): δ 0.90 (t, J = 7.3 Hz, 3H, CH3), 1.31–1.39 (m, 2H, CH2), 1.47–1.54 (m, 2H, CH2), 1.61–1.68 (m, 1H, CH2), 1.80–1.89 (m, 1H, CH2), 3.94 (m, 1H, SCH), 5.01 (dd, J = 13.9, 8.5 Hz, 1H, NCH2), 5.36 (d, J = 13.9 Hz, 1H, NCH2), 7.88–7.92 (m, 1H, Hquino), 8.12–8.14 (m, 1H, Hquino), 8.18–8.22 (m, 2H, Hquino), 9.28–9.30 (m, 1H, Hquino), 9.39–9.41 (m, 1H, Hquino).
13C-NMR (101 MHz, DMSO-d6): δ 13.75 (CH3), 21.65 (CH2), 28.03 (CH2), 30.81 (CH2), 36.21 (SCH), 62.05 (NCH2), 122.25 (Cquino), 126.40 (Cquino), 126.87 (Cquino), 129.39 (Cquino), 130.59 (Cquino), 133.14 (Cquino), 133.61 (Cquino), 148.65 (Cquino), 150.23 (Cquino).
Anal. Calcd for C15H18BrNS: C 55.56, H 5.59, N 4.32, Br 24.64, S 9.89. Found: C 55.69, H 5.96, N 4.44, Br 24.86, S 10.10.
2-Pentyl-2H,3H-[1,4]thiazino[2,3,4-ij]quinolin-4-ium bromide (6). A solution of bromine (0.130 g, 0.80 mmol) in methylene chloride (10 mL) was added dropwise to a solution of di(8-quinolinyl) disulfide (0.261 g, 0.80 mmol) in methylene chloride (10 mL), and the mixture was stirred for 10 min at room temperature. A solution of heptene-1 (0.157 g, 1.6 mmol) in methylene chloride (10 mL) was added dropwise. The reaction mixture was stirred for 48 h at room temperature and left overnight. The mixture was filtered, and the solvent was removed by rotary evaporator. The residue was washed with cold hexane and dried in vacuum, giving the product (0.438 g, 81% yield) in the form of a yellow powder, mp 219–220 °C.
1H-NMR (400 MHz, DMSO-d6): δ 0.88 (s, 3H, CH3), 1.30–1.32 (m, 4H, CH2), 1.49–1.56 (m, 2H, CH2), 1.58–1.65 (m, 1H, CH2), 1.81–1.84 (m, 1H, CH2), 3.94 (m, 1H, SCH), 5.01 (dd, J = 13.7, 8.3 Hz, 1H, NCH2), 5.35 (d, J = 13.7 Hz, 1H, NCH2), 7.88–7.92 (m, 1H, Hquino), 8.12–8.13 (m, 1H, Hquino), 8.18–8.22 (m, 2H, Hquino), 9.27–9.30 (m, 1H, Hquino), 9.39–9.41 (m, 1H, Hquino).
13C-NMR (101 MHz, DMSO-d6): δ 13.78 (CH3), 21.82 (CH2), 25.48 (CH2), 30.60 (CH2), 31.01 (CH2), 36.18 (SCH), 61.97 (NCH2), 122.21 (Cquino), 126.36 (Cquino), 126.81 (Cquino), 129.33 (Cquino), 130.54 (Cquino), 132.54 (Cquino), 133.10 (Cquino), 148.60 (Cquino), 150.19 (Cquino).
Anal. Calcd for C16H20BrNS: C 56.80, H 5.96, N 4.14, Br 23.62, S 9.48. Found: C 57.02, H 6.11, N 4.24, Br 23.97, S 9.72.
