Fluorescence Spectroscopy of Enantiomeric Amide Compounds Enforced by Chiral Light
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
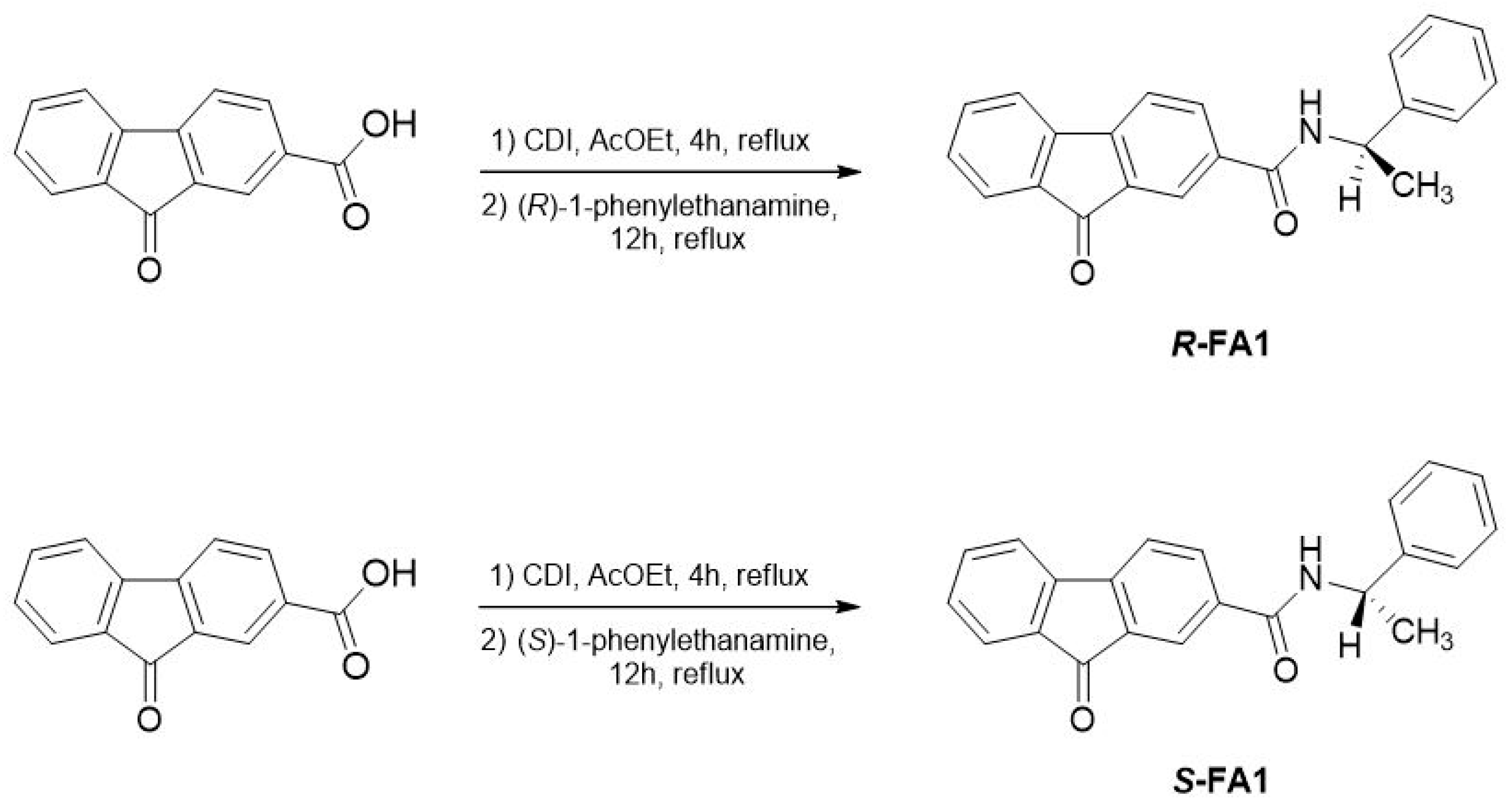
2.1. Chemical Synthesis of Enantiomeric Amide Compounds
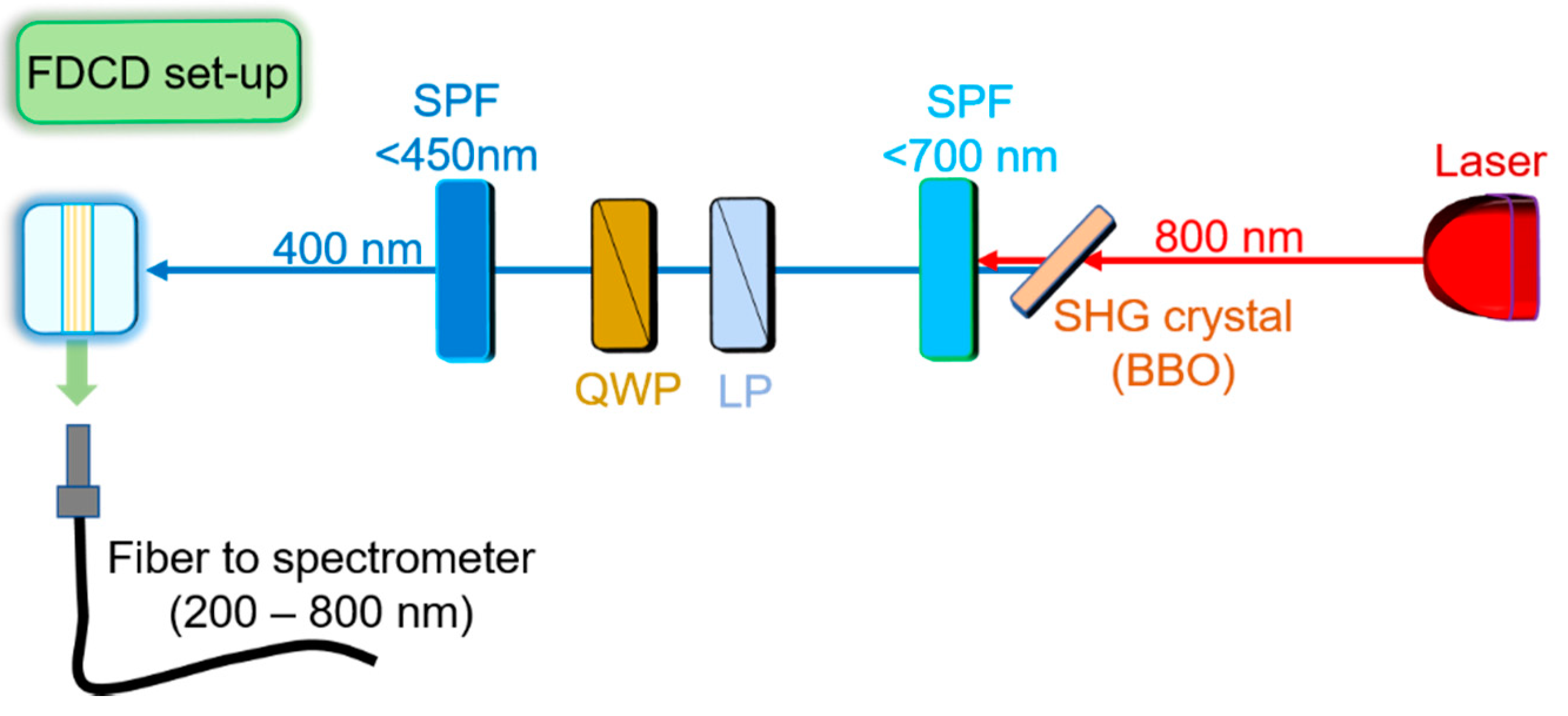
2.2. Fluorescence Spectroscopy Setup
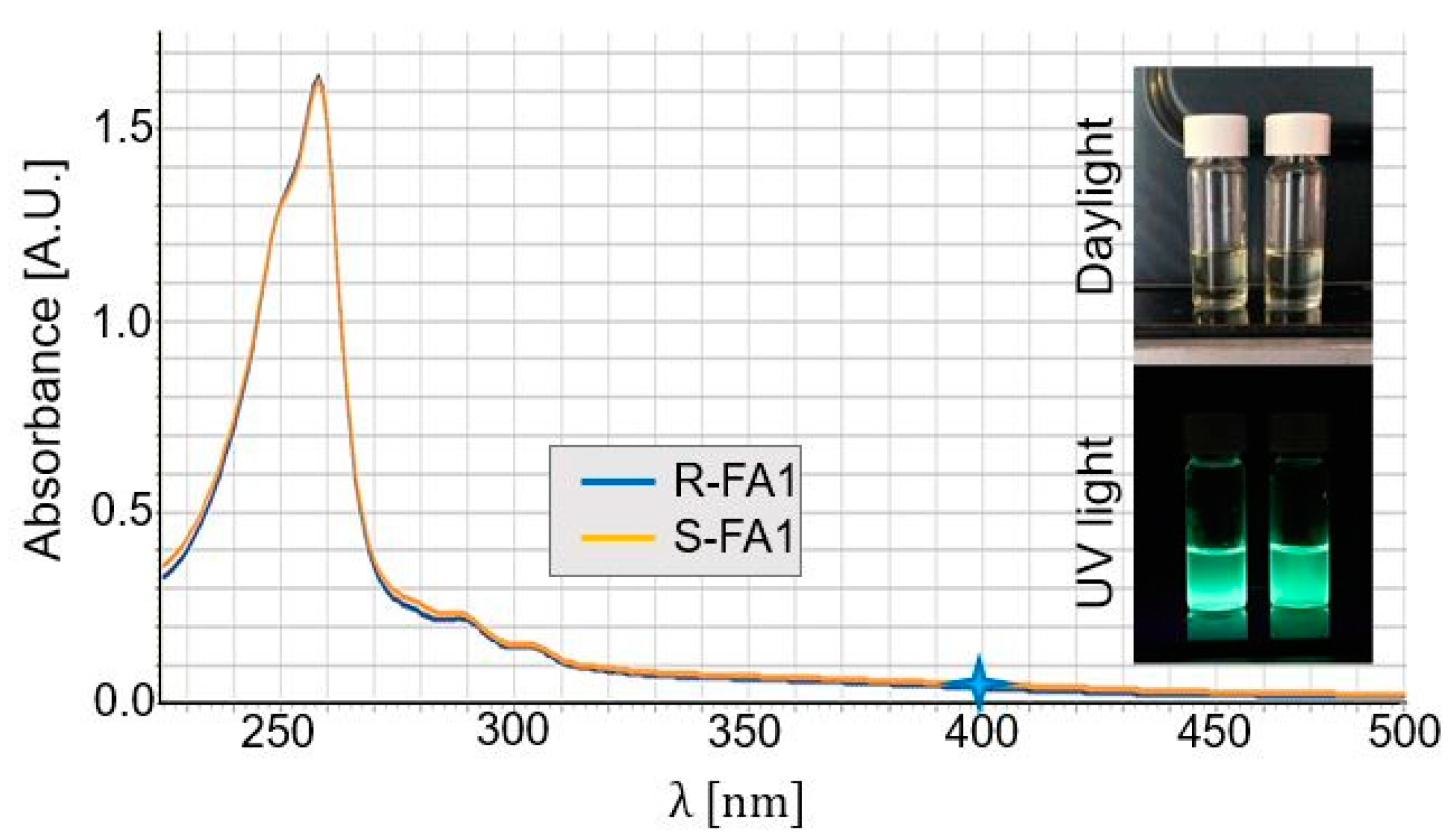
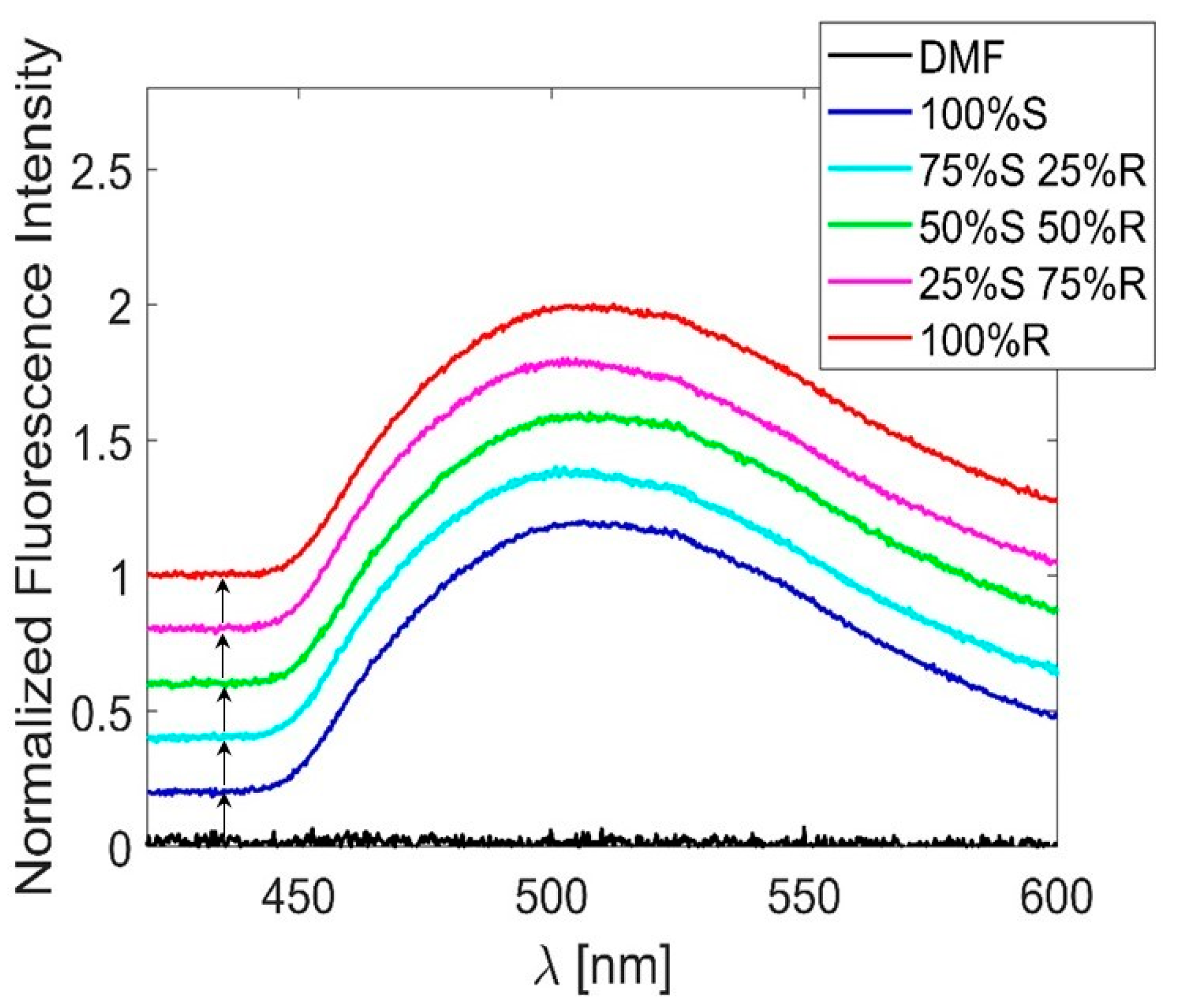
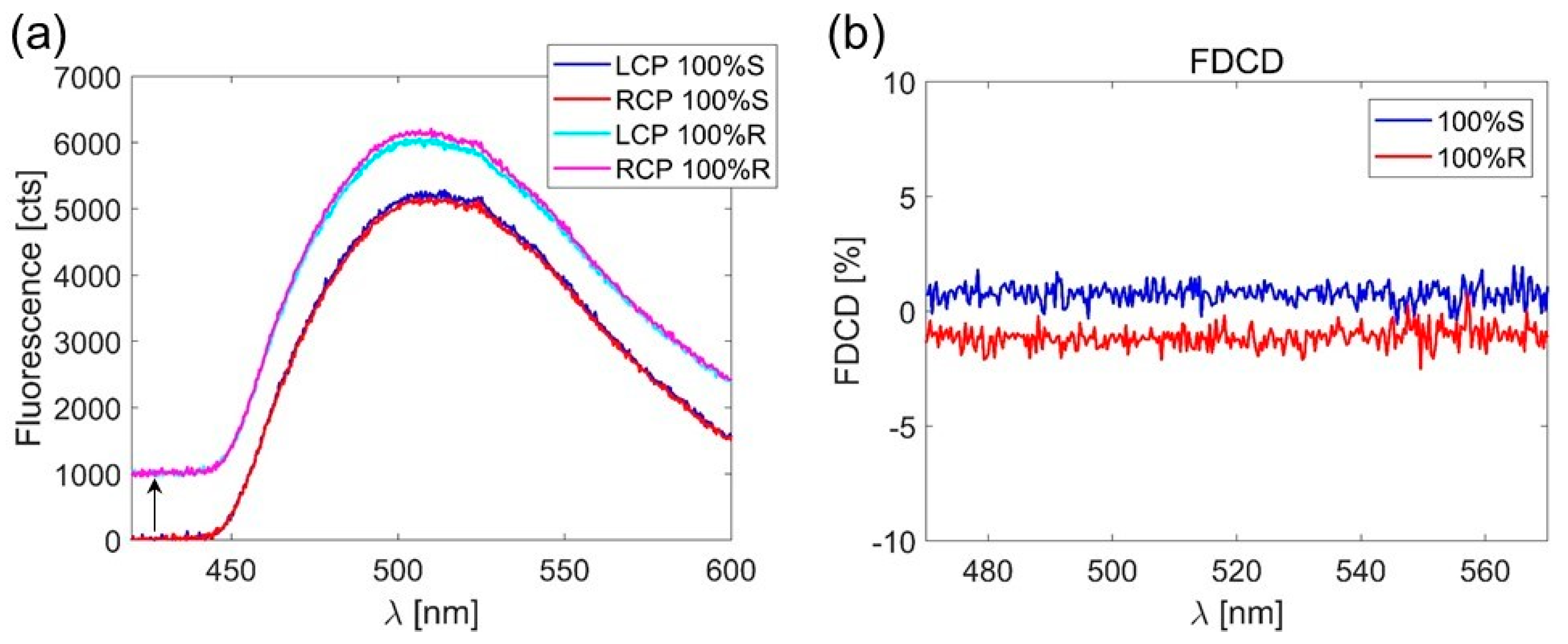
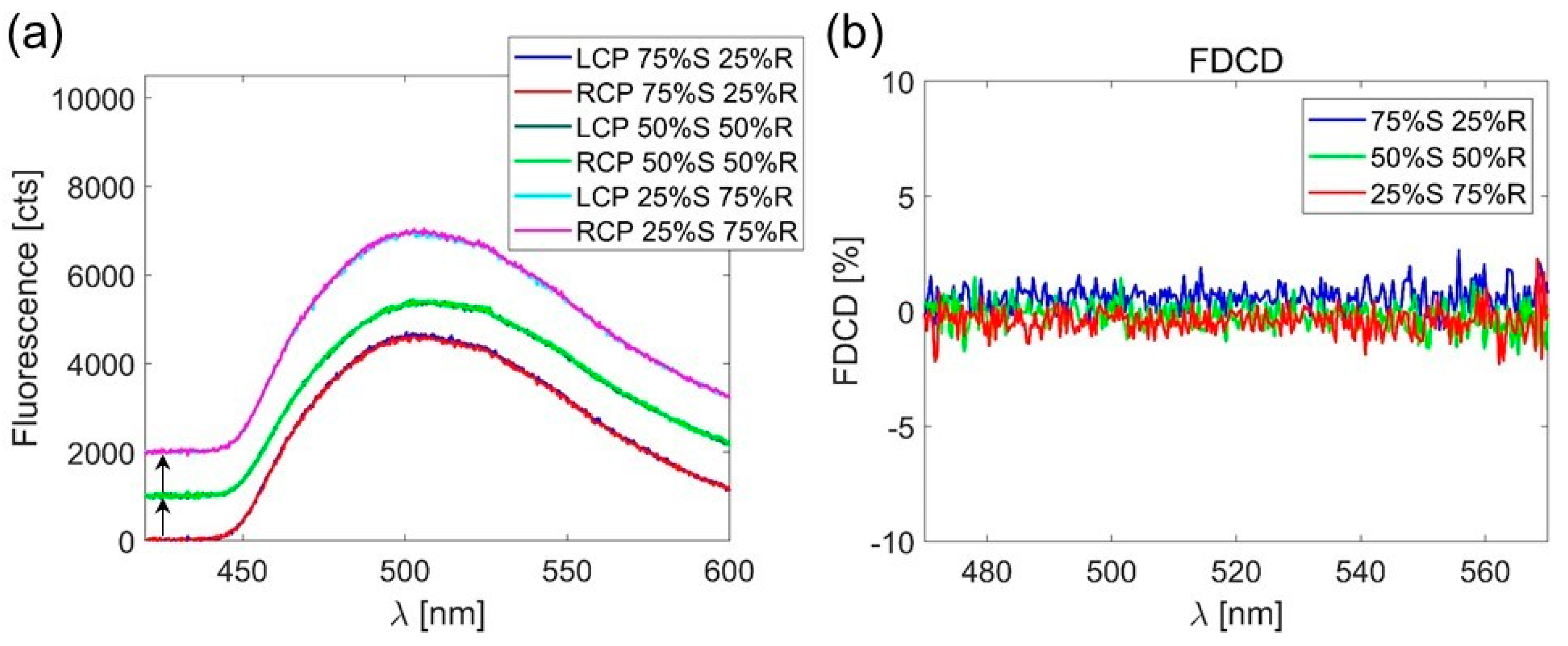
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Adv. Nat. Sci. Nanosci. Nanotechnol. 2006, 2, 85–100. [Google Scholar]
- Smith, S.W. Chiral Toxicology: It’s the Same Thing…Only Different. Toxicol. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef]
- Berova, N.; Di Bari, L.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef]
- Pescitelli, G.; Di Bari, L.; Berova, N. Conformational aspects in the studies of organic compounds by electronic circular dichroism. Chem. Soc. Rev. 2011, 40, 4603–4625. [Google Scholar] [CrossRef]
