Phenolic Compound Composition and Biological Activities of Fractionated Soybean Pod Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
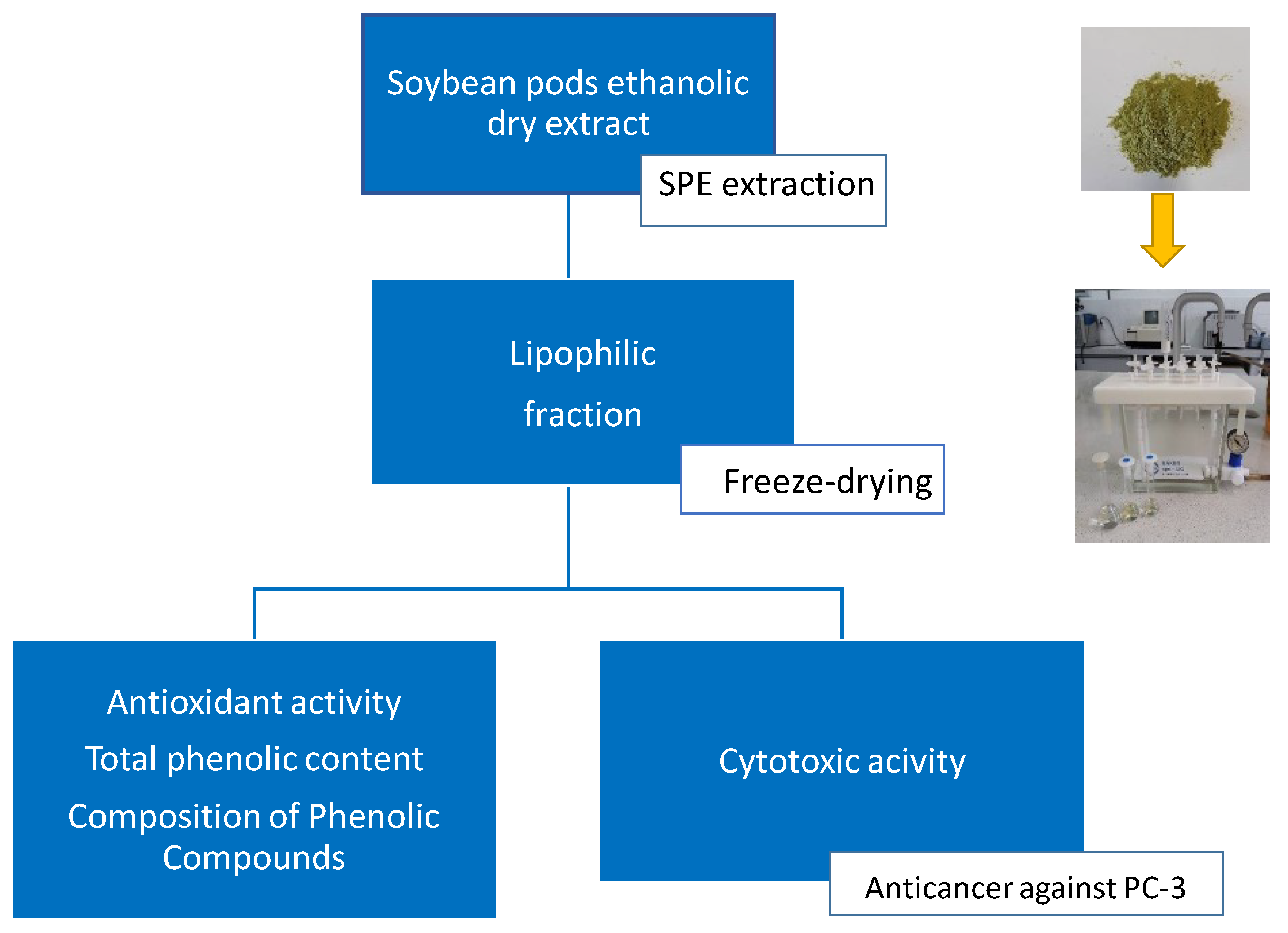
2.2. Extract Preparation
2.3. Determination of Polyphenol Content and Antioxidant Activity
2.4. Composition of Phenolic Compounds
2.5. Cytotoxic Activity
2.5.1. Cell Lines
2.5.2. MTT Cytotoxicity Test
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pabich, M.; Materska, M. Biological effect of soy isoflavones in the prevention of civilization diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. In Global Report on Diabetes; WHO Press: Geneva, Switzerland, 2016; pp. 1–88. ISBN 978 92 4 156525 7.
