Evaluation of Antioxidant and Anticorrosive Activities of Ceriops tagal Plant Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metal Preparation
2.2. Plant Extract Preparation
2.3. Total Phenolic Content (TPC) and Total Flavonoid Content Analysis
2.4. Antioxidant Studies
2.4.1. DPPH Free-Radical Scavenging Assay
2.4.2. Nitric Oxide Inhibition
2.4.3. Hydrogen Peroxide Free-Radical Scavenging
2.4.4. Reducing Potential
2.4.5. Phosphomolybdate Method
2.5. Corrosion Inhibition
2.5.1. Weight Loss Method
Effect of Concentration on Corrosion Inhibition
Effect of Temperature on Corrosion Inhibition
2.5.2. Atomic Adsorption Spectrometric (AAS)
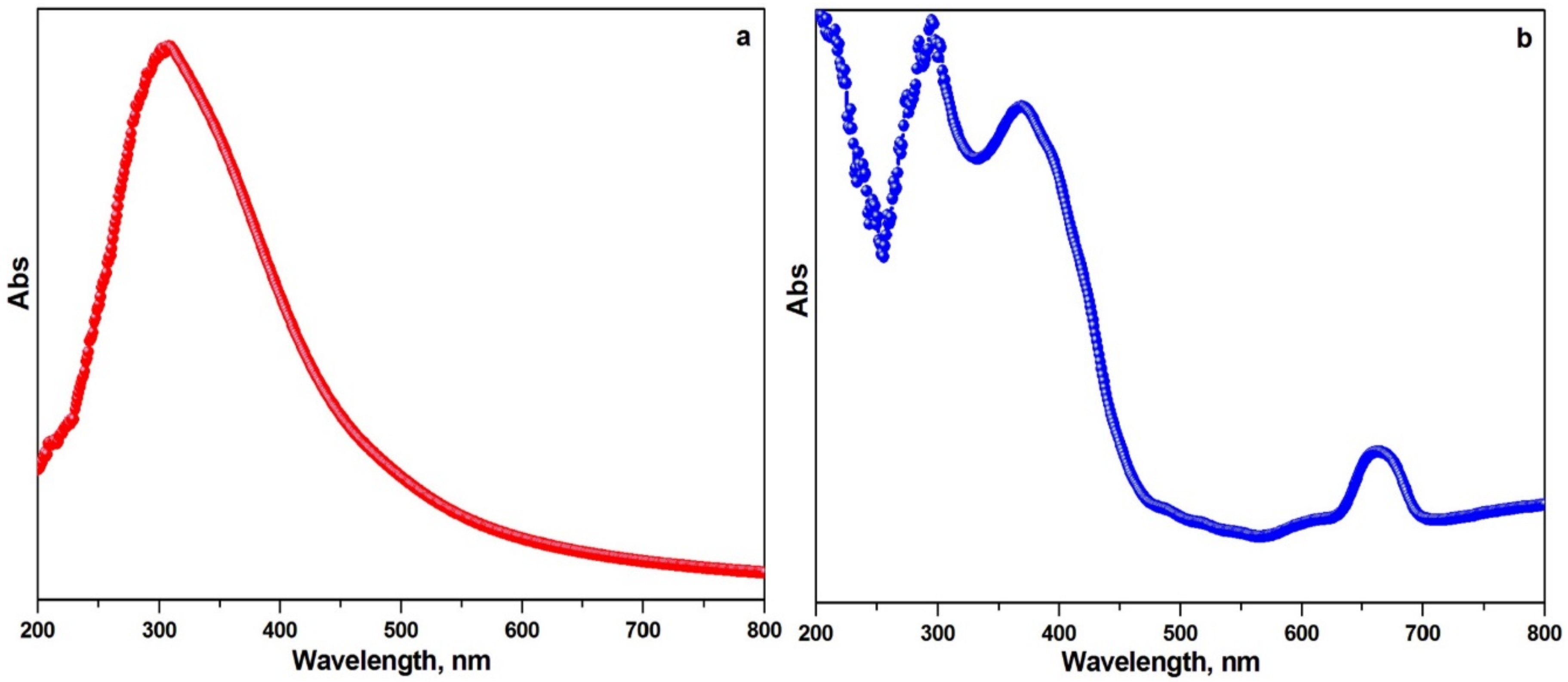
2.5.3. UV-Visible Spectroscopy Analysis
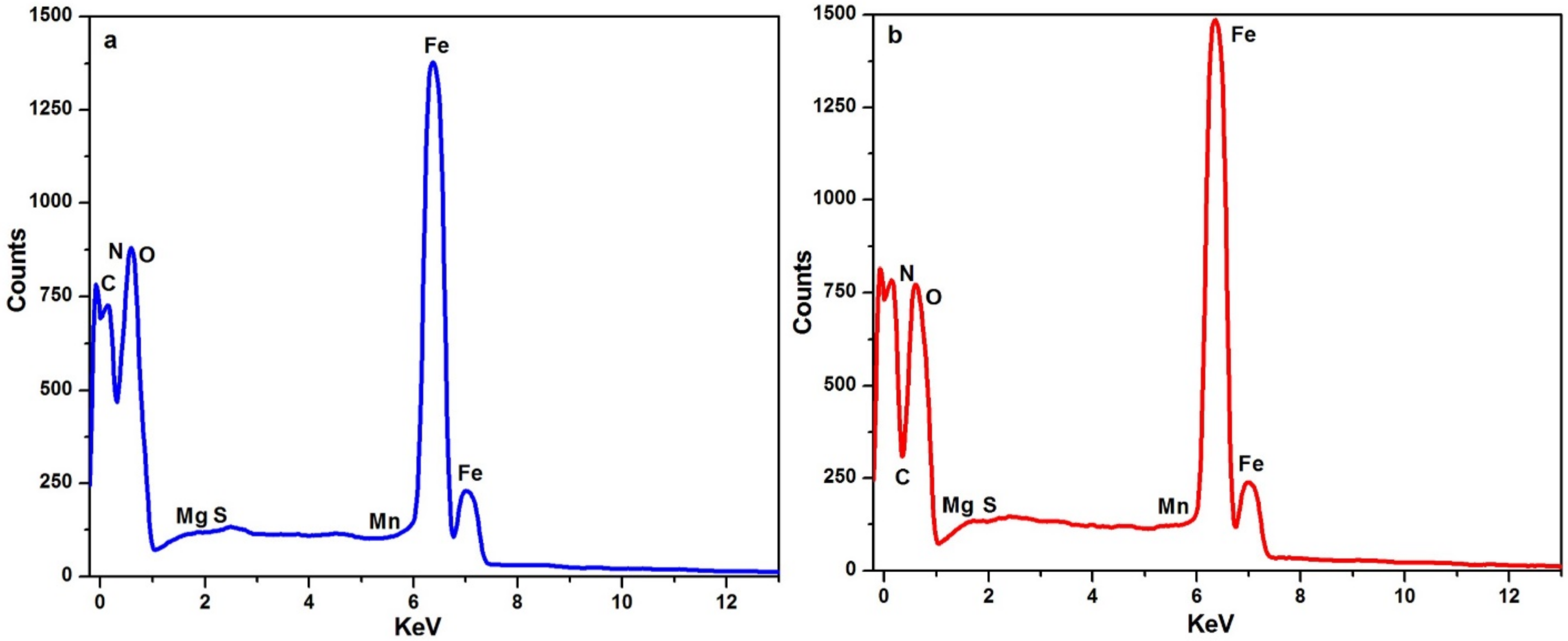
2.5.4. Surface Analysis by SEM-EDX
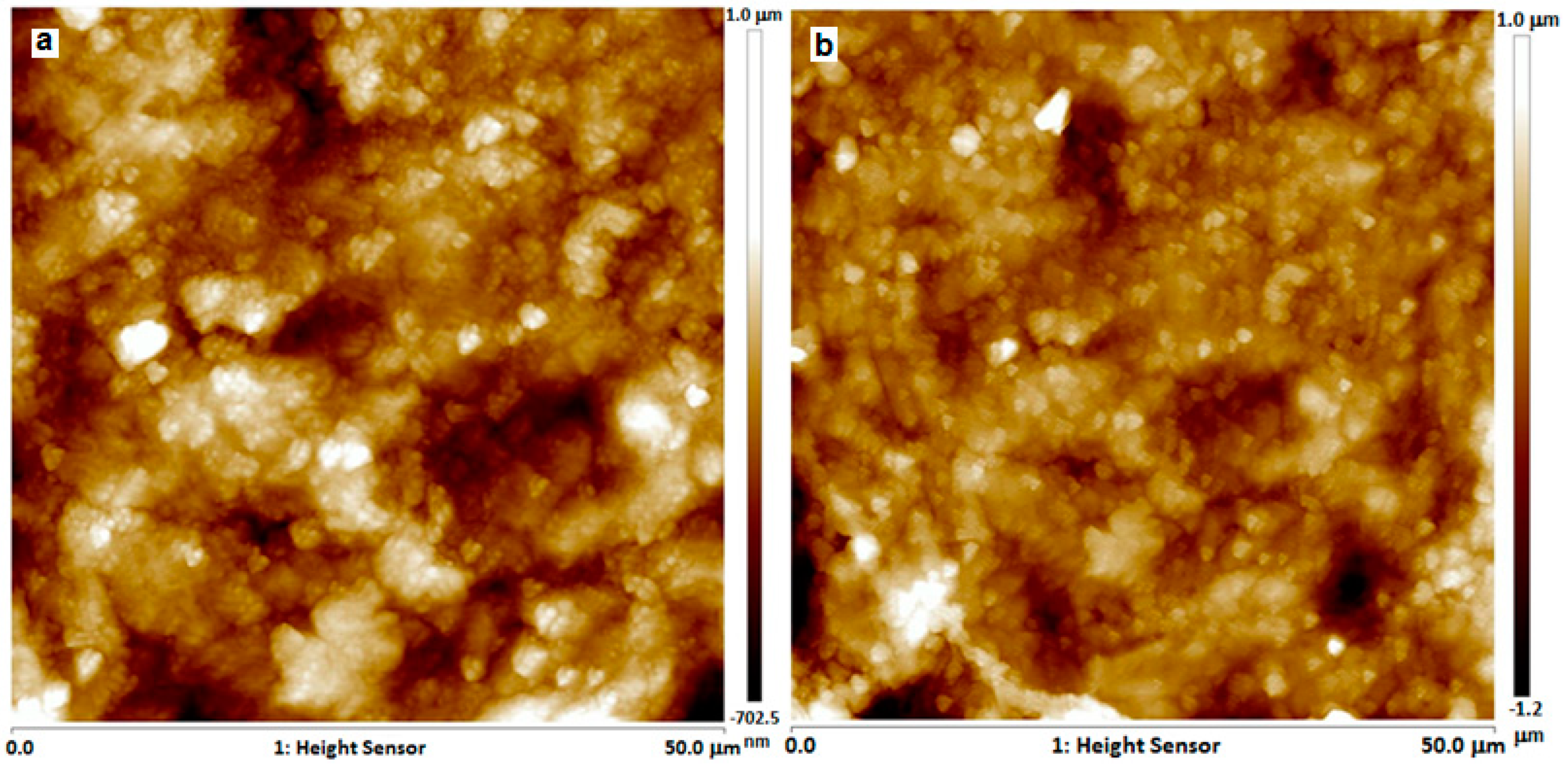
2.5.5. Atomic Force Microscopy (AFM)
3. Results and Discussion
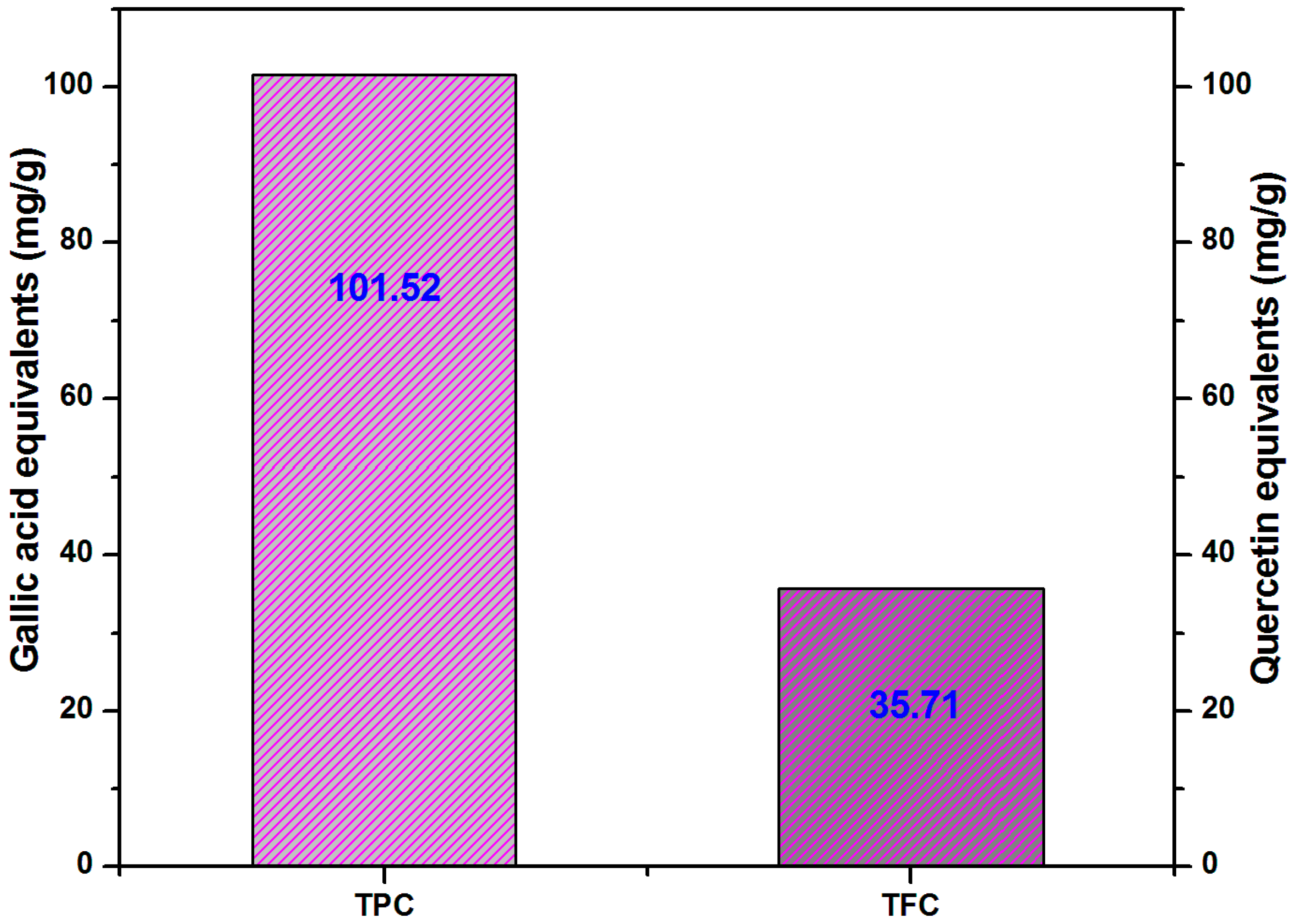
3.1. TPC-TFC
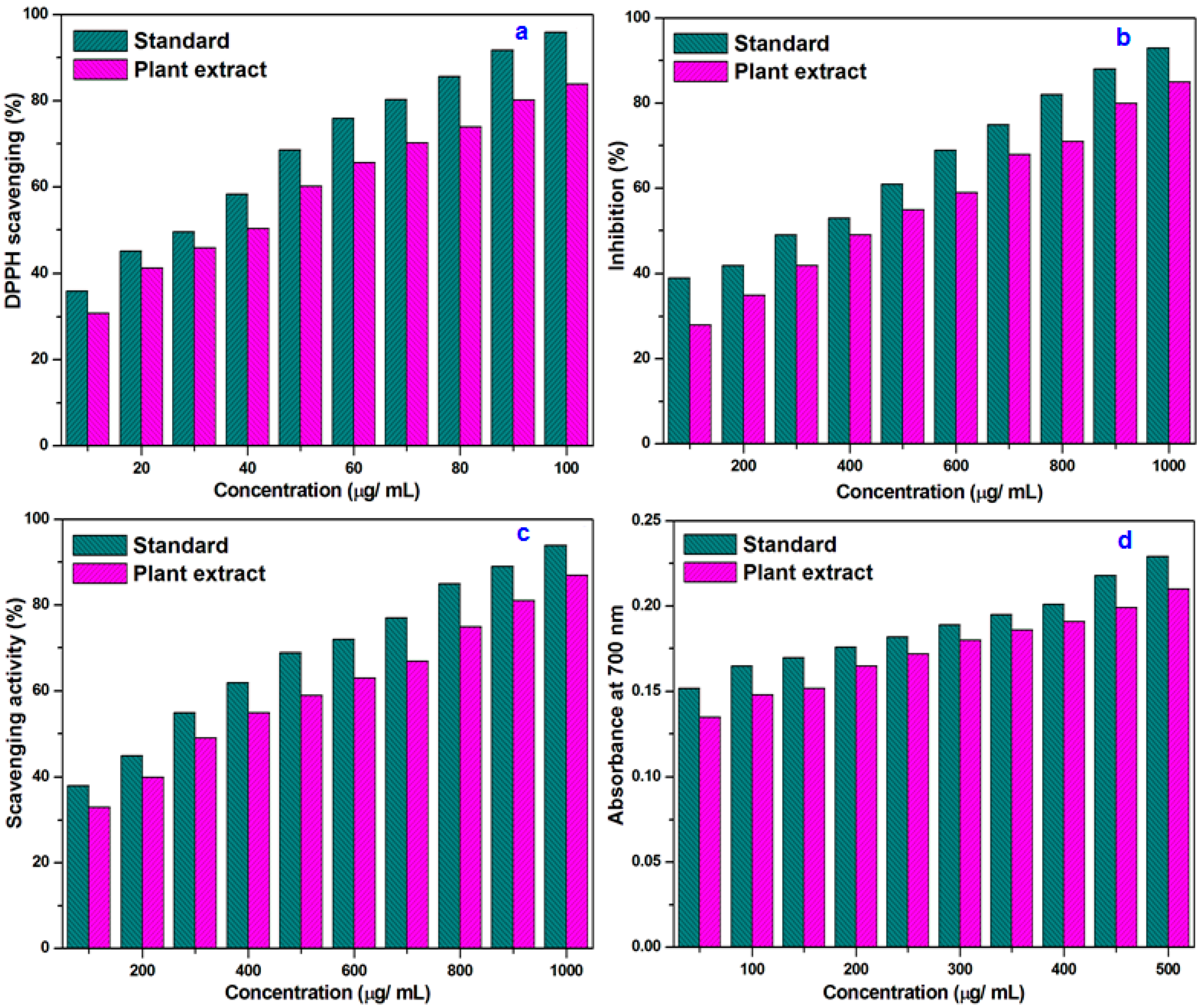
3.2. Antioxidant Studies
3.2.1. DPPH Free Radical Scavenging Assay
