Closed-Loop Modeling to Evaluate the Performance of a Scaled-Up Lithium–Sulfur Battery in Electric Vehicle Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Simulation Method
2.2. Battery Modeling
2.3. Vehicle Modeling
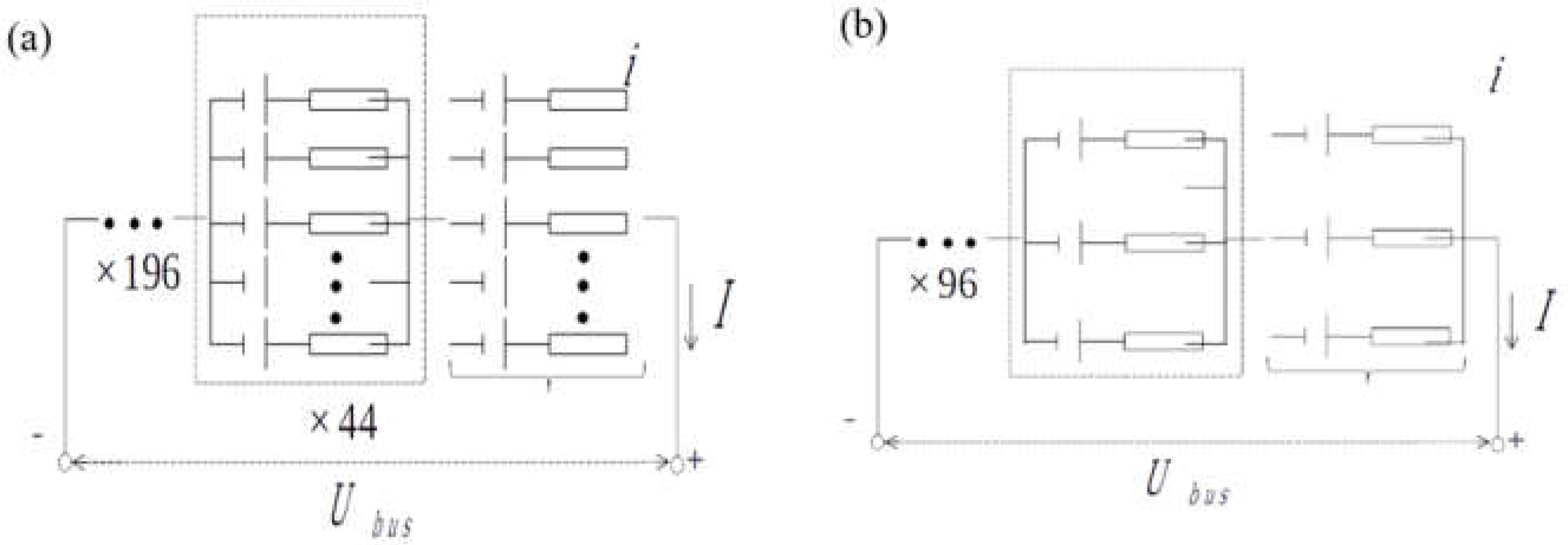
2.4. Battery Configuration
2.5. Vehicle Configuration
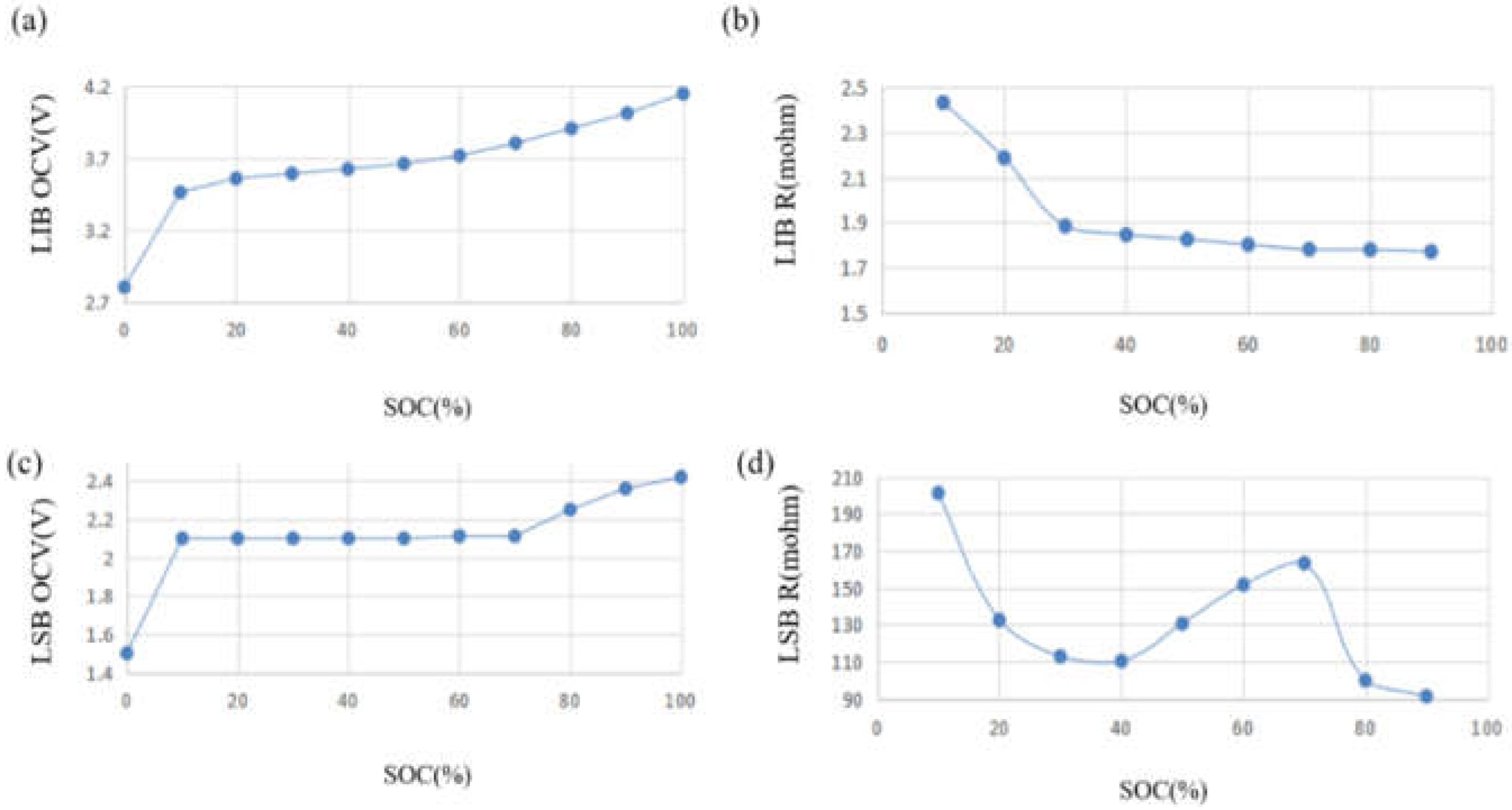
2.6. Test and Parameter Identification
2.7. Pack Parameter Identification
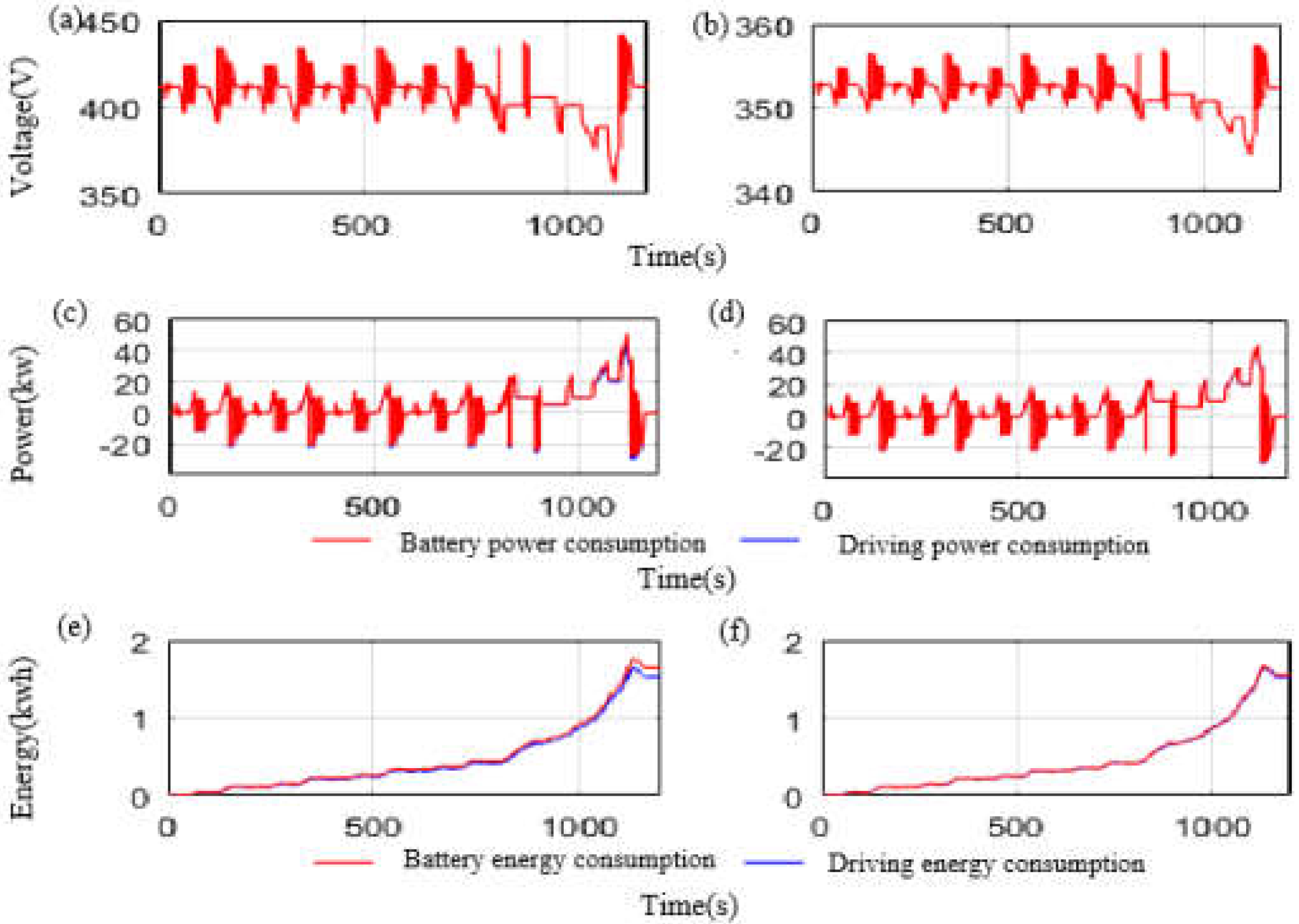
