Strongly Adhesive and Antimicrobial Peptide-Loaded, Alginate–Catechol-Based Gels for Application against Periimplantitis
Abstract
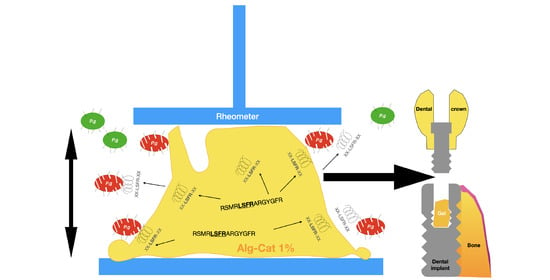
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Gel Preparation
2.2. Mechanical Evaluation
2.3. Antimicrobial Properties
2.3.1. Antimicrobial Activity of Gels Loaded with D-CTL
2.3.2. Analysis with HPLC and Mass Spectrometry of Remaining CTL Inside the Gel and Its Released Derived Fragments
2.3.3. Bacterial Colonization of the Gel
3. Results and Discussion
Gelation Kinetics and Mechanical Properties of the Alg–Cat Based Gels
Gelation Kinetics
4. Percentage of P. gingivalis Inhibition with D-CTL Inside the Gel
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




References
- Bernardi, S.; Bianchi, S.; Tomei, A.R.; Continenza, M.A.; Macchiarelli, G. Microbiological and SEM-EDS evaluation of titanium surfaces exposed to periodontal gel: In vitro study. Materials 2019, 12, 1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Gatto, R.; Monaco, A. Periodontitis, Implant loss and peri-implantitis. A meta-analysis. Clin. Oral Implant Res. 2015, 26, e8–e16. [Google Scholar] [CrossRef] [PubMed]
- Oji, C.; Chukwuneke, F. Poor oral hygiene may be the sole cause of oral cancer. J. Maxillofac. Oral Surg. 2012, 11, 379–383. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, S.; Fantozzi, G.; Bernardi, S.; Antonouli, S.; Continenza, M.A.; Macchiarelli, G. Commercial oral hygiene products and implant collar surfaces: Scanning electron microscopy observations. Can. J. Dent. Hyg. 2020, 54, 26–31. [Google Scholar]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [Green Version]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontology 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Jansen, V.K.; Conrads, G.; Richter, E.J. Microbial leakage and marginal fit of the implant-abutment interface. Int. J. Oral Maxillofac. Implant. 1997, 12, 527–540. [Google Scholar]
- Cosyn, J.; Van Aelst, L.; Collaert, B.; Persson, G.R.; De Bruyn, H. The peri-implant sulcus compared with internal implant and suprastructure components: A microbiological analysis. Clin. Implant. Dent. Relat. Res. 2011, 13, 286–295. [Google Scholar] [CrossRef]
- Sasada, Y.; Cochran, D.L. Implant-abutment connections: A review of biologic consequences and peri-implantitis implications. Int. J. Oral Maxillofac. Implant. 2017, 32, 1296–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauritano, D.; Moreo, G.; Lucchese, A.; Viganoni, C.; Limongelli, L.; Carinci, F. The impact of implant-abutment connection on clinical outcomes and microbial colonization: A narrative review. Materials 2020, 13, 1131. [Google Scholar] [CrossRef] [Green Version]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Wang, J.L.; Han, J.L.; Hsieh, K.H. Antibacterial activity and biocompatibility of a chitosan-gamma-poly(glutamic acid) polyelectrolyte complex hydrogel. Carbohydr. Res. 2010, 345, 1774–1780. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Biocompatible and biodegradable poly(Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016, 82, 150–159. [Google Scholar] [CrossRef]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial hydrogels: Promising materials for medical application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef] [Green Version]
- D’Ercole, S.; Tete, S.; Catamo, G.; Sammartino, G.; Femminella, B.; Tripodi, D.; Spoto, G.; Paolantonio, M. Microbiological and biochemical effectiveness of an antiseptic gel on the bacterial contamination of the inner space of dental implants: A 3-month human longitudinal study. Int. J. Immunopathol. Pharmacol. 2009, 22, 1019–1026. [Google Scholar] [CrossRef]
- Alós, J.I. Antibiotic resistance: A global crisis. Enferm. Infecc. Microbiol. Clin. 2015, 33, 692–699. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Peter, J.G.; Trubiano, J.A.; Phillips, E.J. Antibiotic allergy. Lancet 2019, 393, 183–198. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef]
