Effect of Amino Acid and Titanium Foliar Application on Smooth-Stalked Meadow Grass (Poa pratensis L.) Macronutrient Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Analysis
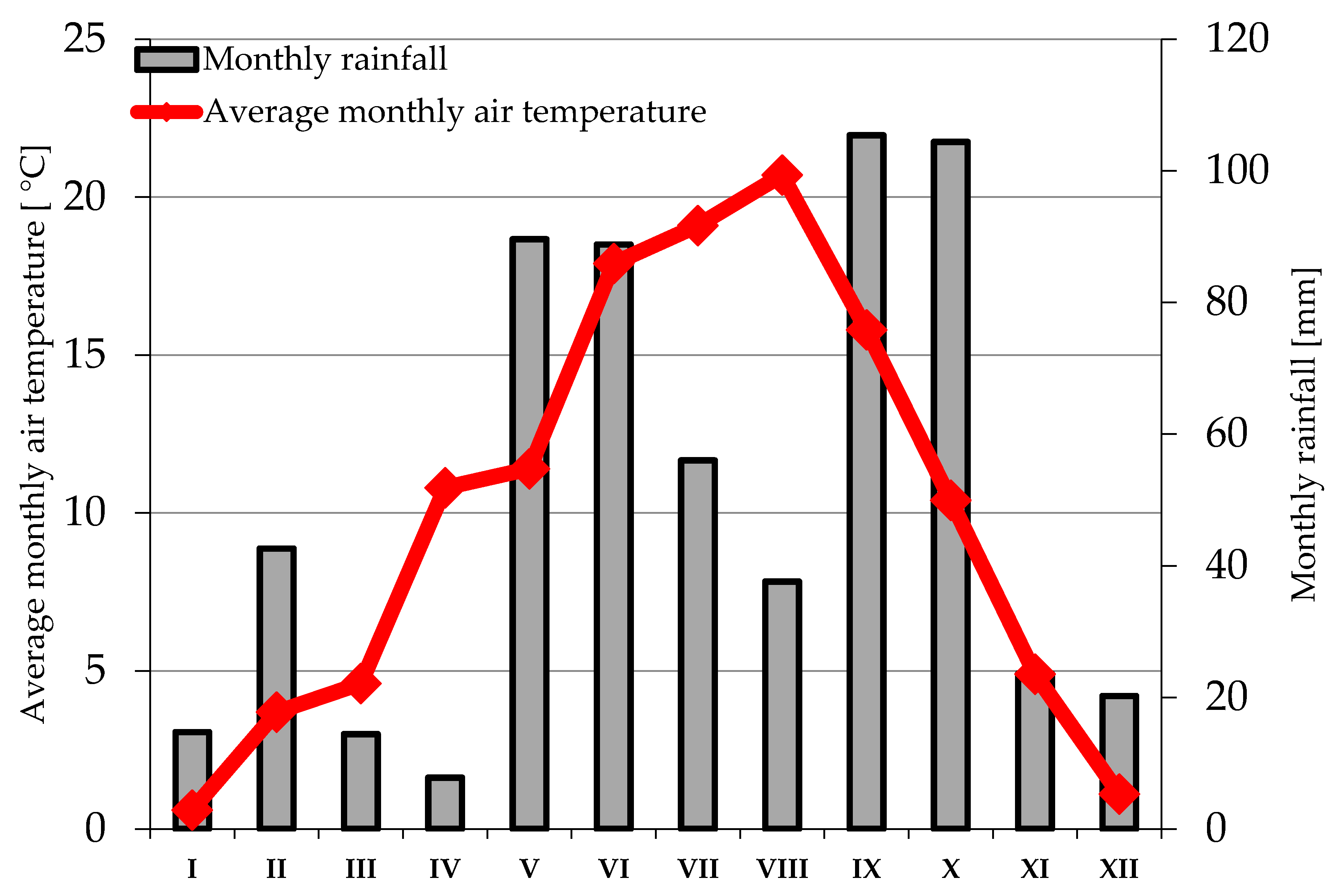
2.2. Weather Conditions
2.3. Materials and Experimental Design
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kocira, S.; Kocira, A.; Szmigielski, M.; Piecek, A.; Sagan, A.; Malaga-Tobola, U. Effect of an amino acids-containing biostimulator on common bean crop. Przem. Chem. 2015, 94, 1732–1736. [Google Scholar] [CrossRef]
- Kocira, S. Effect of amino acid biostimulant on the yield and nutraceutical potential of soybean. Chil. J. Agric. Res. 2019, 79, 17–25. [Google Scholar] [CrossRef]
- Kocira, A.; Lamorska, J.; Kornas, R.; Nowosad, N.; Tomaszewska, M.; Leszczyńska, D.; Kozłowicz, K.; Tabor, S. Changes in biochemistry and yield in response to biostimulants applied in bean (Phaseolus vulgaris L.). Agronomy 2020, 10, 189. [Google Scholar] [CrossRef] [Green Version]
- Nardi, S.; Carletti, P.; Pizzeghello, D.; Muscolo, A. Biological activities of humic substances. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; Senesi, N., Xing, B., Huang, P.M., Eds.; Wiley: Hoboken, NJ, USA, 2009; Volume 2, pp. 305–340. [Google Scholar]
- Heckman, J.R. Effect of an organic bio-stimulant on cabbage yield. J. Home Consum. Hortic. 1994, 1, 11–113. [Google Scholar] [CrossRef]