2.8. Model Accuracy Verification
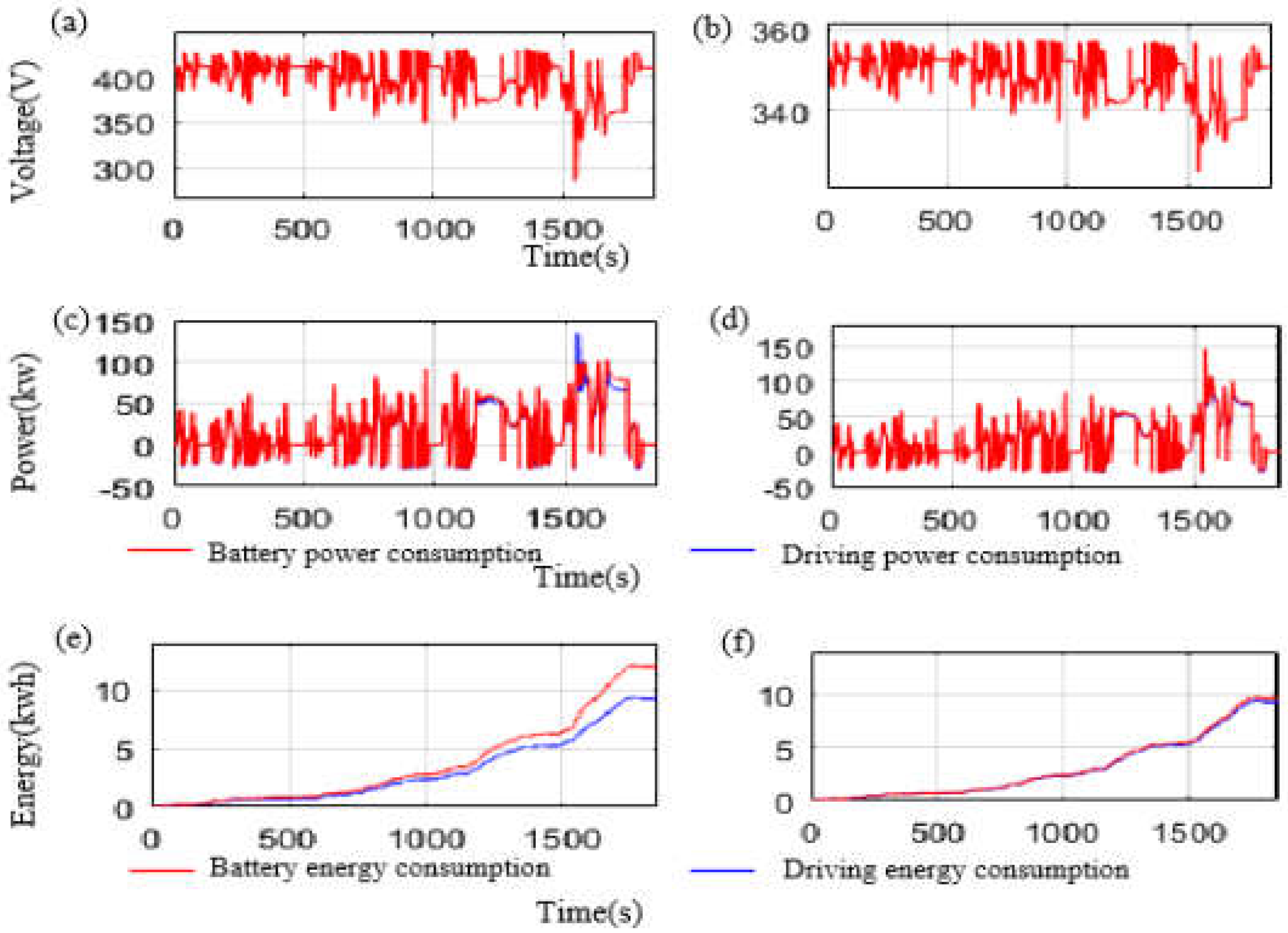
3. Results and Discussion
- Charge start: start the charge when the battery SOC is 20%.
- Charge current ramp: ramp the charge current 1 A per second, until the charge current reaches the configured constant-charge current value.
- Constant current charge: continuously charge with the configured current and decrease the charge current accordingly to avoid the battery output voltage reaching the voltage limit.