- Park, J.P.; Song, I.T.; Lee, J.; Ryu, J.H.; Lee, Y.; Lee, H. Vanadyl-catecholamine hydrogels inspired by ascidians and mussels. Chem. Mater. 2015, 27, 105–111. [Google Scholar] [CrossRef]
- Park, H.J.; Jin, Y.; Shin, J.; Yang, K.; Lee, C.; Yang, H.S.; Cho, S.W. Catechol-functionalized hyaluronic acid hydrogels enhance angiogenesis and osteogenesis of human adipose-derived stem cells in critical tissue defects. Biomacromolecules 2016, 17, 1939–1948. [Google Scholar] [CrossRef]
- Fan, C.; Fu, J.; Zhu, W.; Wang, D.A. A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta Biomater. 2016, 33, 51–63. [Google Scholar] [CrossRef]
- Li, Y.; Meng, H.; Liu, Y.; Narkar, A.; Lee, B.P. Gelatin microgel incorporated poly(ethylene glycol)-based bioadhesive with enhanced adhesive property and bioactivity. ACS Appl. Mater. Interfaces 2016, 8, 11980–11989. [Google Scholar] [CrossRef]
- Cencer, M.; Liu, Y.; Winter, A.; Murley, M.; Meng, H.; Lee, B.P. Effect of pH on the rate of curing and bioadhesive properties of dopamine functionalized poly(ethylene glycol) hydrogels. Biomacromolecules 2014, 15, 2861–2869. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Ton, X.A.; Zhao, S.; Paez, J.I.; Del Campo, A. Mechanically reinforced catechol-containing hydrogels with improved tissue gluing performance. Biomimetics 2017, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Krogsgaard, M.; Behrens, M.A.; Pedersen, J.S.; Birkedal, H. Self-healing mussel-inspired multi-pH-responsive hydrogels. Biomacromolecules 2013, 14, 297–301. [Google Scholar] [CrossRef]
- Grijalvo, S.; Nieto-Díaz, M.; Maza, R.M.; Eritja, R.; Díaz, D.D. Alginate hydrogels as scaffolds and delivery systems to repair the damaged spinal cord. Biotechnol. J. 2019, 14, e1900275. [Google Scholar] [CrossRef] [Green Version]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A.; Larsson, A. Drug delivery from hydrogels: A general framework for the release modeling. Curr Drug Deliv. 2017, 14, 179–189. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Darabi, M.A.; Yuan, Q.; Xing, M. Mussel-inspired alginate gel promoting the osteogenic differentiation of mesenchymal stem cells and anti-infection. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Shin, J.; Lee, J.S.; Byun, E.; Ryu, J.H.; Um, S.H.; Kim, D.I.; Lee, H.; Cho, S.W. Bioinspired, Calcium-free alginate hydrogels with tunable physical and mechanical properties and improved biocompatibility. Biomacromolecules 2013, 14, 2004–2013. [Google Scholar] [CrossRef]
- Longo, J.; Garnier, T.; Mateescu, M.; Ponzio, F.; Schaaf, P.; Jierry, L.; Ball, V. Stable bioactive enzyme-containing multilayer films based on covalent cross-linking from mussel-inspired adhesives. Langmuir 2015, 31, 12447–12454. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, M.; Baixe, S.; Garnier, T.; Jierry, L.; Ball, V.; Haikel, Y.; Metz-Boutigue, M.H.; Nardin, M.; Schaaf, P.; Etienne, O.; et al. Antibacterial peptide-based gel for prevention of medical implanted-device infection. PLoS ONE 2015, 10, e0145143. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.E. Antimicrobial peptides. Surg. Infect. 2018, 19, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; de Jesus, M.B.; Kapila, Y.L. Bacterial anti-microbial peptides and nano-sized drug delivery systems: The state of the art toward improved bacteriocins. J. Control. Release 2020, 321, 100–118. [Google Scholar] [CrossRef]
- Wiesner, J.; Vilcinskas, A. Antimicrobial peptides: The ancient arm of the human immune system. Virulence 2010, 1, 440–464. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini review on antimicrobial peptides, sources, mechanism and recent applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Mahata, S.K.; Mahata, M.; Wakade, A.R.; O’Connor, D.T. Primary structure and function of the catecholamine release inhibitory peptide catestatin (chromogranin A344-364): Identification of amino acid residues crucial for activity. Mol. Endocrinol. 2000, 14, 1525–1535. [Google Scholar] [CrossRef] [Green Version]