- Glińska, S.; Bartczak, M.; Oleksiak, S.; Wolska, A.; Gabara, B.; Posmyk, M.M. Effects of anthocyanin-rich extract from red cabbage leaves on meristematic cells of Allium cepa L. roots treated with heavy metals. Ecotoxicol. Environ. Saf. 2007, 68, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- El-Said, M.A.A.; Mahdy, A.Y. Response of two wheat cultivars to foliar application with amino acids under low levels of nitrogen fertilization. Middle East J. Agric. Res. 2016, 5, 462–472. [Google Scholar]
- Wahba, H.E.; Motawe, H.M.; Ibrahim, A.Y. Growth and chemical composition of Urtica pilulifera L. plant as influenced by foliar application of some amino acids. J. Mater. Environ. Sci. 2015, 6, 499–506. [Google Scholar]
- Hounsome, N.; Hounsome, B.; Tomos, D.; Edwards-Jones, G. Plant metabolites and nutritional quality of vegetables. J. Food Sci. 2008, 73, R48–R65. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010; p. 782. [Google Scholar]
- Hull, R.; Liu, H. Turfgrass nitrogen: Physiology and environmental impacts. Int. Turfgrass Soc. Res. J. 2005, 10, 962–975. [Google Scholar]
- Kocira, S. Effect of applying a biostimulant containing seaweed and amino acids on the content of fiber fractions in three soybean cultivars. Legume Res. 2019, 42, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Ervin, E.H.; Zhang, X. Applied physiology of natural and synthetic plant growth regulators. In Handbook of Turfgrass Management and Physiology; Pessarakli, M., Ed.; CRC Press: New York, NY, USA, 2008; pp. 171–202. [Google Scholar]
- Schmidt, R.E.; Ervin, E.H.; Zhang, X. Questions and answers about biostimulants. Golf Course Manag. 2003, 17, 91–94. Available online: https://hitechag.com.au/wp-content/uploads/sites/160/2015/10/QAbiostimulants.pdf (accessed on 7 October 2021).