- Zinna, F.; Resta, C.; Górecki, M.; Pescitelli, G.; Di Bari, L.; Jávorfi, T.; Hussain, R.; Siligardi, G. Circular Dichroism Imaging: Mapping the Local Supramolecular Order in Thin Films of Chiral Functional Polymers. Macromolecules 2017, 50, 2054–2060. [Google Scholar] [CrossRef]
- Homberg, A.; Brun, E.; Zinna, F.; Pascal, S.; Górecki, M.; Monnier, L.; Besnard, C.; Pescitelli, G.; Di Bari, L.; Lacour, J. Combined reversible switching of ECD and quenching of CPL with chiral fluorescent macrocycles. Chem. Sci. 2018, 9, 7043. [Google Scholar] [CrossRef] [Green Version]
- Albano, G.; Górecki, M.; Pescitelli, G.; Di Bari, L.; Jávorfi, T.; Hussain, R.; Siligardi, G. Electronic circular dichroism imaging (CDi) maps local aggregation modes in thin films of chiral oligothiophenes. New J. Chem. 2019, 43, 14584–14593. [Google Scholar] [CrossRef]
- Matsuo, K.; Yonehara, R.; Gekko, K. Secondary-structure analysis of proteins by vacuum-ultraviolet circular dichroism spectroscopy. J. Biochem. 2004, 135, 405–411. [Google Scholar] [CrossRef]
