Preharvest Foliar Salicylic Acid Sprays Reduce Cracking of Fig Fruit at Harvest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Fruit Cracking Incidence and Severity (Years 1 and 2)
2.3. Color Measurement (Years 1 and 2)
2.4. Fruit Weight, Total Soluble Solids, pH, and Titratable Acidity (Years 1 and 2)
2.5. Total Anthocyanins and Cyanidin-3-Rutinoside Contents (Year 1)
2.6. qPCR Expression Analysis (Year 1)
2.7. Statistical Analysis
3. Results
3.1. SA Application Decreased Fruit Cracking Incidence and Severity
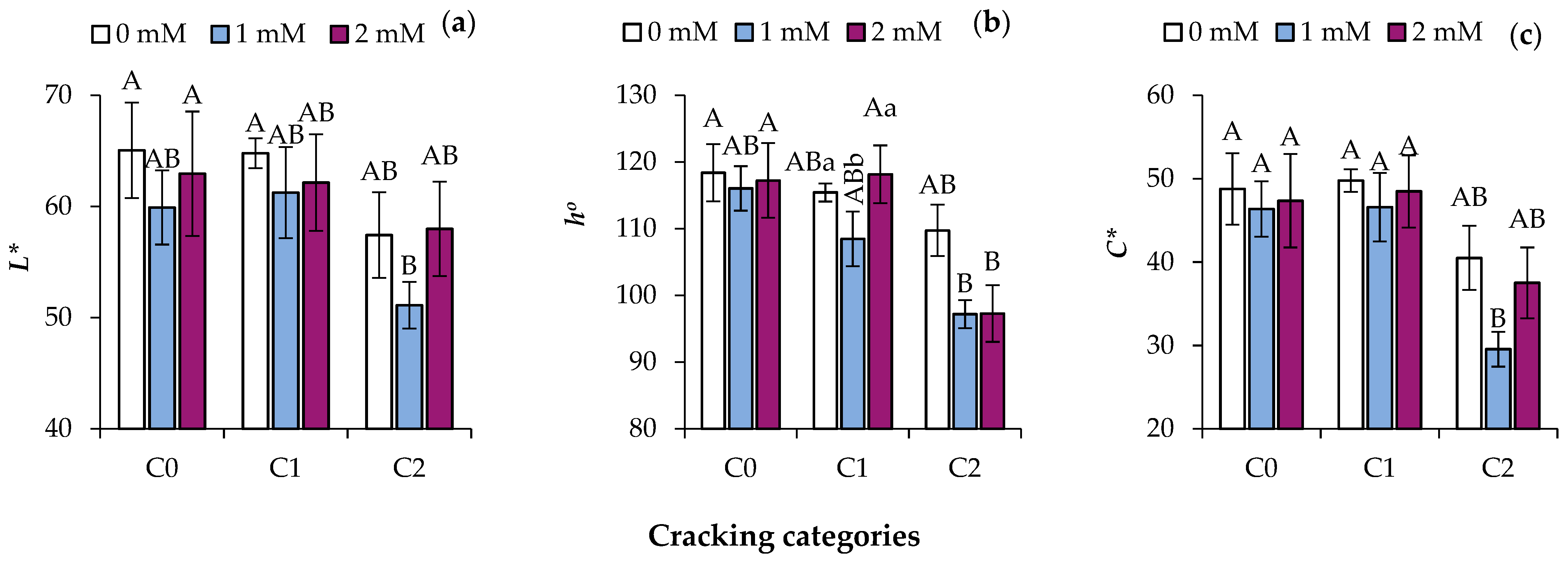
3.2. SA Application Limits Color Development
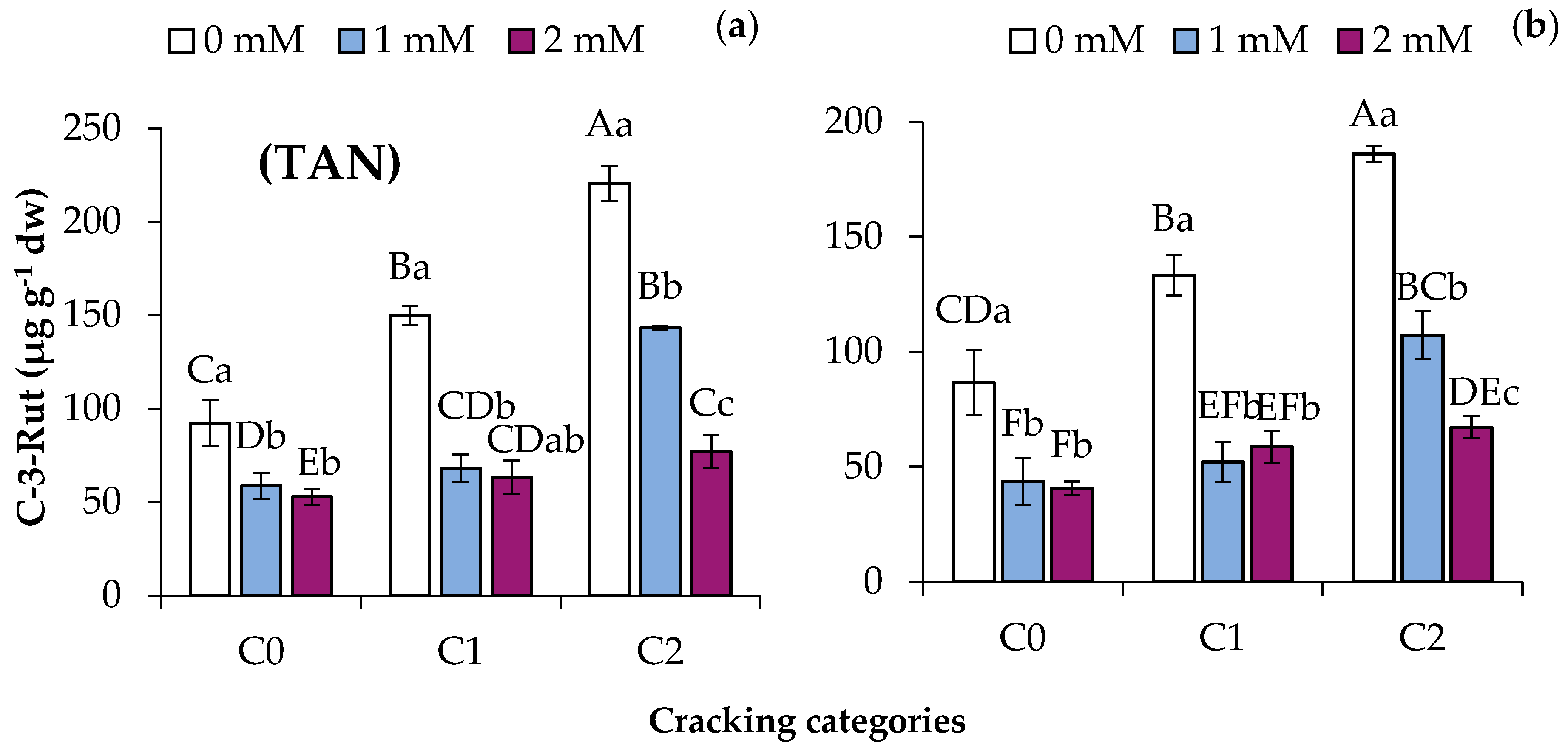
3.3. SA Application Decreases Total Anthocyanins and Cyanidin-3-Rutinoside Contents
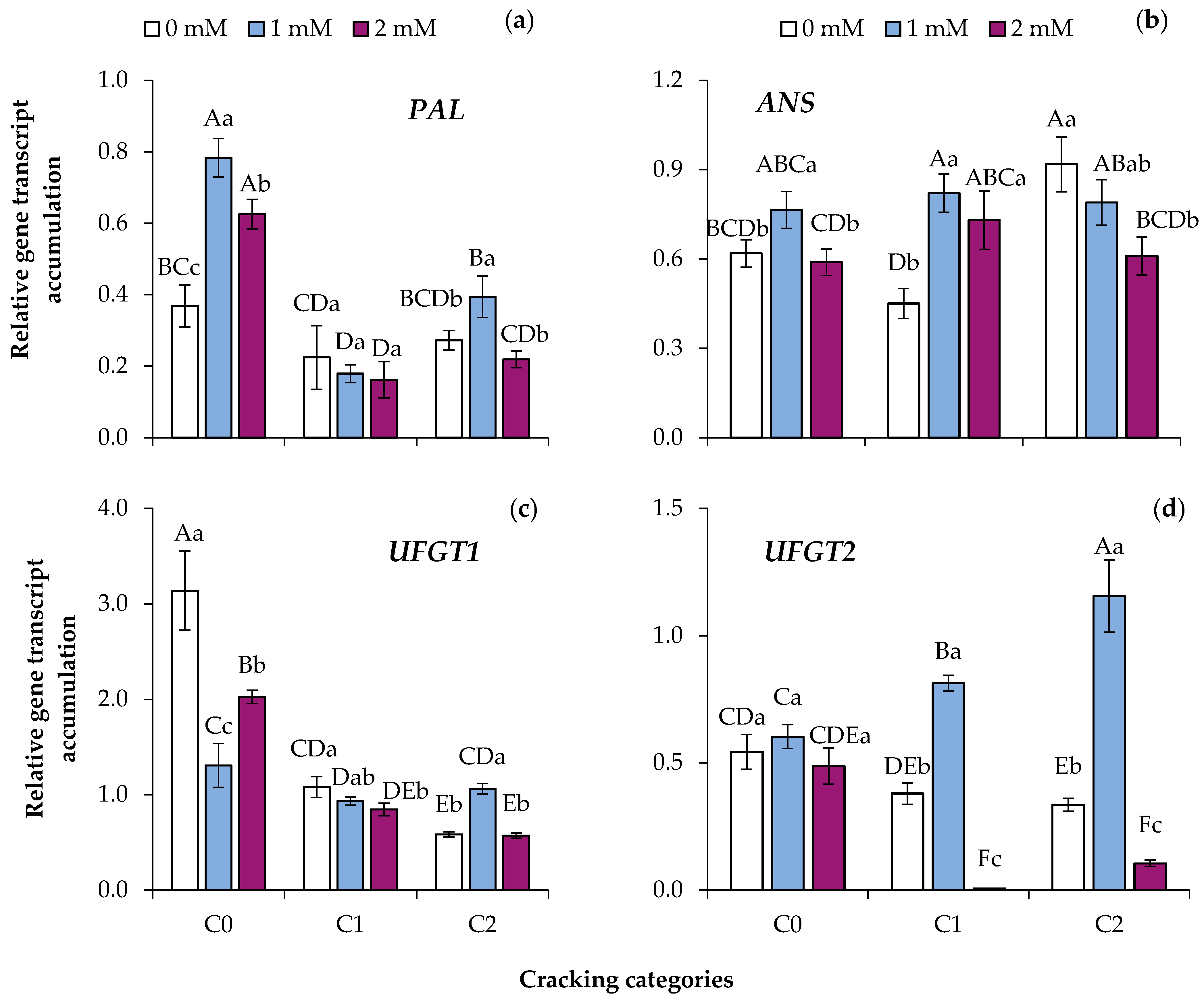
3.4. Expression Analysis of Genes Coding for Regulatory Enzymes of the Phenylpropanoid Biosynthetic Pathway
3.5. Total Soluble Solids (TSS), pH and Titratable Acidity (TA)
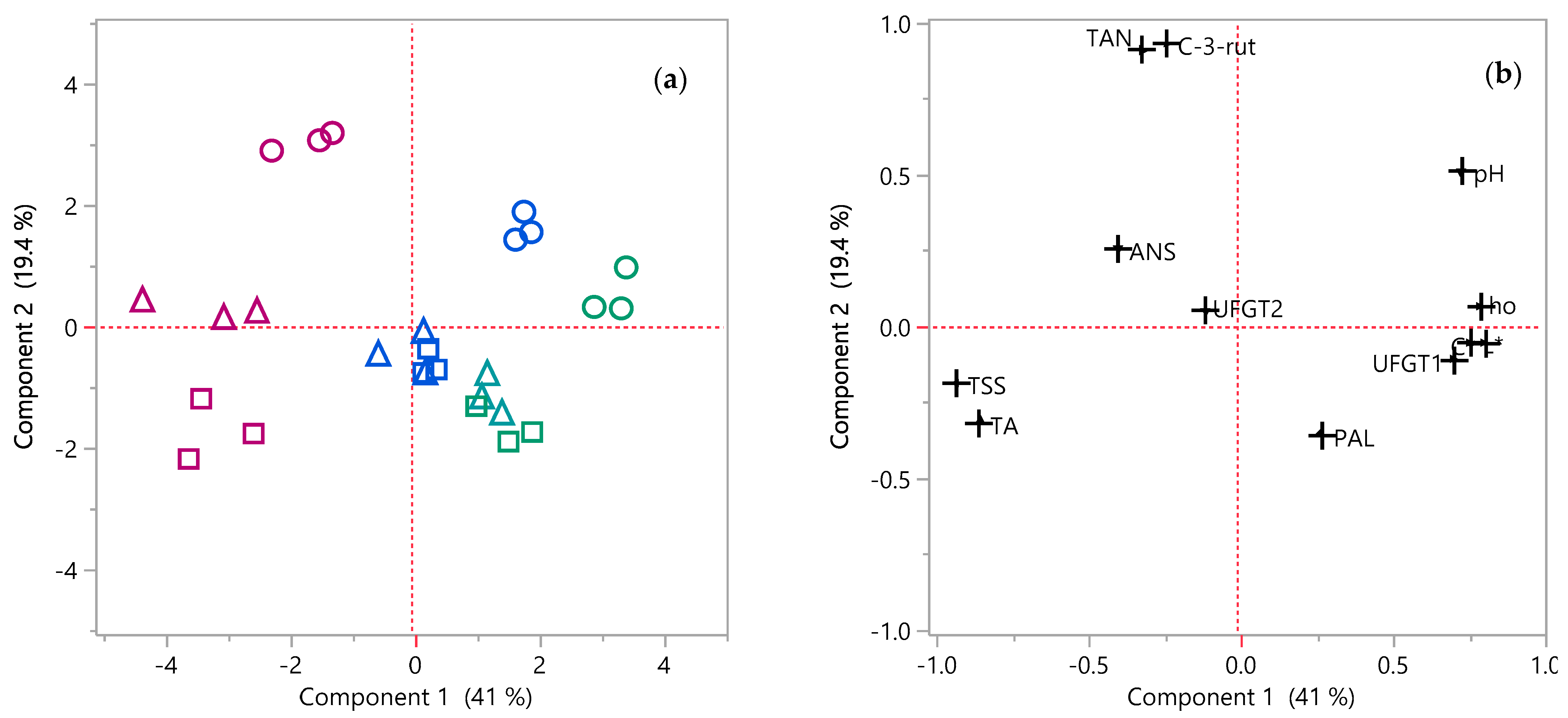
3.6. Principal Components Analysis (PCA) and Pairwise Correlations
4. Discussion
4.1. SA Application Decreased Fruit Cracking Incidence and Severity
4.2. Color Development in Peel and Transcription of Related Enzymes
4.3. Ripening Indices Other than Color
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flaishman, M.A.; Rodov, V.; Stover, E. The fig: Botany, horticulture, and breeding. Hortic. Rev. 2008, 34, 113–196. [Google Scholar] [CrossRef]
- Kong, M.; Lampinen, B.; Shackel, K.; Crisosto, C.H. Fruit skin side cracking and ostiole-end splitting shorten postharvest life in fresh figs (Ficus carica L.), but are reduced by deficit irrigation. Postharvest Biol. Technol. 2013, 85, 154–161. [Google Scholar] [CrossRef]
- Colelli, C.; Amodio, M.L. Subtropical fruits: Fig. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Gil, M., Beaudry, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 427–434. [Google Scholar] [CrossRef]