- Briolat, J.; Wu, S.D.; Mahata, S.K.; Gonthier, B.; Bagnard, D.; Chasserot-Golaz, S.; Helle, K.B.; Aunis, D.; Metz-Boutigue, M.H. New antimicrobial activity for the catecholamine release-inhibitory peptide from chromogranin A. Cell. Mol. Life Sci. 2005, 62, 377–385. [Google Scholar] [CrossRef]
- Akaddar, A.; Doderer-Lang, C.; Marzahn, M.R.; Delalande, F.; Mousli, M.; Helle, K.; Van Dorsselaer, A.; Aunis, D.; Dunn, B.M.; Metz-Boutigue, M.H.; et al. Catestatin, An endogenous chromogranin A-derived peptide, inhibits in vitro growth of Plasmodium falciparum. Cell. Mol. Life Sci. 2010, 67, 1005–1015. [Google Scholar] [CrossRef] [Green Version]
- Özçelik, H.; Vrana, N.E.; Gudima, A.; Riabov, V.; Gratchev, A.; Haikel, Y.; Metz-Boutigue, M.H.; Carradò, A.; Faerber, J.; Roland, T.; et al. Harnessing the multifunctionality in nature: A bioactive agent release system with self-antimicrobial and immunomodulatory properties. Adv. Healthc. Mater. 2015, 4, 2026–2036. [Google Scholar] [CrossRef]
- Egger, M.; Beer, A.G.; Theurl, M.; Schgoer, W.; Hotter, B.; Tatarczyk, T.; Vasiljevic, D.; Frauscher, S.; Marksteiner, J.; Patsch, J.R.; et al. Monocyte migration: A novel effect and signaling pathways of catestatin. Eur. J. Pharmacol. 2008, 598, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shooshtarizadeh, P.; Laventie, B.J.; Colin, D.A.; Chich, J.F.; Vidic, J.; de Barry, J.; Chasserot-Golaz, S.; Delalande, F.; Van Dorsselaer, A.; et al. Two chromogranin a-derived peptides induce calcium entry in human neutrophils by calmodulin-regulated calcium independent phospholipase A2. PLoS ONE 2009, 4, e4501. [Google Scholar] [CrossRef] [Green Version]
- Aung, G.; Niyonsaba, F.; Ushio, H.; Kajiwara, N.; Saito, H.; Ikeda, S.; Ogawa, H.; Okumura, K. Catestatin, A neuroendocrine antimicrobial peptide, induces human mast cell migration, degranulation and production of cytokines and chemokines. Immunology 2011, 132, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Rabbi, M.F.; Labis, B.; Metz-Boutigue, M.H.; Bernstein, C.N.; Ghia, J.E. Catestatin decreases macrophage function in two mouse models of experimental colitis. Biochem. Pharmacol. 2014, 89, 386–398. [Google Scholar] [CrossRef]
- Eissa, N.; Hussein, H.; Mesgna, R.; Bonin, S.; Hendy, G.N.; Metz-Boutigue, M.H.; Bernstein, C.N.; Ghia, J.E. Catestatin regulates epithelial cell dynamics to improve intestinal inflammation. Vaccines 2018, 6, 67. [Google Scholar] [CrossRef] [Green Version]
- Muntjewerff, E.M.; Dunkel, G.; Nicolasen, M.J.T.; Mahata, S.K.; van den Bogaart, G. Catestatin as a target for treatment of inflammatory diseases. Front. Immunol. 2018, 9, 2199. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, X.; Yang, C.; Su, X.; Yang, W.; Dai, Y.; Han, H.; Jiang, J.; Lu, L.; Wang, H.; et al. Decreased circulating catestatin levels are associated with coronary artery disease: The emerging anti-inflammatory role. Atherosclerosis 2019, 281, 78–88. [Google Scholar] [CrossRef]
- Sugawara, M.; Resende, J.M.; Moraes, C.M.; Marquette, A.; Chich, J.F.; Metz-Boutigue, M.H.; Bechinger, B. Membrane structure and interactions of human catestatin by multidimensional solution and solid-state NMR spectroscopy. FASEB J. 2010, 24, 1737–1746. [Google Scholar] [CrossRef] [Green Version]
- Jean-François, F.; Elezgaray, J.; Berson, P.; Vacher, P.; Dufourc, E.J. Pore formation induced by an antimicrobial peptide: Electrostatic effects. Biophys. J. 2008, 95, 5748–5756. [Google Scholar] [CrossRef] [Green Version]
- Zaet, A.; Dartevelle, P.; Daouad, F.; Ehlinger, C.; Quilès, F.; Francius, G.; Boehler, C.; Bergthold, C.; Frisch, B.; Prévost, G.; et al. D-Cateslytin, A new antimicrobial peptide with therapeutic potential. Sci. Rep. 2017, 7, 15199. [Google Scholar] [CrossRef]