- Rizzo, G.; Baroni, L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free. Rad. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Int. J. Pharm. Sci. Technol. 2011, 6, 12–35. [Google Scholar]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, polyphenols and tannins: An overview. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Crozier, A., Clifford, M., Ashihara, H., Eds.; Blackwell: Oxford, UK, 2006; pp. 1–24. [Google Scholar]
- Subbiah, V.; Zhong, B.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Screening of Phenolic Compounds in Australian Grown Berries by LC-ESI-QTOF-MS/MS and Determination of Their Antioxidant Potential. Antioxidants 2021, 10, 26. [Google Scholar] [CrossRef]
- Duenas, M.; Hernandez, T.; Robredo, S.; Lamparski, G.; Estrella, I.; Munoz, R. Bioactive Phenolic Compounds of Soybean (Glycine max cv. Merit): Modifications by Different Microbiological Fermentations. Pol. J. Food Nutr. Sci. 2012, 62, 241–250. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, Y.; Wang, B.; Li, S.; Guo, F.; Hua, H.; Zhao, Y.; Yu, Z. Comparison of phenolic compounds, antioxidant and antidiabetic activities between selected edible beans and their different growth periods leaves. J. Funct. Foods 2017, 35, 694–702. [Google Scholar] [CrossRef]
- Prakash, D.; Upadhyay, G.; Singh, B.N.; Singh, H. Antioxidant and free radical-scavenging activities of seedsand agri-wastes of some varieties of soybean (Glycine max). Food Chem. 2007, 104, 783–790. [Google Scholar] [CrossRef]
- Yuk, H.J.; Lee, J.H.; Curtis-Long, M.J.; Lee, J.W.; Kim, Y.S.; Ryu, H.W.; Park, C.G.; Jeong, T.-S.; Park, K.H. The most abundant polyphenol of soy leaves, coumestrol, displays potent α-glucosidase inhibitory activity. Food Chem. 2011, 126, 1057–1063. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Penninton, J.S. Stage of development descriptions for soybeans, Glycine max (L.) Merril. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Mujić, I.; Šertović, E.; Jokić, S.; Sarić, Z.; Alibabić, V.; Vidović, S.; Živković, J. Isoflavone content and antioxidant properties of soybean seeds. Croat. J. Food Sci. Technol. 2011, 3, 16–20. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT–Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- European/Polish Standard PN-EN ISO 10993-5:2009 Norm. In Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; Polish Committee for Standardization: Warszawa, Poland, 2009.