- Byeon, S.E.; Lee, J. Fruit maturity differentially affects fruit quality and responses of targeted metabolites in cold-stored figs (Ficus carica L.). J. Sci. Food Agric. 2021, 101, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Khadivi-Khub, A. Physiological and genetic factors influencing fruit cracking. Acta Physiol. Plant 2015, 37, 1718. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, N.; Singh, H. Pericarp and pedicel anatomy in relation to fruit cracking in lemon (Citrus limon L Burm.). Sci. Hortic. 2019, 246, 462–468. [Google Scholar] [CrossRef]
- Chessa, I. Fig. In Postharvest Physiology and Storage of Tropical and Subtropical Fruits; Mitra, S., Ed.; CAB International: Wallingford, UK, 1997; pp. 245–268. [Google Scholar]
- Crisosto, C.H.; Ferguson, L.; Bremer, V. Fig. In Postharvest Biology and Technology of Tropical and Subtropical Fruits: Fundamental Issues; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 134–158. [Google Scholar] [CrossRef]
- OECD. Fresh Figs. In International Standards for Fruit and Vegetables; OECD Publishing: Paris, France, 2015. [Google Scholar] [CrossRef]
- Crane, J.C.; Blondeau, R. The use of growth-regulating chemicals to induce parthenocarpic fruit in the Calimyrna fig. Plant Physiol. 1949, 24, 44. [Google Scholar] [CrossRef] [PubMed]
- Holstein, H.; Crisosto, G.; Crisosto, C. Fertigation with potassium increases size and yield in fresh figs growing in California. Acta Hortic. 2017, 1173, 177–181. [Google Scholar] [CrossRef]
- Gaaliche, B.; Ladhari, A.; Zarrelli, A.; Mimoun, M.B. Impact of foliar potassium fertilization on biochemical composition and antioxidant activity of fig (Ficus carica L.). Sci. Hortic. 2019, 253, 111–119. [Google Scholar] [CrossRef]
- Irfan, P.K.; Vanjakshi, V.; Prakash, M.K.; Ravi, R.; Kudachikar, V.B. Calcium chloride extends the keeping quality of fig fruit (Ficus carica L.) during storage and shelf-life. Postharvest Biol. Technol. 2013, 82, 70–75. [Google Scholar] [CrossRef]
- Dempsey, D.M.A.; Klessig, D.F. How does the multifaceted plant hormone salicylic acid combat disease in plants and are similar mechanisms utilized in humans? BMC Biol. 2017, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Tian, S. Effects of pre-and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage. Postharvest Biol. Technol. 2005, 35, 253–262. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.; Wang, J.; Wang, P.; Lu, D.; Deng, S.; Lei, H.; Gao, Y.; Tao, Y. Treatment with exogenous salicylic acid maintains quality, increases bioactive compounds, and enhances the antioxidant capacity of fresh goji (Lycium barbarum L.) fruit during storage. LWT 2021, 140, 110837. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Kong, W.; Li, S.; Archbold, D.D. Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage. Postharvest Biol. Technol. 2006, 41, 244–251. [Google Scholar] [CrossRef]
- Asghari, M.; Aghdam, M.S. Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends Food Sci. Technol. 2010, 21, 502–509. [Google Scholar] [CrossRef]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D. Vapour treatments with methyl salicylate or methyl jasmonate alleviated chilling injury and enhanced antioxidant potential during postharvest storage of pomegranates. Food Chem. 2011, 124, 964–970. [Google Scholar] [CrossRef]
- Drogoudi, P.; Pantelidis, G.E.; Vekiari, S.A. Physiological disorders and fruit quality attributes in pomegranate: Effects of meteorological parameters, canopy position and acetylsalicylic acid foliar sprays. Front. Plant Sci. 2021, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liu, R.; Fang, X.; Tong, C.; Chen, H.; Gao, H. Effects of salicylic acid treatment on fruit quality and wax composition of blueberry (Vaccinium virgatum Ait). Food Chem. 2022, 368, 130757. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Serrano, M.; Valero, D.; Martínez-Romero, D.; Castillo, S.; Zapata, P.J. Enhancement of antioxidant systems and storability of two plum cultivars by preharvest treatments with salicylates. Int. J. Mol. Sci. 2017, 18, 1911. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Giménez, M.J.; Zapata, P.J.; Guillén, F.; Valverde, J.M.; Serrano, M.; Valero, D. Preharvest application of methyl salicylate, acetyl salicylic acid and salicylic acid alleviated disease caused by Botrytis cinerea through stimulation of antioxidant system in table grapes. Int. J. Food Microbiol. 2020, 334, 108807. [Google Scholar] [CrossRef]