3.3. Synthesis of Compounds 7–10 from Natural Products
3-(4-Hydroxy-3-methoxyphenyl)-2-methyl-2H,3H-[1,4]thiazino[2,3,4-ij]quinolin-4-ium chloride (7). A solution of sulfuryl chloride (0.113 g, 0.83 mmol) in chloroform (10 mL) was added dropwise to a solution of di(8-quinolinyl) disulfide (0.268 g, 0.83 mmol) in chloroform (10 mL), and the mixture was stirred for 10 min at room temperature. A solution of isoeugenol (0.274 g, 1.6 mmol) in chloroform (10 mL) was added dropwise, and the reaction mixture was stirred for 45 h at room temperature and left overnight. The formed precipitate was filtered off, washed with hexane and dried in vacuum, giving the product (0.54 g, 90% yield) in the form of a yellow powder, mp 156–159 °C.
1H-NMR (400 MHz, DMSO-d6): δ 1.40 (d, J = 7.0 Hz, 3H, CH3,), 3.70 (s, 3H, OCH3), 3.96 (m, 1H, SCH), 5.73 (m, 1H, NCH), 6.58 (m, 1H, Ar), 6.61 (m, 1H, Ar), 6.80 (m, 1H, Ar), 7.94–7.98 (m, 1H, Hquino), 8.14–8.16 (m, 1H, Hquino), 8.24–8.27 (m, 1H, Hquino), 8.32–8.34 (m, 1H, Hquino), 9.43–9.45 (m, 1H, Hquino), 9.48–9.50 (m, 1H, Hquino).
13C-NMR (101 MHz, DMSO-d6): δ 20.04 (CH3), 37.46 (SCH2), 55.74 (OCH3), 73.69 (NCH), 110.39, 115.40, 117.77, 122.94, 124.85, 127.68, 129.08, 129.70, 130.87, 132.96, 133.95, 147.17, 148.03, 150.25, 151.10.
Anal. Calcd for C19H18NClO2S: C 63.41, H 5.04, N 3.89, Cl 9.85, S 8.91. Found: C 63.69, H 5.23, N 4.07, Cl 10.09, S 9.21.
2-[(4-Hydroxy-3-methoxyphenyl)methyl]-2H,3H-[1,4]thiazino[2,3,4-ij]quinolin-4-ium chloride (8). A solution of sulfuryl chloride (0.167 g, 1.2 mmol) in chloroform (10 mL) was added dropwise to a solution of di(8-quinolinyl) disulfide (0.396 g, 1.2 mmol) in chloroform (10 mL), and the mixture was stirred for 10 min at room temperature. A solution of eugenol (0.394 g, 2.4 mmol) in chloroform (10 mL) was added dropwise. The reaction mixture was stirred for 1 h at room temperature and 7 h at reflux and left overnight. The formed precipitate was filtered off, washed with hexane and dried in vacuum, giving the product (0.648 g, 75% yield) in the form of a yellow powder, mp 195–197 °C.
1H-NMR (400 MHz, D2O): δ 2.88–2.94 (m, 1H, CH2), 3.02 (dd, J = 14.2, 6.0 Hz, 1H, CH2), 3.68 (s, 3H, CH3), 3.92–3.97 (m, 1H, SCH), 4.94 (dd, J = 14.4, 6.8 Hz, 1H, NCH2), 5.21 (dd, J = 14.4, 2.4 Hz, 1H, NCH2), 6.48–6.53 (m, 2H, Ar), 6.68 (s, 1H, Ar) 7.69–7.73 (m, 1H, Hquino), 7.80–7.82 (m, 1H, Hquino), 7.92–7.98 (m, 2H, Hquino), 8.96–9.00 (m, 2H, Hquino).
13C-NMR (101 MHz, D2O): δ 36.84 (CH2), 37.18 (SCH), 55.34 (CH3), 61.78 (CH2N), 112.80, 114.59, 121.35, 121.70, 125.61, 126.51, 127.91, 129.14, 130.46, 132.69, 133.07, 143.36, 146.56, 148.55, 148.68.
Anal. Calcd for C19H18NClO2S: C 63.41, H 5.04, N 3.89, Cl 9.85, S 8.91. Found: C 63.73, H 5.21, N 4.01, Cl 10.31, S 9.29.
