Metal-Organic Frameworks Characterization via Inverse Pulse Gas Chromatography
Abstract
:1. Introduction
2. Fundamentals of Inverse Gas Chromatography (IGC)
2.1. Instrumentation and Methods
2.2. Theory
2.2.1. Dispersive Properties of the Surface
2.2.2. Specific Properties of the Surface
3. Applications of IGC for the Characterization of MOFs
3.1. HKUST-1
3.2. MIL-Based MOFs
3.3. Isoreticular Metal-Organic Frameworks (IRMOF)
3.4. Zeolitic Imidazole Frameworks (ZIFs)
3.5. UiO-66 MOF
3.6. Other MOFs
4. Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barrer, R.M.; Sutherland, J.W. Inclusion complexes of faujasite with paraffins and permanent gases. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1956, 237, 439–463. [Google Scholar] [CrossRef]
- Eder, F.; Lercher, J.A. On the role of the pore size and tortuosity for sorption of alkanes in molecular sieves. J. Phys. Chem. B 1997, 101, 1273–1278. [Google Scholar] [CrossRef]
- Hufton, J.R.; Danner, R.P. Chromatographic study of alkanes in silicalite: Equilibrium properties. AIChE J. 1993, 39, 954–961. [Google Scholar] [CrossRef]
- Jänchen, J.; Stach, H. Dependence of the adsorption equilibrium of n-decane on the Si Al-ratio of faujasite-zeolites. Zeolites 1985, 5, 57–59. [Google Scholar] [CrossRef]
- Richards, R.E.; Rees, L.V.C. Sorption and Packing of n-Alkane Molecules in ZSM-5+. Langmuir 1987, 3, 335–340. [Google Scholar] [CrossRef]
- Stach, H.; Lohse, U.; Thamm, H.; Schirmer, W. Adsorption equilibria of hydrocarbons on highly dealuminated zeolites. Zeolites 1986, 6, 74–90. [Google Scholar] [CrossRef]
- Sun, M.S.; Talu, O.; Shah, D.B. Adsorption equilibria of C5-C10 normal alkanes in silicalite crystals. J. Phys. Chem. 1996, 100, 17276–17280. [Google Scholar] [CrossRef]
- Thamm, H.; Stach, H.; Fiebig, W. Calorimetric study of the absorption of n-butane and but-l-ene on a highly dealuminated Y-type zeolite and on silicalite. Zeolites 1983, 3, 95–97. [Google Scholar] [CrossRef]
- Eder, F.; Lercher, J.A. Alkane sorption on siliceous and aluminophosphate molecular sieves. A comparative study. J. Phys. Chem. 1996, 100, 16460–16462. [Google Scholar] [CrossRef]
- Liu, B.; Smit, B.; Rey, F.; Valencia, A.S.; Calero, S. A new united atom force field for adsorption of alkenes in zeolites. J. Phys. Chem. C 2008, 112, 2492–2498. [Google Scholar] [CrossRef] [Green Version]
- De Meyer, K.M.A.; Chempath, S.; Denayer, J.F.M.; Martens, J.A.; Snurr, A.R.Q.; Baron, G.V. Packing effects in the liquid-phase adsorption of C5–C22 n-alkanes on ZSM-5. J. Phys. Chem. B 2003, 107, 10760–10766. [Google Scholar] [CrossRef]
- Daems, I.; Baron, G.V.; Punnathanam, S.; Snurr, A.R.Q.; Denayer, J.F.M. Molecular cage nestling in the liquid-phase adsorption of n-Alkanes in 5A zeolite. J. Phys. Chem. C 2007, 111, 2191–2197. [Google Scholar] [CrossRef]
- Stapley, J.; Buckton, G.; Merrifield, D. Investigation to find a suitable reference material for use as an inverse gas chromatography system suitability test. Int. J. Pharm. 2006, 318, 22–27. [Google Scholar] [CrossRef]
- Díaz, E.; Ordóñez, S.; Auroux, A. Comparative study on the gas-phase adsorption of hexane over zeolites by calorimetry and inverse gas chromatography. J. Chromatogr. A 2005, 1095, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, F. Introduction into the characterisation of porous materials by inverse gas chromatography. J. Chromatogr. A 2004, 1037, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, A.; Strzemiecka, B.; Adamska, K.; Milczewska, K. Inverse gas chromatography as a source of physiochemical data. J. Chromatogr. A 2009, 1216, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Vista, M. LAYLIN K. JAMES (ed.), Nobel Laureates in Chemistry, 1901–1992. Washington, American Chemical Society and the Chemical Heritage Foundation, 1993, XVIII + 798 pp. (History of Modern Chemical Sciences). Nuncius 2012, 9, 940–942. [Google Scholar] [CrossRef]
- Butler, D.; Williams, D.R. Particulate characterization: Inverse gas chromatography. In Encyclopedia of Separation Science; Elsevier: Amsterdam, The Netherlands, 2000; pp. 3609–3614. [Google Scholar] [CrossRef]
- Yashin, Y.I.; Andrej, V. Kiselev’s contributions to the science of adsorption, molecular interaction and chromatography. Pure Appl. Chem. 1989, 61, 1829–1834. [Google Scholar] [CrossRef]
- Smidsrϕd, O.; Guillet, J.E. Study of polymer–solute interactions by gas chromatography. Macromolecules 1969, 2, 272–277. [Google Scholar] [CrossRef]
- Novák, J. Physicochemical measurement by gas chromatography. J. Chromatogr. A 1980, 187, 283–284. [Google Scholar] [CrossRef]