- Ding, C.K.; Wang, C.Y. The dual effects of methyl salicylate on ripening and expression of ethylene biosynthetic genes in tomato fruit. Plant Sci. 2003, 164, 589–596. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Castillo, S.; Serrano, M.; Valero, D. Changes in bioactive compounds, antioxidant activity, and nutritional quality of blood orange cultivars at different storage temperatures. Antioxidants 2020, 9, 1016. [Google Scholar] [CrossRef]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Serrano, M.; Valero, D. Effect of salicylic acid treatment on reducing chilling injury in stored pomegranates. Postharvest Biol. Technol. 2009, 53, 152–154. [Google Scholar] [CrossRef]
- Pappi, P.; Nikoloudakis, N.; Fanourakis, D.; Zambounis, A.; Delis, C. Differential triggering of the phenylpropanoid biosynthetic pathway key genes transcription upon cold stress and viral infection in tomato leaves. Horticulturae 2021, 7, 448. [Google Scholar] [CrossRef]
- Ahmadi-Majd, M.; Rezaei Nejad, A.; Mousavi-Fard, S.; Fanourakis, D. Postharvest application of single, multi-walled carbon nanotubes and graphene oxide stimulates rose keeping quality. J. Hortic. Sci. Biotechnol. 2021, in press. [Google Scholar] [CrossRef]
- Ahmed, F.F.; Mohamed, M.M.; Abou El-Khashab, A.M.A.; Aeed, S.H.A. Controlling fruit splitting and improving productivity of Manfalouty pomegranate trees by using salicylic acid and some nutrients. World Rural Observ. 2014, 6, 87–93. [Google Scholar]
- Durst, R.W.; Wrolstad, R.E. Separation and characterization of anthocyanins by HPLC. Curr. Protoc. Food Anal. Chem. 2001, 1, F1-3. [Google Scholar] [CrossRef]
- Karantzi, A.D.; Kafkaletou, M.; Christopoulos, M.V.; Tsantili, E. Peel colour and flesh phenolic compounds at ripening stages in pollinated commercial varieties of fig (Ficus carica L.) fruit grown in Southern Europe. J. Food Meas. Charact. 2021, 15, 2049–2063. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1-2. [Google Scholar] [CrossRef]
- Tsantili, E.; Shin, Y.; Nock, J.F.; Watkins, C.B. Antioxidant concentrations during chilling injury development in peaches. Postharvest Biol. Technol. 2010, 57, 27–34. [Google Scholar] [CrossRef]