3-(4-Methoxyphenyl)-2-methyl-2H,3H-[1,4]thiazino[2,3,4-ij]quinolin-4-ium chloride (9). A solution of sulfuryl chloride (0.103 g, 0.76 mmol) in methylene chloride (10 mL) was added dropwise to a solution of di(8-quinolinyl) disulfide (0.243 g, 0.76 mmol) in methylene chloride (10 mL), and the mixture was stirred for 10 min at room temperature. A solution of trans-anethole (0.226 g, 1.52 mmol) in methylene chloride (10 mL) was added dropwise, and the reaction mixture was stirred for 48 h at room temperature. The solvent was removed by rotary evaporator. The residue was dried in vacuum, giving the product (0.523 g, quantitative yield) in the form of a yellow powder, mp 97–100 °C.
1H-NMR (400 MHz, D2O): δ 1.43 (d, J = 7.0 Hz, 3H, CH3), 3.65 (s, 3H, OCH3), 3.92 (dd, J = 7.0, 3.1 Hz, 1H, SCH), 6.39 (d, J = 3.1 Hz, 1H, NCH), 6.70 (d, J = 9.0 Hz, 2H, Ar), 6.74 (d, J = 9.0 Hz, 2H, Ar), 7.84–7.88 (m, 1H, Hquino), 7.93–7.95 (m, 1H, Hquino), 8.04–8.08 (m, 1H, Hquino), 8.18–8.20 (m, 1H, Hquino), 9.15–9.16 (m, 1H, Hquino), 9.21–9.23 (m, 1H, Hquino).
13C-NMR (101 MHz, D2O): δ 19.25 (CH3), 38.18 (SCH), 55.23 (OCH3), 74.33 (NCH), 114.17, 122.18, 124.12, 126.68, 127.75, 129.65, 130.30, 131.09, 133.18, 134.52, 150.10, 150.25, 159.10.
Anal. Calcd for C19H18NClOS: C 66.36, H 5.28, N 4.07, Cl 10.31, S 9.33. Found: C 66.46, H 5.34, N 4.15, Cl 10.84, S 9.91.
2-[(3,4-Dimethoxyphenyl)methyl]-2H,3H-[1,4]thiazino[2,3,4-ij]quinolin-4-ium chloride (10). A solution of sulfuryl chloride (0.067 g, 0.50 mmol) in chloroform (10 mL) was added dropwise to a solution of di(8-quinolinyl) disulfide (0.160 g, 0.50 mmol) in chloroform (10 mL), and the mixture was stirred for 10 min at room temperature. A solution of methyl eugenol (0.178 g, 1.0 mmol) in chloroform (10 mL) was added dropwise, and the reaction mixture was stirred for 1 h at room temperature and 3 h at reflux temperature. The mixture was filtered, and the solvent was removed by rotary evaporator. The residue was washed with cold hexane and dried in vacuum, giving the product (0.366 g, 98% yield) in the form of a yellow powder, mp 179–180 °C.
1H-NMR (400 MHz, D2O): δ 2.76–2.82 (m, 1H, CH2), 2.93–2.98 (m, 1H, CH2), 3.56 (s, 3H, OCH3), 3.57 (s, 3H, OCH3), 3.83–3.86 (m, 1H, SCH), 4.82–4.85 (m, 1H, NCH2), 5.12–5.16 (m, 1H, NCH2), 6.45–6.51 (m, 2H, Ar), 6.56 (s, 1H, Ar) 7.56–7.65 (m, 2H, Hquino), 7.80–7.82 (m, 1H, Hquino), 7.90–7.92 (m, 1H, Hquino), 8.89–8.94 (m, 2H, Hquino).
13C-NMR (101 MHz, D2O): δ 37.20 (CH2), 37.56 (SCH), 55.43 (OCH3), 55.53 (OCH3), 62.18 (CH2N), 111.22, 112.45, 121.79, 121.92, 125.95, 126.85, 128.63, 129.49, 130.72, 132.92, 133.20, 146.92, 147.55, 148.92, 149.03.
Anal. Calcd for C20H20NClO2S: C 64.25, H 5.39, N 3.75, Cl 9.48, S 8.58. Found: C 63.73, H 5.21, N 4.01, Cl 10.01, S 8.99.