- Charge stop: when battery SOC reaches 80%, stop charging.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SOC | Battery’s state of charge |
| I | Battery’s current |
| Output voltage of the battery cell | |
| Open-circuit voltage of the battery cell | |
| T | Temperature of the battery |
| Internal resistance of the battery | |
| Driving force of a vehicle | |
| Vehicle’s wind drag force (air resistance) | |
| Friction resistance of a vehicle | |
| Gradient resistance | |
| Acceleration resistance | |
| Rolling friction coefficient | |
| Air resistance coefficient | |
| A | Frontal area of the vehicle |
| Vehicle’s longitudinal speed | |
| m | Vehicle’s mass |
| G | Acceleration of gravity |
| Slope indexes | |
| Rotating mass conversion factor | |
| Inertia of a single wheel | |
| Inertia on the flywheel | |
| Inertia of the traction motor | |
| r | Wheel’s radius |
| Transmission ratio of the gearbox | |
| Transmission ratio of the main reducer box |
References
- Von Schneidemesser, E.; Steinmar, K.; Weatherhead, E.C.; Bonn, B.; Gerwig, H.; Quedenau, J. Air pollution at human scales in an urban environment: Impact of local environment and vehicles on particle number concentrations. Sci. Total Environ. 2019, 688, 691–700. [Google Scholar] [CrossRef]
- Ventura, L.M.B.; Pinto, F.D.O.; Gioda, A.; D’Agosto, M.D.A. Inspection and maintenance programs for in-service vehicles: An important air pollution control tool. Sustain. Cities Soc. 2019, 53, 101956. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.; Gu, F.; Zhang, H.; Fan, Y. Does air pollution stimulate electric vehicle sales? Empirical evidence from twenty major cities in China. J. Clean. Prod. 2019, 249, 119372. [Google Scholar] [CrossRef]
- Tie, S.F.; Tan, C.W. A review of energy sources and energy management system in electric vehicles. Renew. Sustain. Energy Rev. 2013, 20, 82–102. [Google Scholar] [CrossRef]
- BloombergNEF. 2019. Available online: https://about.bnef.com/blog/battery-pack-prices-fall-as-market-ramps-up-with-market-average-at-156-kwh. (accessed on 25 May 2021).
- Tiwari, V.K.; Song, H.; Oh, Y.; Jeong, Y. Synthesis of sulfur-co-polymer/porous long carbon nanotubes composite cathode by chemical and physical binding for high performance lithium-sulfur batteries. Energy 2020, 195, 117034. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Fronczek, D.N.; Bessler, W.G. Mechanistic modeling of polysulfide shuttle and capacity loss in lithium–sulfur batteries. J. Power Sources 2014, 259, 300–310. [Google Scholar] [CrossRef] [Green Version]