3.2.2. Nitric Oxide Inhibition
3.2.3. Hydrogen Peroxide Free Radical Scavenging
3.2.4. Reducing Potential of Plant Extract
3.2.5. Phosphomolybdate Method
3.3. Corrosion Inhibition
3.3.1. Weight Loss Measurement
Effect of Concentration
Effect of Temperature
3.3.2. Atomic Adsorption Spectrometric (AAS)
3.3.3. UV-Visible Spectroscopy Analysis
3.3.4. Surface Analysis by SEM-EDX
3.3.5. AFM
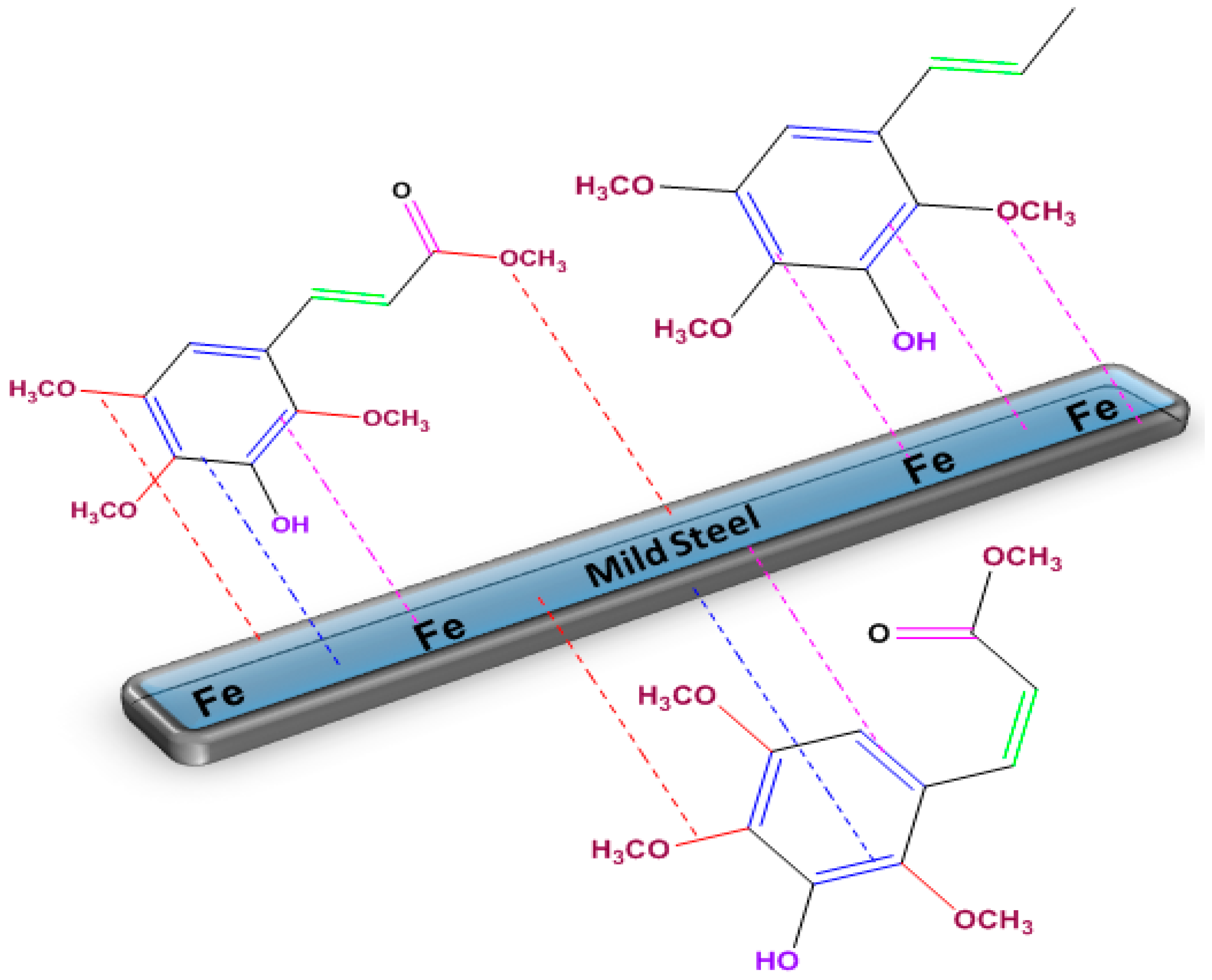
3.3.6. Corrosion Mechanism
3.3.7. Corrosion Comparison
4. Conclusions
- This study mainly focuses on emphasizing the eminent role of the incredible mangrove plant C. tagal in preventing oxidation and corrosion.
- The amount of TPC and TFC highlights the need to focus on other active ingredient in C. tagal to learn more about the plant in the future.
- The scavenging activity on DPPH, nitric oxide, and hydrogen peroxide portraits the performance of C. tagal as a successful antioxidant.
- The inhibition efficiency of the plants is more evident in weight loss, ASS, UV-visible, SEM-EDX, and AFM for corrosion inhibition.
