Upgrading the Hydrogen Storage of MOF-5 by Post-Synthetic Exchange with Divalent Metal Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of MOF-5 Using Solvothermal Method
2.2.2. Post-Synthetic Exchange
2.2.3. Hydrogen and Nitrogen Adsorption
2.2.4. Characterization
3. Results
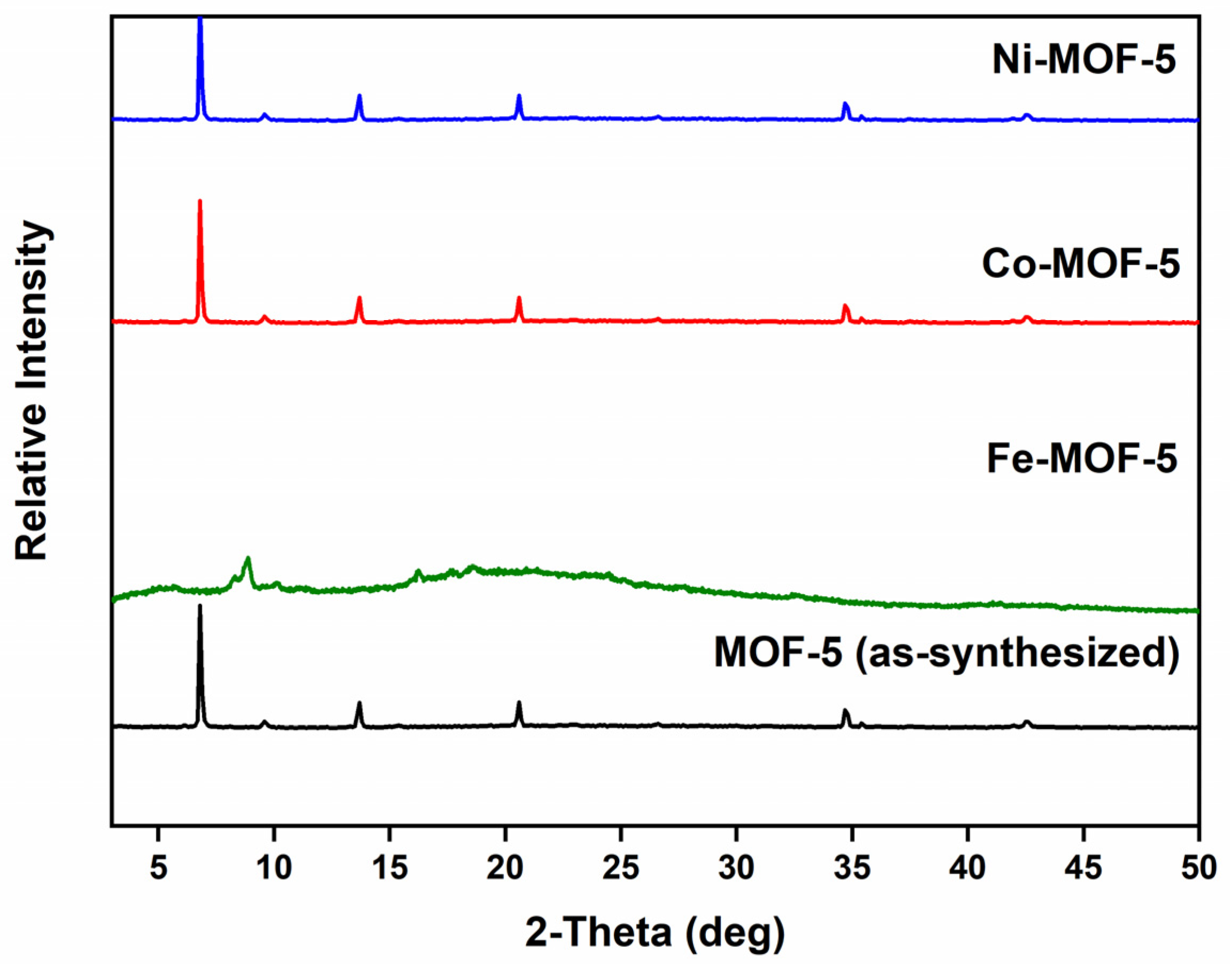
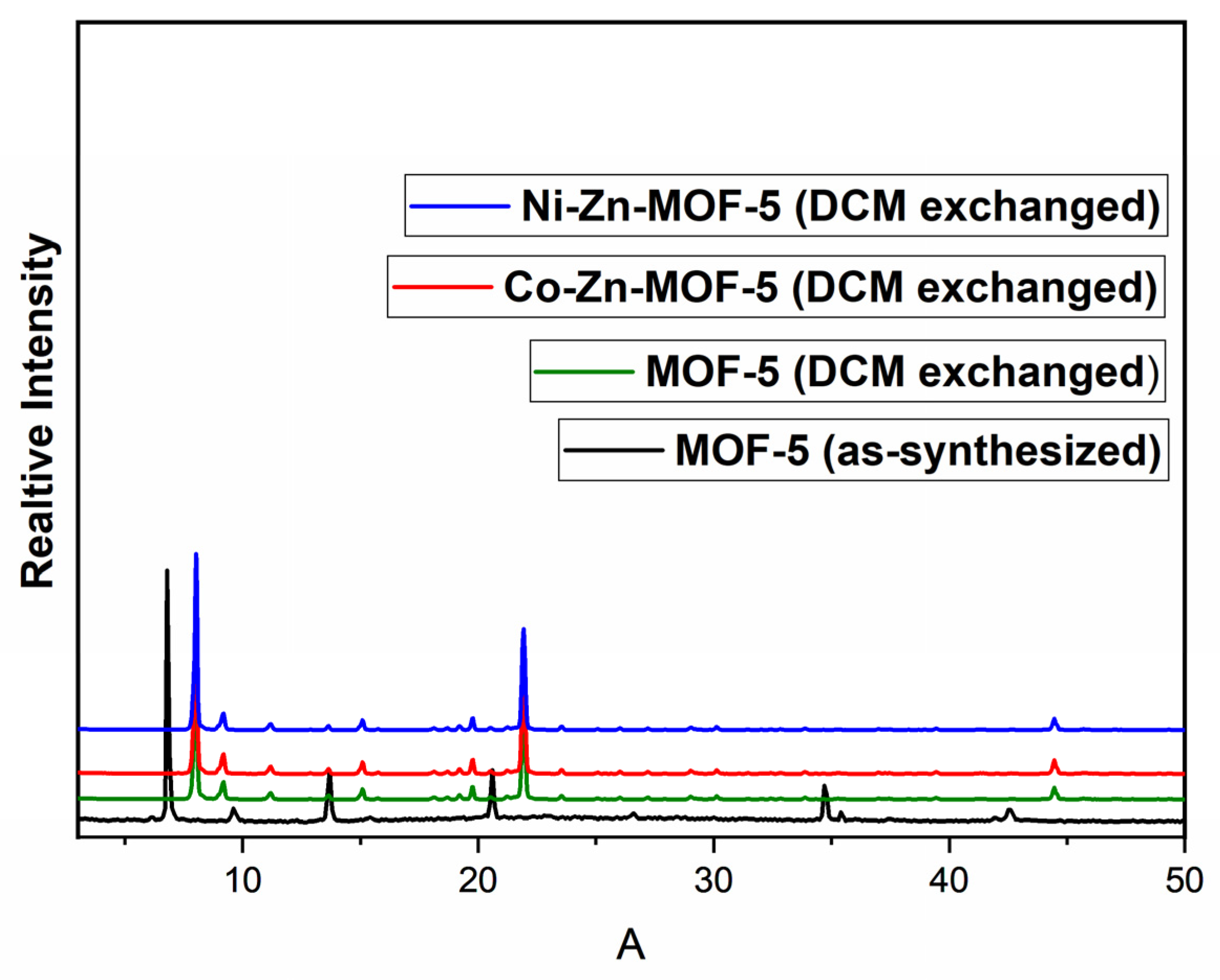
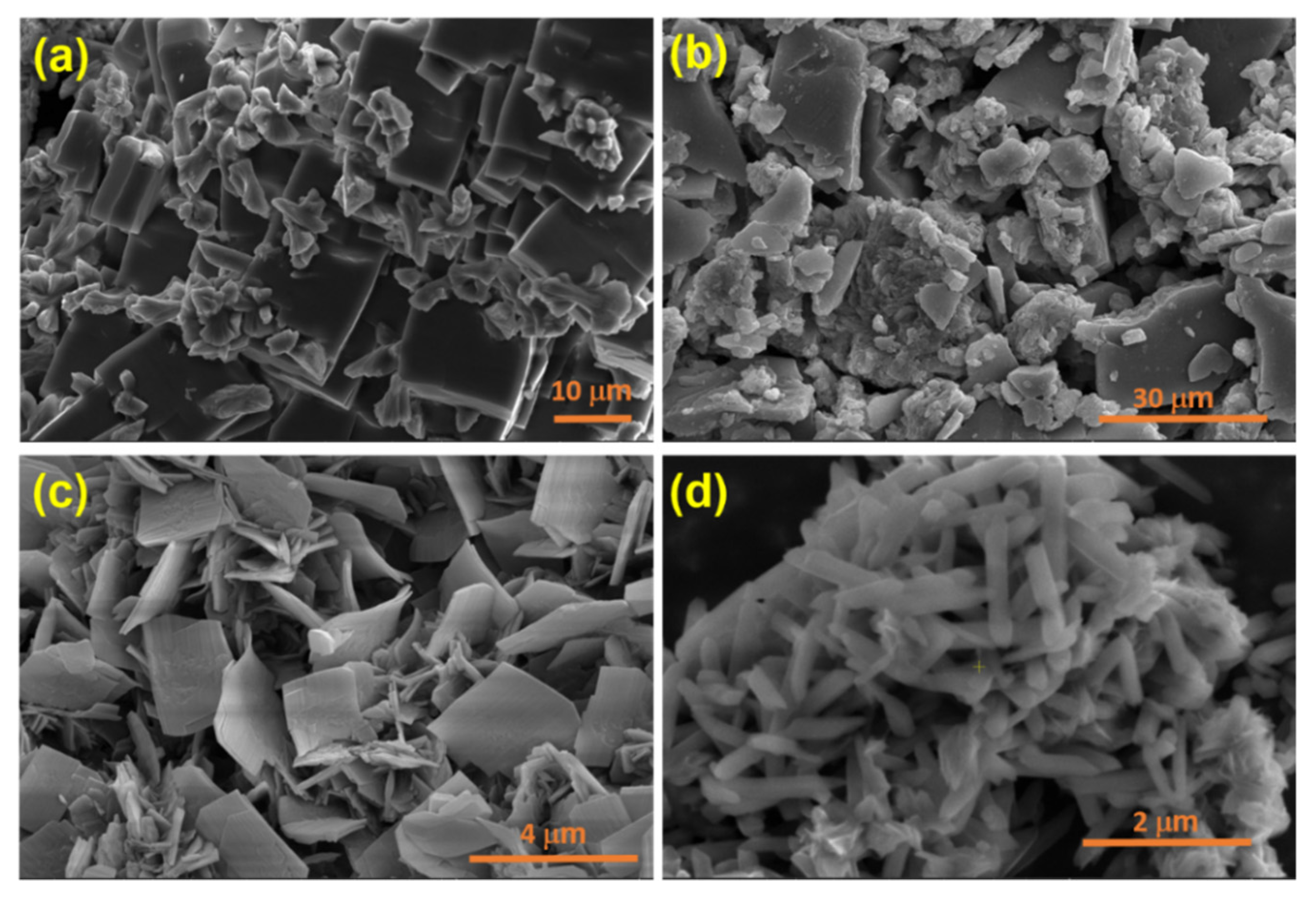
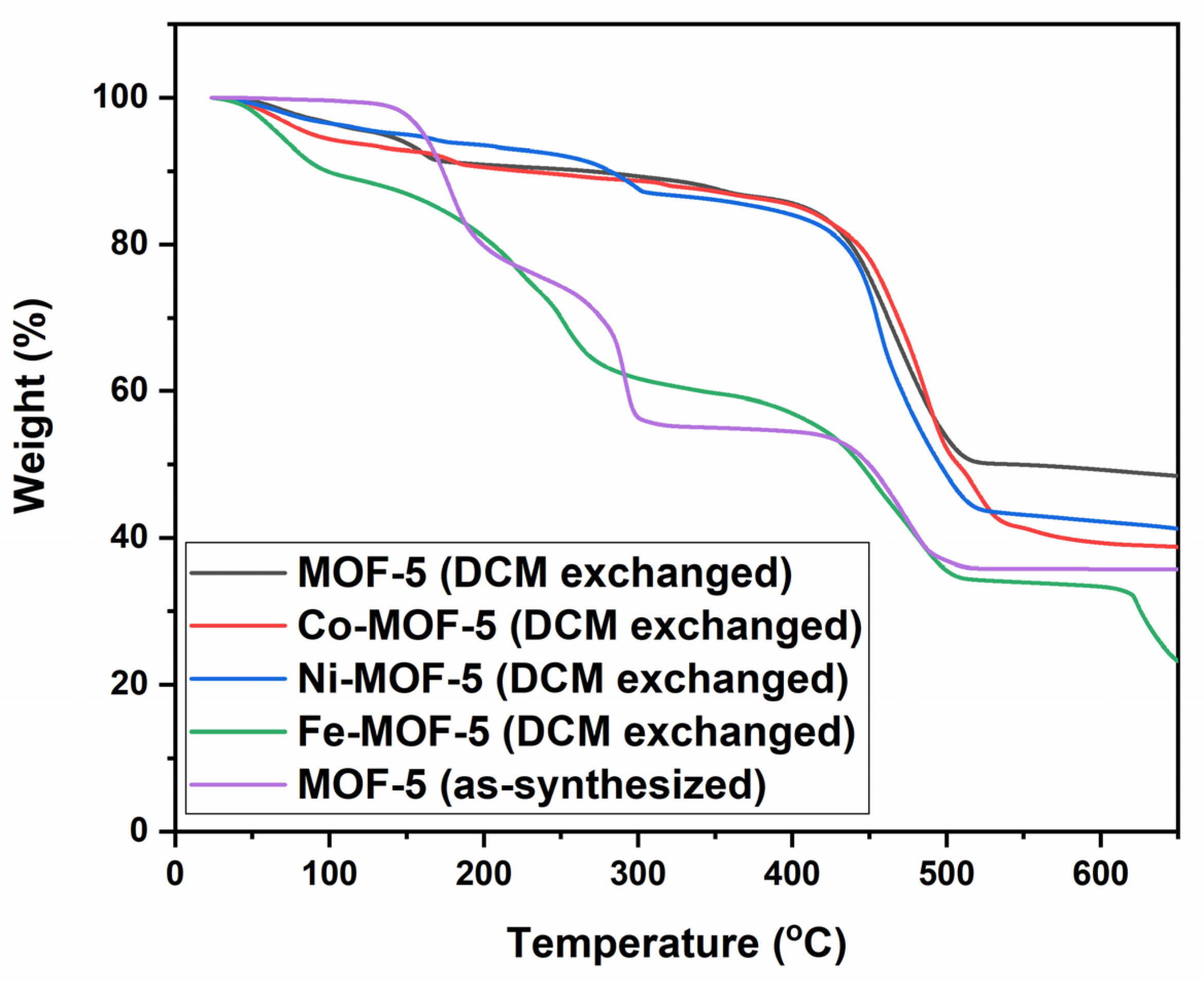
3.1. Characterization of the Prepared MOFs
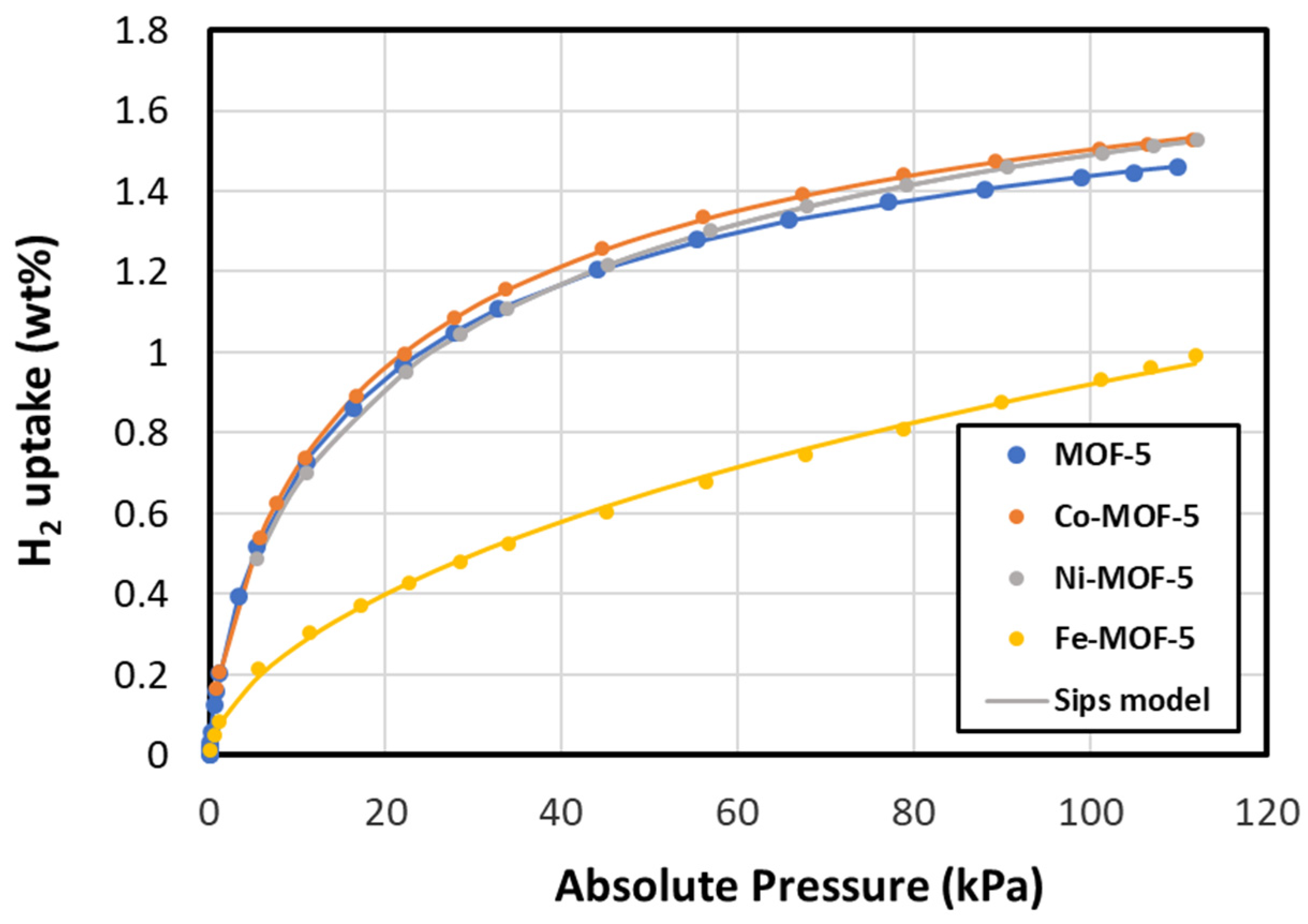
3.2. Surface Area and Hydrogen Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Basile, A.; Iulianelli, A. Advances in Hydrogen Production, Storage and Distribution; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- US DOE. Target Explanation Document: Onboard Hydrogen Storage for Light-Duty Fuel Cell Vehicles; U.S. Drive: Washington, DC, USA, 2017. [Google Scholar]
- He, Y.; Chen, F.; Li, B.; Qian, G.; Zhou, W.; Chen, B. Porous metal–organic frameworks for fuel storage. Coord. Chem. Rev. 2018, 373, 167–198. [Google Scholar] [CrossRef]
- Hirscher, M.; Panella, B.; Schmitz, B. Metal-organic frameworks for hydrogen storage. Microporous Mesoporous Mater. 2010, 129, 335–339. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, e1704303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, L.; Zhou, H.C. Hydrogen storage in metal-organic frameworks. In Nanostructured Materials for Next-Generation Energy Storage and Conversion: Hydrogen Production, Storage, and Utilization; Springer-Verlag GmbH: Berlin, Germany, 2017; pp. 143–170. [Google Scholar]