- Mohammadi-Jam, S.; Waters, K.E. Inverse gas chromatography applications: A review. Adv. Colloid Interface Sci. 2014, 212, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, F.M.; Eggertsen, F.T. Determination of surface area: Adsorption measurements by a continuous flow method. Anal. Chem. 1958, 30, 1387–1390. [Google Scholar] [CrossRef]
- Münch, A.S.; Mertens, F.O.R.L. The Lewis acidic and basic character of the internal HKUST-1 surface determined by inverse gas chromatography. CrystEngComm 2015, 17, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Luner, P.E.; Zhang, Y.; Abramov, Y.A.; Carvajal, M.T. Evaluation of milling method on the surface energetics of molecular crystals using inverse gas chromatography. Cryst. Growth Des. 2012, 12, 5271–5282. [Google Scholar] [CrossRef]
- Menzel, R.; Bismarck, A.; Shaffer, M.S.P. Deconvolution of the structural and chemical surface properties of carbon nanotubes by inverse gas chromatography. Carbon N. Y. 2012, 50, 3416–3421. [Google Scholar] [CrossRef]
- Voelkel, A. Inverse gas chromatography: Characterization of polymers, fibers, modified silicas, and surfactants. Crit. Rev. Anal. Chem. 1991, 22, 411–439. [Google Scholar] [CrossRef]
- Wang, W.; Kale, V.S.; Cao, Z.; Kandambeth, S.; Zhang, W.; Ming, J.; Parvatkar, P.T.; Abou-Hamad, E.; Shekhah, O.; Cavallo, L.; et al. Phenanthroline covalent organic framework electrodes for high-performance zinc-ion supercapattery. ACS Energy Lett. 2020, 5, 2256–2264. [Google Scholar] [CrossRef]
- Yuvaraja, S.; Surya, S.G.; Chernikova, V.; Vijjapu, M.T.; Shekhah, O.; Bhatt, P.M.; Chandra, S.; Eddaoudi, M.; Salama, K.N. Realization of an ultrasensitive and highly selective OFET NO2 sensor: The synergistic combination of PDVT-10 polymer and porphyrin-MOF. ACS Appl. Mater. Interfaces 2020, 12, 18748–18760. [Google Scholar] [CrossRef]
- Chernikova, V.; Shekhah, O.; Belmabkhout, Y.; Eddaoudi, M. Nanoporous fluorinated metal-organic framework-based membranes for CO2 capture. ACS Appl. Nano Mater. 2020, 3, 6432–6439. [Google Scholar] [CrossRef]
- Jiang, H.; Alezi, D.; Eddaoudi, M. A reticular chemistry guide for the design of periodic solids. Nat. Rev. Mater. 2021, 6, 466–487. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, H.; Li, M.; O’Keeffe, M.; Eddaoudi, M. Reticular chemistry 3.2: Typical minimal edge-transitive derived and related nets for the design and synthesis of metal-organic frameworks. Chem. Rev. 2020, 120, 8039–8065. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bhatt, P.M.; Li, J.; Eddaoudi, M.; Liu, Y. Recent progress on microfine design of metal–organic frameworks: Structure regulation and gas sorption and separation. Adv. Mater. 2020, 32. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.H.; Shekhah, O.; Lee, G.; Mallick, A.; Jiang, H.; Li, F.; Chen, B.; Wicks, J.; Eddaoudi, M.; Sargent, E.H. Intermediate binding control using metal-organic frameworks enhances electrochemical CO2 reduction. J. Am. Chem. Soc. 2020, 142, 21513–21521. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wu, G. Metal-organic frameworks based mixed matrix membranes for pervaporation. Microporous Mesoporous Mater. 2016, 235, 151–159. [Google Scholar] [CrossRef]
- Cui, W.G.; Hu, T.L.; Bu, X.H. Metal–organic framework materials for the separation and purification of light hydrocarbons. Adv. Mater. 2020, 32, 1806445. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Xu, J.; Gu, Z. Metal–organic-framework-based gas chromatographic separation. Chem. Asian J. 2019, 14, 3462–3473. [Google Scholar] [CrossRef]
- Rieger, M.; Wittek, M.; Scherer, P.; Löbbecke, S.; Müller-Buschbaum, K. Preconcentration of nitroalkanes with archetype metal–organic frameworks (MOFs) as concept for a sensitive sensing of explosives in the gas phase. Adv. Funct. Mater. 2018, 28, 1–11. [Google Scholar] [CrossRef]
- Thielmann, F.; Butler, D.A.; Williams, D.R.; Baumgarten, E. Characterisation of microporous materials by dynamic sorption methods. Stud. Surf. Sci. Catal. 2000, 129, 633–638. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Schreiber, H.P. Aspects of acid-base interactions and use of inverse gas chromatography. Colloids Surf. A Physicochem. Eng. Asp. 1995, 100, 47–71. [Google Scholar] [CrossRef]
- Tijburg, I.; Jagiello, J.; Vidal, A.; Papirer, E. Inverse gas chromatographic studies on silica: Infinite dilution and finite concentration measurements. Langmuir 1991, 7, 2243–2247. [Google Scholar] [CrossRef]
- Balard, H. Estimation of the surface energetic heterogeneity of a solid by inverse gas chromatography. Langmuir 1997, 13, 1260–1269. [Google Scholar] [CrossRef]
