Impact of Ferment Processing Parameters on the Quality of White Pan Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Evaluation of Flour Quality
2.3. Ferment Preparation
2.4. Evaluation of Ferment Quality
2.5. Bread Processing
2.6. Evaluation of Bread Quality
2.7. Statistical Analysis
3. Results and Discussion
3.1. Flour Quality
3.2. Ferment Storage Time
3.2.1. Effect of Storage Time on Ferment Quality
3.2.2. Effect of Ferment Storage Time on Bread Processing
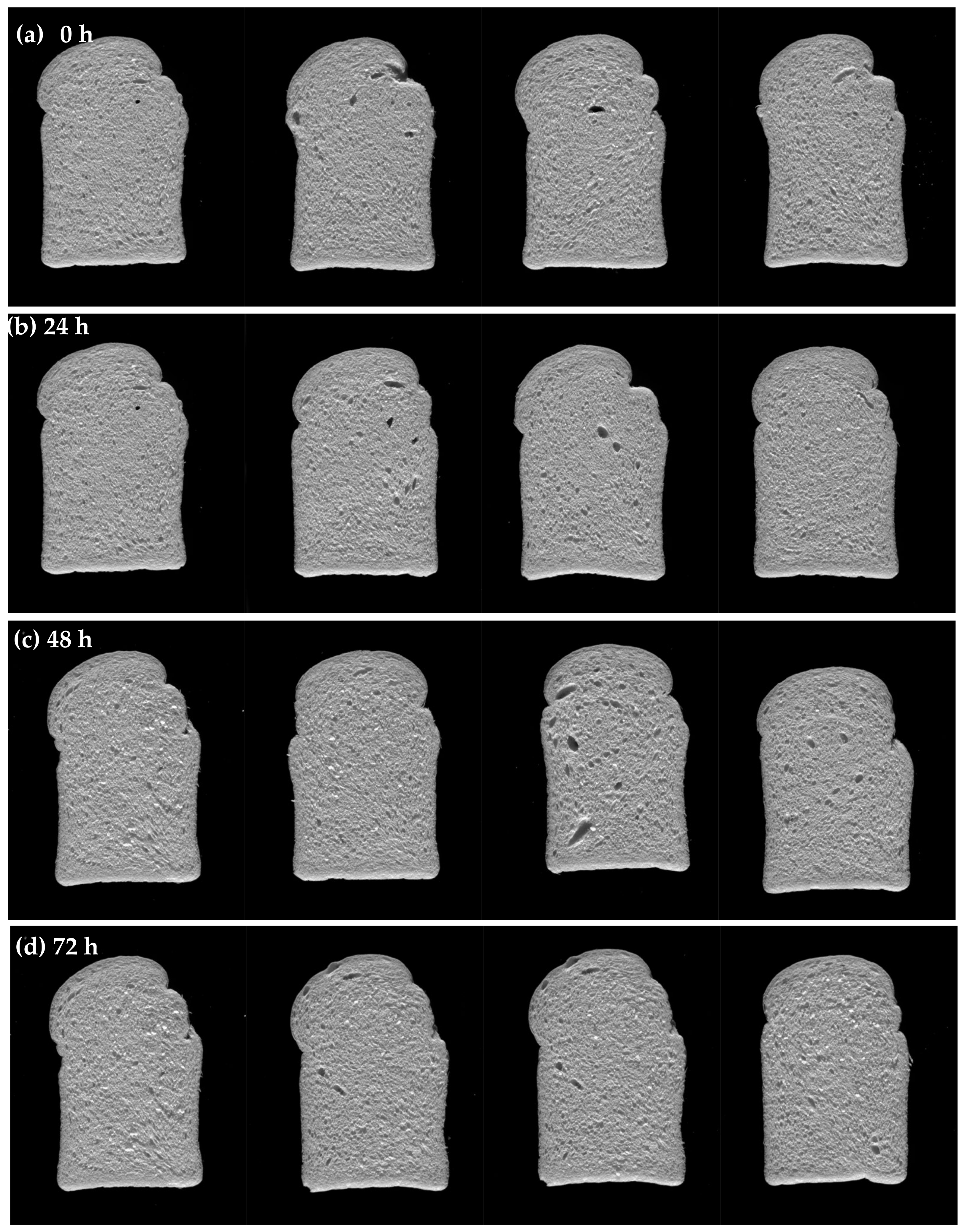
3.2.3. Effect of Ferment Storage Time on Bread Quality
3.3. Fermentation Time
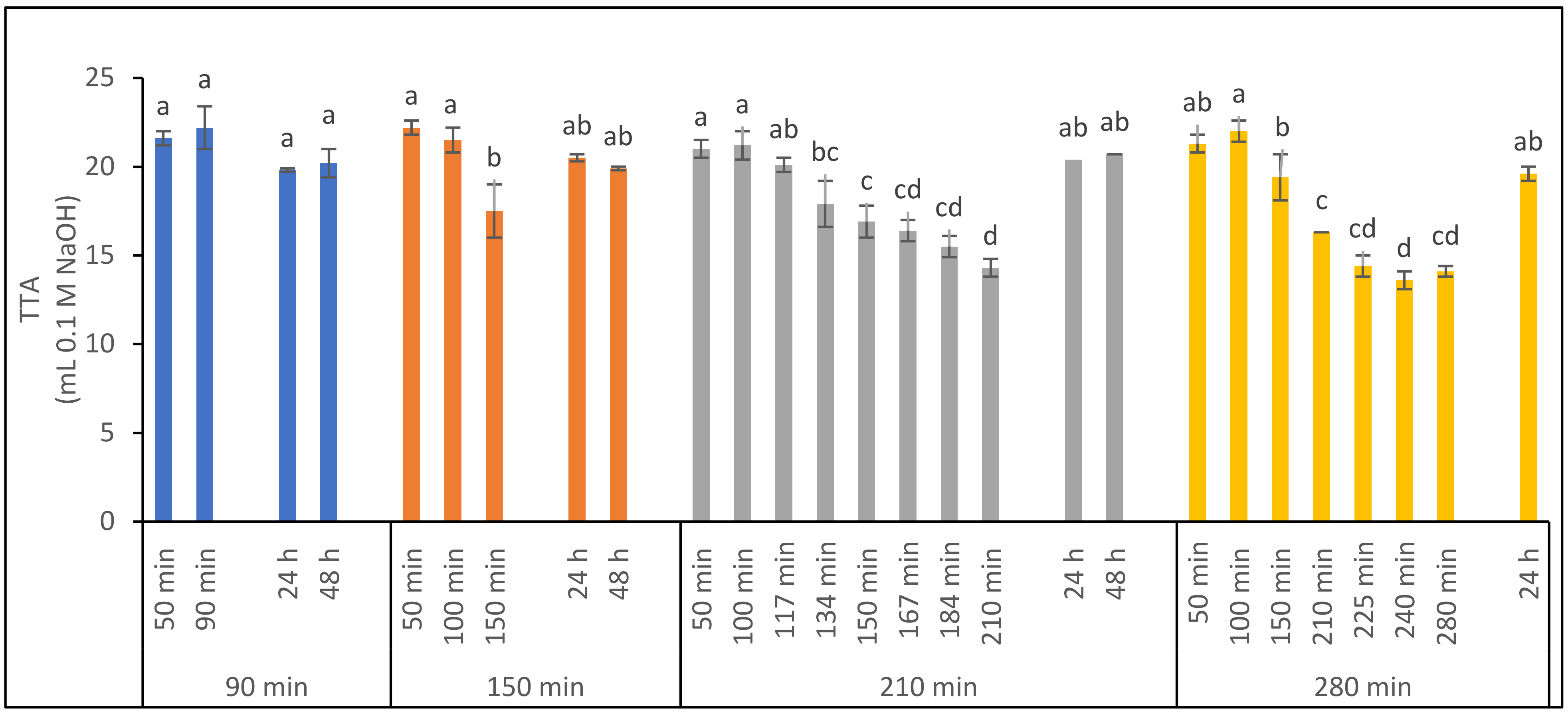
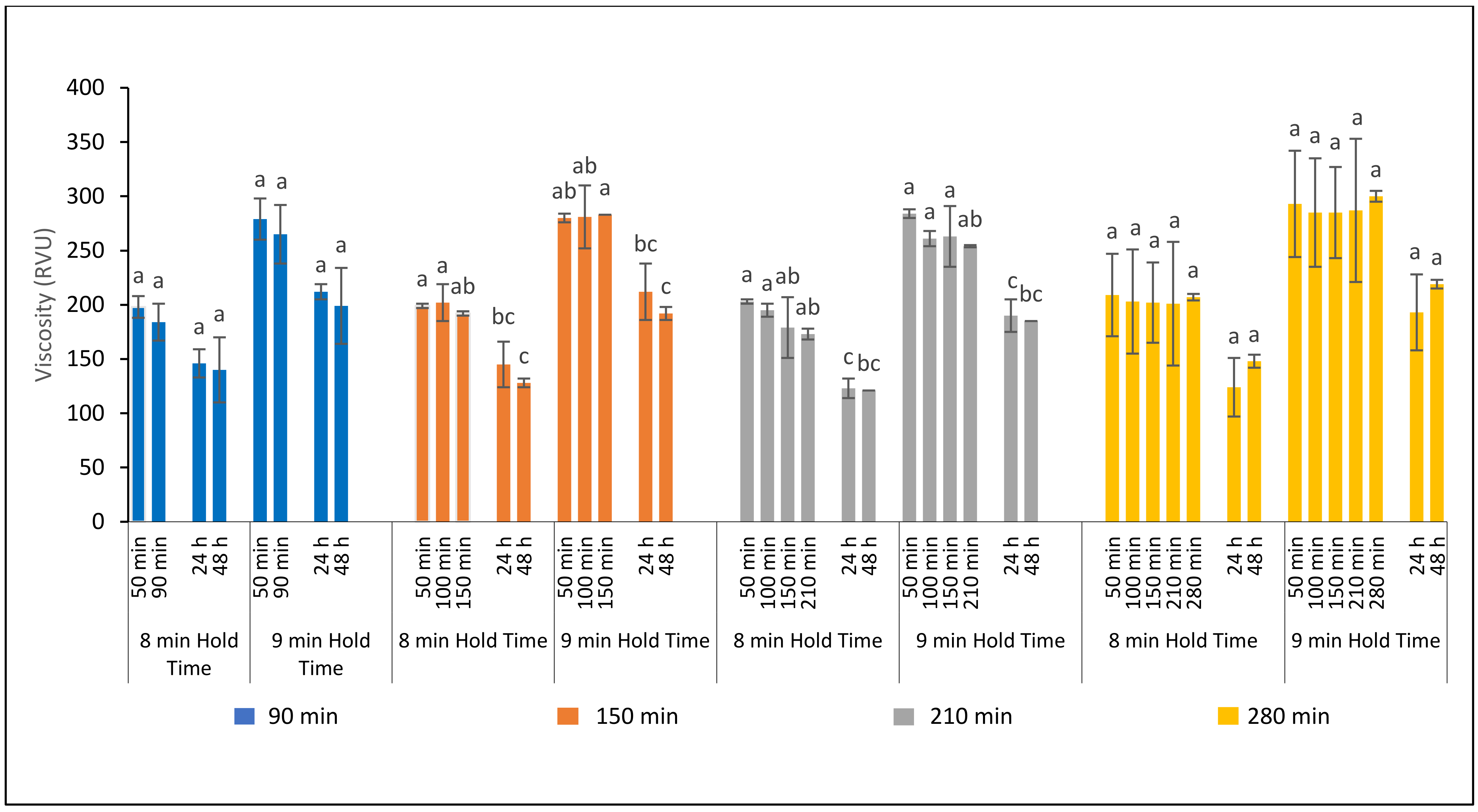
3.3.1. Effect of Fermentation Time on Ferment Quality
3.3.2. Effect of Fermentation Time on Bread Processing
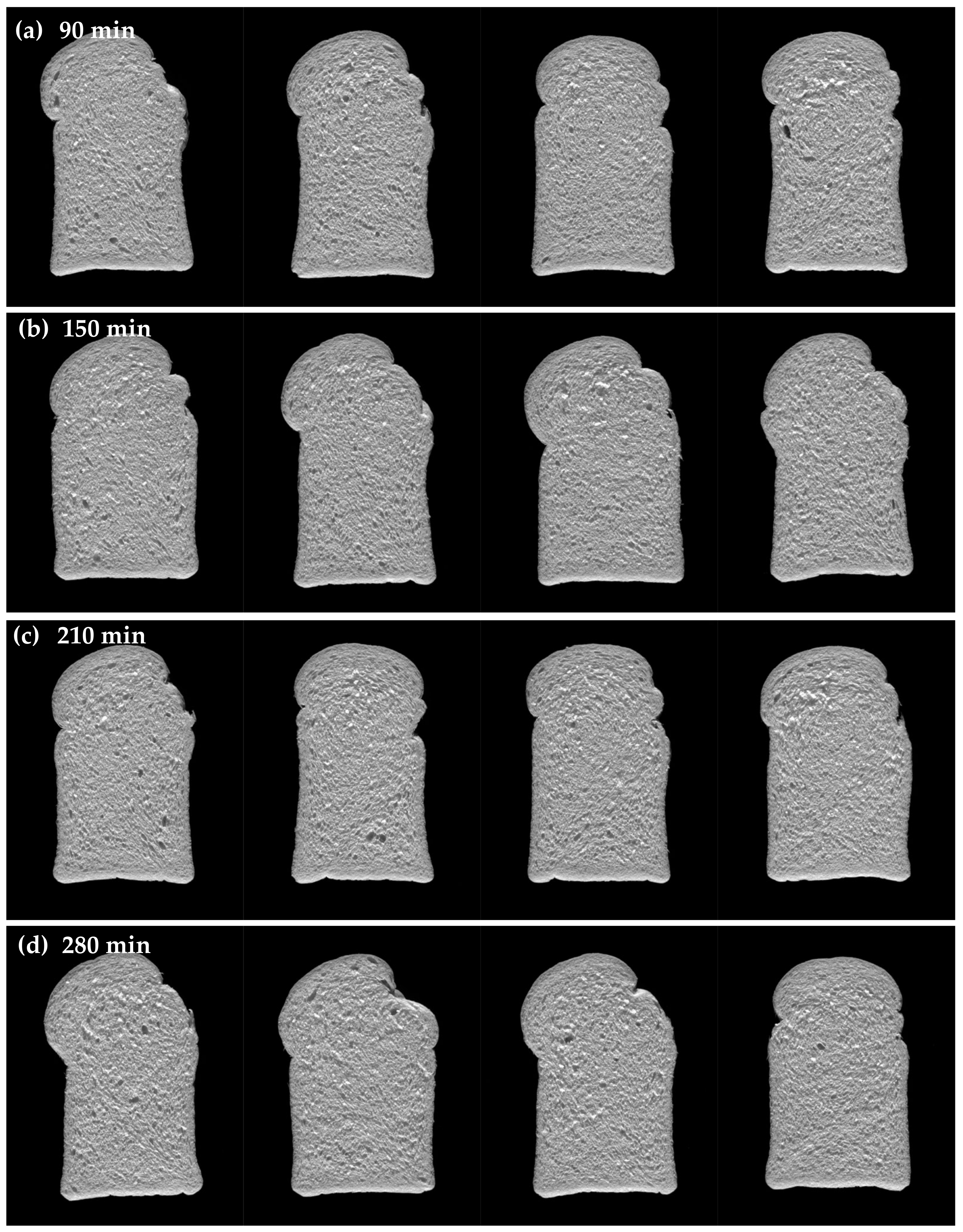
3.3.3. Effect of Fermentation Time on Bread Quality
3.4. Fermentation Temperature
3.4.1. Effect of Fermentation Temperature on Ferment Quality
3.4.2. Effect of Fermentation Temperature on Bread Processing
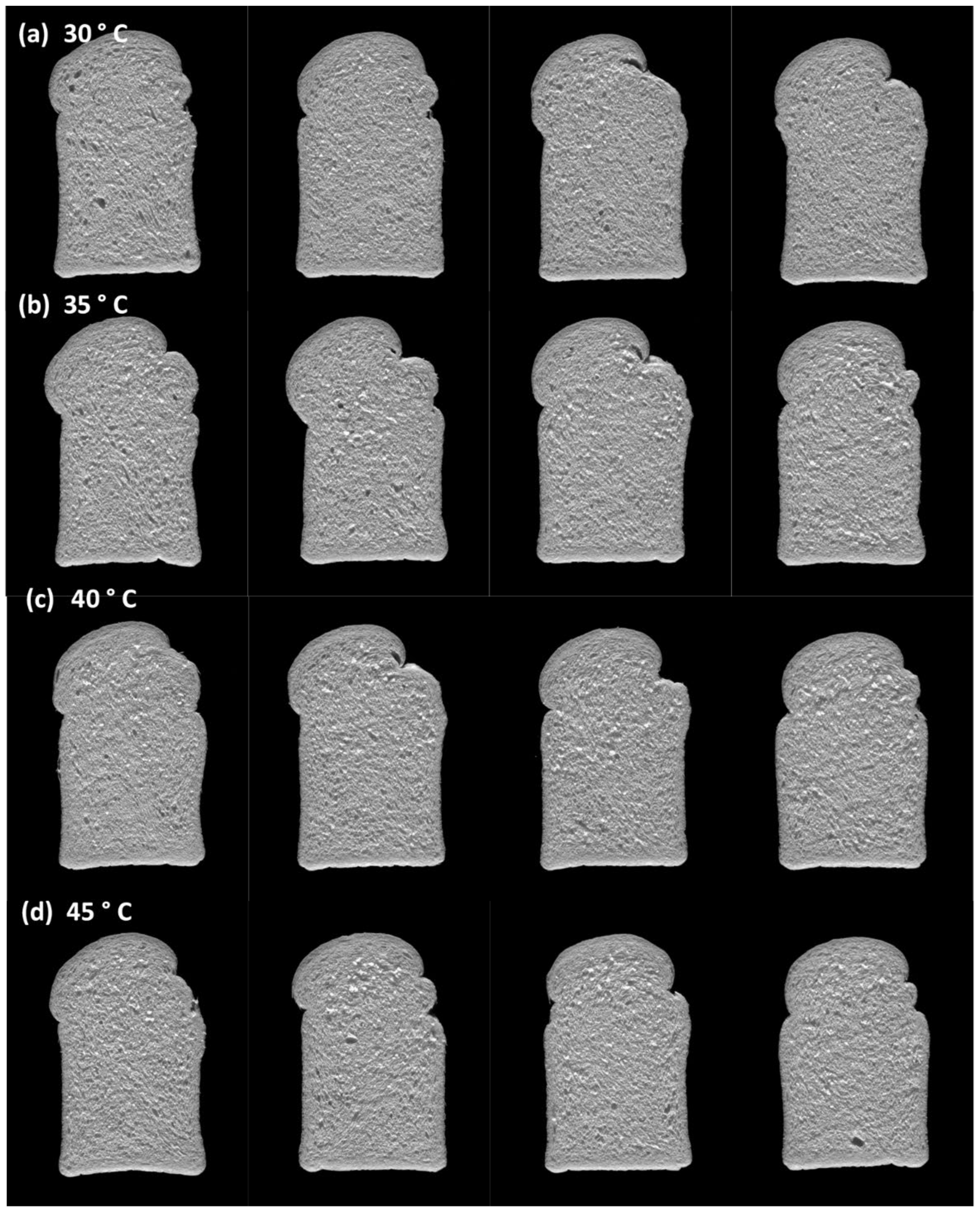
3.4.3. Effect of Fermentation Temperature on Bread Quality
3.5. Validation Trial
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [Green Version]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-promoting components in fermented foods: An up-to-date systematic review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Maloney, D.H.; Foy, J.J. Yeast fermentations. In Handbook of Dough Fermentations; Kulp, K., Lorenz, K., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2003; ISBN 0-8247-4264-8. [Google Scholar]
- Gorton, L.A. Fundamental bakery dough processes. In Baking Science and Technology, 4th ed.; Pyler, E.J., Gorton, L.A., Eds.; Sosland Publishing Co.: Kansas City, MO, USA, 2009; Volume II, pp. 1–124. ISBN 978-0-9820239-1-4. [Google Scholar]
- Sluimer, P. Principles in brief. In Principles of Breadmaking. Functionality of Raw Materials and Process Steps; Sluimer, P., Ed.; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 2005; pp. 1–15. ISBN 1-891127-45-4. [Google Scholar]
- O’Donnell, K. Methods of bread dough making. AIB Tech. Bull. 1996, XVIII, 1–6. [Google Scholar]
- Gorton, L.A.; Bakhoum, M.; van der Maarel, H. Formulating. In Baking Science and Technology, 4th ed.; Pyler, E.J., Gorton, L.A., Eds.; Sosland Publishing Co.: Kansas City, MO, USA, 2009; Volume II, pp. 167–369. ISBN 978-0-9820239-1-4. [Google Scholar]
- Strouts, B. Secondary ingredients for baking: Dough conditioners, enzymes, dough strengtheners, crumb softeners and preservatives. AIB Tech. Bull. 2012, XXXIV, 1–11. [Google Scholar]
- Kulp, K. Technology of brew system in bread production. Bak. Dig. 1983, 57, 20–23. [Google Scholar]
- Pyler, E.J. Liquid ferment process. Their technical evolution and practical adaptation. Bak. Dig. 1970, 44, 34–40. [Google Scholar]
- Fields, E.T. Flour pre-ferments. In BakingTech Presentations, Proceedings of the American Society of Baking 61st Annual Meeting, Chicago Marriot Hotel, Chicago, IL, USA, 3–6 March 1985; American Society of Bakery Engineers: Chicago, IL, USA, 1986. [Google Scholar]