- Zhang, X.; Lin, R.B.; Wang, J.; Wang, B.; Liang, B.; Yildirim, T.; Zhang, J.; Zhou, W.; Chen, B. Optimization of the Pore Structures of MOFs for Record High Hydrogen Volumetric Working Capacity. Adv. Mater. 2020, 32, e1907995. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Gohari Derakhshandeh, P.; Depauw, H.; Coudert, F.X.; Vrielinck, H.; Van Der Voort, P.; Leus, K. Mixed-metal metal-organic frameworks. Chem. Soc. Rev. 2019, 48, 2535–2565. [Google Scholar] [CrossRef] [Green Version]
- Brozek, C.K.; Dincǎ, M. Cation exchange at the secondary building units of metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5456–5467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dincǎ, M.; Long, J.R. High-enthalpy hydrogen adsorption in cation-exchanged variants of the microporous metal-organic framework Mn3[(Mn4CI) 3(BTT)8(CH3OH)10]2. J. Am. Chem. Soc. 2007, 129, 11172–11176. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.W. Hydrogen storage in metal-organic frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Regli, L.; Chavan, S.; Ricchiardi, G.; Spoto, G.; Dietzel, P.D.C.; Bordiga, S.; Zecchina, A. Role of exposed metal sites in hydrogen storage in MOFs. J. Am. Chem. Soc. 2008, 130, 8386–8396. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.M.; Jeong, H.M.; Park, J.H.; Zhang, Y.B.; Kang, J.K.; Yaghi, O.M. Supercapacitors of nanocrystalline metal-organic frameworks. ACS Nano 2014, 8, 7451–7457. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A.; Dhakshinamoorthy, A.; Garcia, H. Mixed-Metal MOFs: Unique Opportunities in Metal–Organic Framework (MOF) Functionality and Design. Angew. Chem. 2019, 131, 15330–15347. [Google Scholar] [CrossRef]
- Kim, M.; Cahill, J.F.; Fei, H.; Prather, K.A.; Cohen, S.M. Postsynthetic Ligand and Cation Exchange in Robust Metal-Organic Frameworks. J. Am. Chem. Soc. 2012, 134, 18082–18088. [Google Scholar] [CrossRef]
- Peedikakkal, A.M.P.; Aljundi, I.H. Mixed-Metal Cu-BTC Metal-Organic Frameworks as a Strong Adsorbent for Molecular Hydrogen at Low Temperatures. ACS Omega 2020, 5, 28493–28499. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal- organic framework. Nat. Cell Biol. 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen storage in microporous metal-organic frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef] [Green Version]
- Kaye, S.S.; Dailly, A.; Yaghi, O.M.; Long, J.R. Impact of preparation and handling on the hydrogen storage properties of Zn4O(1,4-benzenedicarboxylate)3 (MOF-5). J. Am. Chem. Soc. 2007, 129, 14176–14177. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Deng, S.; Yang, Z. Hydrogen adsorption on metal-organic framework (MOF-5) synthesized by DMF approach. J. Porous Mater. 2009, 16, 141–149. [Google Scholar] [CrossRef]