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Immunol. J. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitrocytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Łudzik, K.; Kustrzepa, K.; Kowalewicz-Kulbat, M.; Kontek, R.; Kontek, B.; Wróblewska, A.; Joźwiak, M.; Lulo, D. Antimicrobial and cytotoxic properties of bisquaternary ammonium bromides of different spacer length. J. Surfactants Deterg. 2018, 21, 91–99. [Google Scholar] [CrossRef]
- Hidayat, D.A.; Dwira, S. Phytochemical analysisand in vitro cytotoxicity test of black soybean (Glycine soja L.) ethanolic extract as a growth inhibitor of the HCT-116 colon carcinoma cell line. J. Phys. Conf. Ser. 2018, 1073, 032041. [Google Scholar] [CrossRef]
- Correa, C.R.; Li, L.; Aldini, G.; Carini, M.; Chen, C.-Y.O.; Chun, H.-K.; Cho, S.-M.; Park, K.-M.; Russell, R.M.; Blumberg, J.B. Composition and stability of phytochemicals in five varieties of black soybeans (Glycine max). Food Chem. 2010, 123, 1176–1184. [Google Scholar] [CrossRef]
- Romani, A.; Vignolini, P.; Galardi, C.; Aroldi, C.; Vazzana, C.; Heimler, D. Polyphenolic content in different plant parts if soy cultivars grown under natural conditions. J. Agric. Food Chem. 2003, 51, 5301–5306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carneiro, A.; Moreira, E.; Bragagnolo, F.; Borges, M.; Pilon, A.; Rinaldo, D.; Funari, C. Soya agricultural waste as a rich source of isoflavones. Food Res. Int. 2020, 130, 108949. [Google Scholar] [CrossRef]
- Murakami, S.; Nakata, R.; Aboshi, T.; Yoshinaga, N.; Teraishi, M.; Okumoto, Y.; Ishihara, A.; Morisaka, H.; Huffaker, A.; Schmelz, E.A.; et al. Insect-induced daidzein, formononetin and their conjugates in soybean leaves. Metabolites 2014, 4, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Boué, M.S.; Carter-Wientjes, H.C.; Shih, Y.B.; Cleveland, E.T. Identification of flavone aglycones and glycosides in soybean pods by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2003, 991, 61–68. [Google Scholar] [CrossRef]
- Bragagnolo, F.S.; Funari, C.S.; Ibáñez, E.; Cifuentes, A. Metabolomics as a Tool to Study Underused Soy Parts: In Search of Bioactive Compounds. Foods 2021, 10, 1308. [Google Scholar] [CrossRef]
- Song, H.H.; Ryu, H.W.; Lee, K.J.; Jeong, I.Y.; Kim, D.S.; Oh, S.R. Metabolomics investigation of flavonoid synthesis in soybean leaves depending on the growth stage. Metabolomics 2014, 10, 833–841. [Google Scholar] [CrossRef]
- Lee, C.C.; Dudonné, S.; Dubé, P.; Desjardins, Y.; Kim, J.H.; Kim, J.S.; Kim, J.-E.; Park, J.H.Y.; Lee, K.W.; Lee, C.Y. Comprehensive phenolic composition analysis and evaluation of Yak-Kong soybean (Glycine max) for the prevention of atherosclerosis. Food Chem. 2017, 234, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Hashimoto, S.; Yu, C.; Igarashi, K. Protective effects of dietary kaempferol glycoside components from unripe soybean (Edamame, Glycine max L. Merrill. ‘Jindai’) leaves and their serous metabolite on carbon tetrachloride-induced liver injury mice. J. Food Sci. Technol. 2018, 55, 4515–4521. [Google Scholar] [CrossRef]