- Thielmann, F.; Butler, D.A.; Williams, D.R. Characterization of porous materials by finite concentration inverse gas chromatography. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187–188, 267–272. [Google Scholar] [CrossRef]
- Lavielle, L.; Schultz, J. Surface properties of carbon fibers determined by inverse gas chromatography: Role of pretreatment. Langmuir 1991, 7, 978–981. [Google Scholar] [CrossRef]
- James, A.T.; Martin, A.J. Gas-liquid partition chromatography; the separation and micro-estimation of volatile fatty acids from formic acid to dodecanoic acid. Biochem. J. 1952, 50, 679–690. [Google Scholar] [CrossRef]
- Mäihäniemi-Ylä, P.P.; Williams, D.R. A comparison of frontal and nonfrontal methods for determining solid-liquid adsorption isotherms using inverse liquid chromatography. Langmuir 2007, 23, 4095–4101. [Google Scholar] [CrossRef] [PubMed]
- Littlewood, A.B.; Phillips, C.S.G.; Price, D.T. The chromatography of gases and vapours. Part V. Partition analyses with columns of silicone 702 and of tritolyl phosphate. J. Chem. Soc. 1955, 1480–1489. [Google Scholar] [CrossRef]
- Katsanos, N.A.; Thede, R.; Roubani-Kalantzopoulou, F. Diffusion, adsorption and catalytic studies by gas chromatography. J. Chromatogr. A 1998, 795, 133–184. [Google Scholar] [CrossRef]
- Finsy, V.; de Bruyne, S.; Alaerts, L.; de Vos, D.; Jacobs, P.A.; Baron, G.V.; Denayer, J.F.M. Shape selective adsorption of linear and branched alkanes in the Cu3(BTC)2 metal-organic framework. In Studies in Surface Science and Catalysis; Elsevier B.V.: Amsterdam, The Nehterlands, 2007. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Dorris, G.M.; Gray, D.G. Adsorption of n-alkanes at zero surface coverage on cellulose paper and wood fibers. J. Colloid Interface Sci. 1980, 77, 353–362. [Google Scholar] [CrossRef]
- Schultz, J.; Martin, C. The role of the interface in carbon fibre-epoxy compositest. J. Adhes. 1987, 23, 45–60. [Google Scholar] [CrossRef]
- Pefferkorn, E. Interfacial Phenomena in Chromatography; Routledge: London, UK, 1999. [Google Scholar] [CrossRef]
- Brookman, D.J.; Sawyer, D.T. Specific interactions affecting gas chromatographic retention for modified alumina columns. Anal. Chem. 1968, 40, 106–110. [Google Scholar] [CrossRef]
- Riddle, F.L.; Fowkes, F.M. Spectral shifts in acid-base chemistry. 1. Van der Waals Contributions to Acceptor Numbers. J. Am. Chem. Soc. 1990, 112, 3259–3264. [Google Scholar] [CrossRef]
- Schuster, P. The donor-acceptor approach to molecular interactions. Fluid Phase Equilib. 1979, 3, 251–252. [Google Scholar] [CrossRef]
- Autie-Castro, G.; Autie, M.A.; Rodríguez-Castellón, E.; Aguirre, C.; Reguera, E. Cu-BTC and Fe-BTC metal-organic frameworks: Role of the materials structural features on their performance for volatile hydrocarbons separation. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 351–357. [Google Scholar] [CrossRef]
- Gutiérrez, I.; Díaz, E.; Vega, A.; Ordóñez, S.; Guerrero-Ruiz, A.; Castillejos-López, E.; Rodríguez-Ramos, I. Hydrocarbons adsorption on metal trimesate MOFs: Inverse gas chromatography and immersion calorimetry studies. Thermochim. Acta 2015, 602, 36–42. [Google Scholar] [CrossRef]
- Autié-Castro, G.; Reguera, E.; Cavalcante, C.L.; Araujo, A.S.; Rodríguez-Castellón, E. Surface acid-base properties of Cu-BTC and Fe-BTC MOFs. An inverse gas chromatography and n-butylamine thermo desorption study. Inorg. Chim. Acta 2020, 507, 119590. [Google Scholar] [CrossRef]
- Finsy, V.; Calero, S.; García-Pérez, E.; Merkling, P.J.; Vedts, G.; de Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Low-coverage adsorption properties of the metal-organic framework MIL-47 studied by pulse chromatography and Monte Carlo simulations. Phys. Chem. Chem. Phys. 2009, 11, 3515–3521. [Google Scholar] [CrossRef] [PubMed]
- Duerinck, T.; Couck, S.; Vermoortele, F.; de Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Pulse gas chromatographic study of adsorption of substituted aromatics and heterocyclic molecules on MIL-47 at zero coverage. Langmuir 2012, 28, 13883–13891. [Google Scholar] [CrossRef]
- Couck, S.; Rémy, T.; Baron, G.V.; Gascon, J.; Kapteijn, F.; Denayer, J.F.M. A pulse chromatographic study of the adsorption properties of the amino-MIL-53 (Al) metal-organic framework. Phys. Chem. Chem. Phys. 2010, 12, 9413–9418. [Google Scholar] [CrossRef]
- Sun, T.; Ren, X.; Hu, J.; Wang, S. Expanding pore size of Al-BDC metal-organic frameworks as a way to achieve high adsorption selectivity for CO2/CH4 separation. J. Phys. Chem. C. 2014, 118, 15630–15639. [Google Scholar] [CrossRef]