- Stubbs, A.W.; Braglia, L.; Borfecchia, E.; Meyer, R.J.; Román-Leshkov, Y.; Lamberti, C.; Dincǎ, M. Selective Catalytic Olefin Epoxidation with MnII-Exchanged MOF-5. ACS Catal. 2018, 8, 596–601. [Google Scholar] [CrossRef] [Green Version]
- Brozek, C.K.; Michaelis, V.K.; Ong, T.C.; Bellarosa, L.; López, N.; Griffin, R.G.; Dincǎ, M. Dynamic DMF binding in MOF-5 enables the formation of metastable cobalt-substituted MOF-5 analogues. ACS Cent. Sci. 2015, 1, 252–260. [Google Scholar] [CrossRef]
- Brozek, C.K.; Miller, J.T.; Stoian, S.A.; Dinca, M. NO Disproportionation at a Mononuclear Site-Isolated Fe2+ Center in Fe2+-MOF-5. J. Am. Chem. Soc. 2015, 137, 7495–7501. [Google Scholar] [CrossRef]
- Bellarosa, L.; Brozek, C.K.; García-Melchor, M.; Dincə, M.; López, N. When the solvent locks the cage: Theoretical insight into the transmetalation of MOF-5 lattices and its kinetic limitations. Chem. Mater. 2015, 27, 3422–3429. [Google Scholar] [CrossRef] [Green Version]
- Brozek, C.K.; Dincə, M. Thermodynamic parameters of cation exchange in MOF-5 and MFU-4l. Chem. Commun. 2015, 51, 11780–11782. [Google Scholar] [CrossRef] [PubMed]
- Brozek, C.K.; Dincǎ, M. Ti3+-, V2+/3+-, Cr2+/3+-, Mn 2+-, and Fe2+-substituted MOF-5 and redox reactivity in Cr- and Fe-MOF-5. J. Am. Chem. Soc. 2013, 135, 12886–12891. [Google Scholar] [CrossRef]