- Watkins, F.H. High-flour liquid ferments. In BakingTech Presentations, Proceedings of the American Society of Baking 67th Annual Meeting, Chicago Marriot Hotel, Chicago, IL, USA, 3–6 March 1991; The Society: Chicago, IL, USA, 1991. [Google Scholar]
- Stakley, D.M. Water pre-ferments. In BakingTech Presentations, Proceedings of the American Society of Baking 61st Annual Meeting, Chicago Marriot Hotel, Chicago, IL, USA, 3–6 March 1985; American Society of Bakery Engineers: Chicago, IL, USA, 1986. [Google Scholar]
- Sutherland, R. Hydrogen ion concentration (pH) and total titratable acidity tests. AIB Tech. Bull. 1989, XI, 1–6. [Google Scholar]
- Reed, D.G. pH in liquid ferments. Bak. Dig. 1965, 39, 32–36. [Google Scholar]
- Doerry, W.T.A.; Ross, A.; Baker, A. Liquid preferments: A study of factors affecting fermentation parameters and bread quality. AIB Tech. Bull. 1985, VII, 1–9. [Google Scholar]
- Perrou, E. Liquid ferments vs. sponge and dough. In Proceedings of the American Society of Baking 76th Annual Technical Conference, Chicago Marriot Hotel, Chicago, IL, USA, 5–8 March 2000. [Google Scholar]
- Nascimento, K.O.; Nascimento Dias Paes, S.; Augusta, I.M. A review ‘clean labeling’: Applications of natural ingredients in bakery products. J. Food Nutr. 2018, 6, 285–294. [Google Scholar] [CrossRef]
- Williams, P.; Sobering, D.; Antoniszyn, J. Protein testing methods. In Wheat Protein, Production and Marketing, Proceedings of the Wheat Protein Symposium, Saskatoon, SK, Canada, 9–10 March 1998; Fowler, D.B., Geddes, W.E., Johnston, A.M., Preston, K.R., Eds.; University of Saskatchewan Press: Saskatoon, SK, Canada, 1998; pp. 37–47. [Google Scholar]
- AACC International. Approved Methods of the American Association of Cereal Chemists, 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Hugo, L.; Rooney, L.W.; Taylor, J.R.N. Fermented sorghum as a functional ingredient as composite breads. Cereal Chem. 2003, 80, 495–499. [Google Scholar] [CrossRef]
- Jackel, S.S. Fermentation—today and tomorrow. In Proceedings of the American Society of Baking 45th Annual Meeting, Chicago, IL, USA, 3–5 March 1969. [Google Scholar]
- Doerry, W.T.A. Sour dough and breads. AIB Tech. Bull. 1998, XX, 1–9. [Google Scholar]
- Sluimer, P. Fermentation and proof. In Principles of Breadmaking. Functionality of Raw Materials and Process Steps; Sluimer, P., Ed.; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 2005; pp. 82–139. ISBN 1-891127-45-4. [Google Scholar]
- Kusunose, C.; Fujii, T.; Matsumoto, H. Role of starch granules in controlling expansion of dough during baking. Cereal Chem. 1999, 76, 920–924. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Li, Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-Tixier, A.-S.; Chemat, F. Vegetable oils as alternative solvents for green oleo-extraction, purification and formulation of food and natural products. Molecules 2017, 22, 1474. [Google Scholar] [CrossRef]
- Medcalf, D.G.; Youngs, V.L.; Gilles, K.A. Wheat starches. II. Effect of polar and nonpolar lipid fractions on pasting characteristics. Cereal Chem. 1968, 45, 88–95. [Google Scholar]