- Although there is no direct correlation between the antioxidant and anticorrosive activities, similar studies on several other plants and their components have highlighted the role of secondary metabolites in both cases. Thus, the C. tagal plant can be taken for further research on metal inhibition and other pharmaceutical applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, M.; Nuruddin, A. Soil and Mangrove: A Review. J. Environ. Sci. Technol. 2016, 9, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Basha, S.K.C. An overview on global mangroves distribution. Indian J. Geo-Mar. Sci. 2018, 47, 766–772. [Google Scholar]
- Ricklefs, R.E.; Latham, R.E. Global Patterns of Diversity in Mangrove Floras. Species Diversity in Ecological Communities: Historical and Geographical Perspectives; University of Chicago Press: Chicago, IL, USA, 1993; Chapter 20; pp. 215–229. [Google Scholar]
- Khafagi, I.; Gab-Alla, A.; Salama, W.; Fouda, M. Biological activities and phytochemical constituents of the gray mangrove Avicennia marina (Forssk.) Vierh. Egypt. J. Biol. 2003, 5, 62–69. [Google Scholar]
- Eswaraiah, G.; Peele, K.A.; Krupanidhi, S.; Kumar, R.B.; Venkateswarulu, T. Studies on phytochemical, antioxidant, antimicrobial analysis and separation of bioactive leads of leaf extract from the selected mangroves. J. King Saud Univ.-Sci. 2019, 32, 842–847. [Google Scholar] [CrossRef]
- Cruz, S.M.; Marroquín, N.; Alvarez, L.E.; Chang, D.E.; Cáceres, A. Evaluation of mangrove (Rhizophora mangle L.) products as coloring, antimicrobial and antioxidant agents. Int. J. Phytocosmet. Nat. Ingred. 2015, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, V.V.; Guruvayoorappan, C. Phytochemical screening of methanolic extract of mangrove Avicennia marina (Forssk.) Vierh. Der Pharm. Sin. 2012, 3, 64–70. [Google Scholar]
- Glasenapp, Y.; Korth, I.; Nguyen, X.V.; Papenbrock, J. Sustainable use of mangroves as sources of valuable medicinal compounds: Species identification, propagation and secondary metabolite composition. S. Afr. J. Bot. 2019, 121, 317–328. [Google Scholar] [CrossRef]
- Bibi, S.N.; Fawzi, M.M.; Gokhan, Z.; Rajesh, J.; Nadeem, N.; Kannan, R.; Albuquerque, R.D.D.G.; Pandian, S.K. Ethnopharmacology, phytochemistry, and global distribution of mangroves-A comprehensive review. Mar. Drugs 2019, 17, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahim, A.A.; Rocca, E.; Steinmetz, J.; Kassim, M.J.; Ibrahim, M.S.; Osman, H. Antioxidant activities of mangrove Rhizophora apiculata bark extracts. Food Chem. 2008, 107, 200–207. [Google Scholar] [CrossRef]
- Thatoi, H.; Behera, B.C.; Mishra, R.R.; Dutta, S.K. Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: A review. Ann. Microbiol. 2012, 63, 1–19. [Google Scholar] [CrossRef]
- Kesavan, D.; Gopiraman, M.; Sulochana, N. Green inhibitors for corrosion of metals: A review. Chem. Sci. Rev. Lett. 2012, 1, 1–8. [Google Scholar]
- Chigondo, M.; Chigondo, F. Recent Natural Corrosion Inhibitors for Mild Steel: An Overview. J. Chem. 2016, 2016, 6208937. [Google Scholar] [CrossRef] [Green Version]
- Khan, G.; Md, K.; Newaz, S.; Basirun, W.J.; Ali, H.B.M.; Faraj, F.L.; Khan, G.M. Application of natural product extracts as green corrosion inhibitors for metals and alloys in acid pickling processes—A review. Int. J. Electrochem. Sci. 2015, 10, 6120–6134. [Google Scholar]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Xiao, X.; Hong, Y.; Xia, W.; Feng, S.; Zhou, X.; Fu, X.; Zang, J.; Xiao, Y.; Niu, X.; Li, C.; et al. Transcriptome Analysis of Ceriops tagal in Saline Environments Using RNA-Sequencing. PLoS ONE 2016, 11, e0167551. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.A.; Ammani, K.; Siddhardha, B.; Sreedhar, U.; Kumar, G.A. Differential biological activities of the solvent extracts of Ceriops decandra (Griff.) and their phytochemical investigations. J. Pharm. Res. 2013, 7, 654–660. [Google Scholar] [CrossRef]
- Ni, S.-J.; Li, J.; Li, M.-Y. Two New Dolabrane Diterpenes from the Chinese Mangrove Ceriops tagal. Chem. Biodivers. 2018, 15, e1700563. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Liu, D.; Li-Weber, M.; Shao, B.; Lin, W. Dolabrane-type diterpenes from the mangrove plant Ceriops tagal with antitumor activities. Fitoterapia 2015, 103, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.-J.; Li, J.; Li, M.-Y. Two new phenylpropanoids from the Chinese mangrove Ceriops tagal. Nat. Prod. Res. 2017, 32, 1676–1681. [Google Scholar] [CrossRef]
- Wang, P.; Cui, Y.; Cai, C.; Chen, H.; Dai, Y.; Chen, P.; Kong, F.; Yuan, J.; Song, X.; Mei, W.; et al. Two New Succinimide Derivatives Cladosporitins A and B from the Mangrove-derived Fungus Cladosporium sp. HNWSW-1. Mar. Drugs 2018, 17, 4. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.-T.; Zheng, C.-J.; Zhang, B.; Zhou, X.-M.; Zhou, Q.; Chen, G.-Y.; Zeng, Z.-E.; Xie, J.-L.; Han, C.-R.; Lyu, J.-X. Two new secondary metabolites from a mangrove-derived fungus Cladosporium sp. JJM22. Nat. Prod. Res. 2018, 33, 34–40. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.-H.; Mugila, N.; Hemapriya, V.; Parameswari, K.; Chitra, S.; Chung, I.-M. Aster koraiensis as nontoxic corrosion inhibitor for mild steel in sulfuric acid. J. Ind. Eng. Chem. 2017, 52, 235–242. [Google Scholar] [CrossRef]