- Srepusharawoot, P.; Araújo, C.M.; Blomqvist, A.; Scheicher, R.H.; Ahuja, R. A comparative investigation of H2 adsorption strength in Cd- and Zn-based metal organic framework-5. J. Chem. Phys. 2008, 129, 164104. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Rai, R.K.; Tyagi, D.; Yao, X.; Li, P.Z.; Yang, X.C.; Zhao, Y.; Xu, Q.; Singh, S.K. Room-temperature synthesis of bimetallic Co-Zn based zeolitic imidazolate frameworks in water for enhanced CO2 and H2 uptakes. J. Mater. Chem. A 2016, 4, 14932–14938. [Google Scholar] [CrossRef]
- Botas, J.A.; Calleja, G.; Sánchez-Sánchez, M.; Orcajo, M.G. Cobalt doping of the MOF-5 framework and its effect on gas-adsorption properties. Langmuir 2010, 26, 5300–5303. [Google Scholar] [CrossRef]
- Li, J.; Cheng, S.; Zhao, Q.; Long, P.; Dong, J. Synthesis and hydrogen-storage behavior of metal-organic framework MOF-5. Int. J. Hydrogen Energy 2009, 34, 1377–1382. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.; Zhao, K.; Li, H.; Bing, Y.; Cheng, P. Enhanced Hydrostability in Ni-Doped MOF-5. Inorg. Chem. 2012, 51, 9200–9207. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Q.; Sun, W. Shape and size control and gas adsorption of Ni(II)-doped MOF-5 nano/microcrystals. Microporous Mesoporous Mater. 2014, 190, 26–31. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Q.; Sun, W. Co(II)-doped MOF-5 nano/microcrystals: Solvatochromic behaviour, sensing solvent molecules and gas sorption property. J. Solid State Chem. 2014, 218, 50–55. [Google Scholar] [CrossRef]