- Prabakaran, M.; Lee, J.-H.; Ahmad, A.; Kim, S.-H.; Woo, K.-S.; Kim, M.-J.; Chung, I.-M. Effect of storage time and temperature on phenolic compounds of soybean (Glycine max L.) flour. Molecules 2018, 23, 2269. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef]
- Amarowicz, R.; Shahidi, F. Antioxidant activity of broad bean seed extract and its phenolic composition. J. Func. Foods 2017 38, 656–662. [CrossRef]
- Lee, J.H.; Woo, K.S.; Kim, J.-K.; Kim, M.; Lee, B.W.; Sim, E.; Jeon, Y.H.; Lee, C.-K.; Kim, H.-J. Effects of gamma-irradiated soybean pod extract on oxidative stress, cancer cell viability, and tyrosinase inhibition. J. Food Biochem. 2018, 42, e12459. [Google Scholar] [CrossRef]
- Kwak, Y.; Ju, J. Glycine max Merr. leaf extract possesses anti-oxidant properties, decreases inflammatory mediator production in murine macrophages, and inhibits growth, migration, and adhesion in human cancer cells. Food Sci. Biotechnol. 2017, 26, 245–253. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, V.; Jain, A.; Jain, R.K.; Maikhuri, J.P.; Gupta, G. Synergistic chemoprotective mechanisms of dietary phytoestrogensin a select combination against prostate cancer. J. Nutr. Biochem. 2011, 22, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.; Bray, T.M.; Helferich, W.G.; Doerge, D.R.; Ho, E. Differentialeffects of whole soy extract and soy isoflavones on apoptosis in prostate cancer cells. Exp. Biol. Med. (Maywood) 2010, 235, 90–97. [Google Scholar] [CrossRef] [PubMed]
| Time Retention (min.) | Tentative Identification | Polyphenol Classes | Molecular Formula | Mw Observed | Mw Theroetical | Error (ppm) | m/z | E | F1 | F2 | F3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.288 | 3-feruloqunic acid | Phenolic acid | C17H20O9 | 368.1111 | 368.1107 | 1.09 | 369.1187 | + | + | - | - |
| 0.393 | Dihydrocaffeic acid 3-O-glucuronide | Phenolic acid | C15H18O10 | 358.0909 | 358.09 | 2.64 | 359.1001 | + | + | - | - |
| 0.538 | p-hydroxybenzoic acid | Phenolic acid | C7H6O3 | 138.0310 | 138.0317 | −4.7 | 139.0383 | + | + | - | - |
| 0.588 | p-Coumaric acid | Phenolic acid | C9H8O3 | 164.047 | 164.0473 | −1.5 | 165.0545 | + | + | + | + |
| 0.638 | Ferulic acid | Phenolic acid | C10H10O4 | 194.0578 | 194.0579 | −0.57 | 195.0650 | + | + | - | - |
| 0.654 | Ferulic acid 4-O-glucoside | Phenolic acid | C16H20O9 | 356.1101 | 356.1107 | −1.81 | 357.1092 | + | + | - | - |
| 0.771 | Caffeoylmalic acid | Phenolic acid | C13H12O8 | 296.0546 | 296.0532 | 4.53 | 297.0618 | + | + | - | - |
| 1.686 | Qercetin 3-O-galactoside | Flavonols | C21H20O12 | 464.0951 | 464.0955 | −0.9 | 465.1025 | + | + | - | - |
| 3.154 | Dihydroquercetin (Taxifolin) | Flavonols | C15H12O7 | 304.0587 | 304.0583 | 1.19 | 305.0657 | + | - | + | - |
| 3.321 | Daidzein | Isoflavonoids | C15H10O4 | 254.0580 | 254.0579 | 0.26 | 255.0652 | + | - | + | - |
| 3.321 | Naringenin | Flavanones | C15H12O5 | 272.069 | 272.0685 | 1.81 | 273.0761 | + | - | + | - |