- Majzoobi, M.; Beparva, P. Effects of acetic and lactic acid on physiochemical characteristics of native and cross-linked wheat starches. Food Chem. 2014, 147, 312–317. [Google Scholar] [CrossRef]
- Lund, D.; Lorenz, K.J. Influence of time, temperature, moisture, ingredients, and processing conditions on starch gelatinization. C R C Crit. Rev. Food Sci. Nutr. 1984, 20, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.R. State of the art—Bakery fermentation. Bak. Dig. 1980, 54, 28–31. [Google Scholar]
- Kulp, K. Influence of liquid ferments on quality characteristics of white pan bread. AIB Tech. Bull. 1986, VIII, 1–9. [Google Scholar]
- Lorenz, K.; Bruemmer, J.-M. Preferments and sourdoughs for German breads. In Handbook of Dough Fermentations; Kulp, K., Lorenz, K., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2003; ISBN 0-8247-4264-8. [Google Scholar]
- Komlenić, D.K.; Ugarčić-Hardi, Ź.; Jukić, M.; Planinić, M.; Bucić-Kojić, A.; Strelec, I. Wheat dough rheology and bread quality effected by Lactobacillus brevis preferment, dry sourdough and lactic acid addition. Int. J. Food Sci. Technol. 2010, 45, 1417–1425. [Google Scholar] [CrossRef]
- Biliaderis, C.G.; Maurice, T.J. Starch gelatinization phenomena studied by differential scanning calorimetry. J. Food Sci. 1980, 45, 1669–1674. [Google Scholar] [CrossRef]
- French, D. Organization of starch granules. In Starch: Chemistry and Technology, 2nd ed.; Whistler, R.L., Paschall, E.F., Bemiller, J.N., Eds.; Academic Press: London, UK, 1984; pp. 184–248. ISBN 0-12746207-8. [Google Scholar]
- Mohamed, A.A.; Rayas-Duarte, P. The effect of mixing and wheat protein/gluten on the gelatinization of wheat starch. Food Chem. 2003, 81, 533–545. [Google Scholar] [CrossRef]
- Balestra, F.; Pinnavaia, G.G.; Romani, S. Evaluation of the effects of different fermentation methods on dough characteristics. J. Texture Stud. 2015, 46, 262–271. [Google Scholar] [CrossRef]
- Angioloni, A.; Romani, S.; Pinnavaia, G.G.; Dalla Rosa, M. Characteristics of bread making doughs: Influence of sourdough fermentation on the fundamental rheological properties. Eur. Food Res. Technol. 2006, 222, 54–57. [Google Scholar] [CrossRef]
- Cauvain, S.P. Breadmaking. In Cereals Processing Technology; Owens, D., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2001; pp. 204–230. ISBN 0-8493-1219-1. [Google Scholar]
- Ragaee, S.; Abdel-Aal, E.M. Pasting properties of starch and protein in selected cereals and quality of their food products. Food Chem. 2006, 95, 9–18. [Google Scholar] [CrossRef]
- Clarke, C.I.; Schober, T.J.; O’Sullivan, K.; Arendt, E.K. Wheat sourdough fermentation: Effects of time and acidification on fundamental rheological properties. Cereal Chem. 2004, 81, 409–417. [Google Scholar] [CrossRef]
- Maher Galal, A.; Varriano-Marston, E.; Johnson, J.A. Rheological dough properties as affected by organic acids and salt. Cereal Chem. 1978, 55, 683–691. [Google Scholar]
- Salovaara, H.; Valjakka, T. The effect of fermentation temperature, flour type, and starter on the properties of sour wheat bread. Int. J. Food Sci. Technol. 1987, 22, 591–597. [Google Scholar] [CrossRef]