- Sun, T.; Hu, J.; Ren, X.; Wang, S. Experimental evaluation of the adsorption, diffusion, and separation of CH4/N2 and CH4/CO2 mixtures on Al-BDC MOF. Sep. Sci. Technol. 2015, 50, 874–885. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Li, Z. Adsorption equilibrium and kinetics of p-xylene on chromium-based metal organic framework MIL-101. Chem. Eng. J. 2011, 173, 150–157. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Jiang, D.Q.; Wang, H.F.; Cui, X.Y.; Yan, X.P. Adsorption and separation of xylene isomers and ethylbenzene on two Zn-terephthalate metal-organic frameworks. J. Phys. Chem. C. 2010, 114, 311–316. [Google Scholar] [CrossRef]
- Luebbers, M.T.; Wu, T.; Shen, L.; Masel, R.I. Trends in the adsorption of volatile organic compounds in a large-pore metal-organic framework, IRMOF-1. Langmuir 2010, 26, 11319–11329. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, I.; Díaz, E.; Vega, A.; Ordóñez, S. Consequences of cavity size and chemical environment on the adsorption properties of isoreticular metal-organic frameworks: An inverse gas chromatography study. J. Chromatogr. A 2013, 1274, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Luebbers, M.T.; Wu, T.; Shen, L.; Masel, R.I. Effects of molecular sieving and electrostatic enhancement in the adsorption of organic compounds on the zeolitic imidazolate framework ZIF-8. Langmuir 2010, 26, 15625–15633. [Google Scholar] [CrossRef]
- Virdis, T.; Danilov, V.; Baron, G.V.; Denayer, J.F.M. Nonideality in the adsorption of ethanol/ethyl acetate/water mixtures on ZIF-8 metal organic framework. Ind. Eng. Chem. Res. 2018, 57, 7040–7047. [Google Scholar] [CrossRef]
- Yusuf, K.; Badjah-Hadj-Ahmed, A.Y.; Aqel, A.; Aouak, T.; Othman, Z.A.A.L. Zeolitic imidazolate framework-methacrylate composite monolith characterization by inverse gas chromatography. J. Chromatogr. A 2016, 1443, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Van der Perre, S.; Bozbiyik, B.; Lannoeye, J.; de Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Experimental study of adsorptive interactions of polar and nonpolar adsorbates in the Zeolitic Imidazolate Framework ZIF-68 via pulse gas chromatography. J. Phys. Chem. C 2015, 119, 1832–1839. [Google Scholar] [CrossRef]
- Chang, N.; Yan, X.P. Exploring reverse shape selectivity and molecular sieving effect of metal-organic framework UIO-66 coated capillary column for gas chromatographic separation. J. Chromatogr. A 2012, 1257, 116–124. [Google Scholar] [CrossRef]
- Bozbiyik, B.; Duerinck, T.; Lannoeye, J.; de Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Adsorption and separation of n-hexane and cyclohexane on the UiO-66 metal-organic framework. Microporous Mesoporous Mater. 2014, 183, 143–149. [Google Scholar] [CrossRef]
- Duerinck, T.; Bueno-Perez, R.; Vermoortele, F.; de Vos, D.E.; Calero, S.; Baron, G.V.; Denayer, J.F.M. Understanding hydrocarbon adsorption in the uio-66 metal-organic framework: Separation of (un)saturated linear, branched, cyclic adsorbates, including stereoisomers. J. Phys. Chem. C 2013, 117, 12567–12578. [Google Scholar] [CrossRef]
- Duerinck, T.; Denayer, J.F.M. Unusual chain length dependent adsorption of linear and branched alkanes on UiO-66. Adsorption 2014, 20, 251–259. [Google Scholar] [CrossRef]
- Couck, S.; Liu, Y.Y.; Leus, K.; Baron, G.V.; van der Voort, P.; Denayer, J.F.M. Gas phase adsorption of alkanes, alkenes and aromatics on the sulfone-DUT-5 metal organic framework. Microporous Mesoporous Mater. 2015, 206, 217–225. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Wen, Y.; Zhou, X.; Li, Z. Oxygen-selective adsorption property of ultramicroporous MOF Cu(Qc)2 for air separation. Ind. Eng. Chem. Res. 2020, 59, 6219–6225. [Google Scholar] [CrossRef]
- Assen, A.H.; Virdis, T.; de Moor, W.; Moussa, A.; Eddaoudi, M.; Baron, G.; Denayer, J.F.M.; Belmabkhout, Y. Kinetic separation of C4 olefins using Y-fum-fcu-MOF with ultra-fine-tuned aperture size. Chem. Eng. J. 2021, 413, 127388. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2 (H2O)3](n). Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Peralta, D.; Chaplais, G.; Simon-Masseron, A.; Barthelet, K.; Chizallet, C.; Quoineaud, A.A.; Pirngruber, G.D. Comparison of the behavior of metal-organic frameworks and zeolites for hydrocarbon separations. J. Am. Chem. Soc. 2012, 134, 8115–8126. [Google Scholar] [CrossRef]
- Wu, D.; Guo, X.; Sun, H.; Navrotsky, A. Interplay of confinement and surface energetics in the interaction of water with a metal-organic framework. J. Phys. Chem. C 2016, 120, 7562–7567. [Google Scholar] [CrossRef]
- Dathe, H.; Peringer, E.; Roberts, V.; Jentys, A.; Lercher, J.A. Metal organic frameworks based on Cu2+ and benzene-1,3,5-tricarboxylate as host for SO2 trapping agents. C. R. Chim. 2005, 8, 753–763. [Google Scholar] [CrossRef]