3.4. Synthesis of Compounds 11–14 from Styrene Derivatives and 1H-Indene
3-Phenyl-2H,3H-[1,4]thiazino[2,3,4-ij]quinolin-4-ium chloride (11). A solution of sulfuryl chloride (0.042 g, 0.31 mmol) in methylene chloride (10 mL) was added dropwise to a solution of di(8-quinolinyl) disulfide (0.100 g, 0.31 mmol) in methylene chloride (10 mL), and the mixture was stirred for 10 min at room temperature. A solution of styrene (0.065 g, 0.62 mmol) in methylene chloride (5 mL) was added dropwise, and the reaction mixture was stirred for 24 h at room temperature. The mixture was filtered, and the solvent was removed by rotary evaporator. The residue was washed with cold hexane and dried in vacuum, giving the product (0.18 g, 97% yield) in the form of a light-yellow oil.
1H-NMR (400 MHz, D2O): δ 3.78 (dd, J = 14.3, 3.5 Hz, 1H, SCH2), 3.98 (dd, J = 14.3, 3.0 Hz, 1H, SCH2), 6.79–6.83 (m, 1H, NCH), 6.89–6.96 (m, 2H, Ar), 7.36–7.44 (m, 3H, Ar), 7.91–7.95 (m, 1H, Hquino), 8.08–8.13 (m, 2H, Hquino), 8.23–8.25 (m, 1H, Hquino), 9.21–9.27 (m, 2H, Hquino).
13C-NMR (101 MHz, D2O): δ 29.77 (SCH2), 69.32 (NCH), 121.99, 125.76, 126.34, 128.05, 129.14, 129.23, 129.32, 129.45, 133.91, 134.12, 136.95, 149.62, 150.31.
Anal. Calcd for C17H14ClNS: C 68.10, H 4.71, Cl 11.82, N 4.67, S 10.70. Found: C 67.81, H 4.53, Cl 12.01, N 4.48, S 10.48.
7aH,8H,12bH-Indeno[1′,2′:5,6][1,4]thiazino[2,3,4-ij]quinolin-13-ium chloride (12). A solution of sulfuryl chloride (0.071 g, 0.53 mmol) in methylene chloride (10 mL) was added dropwise to a solution of di(8-quinolinyl) disulfide (0.169 g, 0.53 mmol) in methylene chloride (10 mL), and the mixture was stirred for 10 min at room temperature. A solution of 1H-indene (0.140 g, 1.06 mmol) in methylene chloride (10 mL) was added dropwise, and the reaction mixture was stirred for 65 h at room temperature. The mixture was filtered, and the solvent was removed by rotary evaporator. The residue was washed with cold hexane and dried in vacuum, giving the product (0.266 g, 80% yield) in the form of a yellow powder, mp 158–160 °C.
1H-NMR (400 MHz, D2O): δ 3.07 (d, J = 16.8 Hz, 1H, CH2), 3.61 (d, J = 16.8 Hz, 1H, CH2), 4.52 (d, J = 4.2 Hz, 1H, SCH), 6.71 (s, 1H, NCH), 6.77–6.79 (m, 1H, Ar), 7.13–7.17 (m, 1H, Ar), 7.33–7.36 (m, 1H, Ar), 7.45–7.46 (m, 1H, Ar), 7.68–7.72 (m, 1H, Hquino), 7.81–7.83 (m, 1H, Hquino), 8.04–8.06 (m, 1H, Hquino), 8.19–8.22 (m, 1H, Hquino), 9.20–9.22 (m, 1H, Hquino), 9.43–9.44 (m, 1H, Hquino).
13C-NMR (101 MHz, D2O): δ 36.27 (CH2), 39.61 (SCH), 72.47 (NCH), 121.6, 122.27, 125.14, 126.38, 127.22, 127.33, 129.12, 129.49, 131.04, 132.50, 138.22, 138.68, 138.71, 149.35, 149.81.
Anal. Calcd for C18H14NClS: C 69.33, H 4.53, N 4.49, Cl 11.37, S 10.28. Found: C 69.46, H 4.68, N 4.63, Cl 11.81, S 10.56.