- Barber, B.; Ortolá, C.; Barber, S.; Fernández, F. Storage of packaged white bread. III. Effects of sour dough and addition of acids on bread characteristics. Z. für Lebensm. Unters. Und Forsch. 1992, 194, 442–449. [Google Scholar] [CrossRef]

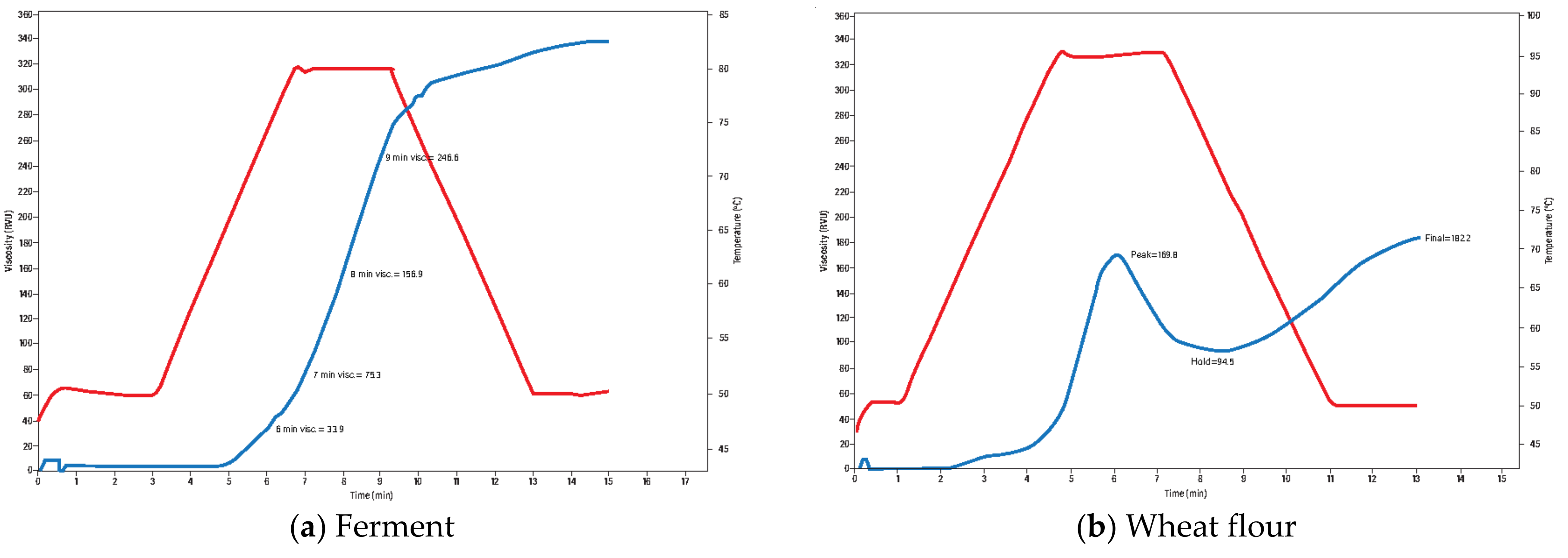






| Processing Parameter | Stage 1 | Stage 2 | Stage 3 | Stage 4 |
|---|---|---|---|---|
| Agitator 1 speed (Hz) | 10 | 35 | 50 | 10 |
| Agitator 2 speed (Hz) | 10 | 35 | 50 | 10 |
| Sparge/air (15 L/min) | ON | |||
| Fermentation time (min) | 1 | 5 + 2 (stoppage) | 5 + 2 (stoppage) | 150–280 1 |
| Fermentation temperature (°C) | 35 | 35 | 35 | 30–45 1 |
| Ingredient | Baker’s % | Weight (g) |
|---|---|---|
| Water | 112 | 8400 |
| Wheat flour | 100 | 7500 |
| Yeast | 3.1 | 235 |
| Oil | 2 | 150 |
| Processing Parameter | Processing Trial | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Ferment storage time (h) | 0, 24, 48, 72 | 0, 24, 48 1 | 0, 24, 48 1 | 0, 24, 48, 72 |
| Fermentation time (min) | 150 | 90, 150, 210, 280 | 240 | 240 |
| Fermentation temperature (°C) | 35 | 35 | 30, 35, 40, 45 | 35 |
| Ferment Processing Trial | Time Intervals for Measuring TTA | Time Intervals for Measuring Viscosity |
|---|---|---|
| Trials 1: Ferment Storage Time | ||
| Fermentation stage (min) | 50, 100, 150 | 50, 100, 150 |
| Ferment storage time (h) | 24, 48, 72 | 24, 48, 72 |
| Trial 2: Fermentation Time | ||
| 90 min | 50, 90 | 50, 90 |
| 150 min | 50, 100, 150 | 50, 100, 150 |
| 210 min | 50, 100, 117, 134, 150, 167, 184, 210 1 | 50, 100, 150, 210 |
| 280 min | 50, 100, 150, 210, 225, 240, 280 1 | 50, 100, 150, 210, 280 |
| Trial 3: Fermentation Temperature | 50, 100, 156, 167, 184, 200, 220, 240 2 | 50, 100, 150, 240 2 |
| Trial 4: Validation | ||
| Fermentation stage (min) | 240 | 240 |
| Ferment storage time (h) | 24, 48, 72 | 24, 48, 72 |
| Ingredient | Baker’s % | Ferment Inclusion Level (%) Ingredient Weight (g) | |||
|---|---|---|---|---|---|
| 0 | 30 | 50 | 70 | ||
| Wheat flour 1 | 100 | 2619 | 2245 | 1995 | 1746 |
| Water | 65–69 2 | 1702–1807 | 1290–1396 | 1016–1121 | 742–847 |
| Ferment | variable | 0 | 786 3 | 1310 | 1833 |
| Yeast | 6.5 | 170 | 170 | 170 | 170 |
| Salt | 1.5 | 39 | 39 | 39 | 39 |
| Dough improver | 1 | 26 | 26 | 26 | 26 |
| Oil | 1 | 26 | 26 | 26 | 26 |
| Sugar | 0.5 | 13 | 13 | 13 | 13 |
| Parameter 1 | Value |
|---|---|
| Protein (%) | 11.6 |
| Moisture (%) | 14.8 |
| Ash (%) | 0.63 |
| Falling number (sec) | 405 |
| Starch damage (UCD) | 25.1 |
| Farinograph Properties | |
| Absorption (%) | 62.7 |
| Dough development time (min) | 2.1 |
| Stability (min) | 15.0 |
| Mixing tolerance index (BU) | 14 |
| RVA Pasting Properties | |
| Peak viscosity (RVU) | 160 |
| Hot paste viscosity (RVU) | 88 |
| Breakdown (RVU) | 72 |
| Final viscosity (RVU) | 177 |
| Setback (RVU) | 89 |
| Pasting time (min) | 5.98 |
| Ferment Storage and Inclusion Level | Mixing Time (min) | Proof Time (min) | Specific Volume (cm3/g) | Crumb Firmness Force (g) | Crumb Colour | ||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| Control | 7.3 ± 0.4 a | 39.8 ± 1.7 ab | 4.7 ± 0.1 a | 264 ± 11 a | 79.9 ± 0.2 a | 1.53 ± 0.02 a | 13.1 ± 0.1 b |