- Cinquanta, L.; Di Matteo, M.; Esti, M. Physical pre-treatment of plums (Prunus domestica). Part 2. Effect on the quality characteristics of different prune cultivars. Food Chem. 2002, 79, 233–238. [Google Scholar] [CrossRef]
- Tsaniklidis, G.; Pappi, P.; Tsafouros, A.; Charova, S.N.; Nikoloudakis, N.; Roussos, P.A.; Paschalidis, K.A.; Delis, C. Polyamine homeostasis in tomato biotic/abiotic stress cross-tolerance. Gene 2020, 727, 144230. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Peng, Z.; Zhao, Y.; Wu, K.; Zhou, N.; Yan, Y.; Ramaswamy, H.S.; Sun, J.; Bai, W. The impact of ultrasonic treatment on blueberry wine anthocyanin color and its In-vitro anti-oxidant capacity. Food Chem. 2020, 333, 127455. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, Y.; Vainstein, A.; Chen, S.; Ma, H. Regulation of Fig (Ficus carica L.) fruit color: Metabolomic and transcriptomic analyses of the flavonoid biosynthetic pathway. Front. Plant Sci. 2017, 8, 1990. [Google Scholar] [CrossRef] [Green Version]
- Hadjipieri, M.; Georgiadou, E.C.; Drogoudi, P.; Fotopoulos, V.; Manganaris, G.A. The efficacy of acetylsalicylic acid, spermidine and calcium preharvest foliar spray applications on yield efficiency, incidence of physiological disorders and shelf-life performance of loquat fruit. Sci. Hortic. 2021, 289, 110439. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar] [CrossRef]
- Valero, D.; Diaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillen, F.; Martinez-Romero, D.; Serrano, M. Postharvest treatments with salicylic acid, acetylsalicylic acid or oxalic acid delayed ripening and enhanced bioactive compounds and antioxidant capacity in sweet cherry. J. Agric. Food Chem. 2011, 59, 5483–5489. [Google Scholar] [CrossRef]
- Rodov, V.; Vinokur, Y.; Horev, B. Brief postharvest exposure to pulsed light stimulates coloration and anthocyanin accumulation in fig fruit (Ficus carica L.). Postharvest Biol. Technol. 2012, 68, 43–46. [Google Scholar] [CrossRef]
- Gomes, E.P.; Borges, C.V.; Monteiro, G.C.; Belin, M.A.F.; Minatel, I.O.; Junior, A.P.; Tecchio, M.A.; Lima, G.P.P. Preharvest salicylic acid treatments improve phenolic compounds and biogenic amines in ‘Niagara Rosada’ table grape. Postharvest Biol. Technol. 2021, 176, 111505. [Google Scholar] [CrossRef]