- Helen, L.Y.S.; Rahim, A.A.; Saad, B.; Saleh, M.I.; Raj, P.B. Aquilaria crassna leaves extracts-a green corrosion inhibitor for mild steel in 1 M HCl medium. Int. J. Electrochem. Sci. 2014, 9, 830–846. [Google Scholar]
- Prabakaran, M.; Kim, S.-H.; Hemapriya, V.; Chung, I.-M. Evaluation of polyphenol composition and anti-corrosion properties of Cryptostegia grandiflora plant extract on mild steel in acidic medium. J. Ind. Eng. Chem. 2016, 37, 47–56. [Google Scholar] [CrossRef]
- Alam, N.; Bristi, N.J. Rafiquzzaman Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2012, 21, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Aiyegoro, O.A.; Okoh, A.I. Preliminary phytochemical screening and In vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement. Altern. Med. 2010, 10, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malathy, R.; Prabakaran, M.; Kalaiselvi, K.; Chung, I.M.; Kim, S.H. Comparative polyphenol composition, antioxidant and anticorrosion properties in various parts of panax ginseng extracted in different solvents. Appl. Sci. 2021, 11, 93. [Google Scholar] [CrossRef]
- Hemapriya, V.; Chung, I.-M.; Kim, S.-H.; Prabakaran, M. Inhibitory effect of biowaste on copper corrosion in 1 M HCl solution. Mater. Today Commun. 2021, 27, 102249. [Google Scholar] [CrossRef]
- Saeed, M.; Saleem, M.; Usmani, S.; Malik, I.A.; Al-Shammari, F.A.; Deen, K.M. Corrosion inhibition of mild steel in 1 M HCl by sweet melon peel extract. J. King Saud Univ. Sci. 2019, 31, 1344–1351. [Google Scholar] [CrossRef]
- Fadare, O.; Okoronkwo, A.E.; Olasehinde, E.F. Assessment of anti-corrosion potentials of extract of Ficus asperifolia -Miq (Moraceae) on mild steel in acidic medium. Afr. J. Pure Appl. Chem. 2016, 10, 8–22. [Google Scholar] [CrossRef]
- Sakunthala, P.; Vivekananthan, S.S.; Gopiraman, M.; Sulochana, N.; Vincent, A.R. Spectroscopic investigations of physicochemical interactions on Umoren mild steel in an acidic medium by environmentally friendly green inhibitors. J. Surfactants Deterg. 2013, 16, 251–263. [Google Scholar] [CrossRef]
- Rani, B.E.A.; Basu, B.B.J. Green Inhibitors for Corrosion Protection of Metals and Alloys: An Overview. Int. J. Corros. 2011, 2012, 380217. [Google Scholar] [CrossRef]
- Lipinski, B. Hydroxyl Radical and Its Scavengers in Health and Disease. Oxidative Med. Cell. Longev. 2011, 2011, 809696. [Google Scholar] [CrossRef] [Green Version]
- Kumar, T.; Jain, V. Appraisal of total phenol, flavonoid contents, and antioxidant potential of folkloric Lannea coromandelica using in vitro and in vivo assays. Scientifica 2015, 2015, 203679. [Google Scholar] [CrossRef] [Green Version]
- Chaouche, T.M.; Haddouchi, F.; Ksouri, R.; Atik-Bekkara, F. Evaluation of antioxidant activity of hydromethanolic extracts of some medicinal species from South Algeria. J. Chin. Med. Assoc. 2014, 77, 302–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Song, Y.; Su, H.; Zhang, L.; Chen, G.; Zhao, J. Investigation of Diospyros Kaki L. f husk extracts as corrosion inhibitors and bactericide in oil field. Chem. Central J. 2013, 7, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Gangwar, M.; Gautam, M.K.; Sharma, A.K.; Tripathi, Y.B.; Goel, R.K.; Nath, G. Antioxidant Capacity and Radical Scavenging Effect of Polyphenol RichMallotus philippenensisFruit Extract on Human Erythrocytes: AnIn VitroStudy. Sci. World J. 2014, 2014, 279451. [Google Scholar] [CrossRef] [Green Version]
- Liang, N.; Kitts, D.D. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [Green Version]
- Habu, J.B.; Ibeh, B.O. In vitro antioxidant capacity and free radical scavenging evaluation of active metabolite constituents of Newbouldia laevis ethanolic leaf extract. Biol. Res. 2015, 48, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boora, F.; Chirisa, E.; Mukanganyama, S. Evaluation of Nitrite Radical Scavenging Properties of Selected Zimbabwean Plant Extracts and Their Phytoconstituents. J. Food Process. 2014, 2014, 918018. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.-X.; Zhang, H.-S.; Yang, S.-F. Phenolic compounds and its antioxidant activities in ethanolic extracts from seven cultivars of Chinese jujube. Food Sci. Hum. Wellness 2014, 3, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug. Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabakaran, M.; Kim, S.-H.; Sasireka, A.; Chandrasekaran, M.; Chung, I.-M. Polyphenol composition and antimicrobial activity of various solvent extracts from different plant parts of Moringa oleifera. Food Biosci. 2018, 26, 23–29. [Google Scholar] [CrossRef]
- Chung, I.-M.; Malathy, R.; Priyadharshini, R.; Hemapriya, V.; Kim, S.-H.; Prabakaran, M. Inhibition of mild steel corrosion using Magnolia kobus extract in sulphuric acid medium. Mater. Today Commun. 2020, 25, 101687. [Google Scholar] [CrossRef]
- Manokarana, G.; Prabakaran, M. Evaluation of antioxidant and anticorrosion activities of Ligularia fischeri plant extract. Chem. Sci. Eng. Res. 2019, 1, 16–24. [Google Scholar] [CrossRef]