| 0 h | |||||||
| 30% | 6.7 ± 0.3 a–c | 40.5 ± 2.1 a,b | 4.9 ± 0.1 a | 207 ± 12 b | 78.8 ± 0.6 b,c | 1.47 ± 0.05 a | 13.4 ± 0.8 a,b |
| 50% | 6.4 ± 0.1 a–c | 38.5 ± 2.1 a,b | 4.8 ± 0.1 a | 191 ± 20 b | 78.9 ± 0.2 b,c | 1.48 ± 0.15 a | 13.5 ± 0.1 a,b |
| 70% | 6.2 ± 0.1 a–c | 35.5 ± 0.7 b | 4.9 ± 0.1 a | 186 ± 15 b | 78.9 ± 0.4 a–c | 1.55 ± 0.14 a | 13.7 ± 0.2 a,b |
| 24 h | |||||||
| 30% | 6.6 ± 0.6 a–c | 36.5 ± 2.1 a,b | 4.9 ± 0.1 a | 214 ± 18 b | 79.2 ± 0.4 a,b | 1.46 ± 0.03 a | 13.6 ± 0.4 a,b |
| 50% | 6.4 ± 0.2 a–c | 37.5 ± 0.7 a,b | 4.9 ± 0.1 a | 217 ± 33 b | 79.2 ± 0.5 a,b | 1.45 ± 0.06 a | 13.7 ± 0.3 a,b |
| 70% | 6.4 ± 0.1 a–c | 39.0 ± 2.8 a,b | 5.0 ± 0.1 a | 200 ± 27 b | 79.0 ± 0.2 a–c | 1.46 ± 0.10 a | 13.9 ± 0.1 a,b |
| 48 h | |||||||
| 30% | 6.2 ± 0.1 a–c | 41.0 ± 4.2 a,b | 4.9 ± 0.3 a | 209 ± 21 b | 78.8 ± 0.6 b,c | 1.32 ± 0.34 a | 14.0 ± 0.7 a,b |
| 50% | 5.9 ± 0.1 b,c | 42.5 ± 0.7 a,b | 4.9 ± 0.1 a | 196 ± 16 b | 78.0 ± 0.2 c | 1.33 ± 0.24 a | 14.4 ± 0.6 a,b |
| 70% | 6.9 ± 0.9 a,b | 39.5 ± 3.5 a,b | 5.0 ± 0.0 a | 207 ± 14 b | 78.4 ± 0.2 b,c | 1.26 ± 0.23 a | 14.3 ± 0.7 a,b |
| 72 h | |||||||
| 30% | 6.1 ± 0.4 b,c | 38.5 ± 2.1 a,b | 5.0 ± 0.0 a | 206 ± 20 b | 79.0 ± 0.3 a–c | 1.29 ± 0.21 a | 13.9 ± 0.7 a,b |
| 50% | 5.9 ± 0.1 b,c | 42.0 ± 4.2 a,b | 5.1 ± 0.1 a | 219 ± 12 b | 78.4 ± 0.7 b,c | 1.26 ± 0.17 a | 14.3 ± 0.7 a,b |
| 70% | 5.5 ± 0.1 c | 46.5 ± 5.0 a | 5.0 ± 0.0 a | 198 ± 16 b | 78.9 ± 0.3 b,c | 1.28 ± 0.21 a | 14.5 ± 0.9 a |
| Ferment Storage and Inclusion Level | Crumb Structure | Bread Crumb Scores | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CD 2 (mm) | NC/SA 2 | CWT 2 | CC 2 | Colour | Texture | Softness | Resilience | Strength | |
| Control | 1.92 ± 0.18 b,c | 0.62 ± 0.04 a | 0.45 ± 0.01 a | 0.79 ± 0.01 a | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 0 h | |||||||||
| 30% | 2.02 ± 0.04 a–c | 0.58 ± 0.02 a,b | 0.46 ± 0.01 a | 0.77 ± 0.01 a,b | 9.3 ± 0.4 | 10.3 ± 0.4 | 10.3± 0.4 | 10.0 ± 0.0 | 10.5 ± 0.7 |
| 50% | 2.03 ± 0.01 a–c | 0.58 ± 0.01 a,b | 0.47 ± 0.00 a | 0.77 ± 0.01 a,b | 9.3 ± 0.4 | 9.3 ± 0.4 | 10.5 ± 0.7 | 10.0 ± 0.0 | 10.5 ± 0.7 |
| 70% | 2.11 ± 0.06 a–c | 0.56 ± 0.01 a,b | 0.47 ± 0.00 a | 0.77 ± 0.01 a,b | 9.5 ± 1.4 | 9.5 ± 1.4 | 10.8 ± 0.4 | 9.5 ± 0.7 | 10.0 ± 1.4 |
| 24 h | |||||||||
| 30% | 1.87 ± 0.70 c | 0.62 ± 0.04 a | 0.45 ± 0.01 a | 0.78 ± 0.01 a,b | 9.0 ± 0.0 | 9.8 ± 0.4 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 50% | 1.93 ± 0.11 a–c | 0.60 ± 0.03 a,b | 0.46 ± 0.01 a | 0.78 ± 0.01 a,b | 9.0 ± 0.0 | 9.5 ± 0.7 | 10.0 ± 0.0 | 9.8 ± 0.4 | 10.0 ± 0.0 |
| 70% | 1.95 ± 0.02 a–c | 0.60 ± 0.02 a,b | 0.46 ± 0.00 a | 0.78 ± 0.00 a,b | 8.8 ± 0.4 | 10.0 ± 0.7 | 10.3 ± 0.4 | 10.0 ± 0.0 | 10.5 ± 0.7 |
| 48 h | |||||||||
| 30% | 2.11 ± 0.13 a–c | 0.57 ± 0.02 a,b | 0.47 ± 0.01 a | 0.77 ± 0.01 a,b | 9.8 ± 0.4 | 10.3 ± 0.4 | 10.3 ± 0.4 | 10.0 ± 0.0 | 9.5 ± 0.7 |
| 50% | 2.09 ± 0.12 a–c | 0.57 ± 0.02 a,b | 0.47 ± 0.01 a | 0.76 ± 0.01 b | 9.5 ± 0.7 | 10.0 ± 0.7 | 10.3 ± 0.4 | 9.5 ± 0.7 | 9.5 ± 0.7 |
| 70% | 2.11 ± 0.08 a–c | 0.56 ± 0.02 a,b | 0.47 ± 0.01 a | 0.77 ± 0.00 a,b | 9.3 ± 0.4 | 9.5 ± 0.0 | 10.8 ± 0.4 | 9.3 ± 0.4 | 9.5 ± 0.7 |