- Rosenman, A.; Markevich, E.; Salitra, G.; Aurbach, D.; Garsuch, A.; Chesneau, F.F. Review on Li-Sulfur Battery Systems: An Integral Perspective. Adv. Energy Mater. 2015, 5, 1500212. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable lithium–sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc. Chem. Res. 2012, 46, 1125–1134. [Google Scholar] [CrossRef]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene Oxide as a Sulfur Immobilizer in High Performance Lithium/Sulfur Cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, S.; Chen, Y.; Xia, X.; Liu, H. Pomegranate-like S@N-doped graphitized carbon spheres as high-performance cathode for lithium-sulfur battery. Mater. Lett. 2019, 263, 127283. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Li, T.; Gao, X.; Yuan, C. Life cycle assessment of lithium sulfur battery for electric vehicles. J. Power Sources 2017, 343, 284–295. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Rincon-Mora, G. Accurate Electrical Battery Model Capable of Predicting Runtime and I–V Performance. IEEE Trans. Energy Convers. 2006, 21, 504–511. [Google Scholar] [CrossRef]
- He, H.; Xiong, R.; Fan, J. Evaluation of Lithium-Ion Battery Equivalent Circuit Models for State of Charge Estimation by an Experimental Approach. Energies 2011, 4, 582–598. [Google Scholar] [CrossRef]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 16132. [Google Scholar] [CrossRef]
- Thele, M.; Bohlen, O.; Sauer, D.U.; Karden, E. Development of a voltage-behavior model for NiMH batteries using an imped-ance-based modeling concept. J. Power Sources 2007, 175, 635–643. [Google Scholar] [CrossRef]
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S. Electric vehicle battery parameter identification and SOC observability analysis: NiMH and Li-S case studies. IET Power Electron. 2017, 10, 1289–1297. [Google Scholar] [CrossRef] [Green Version]
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S.; Wild, M. A review on electric vehicle battery modelling: From Lithium-ion toward Lithium–Sulphur. Renew. Sustain. Energy Rev. 2016, 56, 1008–1021. [Google Scholar] [CrossRef] [Green Version]
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S. Lithium–Sulfur Battery State-of-Charge Observability Analysis and Estimation. IEEE Trans. Power Electron. 2017, 33, 5847–5859. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.; Xiong, B.; Vilathgamuwa, D.M. Adaptive Ensemble-Based Electrochemical-Thermal-Degradation State Estimation of Lithium-Ion Batteries. IEEE Trans. Ind. Electron. 2021, 99, 1. [Google Scholar]