- Krawiec, P.; Kramer, M.; Sabo, M.; Kunschke, R.; Fröde, H.; Kaskel, S. Improved hydrogen storage in the Metal-Organic Framework Cu 3(BTC)2. Adv. Eng. Mater. 2006, 8, 293–296. [Google Scholar] [CrossRef]
- Prestipino, C.; Regli, L.; Vitillo, J.G.; Bonino, F.; Damin, A.; Lamberti, C.; Zecchina, A.; Solari, P.L.; Kongshaug, K.O.; Bordiga, S. Local structure of framework Cu(II) in HKUST-1 metallorganic framework: Spectroscopic characterization upon activation and interaction with adsorbates. Chem. Mater. 2006, 18, 1337–1346. [Google Scholar] [CrossRef]
- Zhu, B.J.; Yu, X.Y.; Jia, Y.; Peng, F.M.; Sun, B.; Zhang, M.Y.; Luo, T.; Liu, J.H.; Huang, X.J. Iron and 1,3,5-benzenetricarboxylic metal-organic coordination polymers prepared by solvothermal method and their application in efficient as(V) removal from aqueous solutions. J. Phys. Chem. C 2012, 116, 8601–8607. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Vodak, D.; Sudik, A.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Geometric requirements and examples of important structures in the assembly of square building blocks. Proc. Natl. Acad. Sci. USA 2002, 99, 4900–4904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, C.E.; Drago, R.S.; Zerner, M.C. Molecular dimensions for adsorptives. J. Am. Chem. Soc. 1998, 120, 5509–5516. [Google Scholar] [CrossRef]
- Barthelet, K.; Marrot, J.; Riou, D.; Férey, G. A breathing hybrid organic-inorganic solid with very large pores and high magnetic characteristics. Angew. Chem. Int. Ed. 2002, 41, 281–284. [Google Scholar] [CrossRef]
- Serre, C.; Millange, F.; Thouvenot, C.; Noguès, M.; Marsolier, G.; Louër, D.; Férey, G. Very large breathing effect in the first nanoporous chromium(III)-based solids: MIL-53 or CrIII(OH)·{O2C-C6H4- CO2}·{HO2C-C6H4-CO2H}x·H2Oy. J. Am. Chem. Soc. 2002, 124, 13519–13526. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S.I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Serre, C.; Bourrelly, S.; Vimont, A.; Ramsahye, N.A.; Maurin, G.; Llewellyn, P.L.; Daturi, M.; Filinchuk, Y.; Leynaud, O.; Barnes, P.; et al. An explanation for the very large breathing effect of a metal-organic framework during CO2 adsorption. Adv. Mater. 2007, 19, 2246–2251. [Google Scholar] [CrossRef]
- Bourrelly, S.; Llewellyn, P.L.; Serre, C.; Millange, F.; Loiseau, T.; Férey, G. Different adsorption behaviors of methane and carbon dioxide in the isotypic nanoporous metal terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519–13521. [Google Scholar] [CrossRef] [PubMed]
- Couck, S.; Denayer, J.F.M.; Baron, G.V.; Rémy, T.; Gascon, J.; Kapteijn, F. An amine-functionalized MIL-53 metal-organic framework with large separation power for CO2 and CH4. J. Am. Chem. Soc. 2009, 131, 6326–6327. [Google Scholar] [CrossRef] [PubMed]
- Devic, T.; Horcajada, P.; Serre, C.; Salles, F.; Maurin, G.; Moulin, B.; Heurtaux, D.; Clet, G.; Vimont, A.; Grenéche, J.M.; et al. Functionalization in flexible porous solids: Effects on the pore opening and the host-guest interactions. J. Am. Chem. Soc. 2010, 132, 1127–1136. [Google Scholar] [CrossRef]
- Ahnfeldt, T.; Gunzelmann, D.; Loiseau, T.; Hirsemann, D.; Senker, J.; Férey, G.; Stock, N. Synthesis and modification of a functionalized 3D open-framework structure with MIL-53 topology. Inorg. Chem. 2009, 48, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Millange, F.; Serre, C.; Férey, G. Synthesis, structure determination and properties of MIL-53as and MIL-53ht: The first CrIII hybrid inorganic-organic microporous solids: CrIII(OH)·(O2C-C6H4-CO2)·(HO2C-C6H4-CO2H)x. Chem. Commun. 2002, 2, 822–823. [Google Scholar] [CrossRef]
- Alaerts, L.; Kirschhock, C.E.A.; Maes, M.; van der Veen, M.A.; Finsy, V.; Depla, A.; Martens, J.A.; Baron, G.V.; Jacobs, P.A.; Denayer, J.F.M.; et al. Selective adsorption and separation of xylene isomers and ethylbenzene with the microporous vanadium(IV) terephthalate MIL-47. Angew. Chem. Int. Ed. 2007, 46, 4293–4297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llewellyn, P.L.; Bourrelly, S.; Serre, C.; Vimont, A.; Daturi, M.; Hamon, L.; de Weireld, G.; Chang, J.S.; Hong, D.Y.; Hwang, Y.K.; et al. High uptakes of CO2 and CH4 in mesoporous metal-organic frameworks MIL-100 and MIL-101. Langmuir 2008, 24, 7245–7250. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal- organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Edgar, M.; Mitchell, R.; Slawin, A.M.Z.; Lightfoot, P.; Wright, P.A. Solid-State transformations of zinc 1,4-benzenedicarboxylates mediated by hydrogen-bond-forming molecules. Chem. Eur. J. 2001, 7, 5168. [Google Scholar] [CrossRef]