| 72 h | |||||||||
| 30% | 2.05 ± 0.14 a–c | 0.58 ± 0.03 a,b | 0.46 ± 0.01 a | 0.78 ± 0.01 a,b | 9.8 ± 0.4 | 10.0 ± 0.0 | 10.3 ± 0.4 | 10.0 ± 0.0 | 9.5 ± 0.7 |
| 50% | 2.17 ± 0.13 a,b | 0.56 ± 0.02 a,b | 0.47 ± 0.01 a | 0.77 ± 0.01 a,b | 9.5 ± 0.7 | 9.8 ± 0.4 | 10.5 ± 0.0 | 10.0 ± 0.0 | 9.3 ± 0.4 |
| 70% | 2.22 ± 0.21 a | 0.55 ± 0.04 b | 0.47 ± 0.01 a | 0.77 ± 0.01 a,b | 9.3 ± 0.4 | 9.5 ± 0.0 | 11.0 ± 0.0 | 10.0 ± 0.0 | 9.3 ± 0.4 |
| Fermentation Time and Inclusion Level | Mixing Time (min) | Proof Time (min) | Specific Volume (cm3/g) | Crumb Firmness Force (g) | Crumb Colour | ||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| Control | 7.4 ± 0.2 a | 39.7 ± 1.7 a,b | 5.0 ± 0.1 a | 227 ± 27 a | 79.9 ± 0.9 a | 1.27 ± 0.16 a | 13.5 ± 0.9 a |
| 90 min | |||||||
| 30% | 6.6 ± 0.1 b | 39.0 ± 1.4 a,b | 5.0 ± 0.1 a | 198 ± 17 a,b | 79.3 ± 0.5 a,b | 1.31 ± 0.17 a | 13.9 ± 0.8 a |
| 50% | 6.2 ± 0.3 b,c | 40.0 ± 2.8 a,b | 5.1 ± 0.1 a | 187 ± 18 b | 79.1 ± 0.2 a,b | 1.29 ± 0.20 a | 14.1 ± 0.6 a |
| 70% | 6.1 ± 0.5 b,c | 41.0 ± 1.4 a,b | 5.0 ± 0.1 a | 187 ± 21 b | 78.7 ± 0.4 b | 1.34 ± 0.11 a | 14.6 ± 0.6 a |
| 150 min | |||||||
| 30% | 6.7 ± 0.2 b | 38.0 ± 0.0 a,b | 5.2 ± 0.1 a | 186 ± 14 b | 79.6 ± 0.6 a,b | 1.27 ± 0.20 a | 13.6 ± 0.7 a |
| 50% | 6.0 ± 0.1 b,c | 37.0 ± 0.0 b | 5.1 ± 0.1 a | 205 ± 19 a,b | 79.5 ± 0.3 a,b | 1.22 ± 0.19 a | 13.9 ± 0.8 a |
| 70% | 5.7 ± 0.1 c | 39.0 ± 1.4 a,b | 5.1 ± 0.1 a | 197 ± 23 a,b | 79.3 ± 0.2 a,b | 1.26 ± 0.26 a | 14.3 ± 0.7 a |
| 210 min | |||||||
| 30% | 6.8 ± 0.1 a,b | 38.5 ± 0.7 a,b | 4.9 ± 0.1 a | 207 ± 18 a,b | 79.1 ± 0.6 a,b | 1.45 ± 0.19 a | 14.0 ± 0.2 a |
| 50% | 6.1 ± 0.1 b,c | 37.0 ± 0.0 b | 4.9 ± 0.1 a | 195 ± 15 a,b | 79.0 ± 0.5 a,b | 1.37 ± 0.18 a | 14.3 ± 0.4 a |
| 70% | 5.7 ± 0.1 c | 42.5 ± 0.7 a | 5.1 ± 0.1 a | 196 ± 16 a,b | 78.8 ± 0.7 a,b | 1.30 ± 0.10 a | 14.4 ± 0.5 a |
| 280 min | |||||||
| 30% | 6.3 ± 0.2 b,c | 38.5 ± 0.7 a,b | 5.1 ± 0.1 a | 183 ± 9 b | 79.2 ± 0.3 a,b | 1.14 ± 0.16 a | 14.2 ± 0.1 a |
| 50% | 5.9 ± 0.1 b,c | 39.5 ± 0.7 a,b | 5.2 ± 0.1 a | 186 ± 10 b | 79.2 ± 0.2 a,b | 1.11 ± 0.02 a | 14.4 ± 0.3 a |
| 70% | 5.5 ± 0.5 c | 42.0 ± 0.0 a,b | 5.0 ± 0.1 a | 197 ± 16 a,b | 79.1 ± 0.1 a,b | 1.20 ± 0.04 a | 14.9 ± 0.2 a |
| Fermentation Time and Inclusion Level | Crumb Structure | Bread Crumb Scores | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CD 2 (mm) | NC/SA 2 | CWT 2 | CC 2 | Colour | Texture | Softness | Resilience | Strength | |
| Control | 2.06 ± 0.05 c | 0.59 ± 0.01 a | 0.46 ± 0.00 b | 0.77 ± 0.00 a | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 90 min | |||||||||
| 30% | 2.38 ± 0.21 a,b | 0.52 ± 0.04 b | 0.49 ± 0.02 a | 0.76 ± 0.01 a | 9.8 ± 0.4 | 9.8 ± 1.1 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 50% | 2.21 ± 0.06 b,c | 0.54 ± 0.01 a,b | 0.48 ± 0.00 a,b | 0.77 ± 0.01 a | 9.8 ± 0.4 | 10.3 ± 0.4 | 10.5 ± 0.0 | 9.8 ± 0.4 | 10.0 ± 0.0 |
| 70% | 2.38 ± 0.02 a,b | 0.52 ± 0.01 b | 0.49 ± 0.00 a | 0.76 ± 0.00 a | 10.3 ± 0.4 | 10.5 ± 0.7 | 11.0 ± 0.7 | 9.3 ± 0.4 | 9.8 ± 0.4 |
| 150 min | |||||||||