- Chung, I.-M.; Kim, S.-H.; Prabakaran, M. Evaluation of Phytochemical, Polyphenol Composition and Anti-corrosion Capacity of Cucumis anguria L. Leaf Extract on Metal Surface in Sulfuric Acid Medium. Prot. Met. Phys. Chem. Surf. 2020, 56, 214–224. [Google Scholar] [CrossRef]
- Nahlé, A.; Abu-Abdoun, I.; Abdel-Rahman, I. Effect of Temperature on the Corrosion Inhibition of Trans-4-Hydroxy-4′-Stilbazole on Mild Steel in HCl Solution. Int. J. Corros. 2012, 2012, 380329. [Google Scholar] [CrossRef] [Green Version]
- Labjar, N.; Bentiss, F.; Lebrini, M.; Jama, C.; El Hajjaji, S. Study of Temperature Effect on the Corrosion Inhibition of C38 Carbon Steel Using Amino-tris(Methylenephosphonic) Acid in Hydrochloric Acid Solution. Int. J. Corros. 2011, 2011, 548528. [Google Scholar] [CrossRef] [Green Version]
- Umoren, S.; Eduok, U.; Solomon, M.; Udoh, A. Corrosion inhibition by leaves and stem extracts of Sida acuta for mild steel in 1M H2SO4 solutions investigated by chemical and spectroscopic techniques. Arab. J. Chem. 2016, 9, S209–S224. [Google Scholar] [CrossRef] [Green Version]
- Júnior, J.M.F.; Silva, M.G.D.; Monteiro, J.A.; Barros, A.D.S.; Falcão, M.J.C.; Morais, S.M.D. Evaluation of antioxidant activity and inhibition of corrosion by brazilian plant extracts and constituents. Int. J. Electrochem. Sci. 2016, 11, 3862–3875. [Google Scholar] [CrossRef]
- Hassan, H.M. Utility of bacterial biomass as antioxidant, anticorrosive, and antifriction additives for lubricating oils. Petrol Sci. Technol. 2011, 29, 2086–2094. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.-H.; Kalaiselvi, K.; Hemapriya, V.; Chung, I.-M. Highly efficient Ligularia fischeri green extract for the protection against corrosion of mild steel in acidic medium: Electrochemical and spectroscopic investigations. J. Taiwan Inst. Chem. Eng. 2016, 59, 553–562. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.-H.; Hemapriya, V.; Chung, I.-M. Tragia plukenetii extract as an eco-friendly inhibitor for mild steel corrosion in HCl 1 M acidic medium. Res. Chem. Intermed. 2015, 42, 3703–3719. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. A combined experimental and theoretical study of green corrosion inhibition of mild steel in HCl solution by aqueous Citrullus lanatus fruit (CLF) extract. J. Mol. Liq. 2019, 279, 603–624. [Google Scholar] [CrossRef]
- Taheri, S.; Delgado, G.P.; Agbaje, O.B.A.; Giri, P.; Clark, S.M. Corrosion inhibitory effects of mullite in concrete exposed to sulfuric acid attack. Corros. Mater. Degrad. 2020, 1, 282–295. [Google Scholar] [CrossRef]
- Baran, E.; Cakir, A.; Yazici, B. Inhibitory effect of Gentiana olivieri extracts on the corrosion of mild steel in 0.5 M HCl: Electrochemical and phytochemical evaluation. Arab. J. Chem. 2016, 12, 4303–4319. [Google Scholar] [CrossRef] [Green Version]
- Patni, N.; Agarwal, S.; Shah, P. Greener Approach towards Corrosion Inhibition. Chin. J. Eng. 2013, 2013, 784186. [Google Scholar] [CrossRef] [Green Version]
- Chitra, S.; Chung, I.-M.; Kim, S.-H.; Prabakaran, M. A study on anticorrosive property of phenolic components from Pachysandra terminalis against low carbon steel corrosion in acidic medium. Pigment Resin Technol. 2019, 48, 389–396. [Google Scholar] [CrossRef]
- Chung, I.-M.; Hemapriya, V.; Kim, S.-H.; Ponnusamy, K.; Arunadevi, N.; Chitra, S.; Prabakaran, M.; Gopiraman, M. Liriope platyphylla extract as a green inhibitor for mild steel corrosion in sulfuric acid medium. Chem. Eng. Commun. 2019, 208, 72–88. [Google Scholar] [CrossRef]
- Mahalakshmi, D.; Unnisa, C.B.N.; Hemapriya, V.; Subramaniam, E.P.; Roopan, S.M.; Chitra, S.; Chung, I.M.; Kim, S.H.; Prabakaran, M. Anticorrosive potential of ethanol extract of Delonix elata for mild steel in 0.5 M H2SO4—A green approach, Bulg. Chem. Commun. 2019, 51, 31–37. [Google Scholar]
- Chung, I.-M.; Malathy, R.; Kim, S.-H.; Kalaiselvi, K.; Prabakaran, M.; Gopiraman, M. Ecofriendly green inhibitor from Hemerocallis fulva against aluminum corrosion in sulphuric acid medium. J. Adhes. Sci. Technol. 2020, 34, 1483–1506. [Google Scholar] [CrossRef]
- Hemapriya, V.; Prabakaran, M.; Chitra, S.; Swathika, M.; Kim, S.-H.; Chung, I.-M. Utilization of biowaste as an eco-friendly biodegradable corrosion inhibitor for mild steel in 1 mol/L HCl solution. Arab. J. Chem. 2020, 13, 8684–8696. [Google Scholar] [CrossRef]
- Anitha, R.; Unnisa, C.B.N.; Hemapriya, V.; Roopan, S.M.; Chitra, S.; Chung, I.-M.; Kim, S.-H.; Mayakrishnan, P. Anti-corrosive potential of Cyperus rotundus as a viable corrosion inhibitor for mild steel in sulphuric acid. Pigment Resin Technol. 2020, 49, 295–304. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Ahmed, M.H.O.; Abdullah, T.A.; Gaaz, T.S.; Kadhum, A.A.H. Electrochemical studies of novel corrosion inhibitor for mild steel in 1 M hydrochloric acid. Results Phys. 2018, 9, 978–981. [Google Scholar] [CrossRef]
- Chung, I.-M.; Kim, S.-H.; Hemapriya, V.; Kalaiselvi, K.; Prabakaran, M. Inhibition behavior of Tragia involucrata L. phenolic compounds against acidic medium corrosion in low carbon steel surface. Chin. J. Chem. Eng. 2018, 27, 717–725. [Google Scholar] [CrossRef]