| 3.487 | 4-hydroxycoumarin | Other polyphenols | C9H6O3 | 162.0326 | 162.0317 | 4.83 | 163.0399 | + | - | + | - |
| 3.571 | Dihydrosinapic acid | Phenolic acid | C11H14O5 | 226.0846 | 226.0841 | 1.98 | 227.0917 | + | - | + | - |
| 3.820 | Phloridzin | Chalconoids | C21H24O10 | 436.1371 | 436.1369 | 0.24 | 437.1443 | + | - | + | - |
| 3.837 | 3-hydroxyphloridzin | Chalconoids | C21H24O11 | 452.1296 | 452.1319 | −5.0 | 453.1366 | + | - | + | - |
| 3.837 | (+)-Catechin/(-)-Epicatechin | Flavanols | C15H14O6 | 290.0788 | 290.079 | −0.78 | 291.0860 | + | - | + | - |
| 3.837 | Coumarin | Other polyphenols | C9H6O2 | 146.0365 | 146.0368 | −1.61 | 147.0438 | + | - | + | + |
| 3.904 | Dihydroquercetin 3-O-rhamnoside | Flavonols | C21H22O11 | 450.1188 | 450.1162 | 4.79 | 451.1274 | + | - | + | - |
| 3.904 | Genistein | Isoflavonoids | C15H10O5 | 270.0531 | 270.0528 | 0.88 | 271.0603 | + | - | + | - |
| 3.970 | Apigenin 6,8-di-C-glucoside | Flavones | C27H30O15 | 594.1570 | 594.1585 | −2.48 | 595.1662 | + | - | + | + |
| 4.017 | Myricetrin | Flavonols | C21H20O12 | 464.0955 | 464.0955 | 0.02 | 465.1031 | + | - | + | - |
| 4.037 | 3-p-Coumaroylquinic acid | Phenolic acid | C16H18O8 | 338.1014 | 338.1002 | 3.57 | 339.1087 | + | - | + | - |
| 4.103 | Daidzin | Isoflavonoids | C21H20O9 | 416.1112 | 416.1107 | 1.21 | 417.1185 | + | - | + | - |
| 4.137 | Luteolin | Flavones | C15H10O6 | 286.0480 | 286.04770 | 0.75 | 287.0551 | + | - | + | + |
| 4.187 | Quercitin | Flavonols | C15H10O7 | 302.0434 | 302.0427 | 2.43 | 303.0505 | + | - | + | + |
| 4.220 | Glycitein | Isoflavonoids | C16H12O5 | 284.0697 | 284.685 | 4.21 | 285.0768 | + | - | + | - |
| 4.253 | Myricetin 3-O-rutinoside | Flavonols | C27H30O17 | 626.1488 | 626.1483 | 0.84 | 627.1558 | + | - | + | + |
| 4.386 | Quercetin 3-O-rutinoside (Rutin) | Flavonols | C27H30O16 | 610.1578 | 610.1534 | 4.29 | 611.1646 | + | - | + | + |
| 4.432 | 3-O-methyl quercetin | Flavonols | C16H12O7 | 316.0606 | 316.0583 | 4.36 | 317.0677 | + | - | - | + |
| 4.565 | Apigenin 7-O-apiosylglucoside | Flavones | C26H28O14 | 564.1483 | 564.1479 | 0.72 | 565.1556 | + | - | - | + |
| 4.603 | Genistin | Isoflavonoids | C21H20O10 | 432.1078 | 432.1056 | 5.0 | 433.1147 | + | - | + | - |
| 4.615 | Apigenin 7-O-glucoside | Flavones | C21H20O10 | 432.1061 | 432.1056 | 0.98 | 433.1134 | + | - | - | + |
| 4.615 | Luteolin 7-O-glucoside | Flavonols | C21H20O11 | 448.1013 | 448.1006 | 1.65 | 449.1083 | + | - | + | + |
| 4.736 | 6”-O-malonyldaidzin | Isoflavonoids | C24H22O12 | 502.1121 | 502.1111 | 1.97 | 503.1195 | + | - | + | - |
| 4.783 | Qercetin 3-O-glucoside | Flavonols | C21H20O12 | 464.0957 | 464.0955 | 0.45 | 465.1043 | + | - | - | + |
| 5.015 | 6′-O-acetylodaidzin | Isoflavonoids | C23H22O10 | 458.1223 | 458.1213 | 2.17 | 459.1294 | + | - | - | + |
| 5.181 | Glycitin | Isoflavonoids | C22H22O10 | 446.1204 | 446.1213 | −1.97 | 447.1296 | + | - | + | - |
| 5.302 | 6′-O-malonylgenistin | Isoflavonoids | C24H20O13 | 516.0909 | 516.0904 | 0.96 | 517.0977 | + | - | + | - |