- Biemmi, E.; Christian, S.; Stock, N.; Bein, T. High-throughput screening of synthesis parameters in the formation of the metal-organic frameworks MOF-5 and HKUST-1. Microporous Mesoporous Mater. 2009, 117, 111–117. [Google Scholar] [CrossRef]
- Choi, J.S.; Son, W.J.; Kim, J.; Ahn, W.S. Metal-organic framework MOF-5 prepared by microwave heating: Factors to be considered. Microporous Mesoporous Mater. 2008, 116, 727–731. [Google Scholar] [CrossRef]
- Hafizovic, J.; Bjørgen, M.; Olsbye, U.; Dietzel, P.D.C.; Bordiga, S.; Prestipino, C.; Lamberti, C.; Lillerud, K.P. The inconsistency in adsorption properties and powder XRD data of MOF-5 is rationalized by framework interpenetration and the presence of organic and inorganic species in the nanocavities. J. Am. Chem. Soc. 2007, 129, 3612–3620. [Google Scholar] [CrossRef] [PubMed]
- Hausdorf, S.; Wagler, J.; Mossig, R.; Mertens, F.O.R.L. Proton and water activity-controlled structure formation in zinc carboxylate-based metal organic frameworks. J. Phys. Chem. A 2008, 112, 7567–7576. [Google Scholar] [CrossRef]
- Jiang, J.; Sandler, S.I. Monte Carlo simulation for the adsorption and separation of linear and branched alkanes in IRMQF-1. Langmuir 2006, 22, 5702–5707. [Google Scholar] [CrossRef] [PubMed]
- Almazán-Almazán, M.C.; Pérez-Mendoza, M.; Domingo-García, M.; Fernández-Morales, I.; del Rey-Bueno, F.; García-Rodríguez, A.; López-Garzón, F.J. The role of the porosity and oxygen groups on the adsorption of n-alkanes, benzene, trichloroethylene and 1,2-dichloroethane on active carbons at zero surface coverage. Carbon N. Y. 2007, 45, 1777–1785. [Google Scholar] [CrossRef]
- Herry, C.; Baudu, M.; Raveau, D. Estimation of the influence of structural elements of activated carbons on the energetic components of adsorption. Carbon N. Y. 2001, 39, 1879–1889. [Google Scholar] [CrossRef]
- Mäihäniemi-Ylä, P.P.; Heng, J.Y.Y.; Thielmann, F.; Williams, D.R. Inverse gas chromatographic method for measuring the dispersive surface energy distribution for particulates. Langmuir 2008, 24, 9551–9557. [Google Scholar] [CrossRef]
- Pearce, H.A. Zeolite molecular sieves—Structure, chemistry and use. J. Chromatogr. A 1975, 106, 499. [Google Scholar] [CrossRef]
- Pérez-Pellitero, J.; Amrouche, H.; Siperstein, F.R.; Pirngruber, G.; Nieto-Draghi, C.; Chaplais, G.; Simon-Masseron, A.; Bazer-Bachi, D.; Peralta, D.; Bats, N. Adsorption of CO2, CH4, and N2 on zeolitic imidazolate frameworks: Experiments and simulations. Chem. Eur. J. 2010, 16, 1560–1571. [Google Scholar] [CrossRef]
- Pantatosaki, E.; Pazzona, F.G.; Megariotis, G.; Papadopoulos, G.K. Atomistic simulation studies on the dynamics and thermodynamics of nonpolar molecules within the zeolite imidazolate framework-8. J. Phys. Chem. B 2010, 114, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, K.; Badjah-Hadj-Ahmed, A.Y.; Aqel, A.; Othman, Z.A.A.L. Fabrication of zeolitic imidazolate framework-8-methacrylate monolith composite capillary columns for fast gas chromatographic separation of small molecules. J. Chromatogr. A 2015, 1406, 299–306. [Google Scholar] [CrossRef]
- Grob, R.L.; Barry, E.F. Modern Practice of Gas Chromatography; Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Katz, S.; Gray, D.G. The adsorption of hydrocarbons on cellophane. I. Zero coverage limit. J. Colloid Interface Sci. 1981, 82, 318–325. [Google Scholar] [CrossRef]
- Kazayawoko, M.; Balatinecz, J.J.; Romansky, M. Thermodynamics of adsorption of n-alkanes on maleated wood fibers by inverse gas chromatography. J. Colloid Interface Sci. 1997, 190, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.J. Additivity methods in molecular polarizability. J. Am. Chem. Soc. 1990, 112, 8533–8542. [Google Scholar] [CrossRef]
- Babarao, R.; Dai, S.; Jiang, D.E. Effect of pore topology and accessibility on gas adsorption capacity in zeolitic-imidazolate frameworks: Bringing molecular simulation close to experiment. J. Phys. Chem. C 2011, 115, 8126–8135. [Google Scholar] [CrossRef]
- van der Perre, S.; van Assche, T.; Bozbiyik, B.; Lannoeye, J.; de Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Adsorptive characterization of the ZIF-68 metal-organic framework: A complex structure with amphiphilic properties. Langmuir 2014, 30, 8416–8424. [Google Scholar] [CrossRef] [PubMed]
- Devriese, L.I.; Martens, J.A.; Thybaut, J.W.; Marin, G.B.; Baron, G.V.; Denayer, J.F.M. A new methodology to probe shape selectivity in porous adsorbents. Microporous Mesoporous Mater. 2008, 116, 607–613. [Google Scholar] [CrossRef]