- Ervin, E.H.; LaBranche, A.; Zhang, X. Kentucky bluegrass and creeping bentgrass responses to foliar application of glycinebetaine at three ET replacement levels. Int. Turfgrass Soc. Res. J. 2009, 11, 755–764. [Google Scholar]
- Ervin, E.H.; Zhang, X.; Askew, S.D.; Goatley, J.M., Jr. Trinexapac-ethyl, propiconazole, iron, and biostimulant effects on shaded creeping bentgrass. Horttechnology 2004, 14, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Pais, I. The biological importance of titanium. J. Plant Nutr. 1983, 6, 3–131. [Google Scholar] [CrossRef]
- Carvajal, M.; Alcaraz, C.F. Why Ti is a beneficial element for plants. J. Plant Nutr. 1998, 21, 655–664. [Google Scholar] [CrossRef]
- Seidler-Łożykowska, K.; Bocianowski, J. Evaluation of variability of morphological traits of selected caraway (Carum carvi L.) genotypes. Ind. Crop. Prod. 2012, 35, 140–145. [Google Scholar] [CrossRef]
- Mahalanobis, P.C. On the generalized distance in statistics. Proc. Natl. Inst. Sci. India 1936, 12, 49–55. [Google Scholar]
- Falkowski, M.; Kukułka, I.; Kozłowski, S. Właściwości Chemiczne Roślin Łąkowych; Wyd. AR Poznań: Poznań, Poland, 2000; p. 132. [Google Scholar]
- El-Zohiri, S.S.M.; ASfour, Y.M. Effect of some organic compounds on growth and productivity of some potato cultivars. Ann. Agric. Sci. Moshtohor 2009, 47, 403–415. [Google Scholar]
- Sadak, M.S.H.; Abdelhamid, M.T.; Schmidhalter, U. Effect of foliar application of aminoacids on plant yield and physiological parameters in bean plants irrigated with seawater. Acta Biol. Colomb. 2015, 20, 141–152. [Google Scholar] [CrossRef]
- Kandil, A.A.; Sharief, A.E.M.; Seadh, S.E.; Altai, D.S.K. Role of humic acid and amino acids in limiting loss of nitrogen fertilizer and increasing productivity of some wheat cultivars grown under newly reclaimed sandy soil. Int. J. Adv. Res. Biol. Sci. 2016, 3, 123–136. [Google Scholar]