- Hafizovic, J.; Bjørgen, M.; Olsbye, U.; Dietzel, P.D.C.; Bordiga, S.; Prestipino, C.; Lamberti, C.; Lillerud, K.P. The inconsistency in adsorption properties and powder XRD data of MOF-5 is rationalized by framework interpenetration and the presence of organic and inorganic species in the nanocavities. J. Am. Chem. Soc. 2007, 129, 3612–3620. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, J.L.C.; Millward, A.R.; Park, K.S.; Yaghi, O.M. Hydrogen Sorption in Functionalized Metal-Organic Frameworks. J. Am. Chem. Soc. 2004, 126, 5666–5667. [Google Scholar] [CrossRef]
- Yang, S.J.; Jung, H.; Kim, T.; Im, J.H.; Park, C.R. Effects of structural modifications on the hydrogen storage capacity of MOF-5. Int. J. Hydrogen Energy 2012, 37, 5777–5783. [Google Scholar] [CrossRef]
- Saha, D.; Wei, Z.; Deng, S. Hydrogen adsorption equilibrium and kinetics in metal-organic framework (MOF-5) synthesized with DEF approach. Sep. Purif. Technol. 2009, 64, 280–287. [Google Scholar] [CrossRef]
- Panella, B.; Hirscher, M. Hydrogen physisorption in metal-organic porous crystals. Adv. Mater. 2005, 17, 538–541. [Google Scholar] [CrossRef]
- Marco-Lozar, J.P.; Juan-Juan, J.; Suárez-García, F.; Cazorla-Amorós, D.; Linares-Solano, A. MOF-5 and activated carbons as adsorbents for gas storage. Int. J. Hydrogen Energy 2012, 37, 2370–2381. [Google Scholar] [CrossRef]