- Wenzel, S.; Buss, V. Circular dichroism and electronic structure calculations on naproxen. J. Phys. Org. Chem. 1992, 5, 748–754. [Google Scholar]
- Okuom, M.O.; Burks, R.; Naylor, C.; Holmes, A.E. Applied Circular Dichroism: A Facile Spectroscopic Tool for Configurational Assignment and Determination of Enantiopurity. J. Anal. Methods Chem. 2015, 2015, 865605. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Kautz, F.; LaPidus, J. Optical rotatory dispersion and circular dichroism of diastereoisomers. I. The ephedrines and chloramphenicols. Can. J. Chem. 1969, 47, 1957–1963. [Google Scholar] [CrossRef]
- Tinoco, I., Jr.; Turner, D.H. Fluorescence detected circular dichroism. Theory. J. Am. Chem. Soc. 1976, 98, 6453–6456. [Google Scholar] [CrossRef]
- Nehira, T.; Ishihara, K.; Matsuo, K.; Izumi, S.; Yamazaki, T.; Ishida, A. A sensitive method based on fluorescence-detected circular dichroism for protein local structure analysis. Anal. Biochem. 2012, 430, 179–184. [Google Scholar] [CrossRef]
- Nehira, T.; Parish, C.A.; Jockusch, S.; Turro, N.J.; Nakanishi, K.; Berova, N. Fluorescence-Detected Exciton-Coupled Circular Dichroism: Scope and Limitation in Structural Studies of Organic Molecules. J. Am. Chem. Soc. 1999, 121, 8681–8691. [Google Scholar] [CrossRef]
- Tanaka, K.; Pescitelli, G.; Nakanishi, K.; Berova, N. Fluorescence Detected Exciton Coupled Circular Dichroism: Development of New Fluorescent Reporter Groups for Structural Studies. Monatsh. Chem. 2005, 136, 367–395. [Google Scholar] [CrossRef]
- Salam, A.; Meath, W.J. On the relative populations of excited state enantiomers, for randomly orientated molecules, obtained through the use of circularly polarized pulsed lasers. Chem. Phys. Lett. 1997, 277, 199–207. [Google Scholar] [CrossRef]
- Hassey, R.; Swain, E.J.; Hammer, N.I.; Venkataraman, D.; Barnes, M.D. Probing the Chiroptical Response of a Single Molecule. Science 2006, 314, 1437. [Google Scholar] [CrossRef]
- Tang, Y.; Cook, T.A.; Cohen, A.E. Limits on Fluorescence Detected Circular Dichroism of Single Helicene Molecules. J. Phys. Chem. A 2009, 113, 6213–6216. [Google Scholar] [CrossRef]