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.E.; Flaishman, M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Samaras, Y.; Gatidou, G.; Thomaidis, N.S.; Stasinakis, A.S. Review on fresh and dried figs: Chemical analysis and occurrence of phytochemical compounds, antioxidant capacity and health effects. Food Res. Int. 2019, 119, 244–267. [Google Scholar] [CrossRef] [PubMed]
- Strack, D. Phenolic metabolism. In Plant Biochemistry; Dey, P.M., Harborne, J.B., Eds.; Academic Press: Cambridge, MA, USA, 1997; pp. 387–434. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Participation of phenylalanine ammonia-lyase (PAL) in increased phenolic compounds in fresh cold stressed walnut (Juglans regia L.) kernels. Postharvest Biol. Technol. 2015, 104, 17–25. [Google Scholar] [CrossRef]
- Dixon, R.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1999, 7, 1085–1097. [Google Scholar] [CrossRef]
- Cao, L.; Xu, X.; Chen, S.; Ma, H. Cloning and expression analysis of Ficus carica anthocyanidin synthase 1 gene. Sci. Hortic. 2016, 211, 369–375. [Google Scholar] [CrossRef]
- Tsaniklidis, G.; Kafkaletou, M.; Delis, C.; Tsantili, E. The effect of postharvest storage temperature on sweet cherry (Prunus avium L.) phenolic metabolism and colour development. Sci. Hortic. 2017, 225, 751–756. [Google Scholar] [CrossRef]
- Li, L.; Ban, Z.J.; Li, X.H.; Wu, M.Y.; Wang, A.L.; Jiang, Y.Q.; Jiang, Y.H. Differential expression of anthocyanin biosynthetic genes and transcription factor PcMYB10 in pears (Pyrus communis L.). PLoS ONE 2012, 7, e46070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freiman, Z.E.; Rosianskey, Y.; Dasmohapatra, R.; Kamara, I.; Flaishman, M.A. The ambiguous ripening nature of the fig (Ficus carica L.) fruit: A gene-expression study of potential ripening regulators and ethylene-related genes. J. Exp. Bot. 2015, 66, 3309–3324. [Google Scholar] [CrossRef] [Green Version]
- Lama, K.; Harlev, G.; Shafran, H.; Peer, R.; Flaishman, M.A. Anthocyanin accumulation is initiated by abscisic acid to enhance fruit color during fig (Ficus carica L.) ripening. J. Plant Physiol. 2020, 251, 153192. [Google Scholar] [CrossRef]