| 30% | 2.31 ± 0.11 a–c | 0.53 ± 0.02 b | 0.49 ± 0.01 a,b | 0.77 ± 0.01 a | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 50% | 2.39 ± 0.13 a,b | 0.53 ± 0.02 b | 0.49 ± 0.01 a,b | 0.77 ± 0.00 a | 9.8 ± 0.4 | 9.8 ± 0.4 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.3 ± 0.4 |
| 70% | 2.30 ± 0.18 a,b | 0.53 ± 0.03 b | 0.49 ± 0.02 a | 0.77 ± 0.01 a | 9.8 ± 0.4 | 9.8 ± 0.4 | 10.3 ± 0.4 | 9.5 ± 0.0 | 9.8 ± 0.4 |
| 210 min | |||||||||
| 30% | 2.42 ± 0.17 a,b | 0.52 ± 0.02 b | 0.49 ± 0.01 a | 0.76 ± 0.01 a | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.3 ± 0.4 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 50% | 2.57 ± 0.16 a | 0.51 ± 0.02 b | 0.50 ± 0.01 a | 0.76 ± 0.01 a | 9.8 ± 0.4 | 9.5 ± 0.7 | 10.3 ± 0.4 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 70% | 2.52 ± 0.09 a,b | 0.51 ± 0.01 b | 0.50 ± 0.01 a | 0.76 ± 0.01 a | 9.8 ± 0.4 | 9.8 ± 0.4 | 10.5 ± 0.7 | 9.5 ± 0.0 | 9.8 ± 0.4 |
| 280 min | |||||||||
| 30% | 2.44 ± 0.12 a,b | 0.51 ± 0.03 b | 0.50 ± 0.02 a | 0.76 ± 0.01 a | 10.0 ± 0.0 | 9.8 ± 1.1 | 10.5 ± 0.7 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 50% | 2.44 ± 0.11 a,b | 0.51 ± 0.02 b | 0.49 ± 0.01 a | 0.76 ± 0.01 a | 10.3 ± 0.4 | 10.0 ± 0.0 | 10.8 ± 0.4 | 9.5 ± 0.0 | 10.0 ± 0.0 |
| 70% | 2.42 ± 0.08 a,b | 0.52 ± 0.02 b | 0.49 ± 0.01 a | 0.77 ± 0.00 a | 9.5 ± 0.0 | 9.0 ± 0.0 | 11.0 ± 0.0 | 9.5 ± 0.0 | 9.8 ± 0.4 |
| Fermentation Temperature and Inclusion Level | Mixing Time (min) | Proof Time (min) | Specific Volume (cm3/g) | Crumb Firmness Force (g) | Crumb Colour | ||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| Control | 6.8 ± 0.4 a | 37.9 ± 1.6 b | 5.0 ± 0.1 a | 209 ± 36 b–d | 78.7 ± 0.3 ab | 1.30 ± 0.11 a | 14.0 ± 0.2 e,f |
| 30 °C | |||||||
| 30% | 6.2 ± 0.2 a,b | 37.5 ± 0.7 a,b | 5.2 ± 0.1 a | 203 ± 23 c,d | 79.4 ± 0.6 a | 1.21 ± 0.09 a,b | 13.8 ± 0.5 f |
| 50% | 5.6 ± 0.2 b,c | 35.5 ± 2.1 b | 5.2 ± 0.1 a | 209 ± 14 b–d | 79.6 ± 0.4 a | 1.12 ± 0.11 a,b | 14.2 ± 0.3 c–f |
| 70% | 5.3 ± 0.4 b,c | 37.5 ± 3.5 a,b | 5.2 ± 0.1 a | 203 ± 17 c,d | 79.4 ± 0.5 a | 1.12 ± 0.08 a,b | 14.5 ± 0.3 a–e |
| 35 °C | |||||||
| 30% | 5.8 ± 0.4 b,c | 36.5 ± 2.1 b | 5.0 ± 0.1 a | 220 ± 26 a–d | 79.0 ± 0.3 a,b | 1.19 ± 0.15 a,b | 14.3 ± 0.2 b–f |
| 50% | 5.4 ± 0.6 b,c | 36.5 ± 2.1 b | 5.2 ± 0.1 a | 220 ± 26 a–d | 79.6 ± 0.1 a | 1.05 ± 0.05 b | 14.3 ± 0.1 b–f |
| 70% | 5.3 ± 0.3 b,c | 39.0 ± 4.2 a,b | 5.0 ± 0.1 a | 237 ± 32 a–c | 79.4 ± 0.1 a | 1.11 ± 0.04 a,b | 14.8 ± 0.2 a–c |
| 40 °C | |||||||
| 30% | 5.8 ± 0.3 b,c | 35.0 ± 1.4 b | 5.2 ± 0.1 a | 186 ± 14 d | 79.2 ± 0.2 a | 1.08 ± 0.05 b | 14.1 ± 0.1 d–f |
| 50% | 5.5 ± 0.0 b,c | 38.5 ± 2.1 a,b | 5.1 ± 0.2 a | 193 ± 15 c,d | 79.4 ± 0.4 a | 1.05 ± 0.03 b | 14.6 ± 0.4 a–e |
| 70% | 5.1 ± 0.1 b,c | 41.5 ± 0.7 a,b | 5.1 ± 0.1 a | 185 ± 11 d | 79.5 ± 0.1 a | 1.08 ± 0.06 a,b | 14.7 ± 0.2 a–d |
| 45 °C | |||||||
| 30% | 5.3 ± 0.4 b,c | 39.5 ± 3.5 a,b | 4.9 ± 0.1 a | 267 ± 29 a | 78.1 ± 0.7 b | 1.20 ± 0.13 a,b | 14.9 ± 0.1 a,b |
| 50% | 5.1 ± 0.1 b,c | 43.5 ± 5.0 a,b | 4.8 ± 0.1 a | 256 ± 13 a,b | 79.2 ± 0.3 a | 1.08 ± 0.08 b | 14.7 ± 0.3 a–e |
| 70% | 4.9 ± 0.2 c | 46.5 ± 5.0 a | 4.8 ± 0.0 a | 264 ± 36 a | 78.9 ± 0.2 a,b | 1.08 ± 0.04 b | 15.1 ± 0.3 a |