- Zhao, H.; Song, H.; Chou, L. Facile synthesis of MOF-5 structure with large surface area in the presence of benzoyl peroxide by room temperature synthesis. Mater. Chem. Phys. 2014, 143, 1005–1011. [Google Scholar] [CrossRef]
- Tzitzios, V.; Kostoglou, N.; Giannouri, M.; Basina, G.; Tampaxis, C.; Charalambopoulou, G.; Steriotis, T.; Polychronopoulou, K.; Doumanidis, C.; Mitterer, C.; et al. Solvothermal synthesis, nanostructural characterization and gas cryo-adsorption studies in a metal–organic framework (IRMOF-1) material. Int. J. Hydrogen Energy 2017, 42, 23899–23907. [Google Scholar] [CrossRef]
- Ma, S.; Sun, D.; Ambrogio, M.; Fillinger, J.A.; Parkin, S.; Zhou, H.C. Framework-catenation isomerism in metal-organic frameworks and its impact on hydrogen uptake. J. Am. Chem. Soc. 2007, 129, 1858–1859. [Google Scholar] [CrossRef] [PubMed]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Andres, H.M.; Orcaja, G.; Martos, C.; Botas, J.A.; Callega, G. Co/Ni mixed-metal expanded IRMOF-74 series and their hydrogen adsorption properties. Int. J. Hydrogen Energy 2019, 44, 18205–18213. [Google Scholar] [CrossRef]


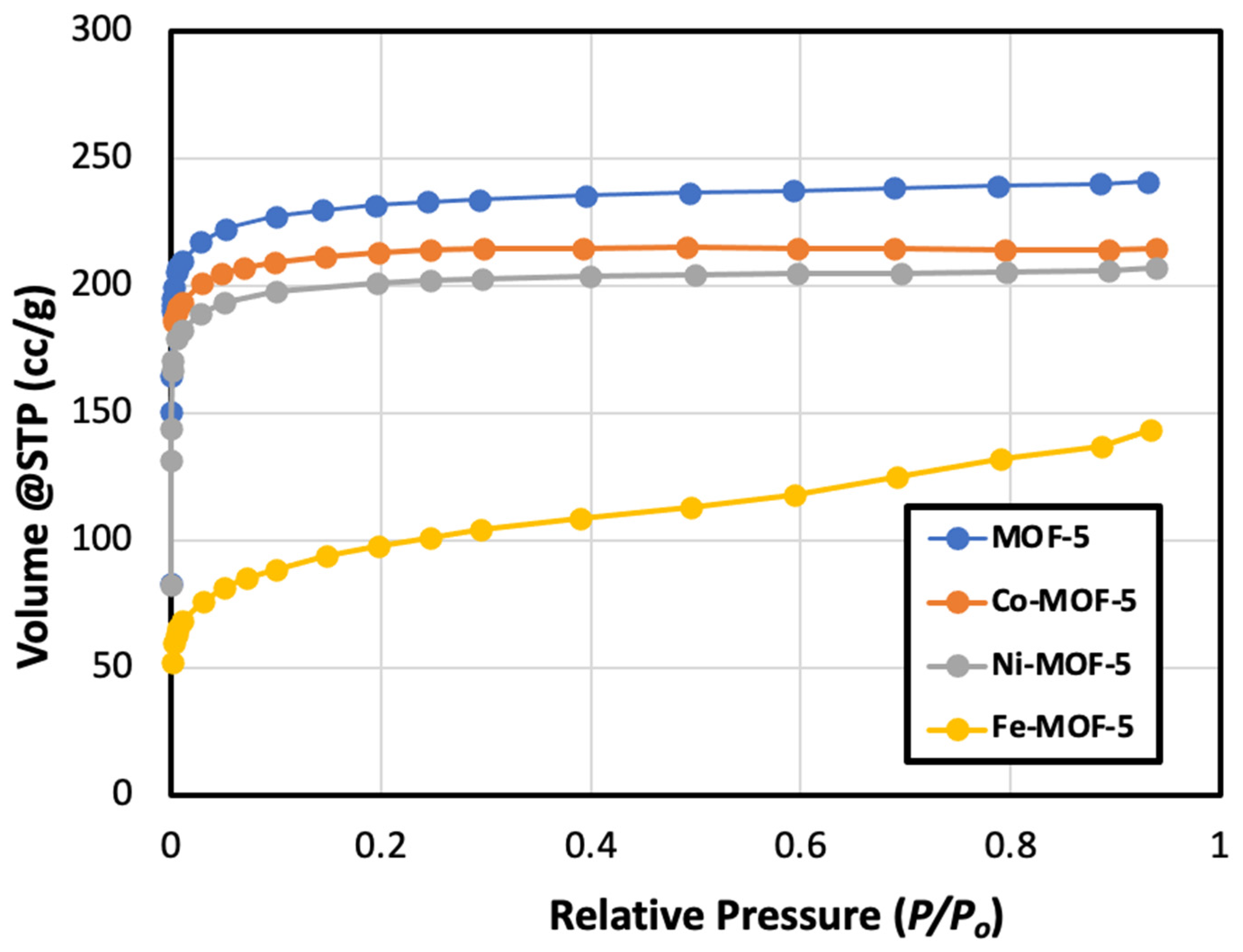
| MOFs | BET Surface Area (m2/g) | Langmuir Surface Area (m2/g) | Micropore Area (m2/g) | Micropore Volume (cm3/g) | Average Pore Diameter (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|---|---|---|
| MOF-5 | 921 | 1043 | 892 | 0.347 | 1.66 | 0.383 |
| Co-MOF-5 | 853 | 934 | 840 | 0.325 | 1.60 | 0.342 |
| Ni-MOF-5 | 802 | 897 | 780 | 0.303 | 1.76 | 0.353 |