- Hassey-Paradise, R.; Cyphersmith, A.; Tilley, A.M.; Mortsolf, T.; Basak, D.; Venkataraman, D.; Barnes, M.D. Dissymmetries in fluorescence excitation and emission from single chiral molecules. Chirality 2009, 21, E265–E276. [Google Scholar] [CrossRef]
- Muller, G.; Muller, F.C.; Maupin, C.L.; Riehl, J.P. The measurement of the fluorescence detected circular dichroism (FDCD) from a chiral Eu (III) system. Chem. Commun. 2005, 2005, 3615–3617. [Google Scholar] [CrossRef] [PubMed]
- Prabodh, A.; Wang, Y.; Sinn, S.; Albertini, P.; Spies, C.; Spuling, E.; Yang, L.-P.; Jiang, W.; Bräse, S.; Biedermann, F. Fluorescence detected circular dichroism (FDCD) for supramolecular host-guest complexes. Chem. Sci. 2021, 12, 9420–9431. [Google Scholar] [CrossRef]
- Chi-Sing Ho, H.; Garcia-Etxarri, A.; Zhao, Y.; Dionne, J. Enhancing Enantioselective Absorption Using Dielectric Nanospheres. ACS Photonics 2017, 4, 197–203. [Google Scholar]
- Petronijevic, E.; Centini, M.; Belardini, A.; Leahu, G.; Hakkarainen, T.; Sibilia, C. Chiral near-field manipulation in Au-GaAs hybrid hexagonal nanowires. Opt. Express 2017, 25, 14148. [Google Scholar] [CrossRef]