- Radkowski, A.; Radkowska, I. Influence of foliar fertilization with amino acid preparations on morphological traits and seed yield of timothy. Plant Soil Environ. 2018, 64, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Radkowski, A.; Radkowska, I.; Godyń, D. Effects of fertilization with amino acid preparation on dry matter yield and chemical composition of meadow plants. J. Elementol. 2018, 23, 947–958. [Google Scholar] [CrossRef]
- Saeed, A.; Akhter, M.; Iqbal, M. Removal and Recovery of Heavy Metals from Aqueous Solution Using Papaya Wood as a New Biosorbent. Sep. Purif. Technol. 2005, 45, 25–31. [Google Scholar] [CrossRef]
- Ahmed, Y.M.; Shalaby, E.A.; Shanan, N.T. The use of organic and inorganic cultures in improving vegetative growth, yield characters and antioxidant activity of roselle plants (Hibiscus sabdariffa L.). Afr. J. Biotechnol. 2011, 10, 1988–1996. [Google Scholar] [CrossRef]
- Koukounaras, A.; Tsouvaltzis, P.; Siomos, A.S. Effect of root and foliar application of amino acids on the growth and yield of greenhouse tomato in different fertilization levels. J. Food Agric. Environ. 2013, 11, 644–648. [Google Scholar]
- Amin, A.A.; Fatma, A.E.; Gharib, M.; El-Awad, A.; El-Sherbeny, M.; Rashad, A. Physiological response of onion plants to foliar application of putrescine and glutamine. Sci. Hortic. 2011, 129, 353–360. [Google Scholar] [CrossRef]
- Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Growth stimulatory effects and genome-wide transcriptional changes produced by protein hydrolysates in maize seedlings. Front. Plant Sci. 2017, 8, 433. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Deng, X.; Wang, B.; Zhang, N.; Zhu, C.; Jiao, Z.; Li, R.; Shen, Q. Effects of foliar application of amino acid liquid fertilizers, with or without Bacillus amyloliquefaciens SQR9, on cowpea yield and leaf microbiota. PLoS ONE 2019, 14, e0222048. [Google Scholar] [CrossRef]
- Abo Sedera, F.; Amany, A.; Abd El-Latif, A.; Bader, L.A.A.; Rezk, S.M. Effect of NPK mineral fertilizer levels and foliar application with humic and amino acids on yield and quality of strawberry. Egypt J. Appl. Sci. 2010, 25, 154–169. [Google Scholar]