- Lama, K.; Yadav, S.; Rosianski, Y.; Shaya, F.; Lichter, A.; Chai, L.; Flaishman, M.A. The distinct ripening processes in the reproductive and non-reproductive parts of the fig syconium are driven by ABA. J. Exp. Bot. 2019, 70, 115–131. [Google Scholar] [CrossRef]
- Leslie, C.A.; Romani, R.J. Salicylic acid: A new inhibitor of ethylene biosynthesis. Plant Cell Rep. 1986, 5, 144–146. [Google Scholar] [CrossRef]
- Babalar, M.; Asghari, M.; Talaei, A.; Khosroshahi, A. Effect of pre-and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chem. 2007, 105, 449–453. [Google Scholar] [CrossRef]
- Kraeva, E.; Andary, C.; Carbonneau, A.; Deloire, A. Salicylic acid treatment of grape berries retards ripening. Vitis 1998, 37, 143–144. [Google Scholar]
- Tajima, Y.; Loo, E.P.I.; Saijo, Y. Plant physiological and molecular mechanisms in cross-regulation of biotic-abiotic stress responses. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Academic Press: Cambridge, MA, USA, 2020; pp. 21–34. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias, I.; Echeverría, G. Differential effect of cultivar and harvest date on nectarine colour, quality and consumer acceptance. Sci. Hortic. 2009, 120, 41–50. [Google Scholar] [CrossRef]

| Gene | Encoded Enzyme | Sequence | Sequence 5′-3′ |
|---|---|---|---|
| FcPAL | Phenylalanine ammonia lyase | Sense primer: | CCCGAGTTTGGATTATGGA |
| Antisense primer: | TTGGCTCACCGTGTTCTT | ||
| FcANS | Anthocyanidin synthase | Sense primer: | GAGCAGTGGGATTCAGGC |
| Antisense primer: | TGGTCATCCGAGTTCACG | ||
| FcUFGT1 | Glycosyltransferase | Sense primer: | CGGAGAACACGGAGAAGAAG |
| Antisense primer: | ATTCCCCTCCAAATTCCAAC | ||
| FcUFGT2 | Glycosyltransferase | Sense primer: | CAGTGTCGTTTGCTGCAGAT |
| Antisense primer: | AAGGAAGTCAACGGCGAGTA | ||
| FcActin | Sense primer: | GCCATTCAAGCCGTGCTTT | |
| Antisense primer: | TGGGAACAGTGTGGCTGACA |
| Probabilities | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration Effect | Category | Variables | |||||||||||
| L* | h° | C* | TSS | pH | TA | TAN | C−-3−Rut | PAL | ANS | UFGT1 | UFGT2 | ||
| psa | C0 | NS | NS | NS | ** | * | *** | *** | ** | *** | * | *** | NS |