- Mohammadi, E.; Tsakmakidis, K.I.; Askarpour, A.N.; Dehkhoda, P.; Tavakoli, A.; Altug, H. Nanophotonic Platforms for Enhanced Chiral Sensing. ACS Photonics 2018, 5, 2669–2675. [Google Scholar] [CrossRef]
- Petronijevic, E.; Sibilia, C. Enhanced near-field chirality in periodic arrays of Si nanowires for chiral sensing. Molecules 2019, 24, 853. [Google Scholar] [CrossRef] [Green Version]
- Petronijevic, E.; Sandoval, E.M.; Ramezani, M.; Ordóňez-Romero, C.L.; Noguez, C.; Bovino, F.A.; Sibilia, C.; Pirruccio, G. Extended Chiro-optical Near-Field Response of Achiral Plasmonic Lattices. J. Phys. Chem. C 2019, 123, 23620–23627. [Google Scholar] [CrossRef]
- Hu, J.; Lawrence, M.; Dionne, J. High Quality Factor Dielectric Metasurfaces for Ultraviolet Circular Dichroism Spectroscopy. ACS Photonics 2020, 7, 36–42. [Google Scholar] [CrossRef]
- Solomon, M.L.; Abendroth, J.M.; Poulikakos, L.V.; Hu, J.; Dionne, J.A. Fluorescence-Detected Circular Dichroism of a Chiral Molecular Monolayer with Dielectric Metasurfaces. J. Am. Chem. Soc. 2020, 142, 18304–18309. [Google Scholar] [CrossRef]