- Shehata, S.M.; Abdel-Azem Heba, S.; Abou El-Yazied, A.; El-Gizawy, A.M. Effect of Foliar Spraying with Amino Acids and Seaweed Extract on Growth Chemical Constitutes, Yield and its Quality of Celeriac Plant. Eur. J. Sci. Res. 2011, 58, 257–265. [Google Scholar]
- El-Din, D.M.; Mokhtar, H.M.O.; Ismael, O. Online Paper Review Analysis. Int. J. Adv. Comput. Sci. Appl. 2015, 6, 220–229. [Google Scholar]
- Bartnik, M.; Wierzchowska-Renke, K.; Głowniak, P.; Głowniak, K. Phenolic acids in Crithmum maritimum L. (Apiaceae) after Tytanit fertilization. Acta Soc. Bot. Pol. 2017, 86, 3560. [Google Scholar] [CrossRef] [Green Version]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Ti as a Beneficial Element for Crop Production. Front. Plant Sci. 2017, 8, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrubý, M.; Cígler, P.; Kuzel, S. Ti in plant nutrition. The contribution to understanding the mechanism of Ti action in plants. J. Plant Nutr. 2002, 25, 577–598. [Google Scholar] [CrossRef]
- Clarkson, D.T.; Hanson, J.B. The mineral nutrition of higher plants. Annu. Rev. Plant Physiol. 1980, 31, 239–298. [Google Scholar] [CrossRef]
- Uren, N.C. Forms, reactions and availability of iron in soils. J. Plant Nutr. 1984, 7, 165–176. [Google Scholar] [CrossRef]
- Ghooshchi, F. Influence of Ti and bio-fertilizers on some agronomic and physiological attributes of triticale exposed to cadmium stress. Glob. NEST J. 2017, 19, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Schmidt, R.E. Hormone-containing products’ impact on antioxidant status of tall fescue and creeping bentgrass subjected to drought. Crop Sci. 2000, 40, 1344–1349. [Google Scholar] [CrossRef]
- Warzecha, T.; Skrzypek, E.; Bocianowski, J.; Sutkowska, A. Impact of Selected PSII Parameters on Barley DH Lines Biomass and Yield Elements. Agronomy 2021, 11, 1705. [Google Scholar] [CrossRef]
- Stachowiak-Wencek, A.; Bocianowski, J.; Waliszewska, H.; Borysiak, S.; Waliszewska, B.; Zborowska, M. Statistical Prediction of Biogas and Methane Yields during Anaerobic digestion Based on the Composition of Lignocellulosic Biomass. BioResources 2021, 16, 7086–7100. [Google Scholar] [CrossRef]