| psa | C1 | NS | *** | NS | *** | *** | *** | *** | *** | NS | ** | * | *** |
| psa | C2 | NS | NS | NS | *** | ** | *** | *** | *** | ** | ** | *** | *** |
| pall (all data differences) | - | ** | ** | ** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Concentration (mM) | Weight (g) | L* | h° | C* | TSS (°Brix) | TAN (μg g−1 dw) |
|---|---|---|---|---|---|---|
| 0 | 62.72 ± 3.64 a | 54.66 ± 1.70 a | 99.39 ± 2.50 b | 31.29 ± 1.70 b | 17.4 ± 0.70 b | 215.3 ± 69.35 a |
| 2 | 65.15 ± 4.52 a | 55.56 ± 2.80 a | 109.59 ± 3.42 a | 39.52 ± 3.02 a | 19.0 ± 0.10 a | 45.37 ± 9.46 b |
| ptr | NS | NS | * | * | * | * |
| Variables | ||||
|---|---|---|---|---|
| Category | Treatment | TSS (°Brix) | pH | TA |
| C0 | 0 mM | 14.93 ± 0.15 Eb | 4.48 ± 0.03 Aa | 1.02 ± 0.10 Fc |
| 1 mM | 15.33 ± 0.12 CDa | 4.31 ± 0.02 Bb | 1.09 ± 0.20 Deb | |
| 2 mM | 15.47 ± 0.06 Ca | 4.28 ± 0.10 BCb | 1.18 ± 0.47 BCa | |
| C1 | 0 mM | 15.12 ± 0.08 DEc | 4.42 ± 0.04 ABa | 1.06 ± 0.15 EFc |
| 1 mM | 15.57 ± 0.15 Cb | 4.26 ± 0.02 BCb | 1.14 ± 0.40 CDb | |
| 2 mM | 15.97 ± 0.12 Ba | 4.32 ± 0.03 Ab | 1.20 ± 0.26 BCa | |
| C2 | 0 mM | 16.00 ± 0.10 Bb | 4.36 ± 0.02 ABa | 1.21 ± 0.57 BCb |
| 1 mM | 16.23 ± 0.12 Bb | 4.13 ± 0.12 CDb | 1.25 ± 0.25 Bb | |
| 2 mM | 16.73 ± 0.15 Aa | 4.06 ± 0.04 Db | 1.39 ± 0.44 Aa | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karantzi, A.D.; Kafkaletou, M.; Tsaniklidis, G.; Bai, J.; Christopoulos, M.V.; Fanourakis, D.; Tsantili, E. Preharvest Foliar Salicylic Acid Sprays Reduce Cracking of Fig Fruit at Harvest. Appl. Sci. 2021, 11, 11374. https://doi.org/10.3390/app112311374
Karantzi AD, Kafkaletou M, Tsaniklidis G, Bai J, Christopoulos MV, Fanourakis D, Tsantili E. Preharvest Foliar Salicylic Acid Sprays Reduce Cracking of Fig Fruit at Harvest. Applied Sciences. 2021; 11(23):11374. https://doi.org/10.3390/app112311374
Chicago/Turabian StyleKarantzi, Athanasia D., Mina Kafkaletou, Giorgios Tsaniklidis, Jinhe Bai, Miltiadis V. Christopoulos, Dimitrios Fanourakis, and Eleni Tsantili. 2021. "Preharvest Foliar Salicylic Acid Sprays Reduce Cracking of Fig Fruit at Harvest" Applied Sciences 11, no. 23: 11374. https://doi.org/10.3390/app112311374