| Fe-MOF-5 | 325 | 525 | 242 | 0.106 | 2.53 | 0.223 |
| BET Surface Area (m2/g) | Hydrogen Adsorption (wt.%) | Reference |
|---|---|---|
| 3362 | 1.32 | [39] |
| 572 | 1.3 | [33] |
| 501–840 | 1.75 | [37] |
| 2050 | 1.2 | [40] |
| 2449 | 1.46 | [41] |
| 572 | 1.3 | [42] |
| 2800 | 1.25 | [43] |
| 261–350 | 0.21–0.5 | [24] |
| 2023–3210 | 0.76–1.24 | [44] |
| 520 | 0.97 | [45] |
| Sips Parameters | |||||
|---|---|---|---|---|---|
| Material | Amount of H2 Adsorbed (wt.%) | C0 | a | n | R2 |
| MOF-5 | 1.46 | 1.87299 | 0.05005 | 1.34862 | 0.999962 |
| Co-MOF-5 | 1.53 | 1.98935 | 0.04594 | 1.35045 | 0.999959 |
| Ni-MOF-5 | 1.53 | 2.12790 | 0.03329 | 1.41521 | 0.999994 |
| Fe-MOF-5 | 0.99 | 4.64781 | 0.00097 | 1.66597 | 0.999365 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peedikakkal, A.M.P.; Aljundi, I.H. Upgrading the Hydrogen Storage of MOF-5 by Post-Synthetic Exchange with Divalent Metal Ions. Appl. Sci. 2021, 11, 11687. https://doi.org/10.3390/app112411687
Peedikakkal AMP, Aljundi IH. Upgrading the Hydrogen Storage of MOF-5 by Post-Synthetic Exchange with Divalent Metal Ions. Applied Sciences. 2021; 11(24):11687. https://doi.org/10.3390/app112411687
Chicago/Turabian StylePeedikakkal, Abdul Malik P., and Isam H. Aljundi. 2021. "Upgrading the Hydrogen Storage of MOF-5 by Post-Synthetic Exchange with Divalent Metal Ions" Applied Sciences 11, no. 24: 11687. https://doi.org/10.3390/app112411687