- Chung, I.-M.; Hemapriya, V.; Ponnusamy, K.; Arunadevi, N.; Chitra, S.; Youn, C.-H.; Kim, S.-H.; Prabakaran, M. Assessment of Low Carbon Steel Corrosion Inhibition by Eco-Friendly Green Chaenomeles sinensis Extract in Acid Medium. J. Electrochem. Sci. Technol. 2018, 9, 238–249. [Google Scholar] [CrossRef] [Green Version]
- Prabakaran, M.; Kim, S.H.; Hemapriya, V.; Gopiraman, M.; Kim, I.S.; Chung, I.M. Rhus verniciflua as a green corrosion inhibitor for mild steel in 1 M H2SO4. RSC Adv. 2016, 6, 57144–57153. [Google Scholar] [CrossRef]
- Anitha, R.; Chitra, S.; Hemapriya, V.; Chung, I.-M.; Kim, S.-H.; Prabakaran, M. Implications of eco-addition inhibitor to mitigate corrosion in reinforced steel embedded in concrete. Constr. Build. Mater. 2019, 213, 246–256. [Google Scholar] [CrossRef]
- Ostovari, A.; Hoseinieh, S.M.; Peikari, M.P.; Shadizadeh, S.R.; Hashemi, J. Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: A comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, alpha-D-Glucose and Tannic acid). Corros. Sci. 2009, 51, 1935–1949. [Google Scholar] [CrossRef]
- Leena, P.; Hukuman, N.H.Z.; Biju, A.R.; Jisha, M. Studies on methanolic extract of Lepidagathis keralensis as green corrosion inhibitor for mild steel in 1M HCl. J. Electrochem. Sci. Technol. 2019, 10, 231–243. [Google Scholar]
- Ramezanzadeh, M.; Bahlakeh, G.; Sanaei, Z.; Ramezanzadeh, B. Corrosion inhibition of mild steel in 1 M HCl solution by ethanolic extract of eco-friendly Mangifera indica (mango) leaves: Electrochemical, molecular dynamics, Monte Carlo and ab initio study. Appl. Surf. Sci. 2019, 463, 1058–1077. [Google Scholar] [CrossRef]
- Bouyanzer, A.; Hammouti, B.; Majidi, L. Pennyroyal oil from Mentha pulegium as corrosion inhibitor for steel in 1M HCl. Mater. Lett. 2006, 60, 2840–2843. [Google Scholar] [CrossRef]
- Benarioua, M.; Mihi, A.; Bouzeghaia, N.; Naoun, M. Mild steel corrosion inhibition by Parsley (Petroselium Sativum) extract in acidic media. Egypt. J. Pet. 2019, 28, 155–159. [Google Scholar] [CrossRef]


| Conc. (ppm) | W (mg·cm−2) | θ | η% | σ a |
|---|---|---|---|---|
| Blank | 0.0989 | - | - | - |
| 100 | 0.0501 | 0.49 | 49 | 0.03 |
| 200 | 0.0432 | 0.56 | 56 | 0.02 |
| 300 | 0.0388 | 0.61 | 61 | 0.01 |
| 400 | 0.0298 | 0.70 | 70 | 0.02 |
| 500 | 0.0102 | 0.90 | 90 | 0.03 |
| 600 | 0.0055 | 0.95 | 95 | 0.01 |
| T (±1 K) | Conc. (ppm) | W (mg·cm−2) | θ | η% | σ a |
|---|---|---|---|---|---|
| Blank | 0.1251 | - | - | - | |
| 100 | 0.0702 | 0.44 | 44 | 0.02 | |
| 200 | 0.0633 | 0.49 | 49 | 0.04 | |
| 313 | 300 | 0.0504 | 0.60 | 60 | 0.03 |
| 400 | 0.0413 | 0.67 | 67 | 0.03 | |
| 500 | 0.0331 | 0.74 | 74 | 0.02 | |
| 600 | 0.0209 | 0.83 | 83 | 0.03 | |
| Blank | 0.1410 | - | - | - | |
| 100 | 0.0857 | 0.39 | 39 | 0.02 | |
| 200 | 0.0758 | 0.46 | 46 | 0.01 | |
| 323 | 300 | 0.0701 | 0.50 | 50 | 0.03 |
| 400 | 0.0598 | 0.58 | 58 | 0.01 | |
| 500 | 0.0503 | 0.64 | 64 | 0.02 | |
| 600 | 0.0328 | 0.77 | 77 | 0.02 | |
| Blank | 0.1787 | - | - | - | |
| 100 | 0.1221 | 0.32 | 32 | 0.02 | |
| 200 | 0.1151 | 0.36 | 36 | 0.03 | |
| 333 | 300 | 0.1020 | 0.43 | 43 | 0.01 |
| 400 | 0.0852 | 0.52 | 52 | 0.01 | |
| 500 | 0.0757 | 0.58 | 58 | 0.02 | |
| 600 | 0.0629 | 0.65 | 65 | 0.02 | |
| Blank | 0.2002 | - | - | - | |
| 100 | 0.1485 | 0.26 | 26 | 0.02 | |
| 343 | 200 | 0.1302 | 0.35 | 35 | 0.03 |
| 300 | 0.1221 | 0.39 | 39 | 0.01 | |
| 400 | 0.1112 | 0.44 | 44 | 0.02 | |
| 500 | 0.1021 | 0.49 | 49 | 0.03 | |
| 600 | 0.0921 | 0.54 | 54 | 0.02 |
| Conc. (ppm) | Amount of Mild Steel Corrodant (mg/I) | η (%) | σ a |
|---|---|---|---|
| Blank | 45.214 | - | - |
| 100 | 32.546 | 28 | 0.01 |
| 200 | 28.541 | 37 | 0.03 |
| 300 | 25.299 | 44 | 0.02 |
| 400 | 20.548 | 55 | 0.04 |
| 500 | 12.571 | 72 | 0.02 |
| 600 | 08.254 | 82 | 0.02 |
| Elements | Composition (Atomic%) | |
|---|---|---|
| Blank | Inhibited | |
| C | 4.34 | 6.37 |
| Fe | 71.54 | 84.21 |
| O | 22.10 | 9.01 |
| S | 0.12 | 0.11 |
| N | 0.18 | 0.12 |
| Mn | 1.51 | 0.07 |
| Mg | 0.21 | 0.11 |
| S. No | Green Inhibitor | η (%) |
|---|---|---|
| 1. | Aster koraiensis | 90.53 [23] |
| 2. | Ceriops tagal# | 95 |
| 3. | Cryptostegia grandiflora | 87.54 [25] |
| 4. | Sweet melon peel | 91.59 [30] |
| 5. | Desmodium triflorum | 92.99 [32] |
| 6. | Polycarpaea corymbosa | 91.78 [32] |
| 7. | Citrullus lanatus | 91.00 [57] |
| 8. | Lepidagathis keralensis | 92.06 [72] |
| 9. | Mangifera indica | 92.73 [73] |
| 10. | Mangifera indica | 92.00 [74] |
| 11. | Mentha pulegium | 80.00 [75] |
| 12. | Petroselium Sativum | 92.39 [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamsuzzaman, M.; Kalaiselvi, K.; Prabakaran, M. Evaluation of Antioxidant and Anticorrosive Activities of Ceriops tagal Plant Extract. Appl. Sci. 2021, 11, 10150. https://doi.org/10.3390/app112110150
Shamsuzzaman M, Kalaiselvi K, Prabakaran M. Evaluation of Antioxidant and Anticorrosive Activities of Ceriops tagal Plant Extract. Applied Sciences. 2021; 11(21):10150. https://doi.org/10.3390/app112110150
Chicago/Turabian StyleShamsuzzaman, Md, Kathirvel Kalaiselvi, and Mayakrishnan Prabakaran. 2021. "Evaluation of Antioxidant and Anticorrosive Activities of Ceriops tagal Plant Extract" Applied Sciences 11, no. 21: 10150. https://doi.org/10.3390/app112110150