| 5.331 | Chrysin | Flavones | C15H10O4 | 254.0582 | 254.0579 | 1.14 | 255.0654 | + | - | - | + |
| 5.416 | Kaempferol 3-O-arabinoside | Flavonols | C20H18O10 | 418.0909 | 418.090 | −0.22 | 419.0985 | + | + | + | + |
| 5.448 | 2′-hydroxyformononetin | Isoflavonoids | C16H12O5 | 284.06888 | 284.0685 | 1.24 | 285.0761 | + | - | - | + |
| 5.602 | 6”-O-malonylglycitin | Isoflavonoids | C25H24O13 | 532.1230 | 532.1217 | 2.39 | 533.1296 | + | - | + | - |
| 5.781 | 6′-O-acetylogenistin | Isoflavonoids | C23H22O11 | 474.1118 | 474.1162 | 3.78 | 475.1250 | + | - | - | + |
| 5.801 | 3,5-O-Dicaffeoylqunic acid | Phenolic acid | C25H24O12 | 516.1272 | 516.1268 | 0.82 | 517.1344 | + | - | + | - |
| 6.230 | Isotrifoliol | Other polyphenols | C16H10O6 | 298.0491 | 298.0477 | 4.57 | 299.0564 | + | - | - | + |
| 6.280 | Apigenin | Flavones | C15H10O5 | 270.0534 | 270.0528 | 2.08 | 271.0606 | + | - | - | + |
| 6.313 | Coumestrol | Other polyphenols | C15H8O5 | 268.0379 | 268.0372 | 2.83 | 269.450 | + | - | - | + |
| 6.946 | Formononetin | Isoflavonoids | C16H12O4 | 268.0740 | 268.0736 | 1.78 | 269.0813 | + | - | - | + |
| 7.911 | 3′-O-methylcatechin | Flavanols | C16H16O6 | 304.0932 | 304.0947 | −4.95 | 305.1004 | + | - | - | + |
| 14.377 | Methyl cinnamate | Other polyphenols | C10H10O2 | 162.0682 | 162.0681 | −0.23 | 163.0755 | + | + | + | + |
| Total Phenolic Content 1 | Antioxidant Activity 2 | Cytotoxic Activity 3 | ||||
|---|---|---|---|---|---|---|
| DPPH | ABTS | HCT116 | PC-3 | L929 | ||
| E | 24.649 a ± 0.618 | 427.3 c ± 4.1 | 73.1 d ± 1.1 | 116 b ± 3.7 | 54 b ± 3.1 | 70 c ± 2.6 |
| F1 | 0.016 d ± 0.019 | 2668.7 a ± 10.3 | 956.5 a ± 8.3 | 102 c ± 3.4 | 56 b ± 2.1 | 56 d ± 2.1 |
| F2 | 14.510 b ± 0.097 | 320.2 d ± 3.5 | 154.7 c ± 2.4 | 156 a ± 3.3 | 66 a ± 2.8 | 112 a ± 2.8 |
| F3 | 6.028 c ± 0.214 | 510.6 b ± 5.2 | 320.2 b ± 4.7 | 120 b ± 3.1 | 70 a ± 2.9 | 80 b ± 3.3 |
| 5-flurouracil 4 | n.a 5 | n.a. | n.a. | 33 d ± 3.2 | 23 c ± 2.58 | 8 e ± 1.1 |
| DPPH | ABTS | TPC | |
|---|---|---|---|
| TPC | −0.99188 | −0.99941 | - |
| Cytoxic activity (PC-3) | 0.97829 | 0.949829 | −0.94401 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pabich, M.; Marciniak, B.; Kontek, R. Phenolic Compound Composition and Biological Activities of Fractionated Soybean Pod Extract. Appl. Sci. 2021, 11, 10233. https://doi.org/10.3390/app112110233
Pabich M, Marciniak B, Kontek R. Phenolic Compound Composition and Biological Activities of Fractionated Soybean Pod Extract. Applied Sciences. 2021; 11(21):10233. https://doi.org/10.3390/app112110233
Chicago/Turabian StylePabich, Marzena, Beata Marciniak, and Renata Kontek. 2021. "Phenolic Compound Composition and Biological Activities of Fractionated Soybean Pod Extract" Applied Sciences 11, no. 21: 10233. https://doi.org/10.3390/app112110233
APA StylePabich, M., Marciniak, B., & Kontek, R. (2021). Phenolic Compound Composition and Biological Activities of Fractionated Soybean Pod Extract. Applied Sciences, 11(21), 10233. https://doi.org/10.3390/app112110233