| Fermentation Temperature and Inclusion Level | Crumb Structure | Bread Crumb Scores | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CD 2 (mm) | NC/SA 2 | CWT 2 | CC 2 | Colour | Texture | Softness | Resilience | Strength | |
| Control | 2.37 ± 0.10 a | 0.51 ± 0.02 b | 0.49 ± 0.01 a | 0.76 ± 0.01 a | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 30 °C | |||||||||
| 30% | 2.34 ± 0.07 a | 0.53 ± 0.02 a,b | 0.48 ± 0.01 a | 0.77 ± 0.00 a | 10.0 ± 0.0 | 9.8 ± 1.1 | 10.0 ± 0.0 | 10.0 ± 0.0 | 9.5 ± 0.7 |
| 50% | 2.30 ± 0.07 a | 0.53 ± 0.02 a,b | 0.48 ± 0.01 a | 0.77 ± 0.00 a | 10.0 ± 0.7 | 10.0 ± 0.7 | 10.8 ± 0.4 | 10.0 ± 0.0 | 8.5 ± 2.1 |
| 70% | 2.28 ± 0.05 a | 0.53 ± 0.01 a,b | 0.48 ± 0.00 a | 0.77 ± 0.00 a | 10.0 ± 0.7 | 10.3 ± 0.4 | 11.0 ± 0.0 | 9.3 ± 0.4 | 7.8 ± 2.5 |
| 35 °C | |||||||||
| 30% | 2.24 ± 0.15 a | 0.55 ± 0.02 a,b | 0.48 ± 0.01 a | 0.77 ± 0.00 a | 10.0 ± 0.0 | 10.3 ± 0.4 | 10.3 ± 0.4 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 50% | 2.33 ± 0.06 a | 0.54 ± 0.01 a,b | 0.48 ± 0.00 a | 0.77 ± 0.00 a | 11.0 ± 0.7 | 10.3 ± 0.4 | 10.8 ± 0.4 | 9.5 ± 0.7 | 9.8 ± 0.4 |
| 70% | 2.41 ± 0.32 a | 0.53 ± 0.05 a,b | 0.49 ± 0.02 a | 0.77 ± 0.01 a | 10.8 ± 0.4 | 10.0 ± 0.7 | 11.0 ± 0.7 | 9.3 ± 0.4 | 9.0 ± 0.0 |
| 40 °C | |||||||||
| 30% | 2.36 ± 0.05 a | 0.52 ± 0.01 a,b | 0.49 ± 0.00 a | 0.76 ± 0.00 a | 10.3 ± 0.4 | 10.3 ± 0.4 | 10.3 ± 0.4 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 50% | 2.33 ± 0.04 a | 0.53 ± 0.01 a,b | 0.49 ± 0.00 a | 0.77 ± 0.01 a | 10.0 ± 1.4 | 10.5 ± 0.0 | 10.8 ± 0.4 | 9.8 ± 0.4 | 8.3 ± 1.8 |
| 70% | 2.49 ± 0.05 a | 0.51 ± 0.01 b | 0.50 ± 0.01 a | 0.77 ± 0.01 a | 10.5 ± 0.7 | 9.3 ± 0.4 | 11.0 ± 0.0 | 9.5 ± 0.0 | 8.0 ± 1.4 |
| 45 °C | |||||||||
| 30% | 2.19 ± 0.23 a | 0.58 ± 0.05 a | 0.47 ± 0.02 a | 0.77 ± 0.01 a | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 50% | 2.34 ± 0.10 a | 0.54 ± 0.01 a,b | 0.49 ± 0.01 a | 0.77 ± 0.00 a | 10.3 ± 1.1 | 10.5 ± 0.7 | 10.5 ± 0.0 | 10.0 ± 0.0 | 9.5 ± 0.7 |
| 70% | 2.35 ± 0.13 a | 0.54 ± 0.02 a,b | 0.48 ± 0.01 a | 0.77 ± 0.00 a | 9.8 ± 1.1 | 9.8 ± 1.1 | 10.8 ± 0.4 | 9.3 ± 0.4 | 9.0 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borsuk, Y.; Bourré, L.; McMillin, K.; Sopiwnyk, E.; Jones, S.; Dyck, A.; Malcolmson, L. Impact of Ferment Processing Parameters on the Quality of White Pan Bread. Appl. Sci. 2021, 11, 10203. https://doi.org/10.3390/app112110203
Borsuk Y, Bourré L, McMillin K, Sopiwnyk E, Jones S, Dyck A, Malcolmson L. Impact of Ferment Processing Parameters on the Quality of White Pan Bread. Applied Sciences. 2021; 11(21):10203. https://doi.org/10.3390/app112110203
Chicago/Turabian StyleBorsuk, Yulia, Lindsay Bourré, Kasia McMillin, Elaine Sopiwnyk, Stuart Jones, Adam Dyck, and Linda Malcolmson. 2021. "Impact of Ferment Processing Parameters on the Quality of White Pan Bread" Applied Sciences 11, no. 21: 10203. https://doi.org/10.3390/app112110203
APA StyleBorsuk, Y., Bourré, L., McMillin, K., Sopiwnyk, E., Jones, S., Dyck, A., & Malcolmson, L. (2021). Impact of Ferment Processing Parameters on the Quality of White Pan Bread. Applied Sciences, 11(21), 10203. https://doi.org/10.3390/app112110203