- James, A.T. Vapour phase chromatography, edited by D.H. Desty. Butterworths scientific publications, London, 1957, 436 pages, price? 3.10s. J. Chromatogr. A 1958, 1, 203. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Bárcia, P.S.; Guimarães, D.; Mendes, P.A.P.; Silva, J.A.C.; Guillerm, V.; Chevreau, H.; Serre, C.; Rodrigues, A.E. Reverse shape selectivity in the adsorption of hexane and xylene isomers in MOF UiO-66. Microporous Mesoporous Mater. 2011, 139, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Senkovska, I.; Hoffmann, F.; Fröba, M.; Getzschmann, J.; Böhlmann, W.; Kaskel, S. New highly porous aluminium based metal-organic frameworks: Al(OH)(ndc) (ndc = 2,6-naphthalene dicarboxylate) and Al(OH)(bpdc) (bpdc = 4,4′-biphenyl dicarboxylate). Microporous Mesoporous Mater. 2009, 122, 93–98. [Google Scholar] [CrossRef]
- Denayer, J.F.; Baron, G.V.; Martens, J.A.; Jacobs, P.A. Chromatographic study of adsorption of n-alkanes on zeolites at high temperatures. J. Phys. Chem. B 1998, 102, 3077–3081. [Google Scholar] [CrossRef]
- Ruthven, D.M.; Kaul, B.K. Compensation theory of adsorption: Correlation and prediction of henry constants for linear paraffins on zeolite adsorbents. Adsorption 1998, 4, 269–273. [Google Scholar] [CrossRef]
- Finsy, V.; Kirschhock, C.E.A.; Vedts, G.; Maes, M.; Alaerts, L.; de Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Framework breathing in the vapour-phase adsorption and separation of xylene isomers with the metal-organic framework MIL-53. Chem. Eur. J. 2009, 15, 7724–7731. [Google Scholar] [CrossRef] [PubMed]
- Finsy, V.; Verelst, H.; Alaerts, L.; de Vos, D.; Jacobs, P.A.; Baron, G.V.; Denayer, J.F.M. Pore-filling-dependent selectivity effects in the vapor-phase separation of xylene isomers on the metal-organic framework MIL-47. J. Am. Chem. Soc. 2008, 130, 7110–7118. [Google Scholar] [CrossRef]
- Chen, K.J.; Madden, D.G.; Pham, T.; Forrest, K.A.; Kumar, A.; Yang, Q.Y.; Xue, W.; Space, B.; Perry, J.J.; Zhang, J.P.; et al. Tuning Pore Size in Square-Lattice Coordination Networks for Size-Selective Sieving of CO2. Angew. Chem. Int. Ed. 2016, 55, 10268–10272. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.X.; Belmabkhout, Y.; Shekhah, O.; Jiang, H.; Adil, K.; Cairns, A.J.; Eddaoudi, M. Tunable rare Earth fcu-MOF platform: Access to adsorption kinetics driven gas/vapor separations via pore size contraction. J. Am. Chem. Soc. 2015, 137, 5034–5040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, D.X.; Cairns, A.J.; Belmabkhout, Y.; Wojtas, L.; Liu, Y.; Alkordi, M.H.; Eddaoudi, M. Tunable rare-earth fcu-MOFs: A platform for systematic enhancement of CO2 adsorption energetics and uptake. J. Am. Chem. Soc. 2013, 135, 7660–7667. [Google Scholar] [CrossRef] [PubMed]
- Assen, A.H.; Belmabkhout, Y.; Adil, K.; Bhatt, P.M.; Xue, D.X.; Jiang, H.; Eddaoudi, M. Ultra-tuning of the rare-earth fcu-MOF aperture size for selective molecular exclusion of branched paraffins. Angew. Chem. Int. Ed. 2015, 54, 14353–14358. [Google Scholar] [CrossRef] [PubMed]
- Shi, B. Problem in the molecular area of polar probe molecules used in inverse gas chromatography. J. Chromatogr. A 2019, 1601, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Brendlé, E.; Papirer, E. A new topological index for molecular probes used in inverse gas chromatography 2. Application for the evaluation of the solid surface specific interaction potential. J. Colloid Interface Sci. 1997, 194, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Legras, A.; Kondor, A.; Heitzmann, M.T.; Truss, R.W. Inverse gas chromatography for natural fibre characterisation: Identification of the critical parameters to determine the Brunauer–Emmett–Teller specific surface area. J. Chromatogr. A 2015, 1425, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
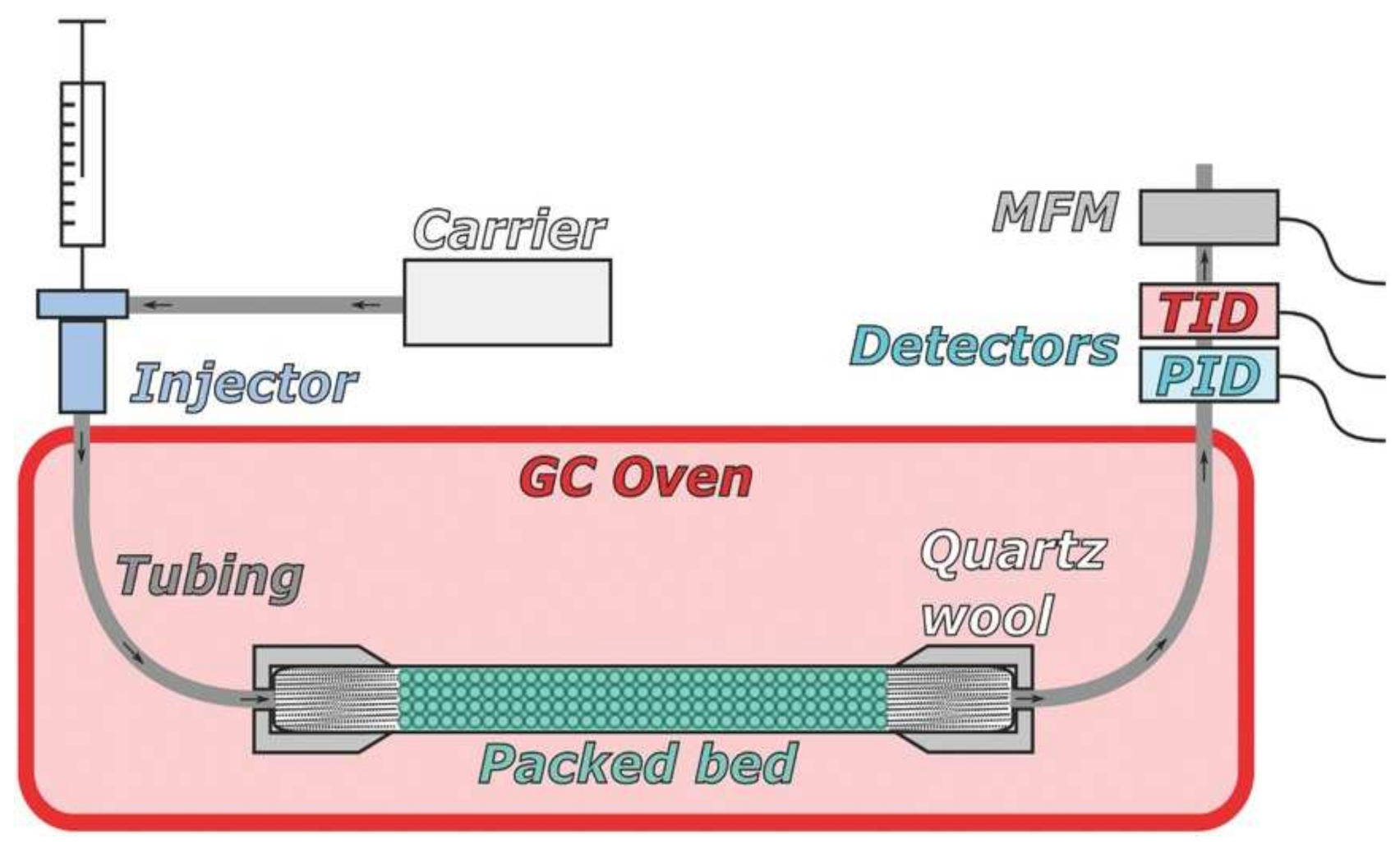
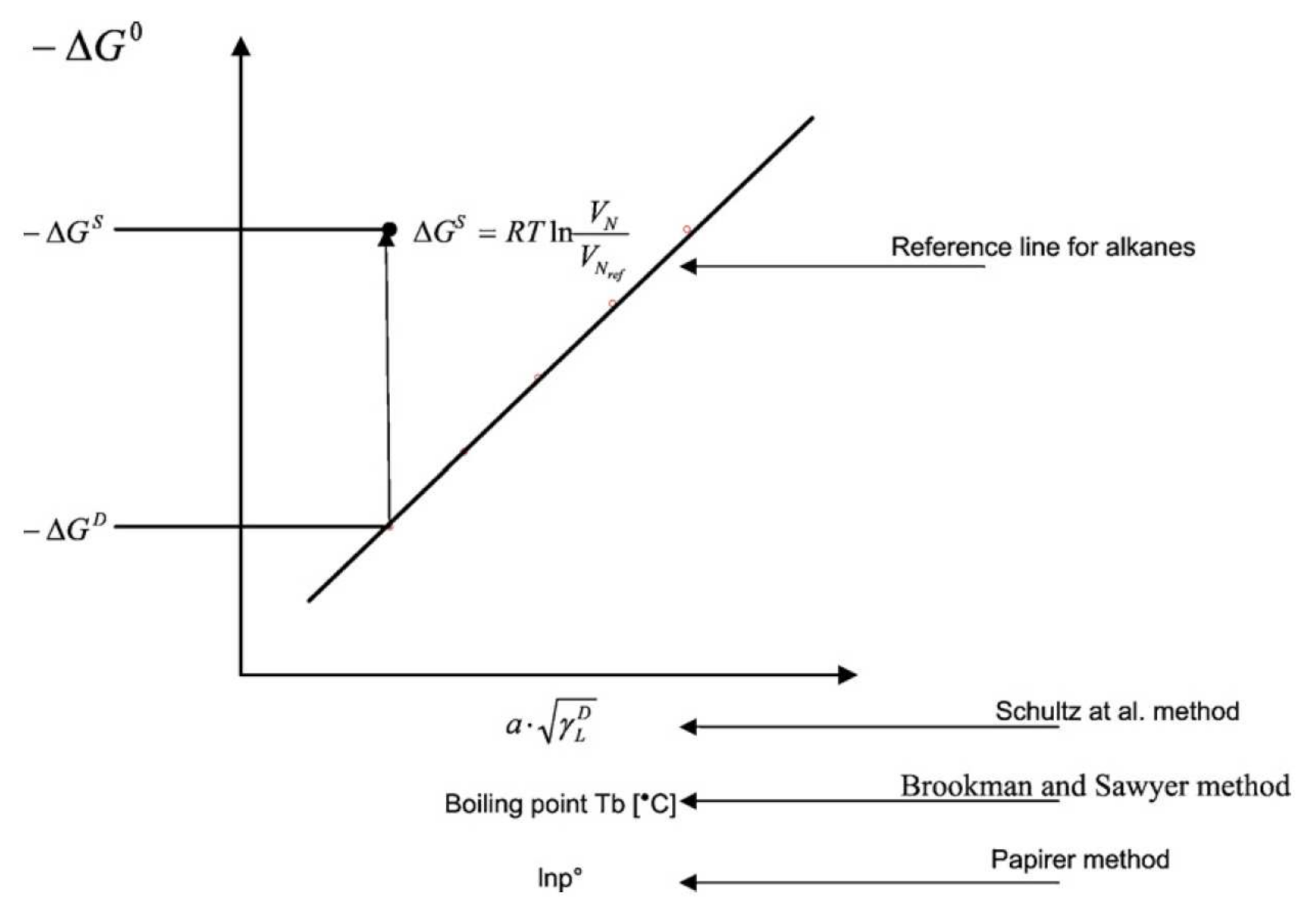

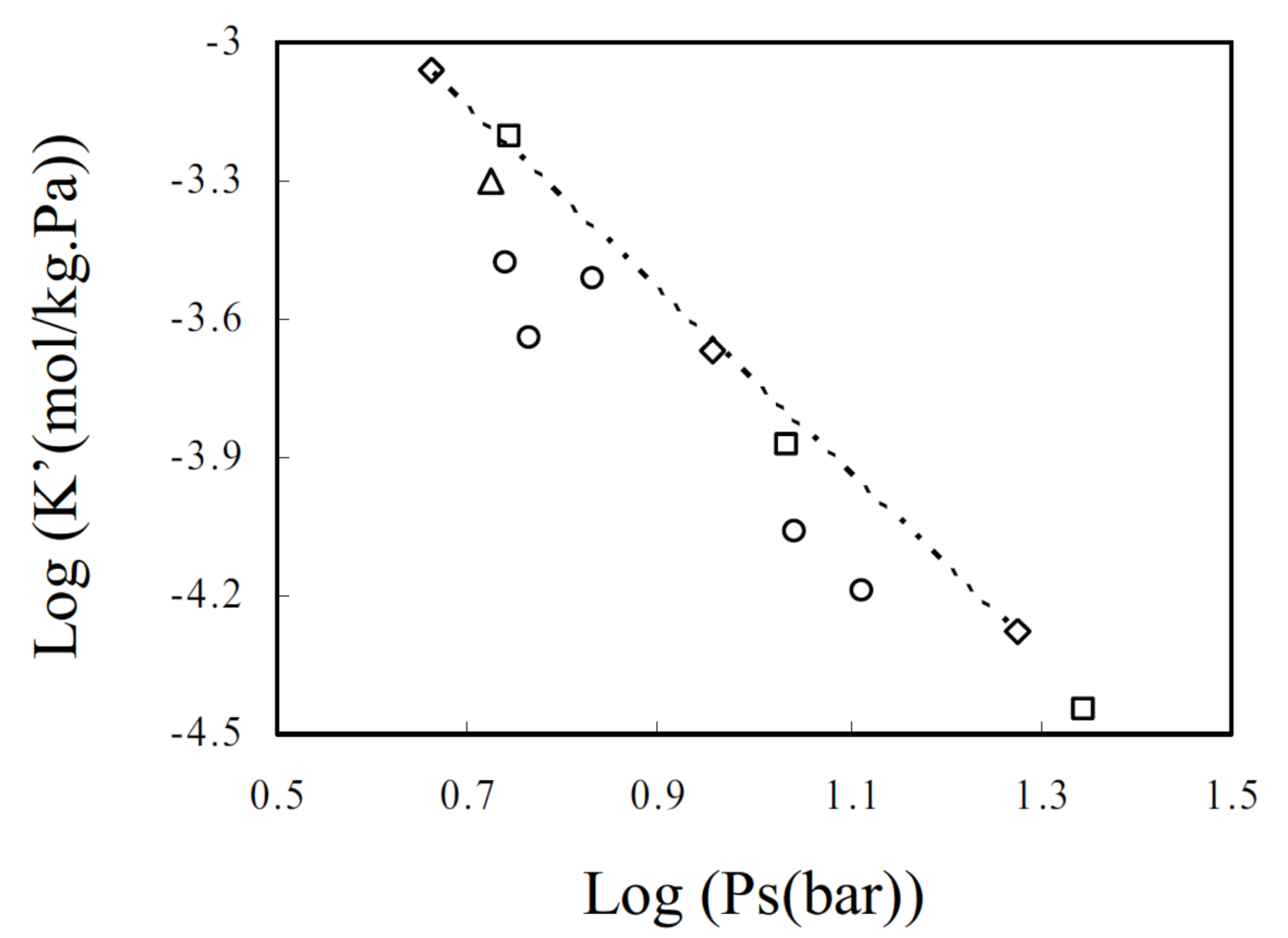
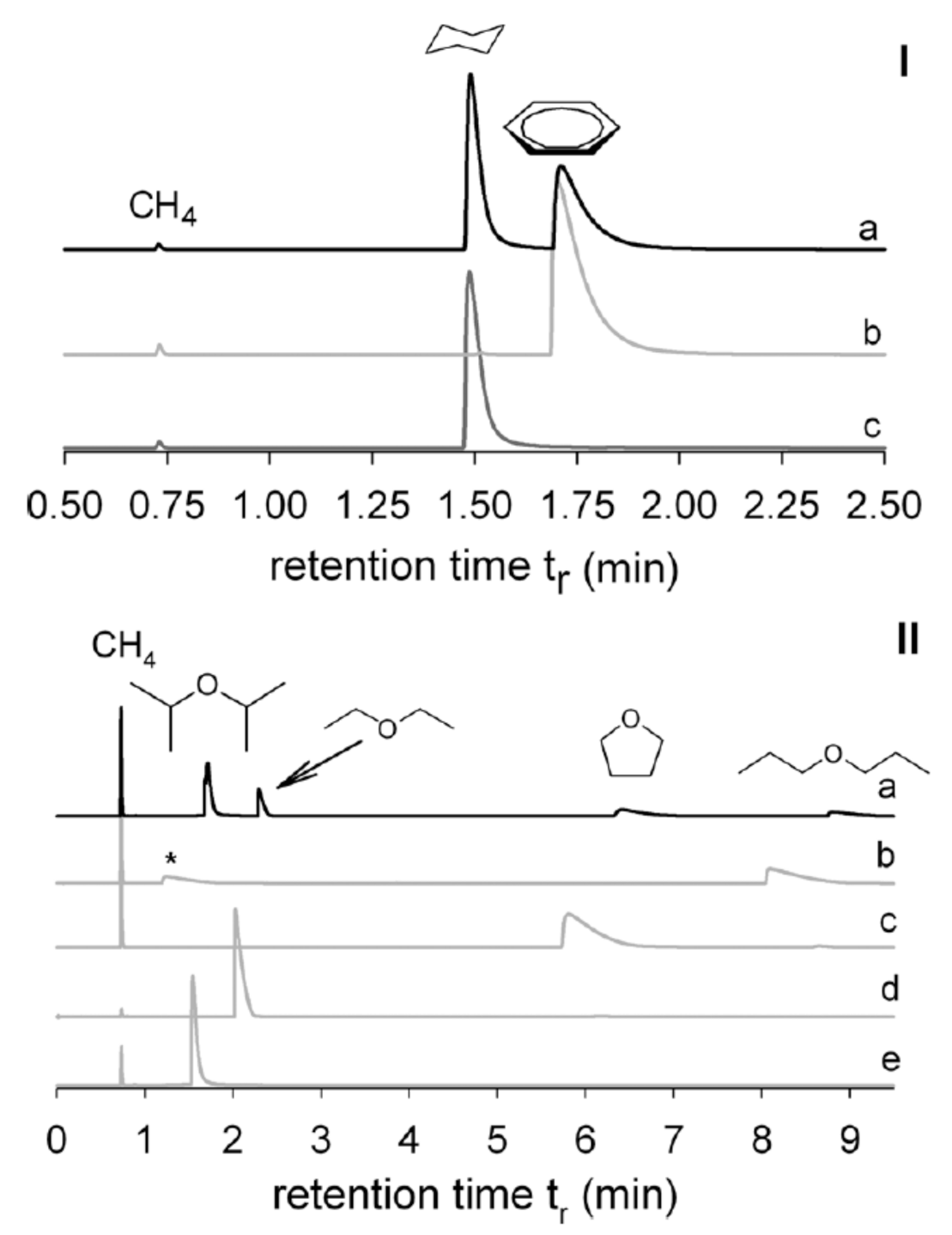
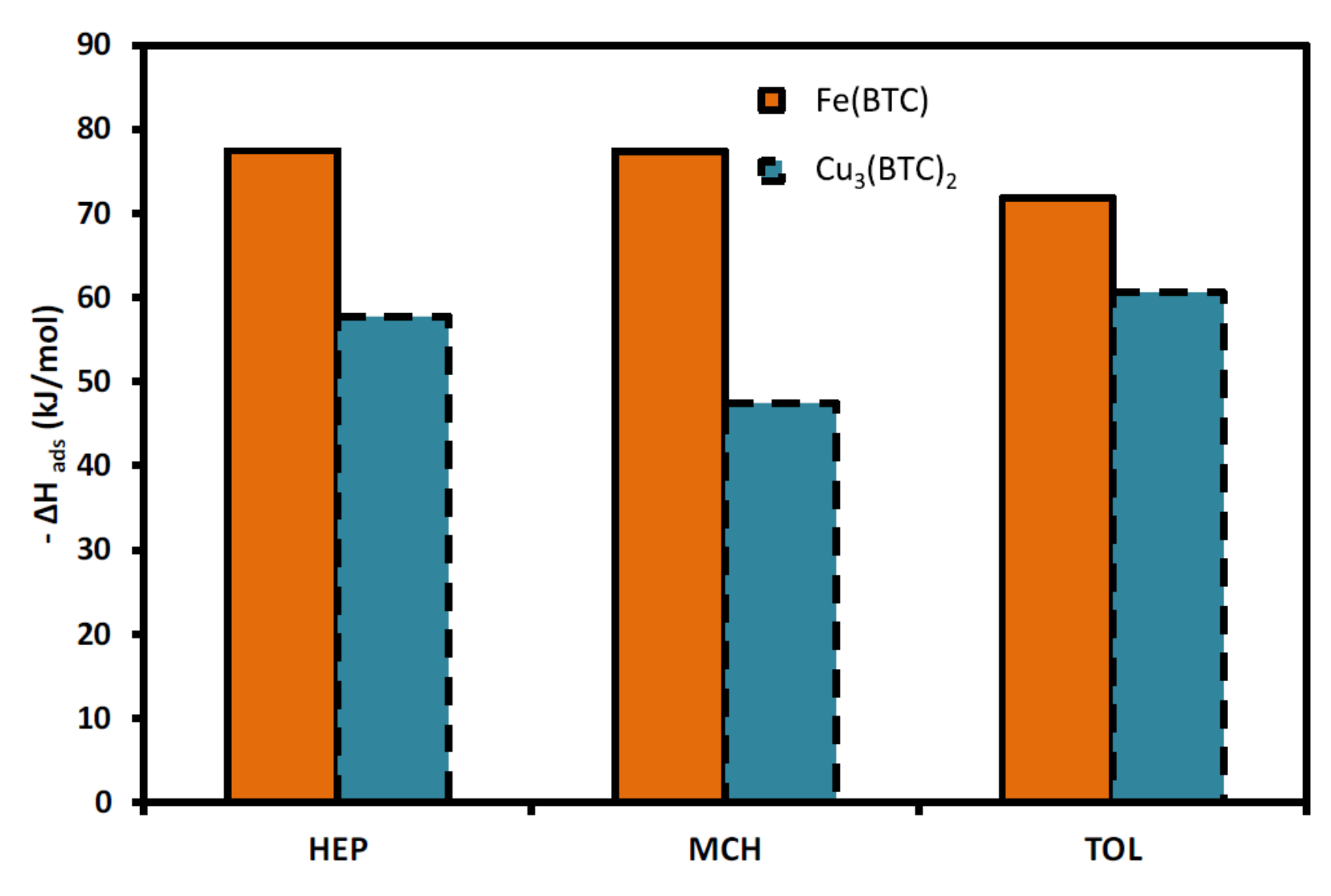
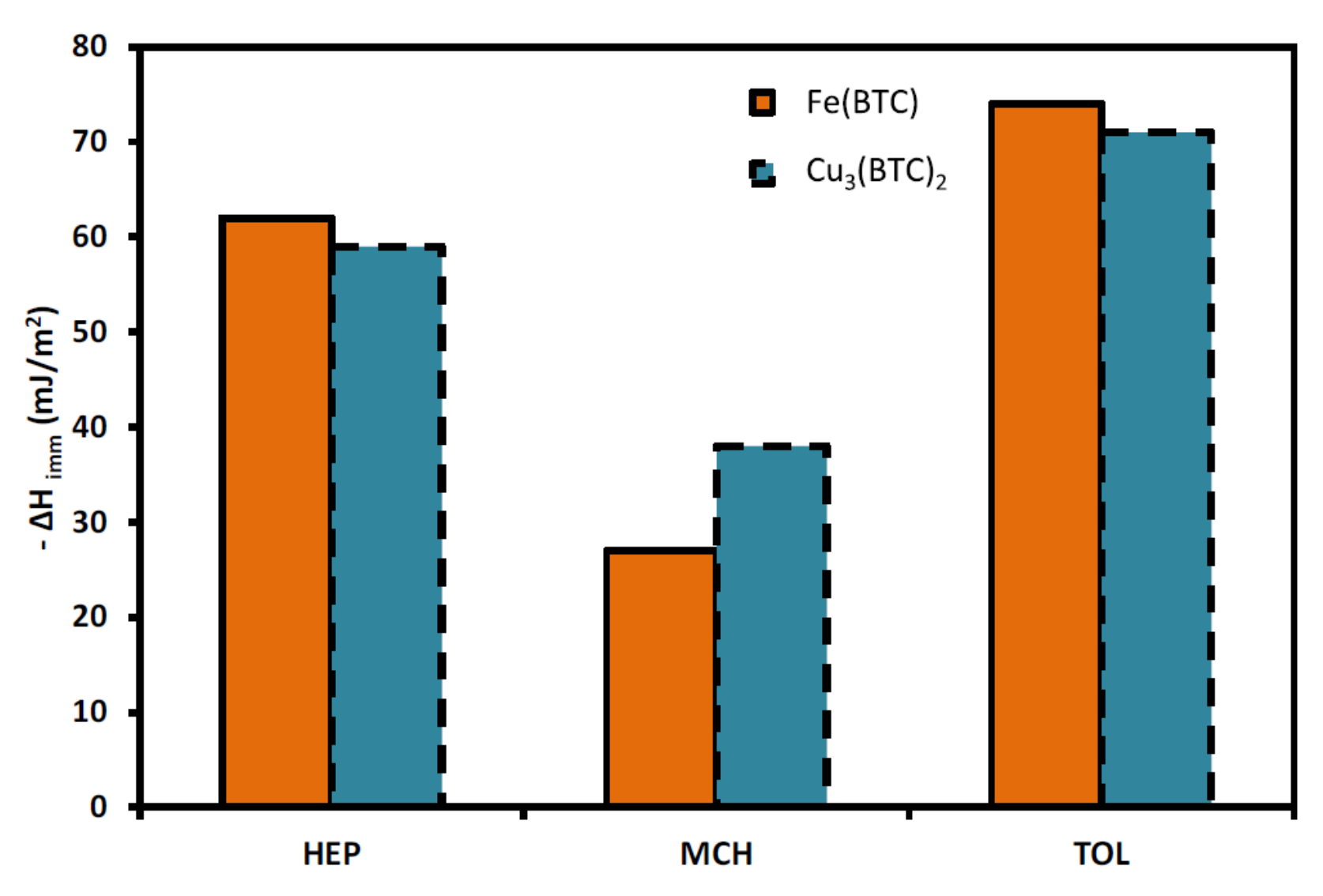


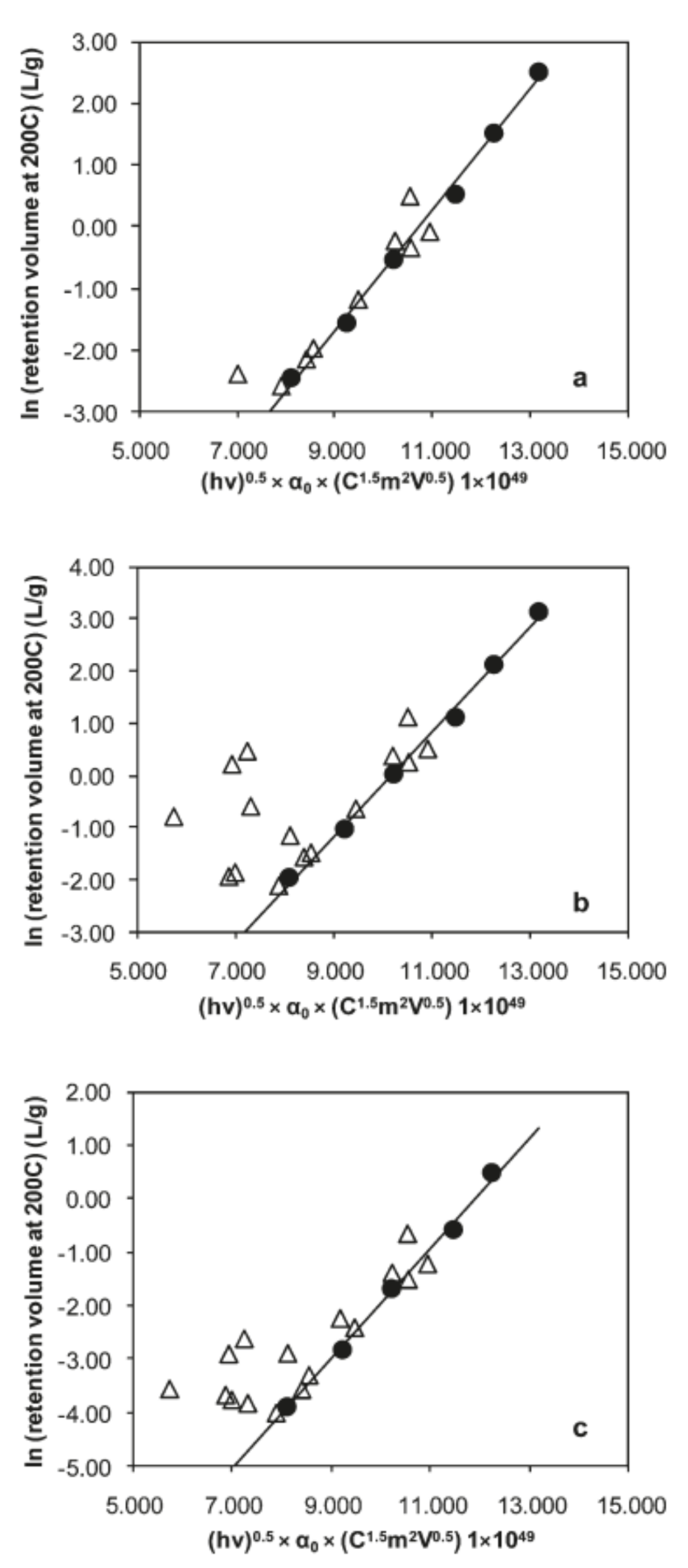
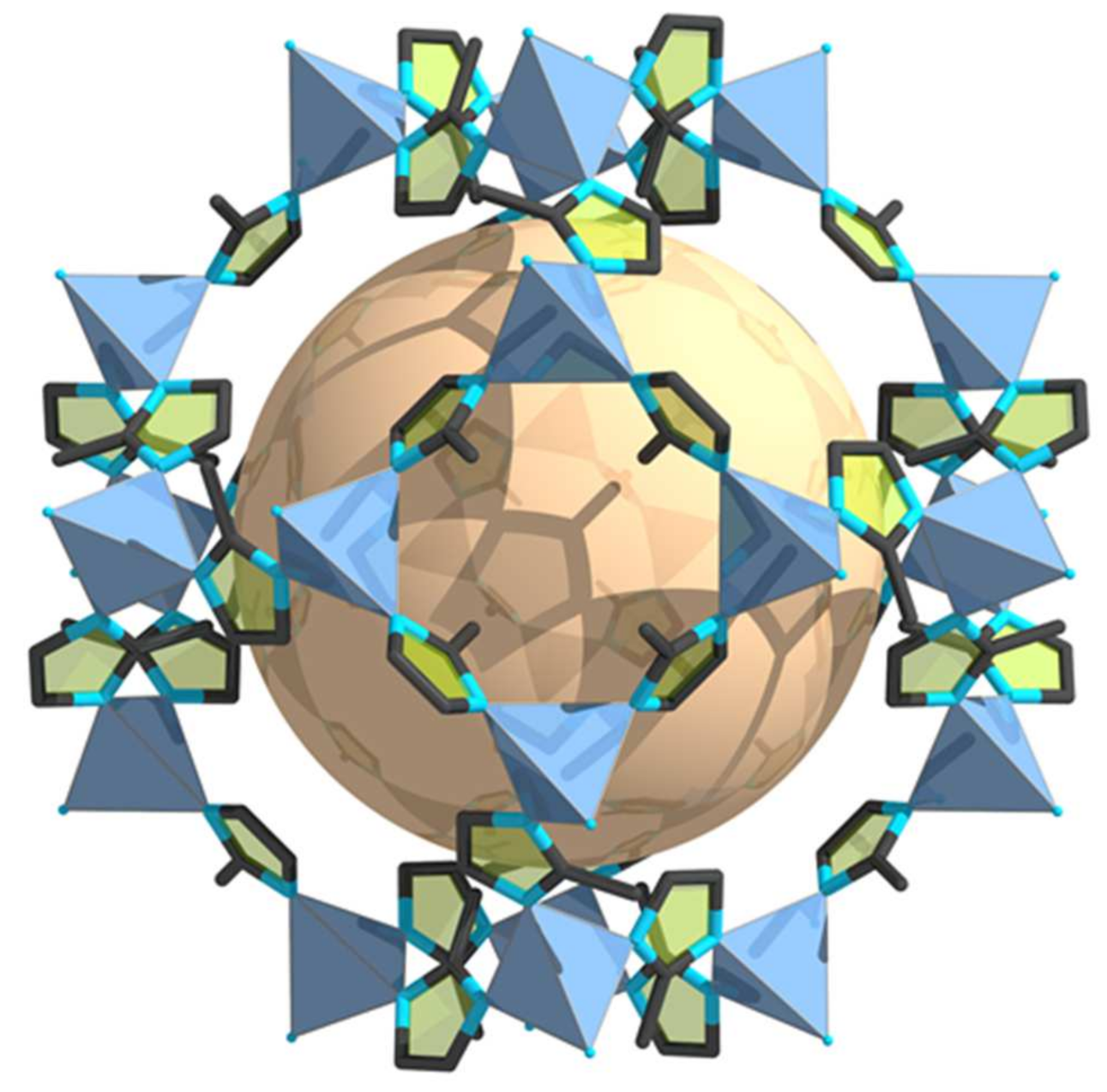
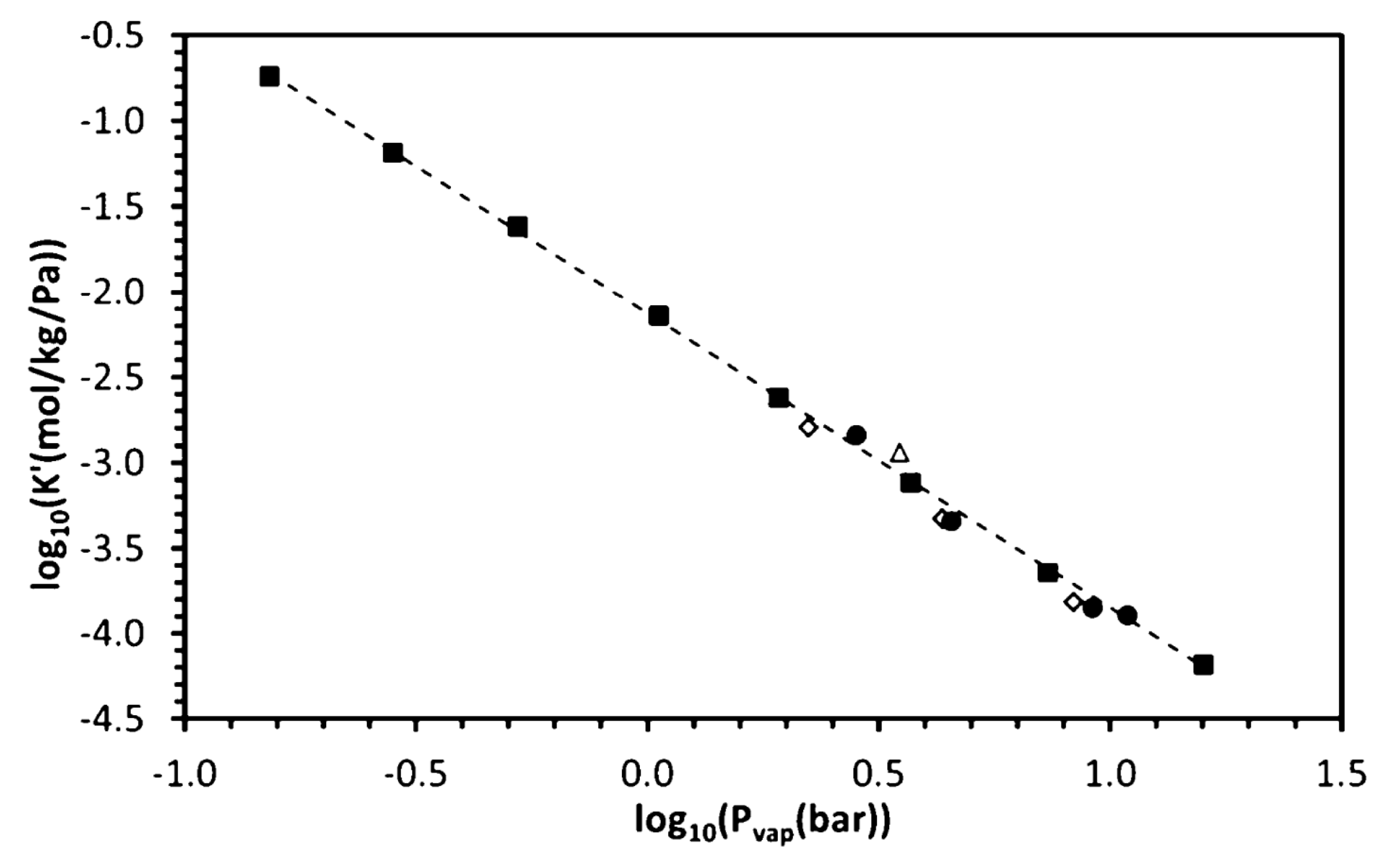
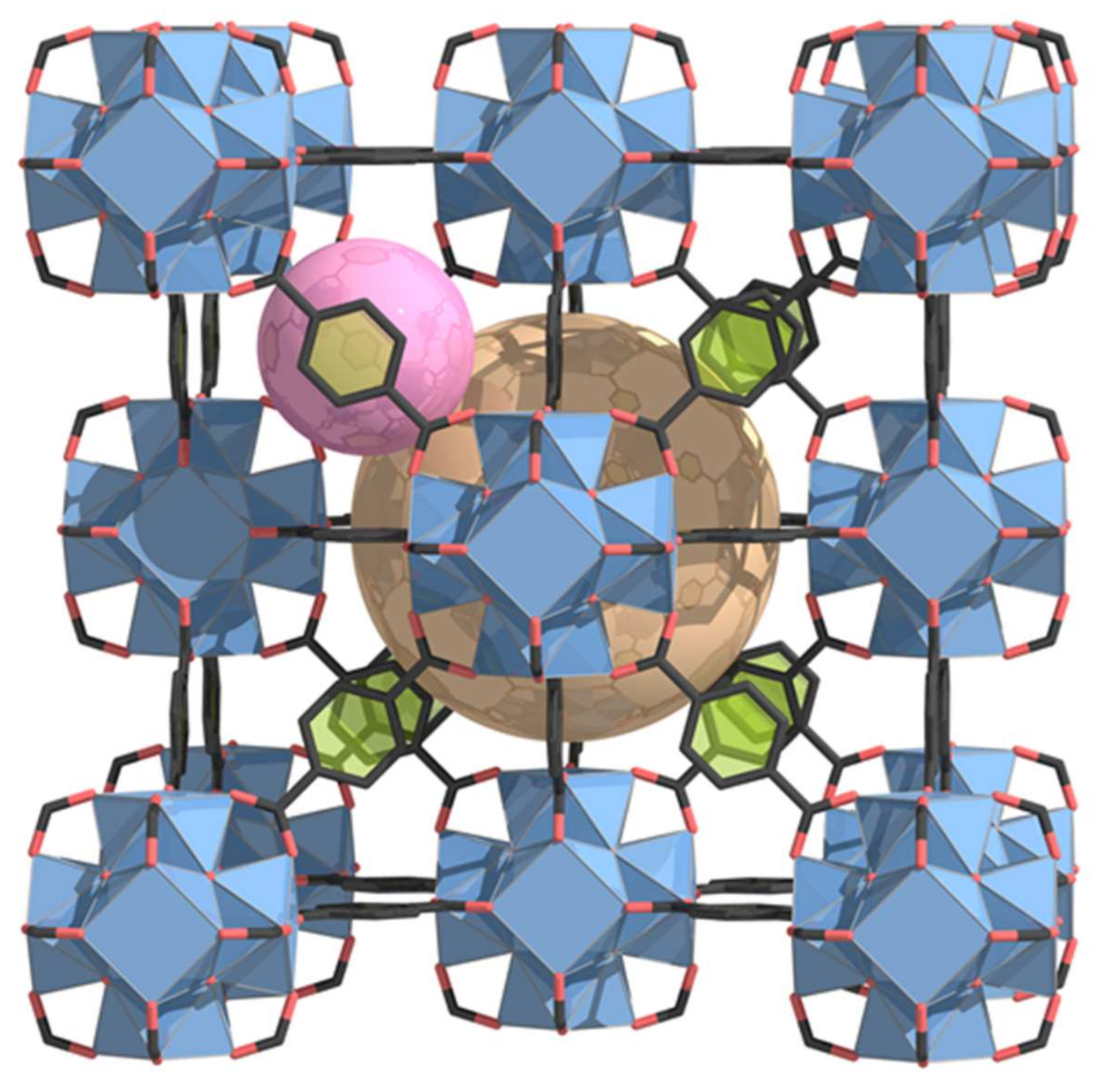


| MOF Material | BET (m2 g−1) | Pore Volume (cm3 g−1) | Pore Opening (Å) | Pore Diameter (Å) | Utilized Probes | Key Parameters | Column Type | Column Dimension | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| HKUST (Hong Kong University of Science and Technology) | |||||||||
| HKUST-1 | n-alkanes Iso-alkanes | , | Packed | 30 cm × 2.16 mm | [49] | ||||
| HKUST-1 | 692.2 | 0.47 | 12.1, 10.3, 4.7 | n-alkanes, Benzene Cyclohexane, THF Ethers | , , , , , , , , , KA, KD | WCOT | 3000 cm × 0.25 mm | [24] | |