- Hu, J.; He, H.; Wei, Z.; Li, Y. Disturbance-Immune and Aging-Robust Internal Short Circuit Diagnostic for Lith-ium-Ion Battery. IEEE Trans. Ind. Electron. 2021, 99, 1. [Google Scholar]
- Ruan, H.; He, H.; Wei, Z.; Quan, Z.; Li, Y. State of Health Estimation of Lithium-ion Battery Based on Constant-Voltage Charging Reconstruction. IEEE Trans. Emerg. Sel. Top. Power Electron. 2021, 70, 1. [Google Scholar]
- Wei, Z.; Zhao, J.; Xiong, R.; Dong, G.; Pou, J.; Tseng, K.J. Online Estimation of Power Capacity with Noise Effect Attenuation for Lithium-Ion Battery. IEEE Trans. Ind. Electron. 2018, 66, 5724–5735. [Google Scholar] [CrossRef]
- Wei, Z.; He, H.; Pou, J.; Tsui, K.L.; Quan, Z.; Li, Y. Signal-disturbance interfacing elimination for unbiased model parameter identi-fication of lithium-ion battery. IEEE Trans. Ind. Inform. 2020, 17, 5887–5897. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Gong, J. A combination Kalman filter approach for State of Charge estimation of lithium-ion battery considering model uncertainty. Energy 2016, 109, 933–946. [Google Scholar] [CrossRef]
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium–sulfur batteries: Electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Nazri, G.-A.; Saif, M. A Bidirectional Power Charging Control Strategy for Plug-in Hybrid Electric Vehicles. Sustainability 2019, 11, 4317. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-S. Energy Efficiency Comparison between Hydraulic Hybrid and Hybrid Electric Vehicles. Energies 2015, 8, 4697–4723. [Google Scholar] [CrossRef] [Green Version]
- Shim, B.J.; Park, K.S.; Koo, J.M.; Jin, S.H. Work and speed based engine operation condition analysis for new European driving cycle (NEDC). J. Mech. Sci. Technol. 2014, 28, 755–761. [Google Scholar] [CrossRef]
- Tsiakmakis, S.; Fontaras, G.; Ciuffo, B.; Samaras, Z. A simulation-based methodology for quantifying European passenger car fleet CO 2 emissions. Appl. Energy 2017, 199, 447–465. [Google Scholar] [CrossRef]
- Pacheco, A.F.; Martins, M.E.S.; Zhao, H. New European Drive Cycle (NEDC) simulation of a passenger car with a HCCI engine: Emissions and fuel consumption results. Fuel 2013, 111, 733–739. [Google Scholar] [CrossRef]
- Tutuianu, M.; Bonnel, P.; Ciuffo, B.; Haniu, T.; Ichikawa, N.; Marotta, A.; Pavlovic, J.; Steven, H. Development of the World-wide harmonized Light duty Test Cycle (WLTC) and a possible pathway for its introduction in the European legislation. Transp. Res. Part D Transp. Environ. 2015, 40, 61–75. [Google Scholar] [CrossRef]
- Cioroianu, C.C.; Marinescu, D.G.; Iorga, A.; Sibiceanu, A.R. Simulation of an electric vehicle model on the new WLTC test cycle using AVL CRUISE software. IOP Conf. Series Mater. Sci. Eng. 2017, 252, 12060. [Google Scholar] [CrossRef]