- Dartevelle, P.; Ehlinger, C.; Zaet, A.; Boehler, C.; Rabineau, M.; Westermann, B.; Strub, J.M.; Cianferani, S.; Haïkel, Y.; Metz-Boutigue, M.H.; et al. D-Cateslytin: A new antifungal agent for the treatment of oral Candida albicans associated infections. Sci. Rep. 2018, 8, 9235. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Vorm, O.; Mann, M. Improved mass accuracy in matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of peptides. J. Am. Soc. Mass Spectrom. 1994, 5, 955–958. [Google Scholar] [CrossRef] [Green Version]
- Couarraze, G.; Grossiord, J. Initiation à la Rhéologie; Lavoisier. Tec et Doc.: Paris, France, 1991. [Google Scholar]
- Cabane, B.; Hénon, S. Liquides, Solutions, Dispersions, Emulsions et Gels; Belin Education: Paris, France, 2003. [Google Scholar]
- Brubaker, C.E.; Messersmith, P.B. Enzymatically degradable mussel-inspired adhesive hydrogel. Biomacromolecules 2011, 12, 4326–4334. [Google Scholar] [CrossRef] [PubMed]
- Renoud, P.; Toury, B.; Benayoun, S.; Attik, G.; Grosgogeat, B. Functionalization of titanium with chitosan via silanation: Evaluation of biological and mechanical performances. PLoS ONE 2012, 7, e39367. [Google Scholar] [CrossRef] [Green Version]
- Kanno, T.; Asada, N.; Yanase, H.; Iwanaga, T.; Yanaihara, N. Salivary secretion of chromogranin a. control by autonomic nervous system. In Chromogranins: Functional and Clinical Aspects; Springer: Boston, MA, USA, 2000; pp. 145–151. [Google Scholar]
- Srithunyarat, T.; Hagman, R.; Höglund, O.V.; Stridsberg, M.; Hanson, J.; Lagerstedt, A.S.; Pettersson, A. Catestatin, Vasostatin, cortisol, and visual analog scale scoring for stress assessment in healthy dogs. Res. Vet. Sci. 2018, 117, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Aslam, R.; Marban, C.; Corazzol, C.; Jehl, F.; Delalande, F.; Van Dorsselaer, A.; Prévost, G.; Haïkel, Y.; Taddei, C.; Schneider, F.; et al. Cateslytin, A chromogranin A derived peptide is active against Staphylococcus aureus and resistant to degradation by its proteases. PLoS ONE 2013, 8, e68993. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Main Peptides Recovered | Experimental Molecular Weight (m/z) | Theorical Molecular Weight (m/z) | Theorical Sequences of Peptide (with/without Oxygen) |
|---|---|---|---|
| CTL | 1859.96 | 186.17 | RSMRLSFRARGYGFR |
| A | 1620.83 | 1620.81 | RSMRLSFRARGYG + 4 oxydations |
| B | 1563.84 * | 1563.78 | -SMRLSFRARGYGF + 1 oxydation |
| C | 1448.78 | 1448.62 | -SMRLSFRARGYG + 3 oxydations |
| D | 1279.64 | 1279.71 | ------RSMRLSFRAR |
| E | 1052.61 | 1052.57 | ------RSMRLSFR |
| F | 989.59 * | 989.49 | ------------------FRARGYGF + 1 oxydation |
| G | 769.44 | 769.41 | ------------------FRARGY |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baixe, S.; Ball, V.; Jierry, L.; Cianférani, S.; Strub, J.-M.; Haikel, Y.; Metz-Boutigue, M.-H.; Etienne, O. Strongly Adhesive and Antimicrobial Peptide-Loaded, Alginate–Catechol-Based Gels for Application against Periimplantitis. Appl. Sci. 2021, 11, 10050. https://doi.org/10.3390/app112110050
Baixe S, Ball V, Jierry L, Cianférani S, Strub J-M, Haikel Y, Metz-Boutigue M-H, Etienne O. Strongly Adhesive and Antimicrobial Peptide-Loaded, Alginate–Catechol-Based Gels for Application against Periimplantitis. Applied Sciences. 2021; 11(21):10050. https://doi.org/10.3390/app112110050
Chicago/Turabian StyleBaixe, Sébastien, Vincent Ball, Loïc Jierry, Sarah Cianférani, Jean-Marc Strub, Youssef Haikel, Marie-Hélène Metz-Boutigue, and Olivier Etienne. 2021. "Strongly Adhesive and Antimicrobial Peptide-Loaded, Alginate–Catechol-Based Gels for Application against Periimplantitis" Applied Sciences 11, no. 21: 10050. https://doi.org/10.3390/app112110050
APA StyleBaixe, S., Ball, V., Jierry, L., Cianférani, S., Strub, J.-M., Haikel, Y., Metz-Boutigue, M.-H., & Etienne, O. (2021). Strongly Adhesive and Antimicrobial Peptide-Loaded, Alginate–Catechol-Based Gels for Application against Periimplantitis. Applied Sciences, 11(21), 10050. https://doi.org/10.3390/app112110050