| HKUST-1 Fe-BTC | ~1800 ~1450 | 10 | n-alkanes, BTX Propane, Propylene | α, | Packed | 12 cm × 2 mm | [57] | ||
| HKUST-1 Fe-BTC | 1514 962 | 0.61 0.38 | 9, 6 | n-heptane, Toluene Methylcyclohexane | , , , Isp | Packed | 27 cm × 5.3 mm | [58] | |
| HKUST-1 Fe-BTC | 1689 470 | 6.9 | 4, 10, 11 25, 29 | n-alkanes Nitroalkanes | , , , , | Packed | 10–15 cm × 2 mm | [38] | |
| HKUST-1 Fe-BTC | n-alkanes, Benzene THF, Acetone Ethylether, EtAc | KA, KD | Packed | 12 cm × 2 mm | [59] | ||||
| MIL (Matériaux de l′Institut Lavoisier) | |||||||||
| MIL-47(V) | 750 | 0.32 | 8.5 | 8.5 | n-alkanes, Benzene Iso-alkanes, Cyclohexane | , , , | Packed | 30 cm × 2.16 mm | [60] |
| MIL-47(V) | 7.9 × 12.0 | Benzenes Heterocyclic | , | Packed | 15 cm × 3.18 mm | [61] | |||
| Amino-MIL-53(Al) | 940–1038 | 0.50 | 8.5 | 8.5 | n-alkanes, 1-alkenes Iso-alkanes Benzene Cyclohexane Cyclohexene | , | Packed | 30 cm × 3.18 mm | [62] |
| AlBDCMicro AlBDCMeso | 1419 700 | 0.497 0.396 | 8.6 25.8 | CO2/CH4 CH4/N2 | Packed | 40 cm × 3 mm | [63,64] | ||
| MIL-53 | 1169 | 2.6 × 13.68.5 × 8.5 | n-alkanes Nitroalkanes | , , , , | Packed | 10–15 cm × 2 mm | [38] | ||
| MIL-101(Cr) | 3054 & 4443 | 2.01 | 14.5 × 16 & ~12 | 29, 34 | n-alkanes, Toluene p-xylene, Acetone | Packed | 5 cm × 3 mm | [65] | |
| IRMOF (Isoreticular Metal-Organic Frameworks) | |||||||||
| MOF-5 MOF-mono | 773 225 | 12 ~7 | xylene isomers and EB | Packed | 80 cm × 1 mm | [66] | |||
| IRMOF-1-1 IRMOF-1-2 IRMOF-1-3 | 1161 781 208 | 7.5 | 15 | More than 30 VOCs n-alkanes | , , , , α0 | Packed | 2–3 cm × 0.53 mm | [67] | |
| IRMOF-1 IRMOF-8 IRMOF-10 | 3046 1362 265 | 10.9 12.5 16.7 | 24.9 41.9 132.6 | n-alkanes, Alkenes Cycloalkane, Aromatics, Chlorinated | , , , , Isp | Packed | 5 cm × 5.3 mm | [68] | |
| ZIF (Zeolitic imidazolate framework) | |||||||||
| ZIF-8 | 1300–1800 | 0.592 | 3.4 | 11.4 | 13 VOCs n-alkanes | , , , , α0 | Packed | 2–3 cm × 0.53 mm | [69] |
| ZIF-8 | 0.66 | 11.6 | EtOH/EtAc/water | , | Packed | 5 cm × 1.59 mm | [70] | ||
| ZIF-8–(BuMA-co-EDMA) | 56 | n-alkanes, Acetone Diethylether, DCM, EtAc, THF Chloroform | , , , , , KA, KD, χ | Monolith | 33.5 cm × 0.25 mm | [71] | |||
| ZIF-8 | 1766 | 3.4 | 11.6 | n-alkanes, Nitroalkanes | , , , , | Packed | 10–15 cm ×2 mm | [38] | |
| ZIF-68 | 11 × 16.5 | n-alkanes, 1-alkenes Iso-alkanes Cycloalkanes Aromatics Polar probes | , | Packed | 20 cm × 3.18 mm | [72] | |||
| UiO-66 (Universitetet i Oslo) | |||||||||
| UiO-66 | 614.3 | 5–8 | 8–11 | Hex. Isomers Benzenes | WCOT | 2000 cm × 0.25 mm | [73] | ||
| UiO-66 | 614.3 | 0.24 | 7.5 | 11 | n-alkanes Cyclohexane Cycloheptane | , , ΔSads | Packed | 30 cm × 3.18 mm | [74] |
| UiO-66-Me, UiO-66-Me2, UiO-66-NO2 | 614.3 | 5–8 | 8–11 | 70 VOCs Alkanes, Alkenes Aromatics | , , | Packed | 35 cm × 3.18 mm | [75] | |
| UiO-66, UiO-66-Me, UiO-66-NO2 | l 7.5 and 11 | n-alkanes Iso-alkanes | , , , | Packed | 30 cm × 3.18 mm | [76] | |||
| Miscellaneous MOFs | |||||||||
| SO2-DUT-5 | 1480 Langmuir | 0.47 | 8.5–10.5 | n-alkanes, 1-alkenes Iso-alkanes Aromatic | , , | Packed | 30 cm × 3.175 mm | [77] | |
| Cu(Qc)2 | 251.4 | 0.132 | 4.7 × 6.6 | O2 and N2 | Packed | 30 cm × 3 mm ID | [78] | ||
| Y-fum-fcu-MOF | 835 | 0.35 | 4.7 | 5.8 Å and 7.6 | C4 olefin isomers | , , | Packed | 5 cm × 3.5 mm | [79] |
| MOF | Pore Size | Window Size | Alkane Separation |
|---|---|---|---|
| ZIF-7 | 4.31 Å | 2.9 Å | Inaccessible to n-alkanes or iso-alkanes No size or shape selectivity |
| ZIF-8 | 11.6 Å | 3.4 Å | Accessible to n-alkanes Inaccessible to iso-alkanes Size selectivity |
| UIO-66 | 11 Å | 7 Å | Accessible to n-alkanes Accessible small branched alkanes Size and reverse shape selectivity |
| MOF-5 | 18.5 Å | 11.2 Å | Accessible to n-alkanes or iso-alkanes No size or shape selectivity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusuf, K.; Shekhah, O.; ALOthman, Z.; Eddaoudi, M. Metal-Organic Frameworks Characterization via Inverse Pulse Gas Chromatography. Appl. Sci. 2021, 11, 10243. https://doi.org/10.3390/app112110243
Yusuf K, Shekhah O, ALOthman Z, Eddaoudi M. Metal-Organic Frameworks Characterization via Inverse Pulse Gas Chromatography. Applied Sciences. 2021; 11(21):10243. https://doi.org/10.3390/app112110243
Chicago/Turabian StyleYusuf, Kareem, Osama Shekhah, Zeid ALOthman, and Mohamed Eddaoudi. 2021. "Metal-Organic Frameworks Characterization via Inverse Pulse Gas Chromatography" Applied Sciences 11, no. 21: 10243. https://doi.org/10.3390/app112110243
APA StyleYusuf, K., Shekhah, O., ALOthman, Z., & Eddaoudi, M. (2021). Metal-Organic Frameworks Characterization via Inverse Pulse Gas Chromatography. Applied Sciences, 11(21), 10243. https://doi.org/10.3390/app112110243