- Petronijevic, E.; Belardini, A.; Leahu, G.; Cesca, T.; Scian, C.; Mattei, G.; Sibilia, C. Circular dichroism in low-cost plasmonics: 2D arrays of nanoholes in silver. Appl. Sci. 2020, 10, 1316. [Google Scholar] [CrossRef] [Green Version]
- Petronijevic, E.; Ghahri, R.; Sibilia, C. Plasmonic Elliptical Nanohole Arrays for Chiral Absorption and Emission in the Near-Infrared and Visible Range. Appl. Sci. 2021, 11, 6012. [Google Scholar] [CrossRef]
- Zhao, Y.; Askarpour, A.N.; Sun, L.; Shi, J.; Li, X.; Alù, A. Chirality detection of enantiomers using twisted optical metamaterials. Nat. Commun. 2017, 8, 14180. [Google Scholar] [CrossRef] [PubMed]
- Petronijević, E.; Leahu, G.; Mussi, V.; Sibilia, C.; Bovino, A.F. Photoacoustic technique for the characterization of plasmonic properties of 2D periodic arrays of gold nanoholes. AIP Adv. 2017, 7, 025210. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belardini, A.; Petronijevic, E.; Ghahri, R.; Rocco, D.; Pandolfi, F.; Sibilia, C.; Mattiello, L. Fluorescence Spectroscopy of Enantiomeric Amide Compounds Enforced by Chiral Light. Appl. Sci. 2021, 11, 11375. https://doi.org/10.3390/app112311375
Belardini A, Petronijevic E, Ghahri R, Rocco D, Pandolfi F, Sibilia C, Mattiello L. Fluorescence Spectroscopy of Enantiomeric Amide Compounds Enforced by Chiral Light. Applied Sciences. 2021; 11(23):11375. https://doi.org/10.3390/app112311375
Chicago/Turabian StyleBelardini, Alessandro, Emilija Petronijevic, Ramin Ghahri, Daniele Rocco, Fabiana Pandolfi, Concita Sibilia, and Leonardo Mattiello. 2021. "Fluorescence Spectroscopy of Enantiomeric Amide Compounds Enforced by Chiral Light" Applied Sciences 11, no. 23: 11375. https://doi.org/10.3390/app112311375
APA StyleBelardini, A., Petronijevic, E., Ghahri, R., Rocco, D., Pandolfi, F., Sibilia, C., & Mattiello, L. (2021). Fluorescence Spectroscopy of Enantiomeric Amide Compounds Enforced by Chiral Light. Applied Sciences, 11(23), 11375. https://doi.org/10.3390/app112311375