- Mock, P.; Kühlwein, J.; Tietge, U.; Franco, V.; Bandivadekar, A.; German, J. The WLTP: How a new test procedure for cars will affect fuel consumption values in the EU. ICCT 2014, 9, 1–20. [Google Scholar]
- Fontaras, G.; Ciuffo, B.; Zacharof, N.; Tsiakmakis, S.; Marotta, A.; Pavlovic, J.; Anagnostopoulos, K. Anagnostopoulos, the difference between re-ported and real-world CO2 emissions: How much improvement can be expected by WLTP introduction? Transp. Res. Procedia 2017, 25, 3933–3943. [Google Scholar] [CrossRef]


| Parameters | LSB Pack | NCM LIB Battery Pack |
|---|---|---|
| Cell Nominal Capacity | 3.3 Ah | 50 Ah |
| Cell Weight | 74.956 g | 961.77 g |
| Cell Size | 90·60·6.36 (mm) | 28.7·148.6·103.7 (mm) |
| Pack Nominal Energy | 60 kWh | 57.6 kWh |
| QTY of Serial cells | 196 Serials | 96 Serials |
| QTY of Parallel cells | 44 Parallel | 3 Parallel |
| Pack Nominal Voltage | 400 v | 384 v |
| Basic Parameter | Unit | Value |
|---|---|---|
| Curb Weight | kg | 1610 |
| Maximum Mass | kg | 1610 + 375 |
| Maximum windward area | m2 | 2.26 |
| Rolling resistance coefficient | / | 0.0132 |
| Air resistance coefficient | / | 0.331 |
| Wheel radius | m | 0.308 |
| Gravitational acceleration | m/s2 | 9.8 |
| Maximum torque of e-motor | N·m | 245 |
| Maximum motor speed | r/min | 8950 |
| Final ratio | / | 7.816 |
| Transmission mechanical efficiency | / | 0.96 |
| Moment of inertia of the wheel | kg·m2 | 3.45 (experience value) |
| Motor inertia | kg·m2 | 0.04 |
| Items | LSB | LIB | |
|---|---|---|---|
| Charging | Acceptable Fast-Charge Current (A) | 131 | 255 |
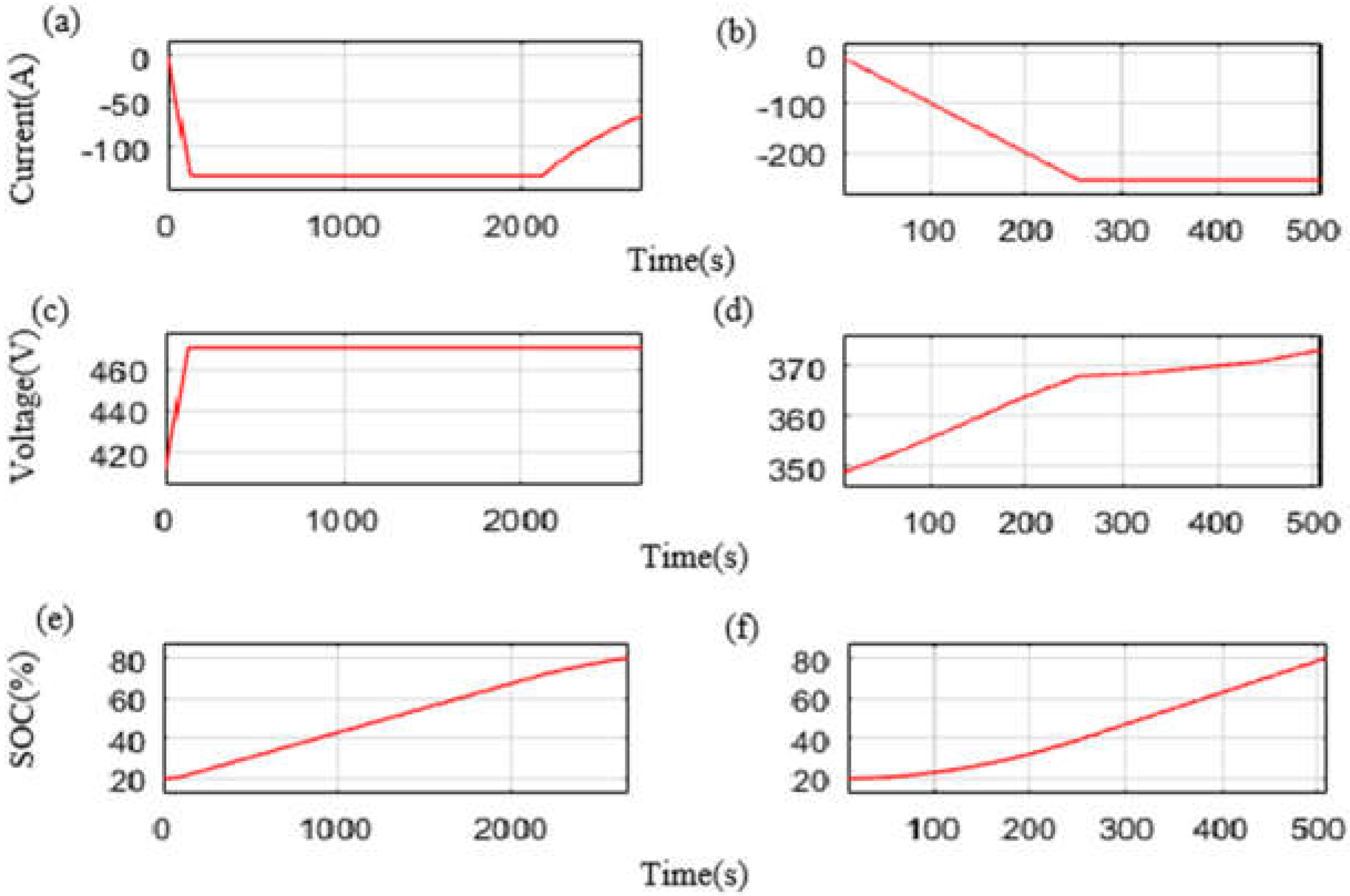
| Charge Time from SOC 20% to SOC 80% (s) | 2681 | 508 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Q.; Zou, Z.; Chen, J.; Jiang, Y.; Zeng, L.; Li, C. Closed-Loop Modeling to Evaluate the Performance of a Scaled-Up Lithium–Sulfur Battery in Electric Vehicle Applications. Appl. Sci. 2021, 11, 9593. https://doi.org/10.3390/app11209593
Zeng Q, Zou Z, Chen J, Jiang Y, Zeng L, Li C. Closed-Loop Modeling to Evaluate the Performance of a Scaled-Up Lithium–Sulfur Battery in Electric Vehicle Applications. Applied Sciences. 2021; 11(20):9593. https://doi.org/10.3390/app11209593
Chicago/Turabian StyleZeng, Qingxin, Zhuo Zou, Jie Chen, Yali Jiang, Lingzhi Zeng, and Changming Li. 2021. "Closed-Loop Modeling to Evaluate the Performance of a Scaled-Up Lithium–Sulfur Battery in Electric Vehicle Applications" Applied Sciences 11, no. 20: 9593. https://doi.org/10.3390/app11209593