- Szwarc, J.; Niemann, J.; Bocianowski, J.; Jakubus, M.; Mrówczyński, M. Connection between Nutrient Content and Resistance to Selected Pests Analyzed in Brassicaceae Hybrids. Agriculture 2021, 11, 94. [Google Scholar] [CrossRef]


| Source of Variation | d.f. | Dry Matter Yiled | P | K | Ca | Mg | Na |
|---|---|---|---|---|---|---|---|
| Block | 3 | 0.046 | 0.022 | 1.450 | 3.175 | 0.196 | 0.0002 |
| Biostimulant | 3 | 1.863 *** | 0.099 ** | 6.631 *** | 2.867 ** | 0.299 ** | 0.005 * |
| Cut | 2 | 39.08 *** | 1.944 *** | 171.8 *** | 24.19 *** | 7.823 *** | 0.155 *** |
| Biostimulant × cut interaction | 6 | 0.586 ** | 0.004 | 0.238 | 0.057 | 0.018 | 0.0002 |
| Residual | 33 | 0.167 | 0.016 | 0.314 | 0.327 | 0.033 | 0.001 |
| Biostimulant | Cut | Control | Ti | Amino Acids | Amino Acids + Ti | Average | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | ||
| Control | I | 5.48c | 0.455 | 6.202b | 0.358 | 6.225b | 0.429 | 6.889a | 0.255 | 6.199 | 0.618 |
| II | 4.552d | 0.391 | 4.669d | 0.248 | 4.996cd | 0.557 | 4.462d | 0.479 | 4.67 | 0.4407 | |
| III | 2.392f | 0.454 | 2.764f | 0.348 | 3.385e | 0.368 | 3.753e | 0.284 | 3.074 | 0.6379 | |
| Average | 4.141 | 1.407 | 4.545 | 1.497 | 4.869 | 1.284 | 5.035 | 1.439 | |||
| LSD0.05 | Biostimulant: 0.328; Cut: 0.284; Biostimulant × Cut interaction: 0.568 | ||||||||||
| Trait | P | K | Ca | Mg | Na | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| Control | 1.784c | 0.337 | 12.85c | 2.737 | 7.437b | 1.215 | 2.77c | 0.6003 | 0.352b | 0.0897 |
| Titanium | 1.867bc | 0.2937 | 13.39b | 2.73 | 7.74b | 1.076 | 2.939b | 0.5948 | 0.369ab | 0.0862 |
| Amino acids | 1.955ab | 0.3151 | 13.86b | 2.754 | 7.997ab | 1.369 | 3.05ab | 0.6135 | 0.387a | 0.0883 |
| Amino acids + Titanium | 1.986a | 0.3331 | 14.6a | 3.187 | 8.589a | 1.318 | 3.136a | 0.702 | 0.3961a | 0.0958 |
| LSD0.05 | 0.107 | 0.524 | 0.617 | 0.178 | 0.029 | |||||
| Cut | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| I | 2.299a | 0.1622 | 17.45a | 1.0668 | 6.533b | 0.4528 | 2.173c | 0.1246 | 0.4889a | 0.0498 |