3-(4-Methylphenyl)-2H,3H-[1,4]thiazino[2,3,4-ij]quinolin-4-ium chloride (13). A solution of sulfuryl chloride (0.097 g, 0.71 mmol) in methylene chloride (10 mL) was added dropwise to a solution of di(8-quinolinyl) disulfide (0.230 g, 0.71 mmol) in methylene chloride (10 mL), and the mixture was stirred for 10 min at room temperature. A solution of 4-methylstyrene (0.168 g, 1.42 mmol) in methylene chloride (10 mL) was added dropwise, and the reaction mixture wasstirred for 24 h at room temperature. The mixture was filtered, and the solvent was removed by rotary evaporator. The residue was dried in vacuum, giving the product (0.445 g, quantitative yield) in the form of a yellow powder, mp 73–75 °C.
1H-NMR (400 MHz, D2O): δ 2.21 (s, 3H, CH3), 3.67 (dd, J = 14.2, 3.2 Hz, 1H, SCH2), 3.89 (dd, J = 14.2, 2.4 Hz, 1H, SCH2), 6.72 (m, 3H, NCH, Ar), 7.10 (d, J = 8.0 Hz, 2H, Ar), 7.84–7.88 (m, 1H, Hquino), 7.98–8.00 (m, 1H, Hquino), 8.04–8.08 (m, 1H, Hquino), 8.19–8.21 (m, 1H, Hquino), 9.15–9.16 (m, 1H, Hquino), 9.22–9.24 (m, 1H, Hquino).
13C-NMR (101 MHz, D2O): δ 20.11 (CH3), 29.67 (SCH2), 69.05 (NCH), 121.93, 125.57, 125.97, 127.93, 129.38, 129.41, 129.57, 131.37, 133.73, 133.90, 139.64, 149.48, 150.16.
Anal. Calcd for C18H16NClS: C 68.89, H 5.14, N 4.46, Cl 11.30, S 10.22. Found: C 69.00, H 5.21, N 4.54, Cl 11.56, S 10.68.
3-Methyl-3-phenyl-2H,3H-[1,4]thiazino[2,3,4-ij]quinolin-4-ium chloride (14). A solution of sulfuryl chloride (0.108 g, 0.8 mmol) in methylene chloride (10 mL) was added dropwise to a solution of di(8-quinolinyl) disulfide (0.256 g, 0.8 mmol) in methylene chloride (10 mL), and the mixture was stirred for 10 min at room temperature. A solution of α-methylstyrene (0.189 g, 1.6 mmol) in methylene chloride (10 mL) was added dropwise, and the reaction mixture was stirred for 24 h at room temperature. The mixture was filtered, and the solvent was removed by rotary evaporator. The residue was washed with cold hexane and dried in vacuum, giving the product (0.436 g, 87% yield) in the form of a yellow oil.
1H-NMR (400 MHz, D2O): δ 1.58 (s, 3H, CH3), 3.57 (d, J = 14.6, 1H, SCH2), 3.78 (d, J = 14.6 1H, SCH2), 6.58–6.64 (m, 1H, Ar), 6.66–6.72 (m, 2H, Ar), 7.00–7.05 (m, 2H, Ar), 7.77–7.62 (m, 1H, Hquino), 7.98–8.03 (m, 1H, Hquino), 8.07–8.13 (m, 1H, Hquino), 8.21–8.25 (m, 1H, Hquino), 8.90–8.94 (m, 1H, Hquino), 9.00–9.04 (m, 1H, Hquino).
13C-NMR (101 MHz, D2O): δ 28.80 (CH3), 50.91 (SCH2), 74.85 (NCH), 122.00, 124.79, 126.84, 127.22, 127.86, 129.28, 129.41, 130.14, 136.99, 141.46, 143.67, 144.20, 148.31.
Anal. Calcd for C18H16NClS: C 68.89, H 5.14, N 4.46, Cl 11.30, S 10.22. Found: C 69.12, H 4.99, N 4.27, Cl 11.19, S 9.96.