| II | 1.728b | 0.1428 | 11.92b | 0.8992 | 8.808a | 1.0599 | 3.466a | 0.3443 | 0.3321b | 0.0318 |
| III | 1.667b | 0.1209 | 11.65b | 0.6602 | 8.481a | 0.7745 | 3.282b | 0.2094 | 0.3071b | 0.0223 |
| LSD0.05 | 0.093 | 0.454 | 0.534 | 0.154 | 0.025 | |||||
| Biostimulant | Cut | Control | Ti | Amino Acids | Amino Acids + Ti |
|---|---|---|---|---|---|
| Cut I | |||||
| Control | 0 | 2.957 | 5.017 | 6.74 | |
| Ti | Cut II | 1.179 | 0 | 2.489 | 4.293 |
| Amino acids | 2.502 | 1.604 | 0 | 3.021 | |
| Amino acids + Ti | 2.988 | 2.024 | 1.646 | 0 | |
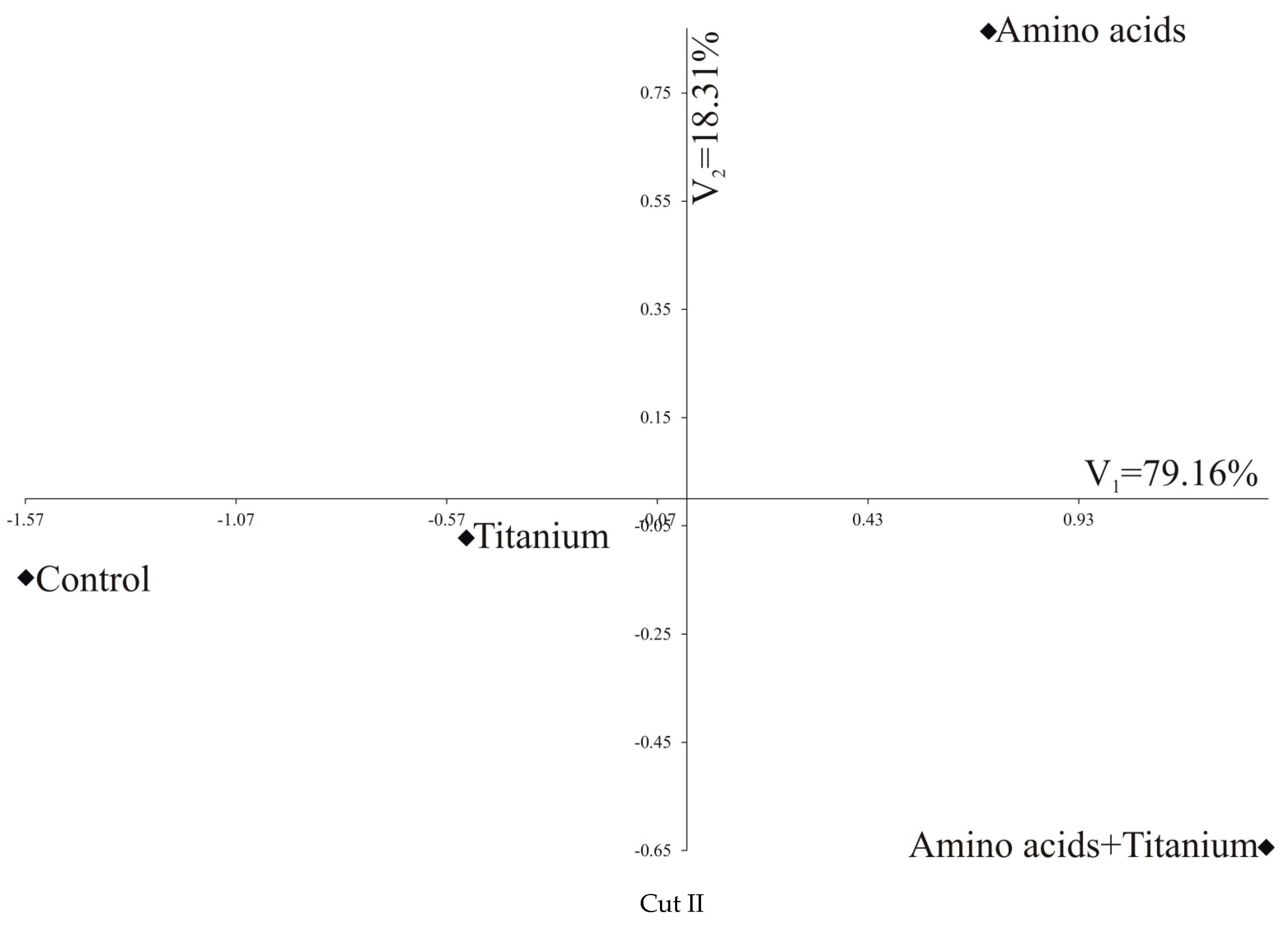
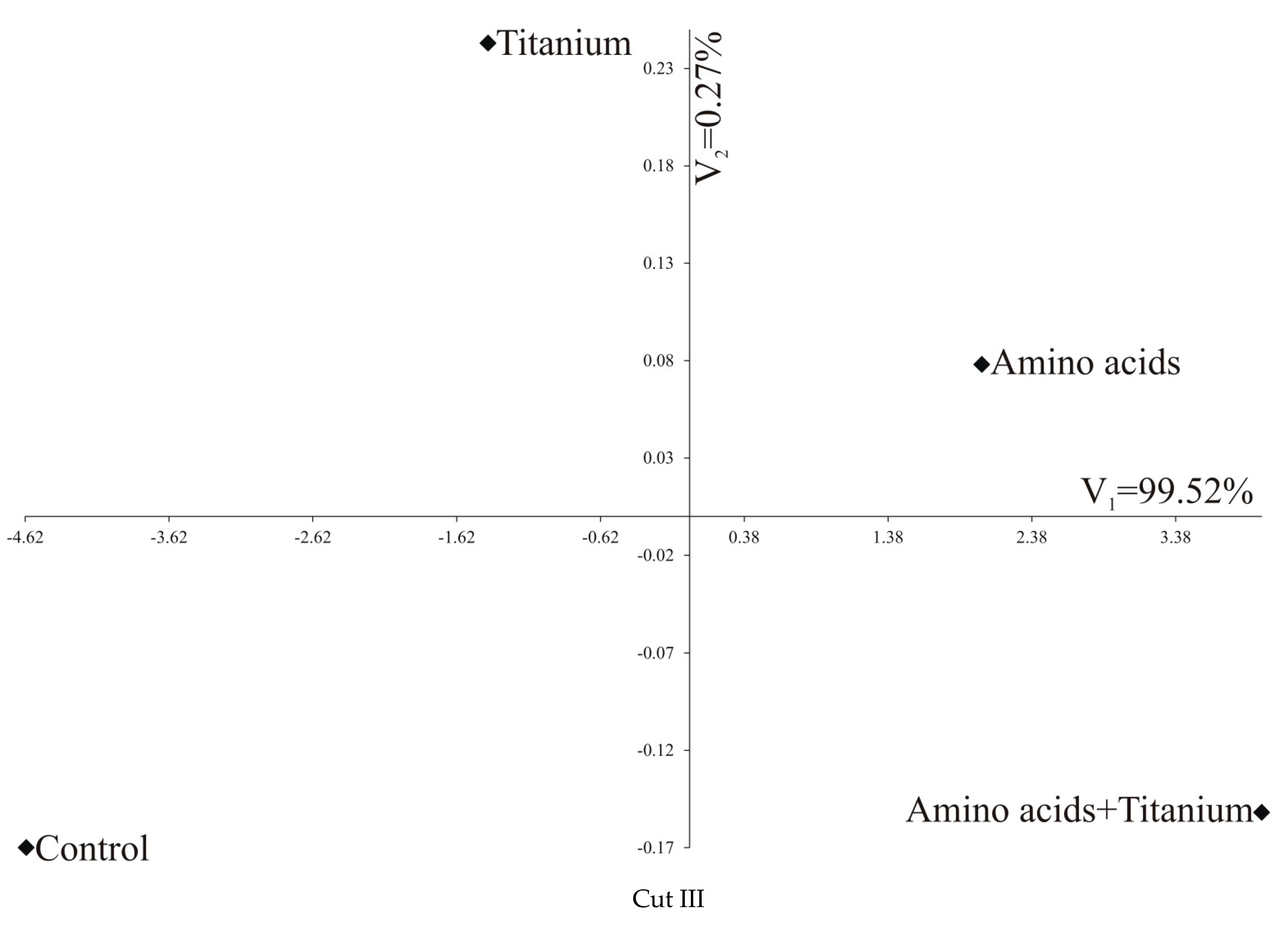
| All three cuts jointly | |||||
| Control | 0 | 3.243 | 6.654 | 8.594 | |
| Ti | Cut III | 5.337 | 0 | 3.457 | 5.395 |
| Amino acids | 9.643 | 4.313 | 0 | 1.994 | |
| Amino acids + Ti | 11.491 | 6.241 | 2.175 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radkowski, A.; Radkowska, I.; Bocianowski, J.; Wolski, K.; Bujak, H. Effect of Amino Acid and Titanium Foliar Application on Smooth-Stalked Meadow Grass (Poa pratensis L.) Macronutrient Content. Appl. Sci. 2021, 11, 11421. https://doi.org/10.3390/app112311421
Radkowski A, Radkowska I, Bocianowski J, Wolski K, Bujak H. Effect of Amino Acid and Titanium Foliar Application on Smooth-Stalked Meadow Grass (Poa pratensis L.) Macronutrient Content. Applied Sciences. 2021; 11(23):11421. https://doi.org/10.3390/app112311421
Chicago/Turabian StyleRadkowski, Adam, Iwona Radkowska, Jan Bocianowski, Karol Wolski, and Henryk Bujak. 2021. "Effect of Amino Acid and Titanium Foliar Application on Smooth-Stalked Meadow Grass (Poa pratensis L.) Macronutrient Content" Applied Sciences 11, no. 23: 11421. https://doi.org/10.3390/app112311421
APA StyleRadkowski, A., Radkowska, I., Bocianowski, J., Wolski, K., & Bujak, H. (2021). Effect of Amino Acid and Titanium Foliar Application on Smooth-Stalked Meadow Grass (Poa pratensis L.) Macronutrient Content. Applied Sciences, 11(23), 11421. https://doi.org/10.3390/app112311421







