Abstract
The aim of this retrospective study was to evaluate the impact of genetic and environmental factors on the impaction of lower third molars using a classical twin study by evaluation of the third molar eruption space and M3 angulation. The study group consisted of 212 twins: 80 dizygotic and 132 monozygotic twins with digital panoramic radiograms and lateral cephalograms. The zygosity of twins was determined using 15 highly polymorphic DNA regions from the venous blood of twins. The results showed that differences between siblings in the dizygotic and monozygotic twin pairs were not statistically significant. The most fitting model for the angulations of lower third molars was AE (additive genetic and specific environmental effect), where the additive genetic factors had up to 88% influence and the specific environment up to 27%. Therefore, the ACE( additive genetic, common and specific environmental effect), model showed higher significance for the lower third molar eruption level where additive genetic estimates reached 71%, a specific environment contributed up to 15% and the common environment reached 32%. The conclusion was that a considerable proportion of the third molar impaction could be attributed to additive genetic effects and the common environment, whereas the specific environment had a lower, but significant impact.
1. Introduction
In clinical dental practice, it is essential to understand the reasons why third molars develop in different ways and what are the factors responsible for the impaction of these teeth.
Impaction of the tooth is considered when the tooth is unable to erupt into its proper position, and it is positioned against another tooth or bone so that its further eruption is unlikely [1]. The impaction of the lower third molars ranged from 25% to 50% and was higher in females than in males [2,3,4]. Although the main treatment option of this pathology was the extraction of these problematic teeth, there was a number of studies that reported that the complications of such treatment were very high and, in some studies, even reached 33% [5,6]. Thus, the knowledge about the etiology of the retention of the lower third molars could help to minimize the number of these complications.
The main reasons for the impaction of lower third molars are lack of space for the eruption and changes in their position during their eruption process [7,8].
The discussions about the position of the lower third molars and their effect on the dentition were always very confusing in the literature. Fifty years ago, it was believed that lower third molars (LM3) could cause anterior crowding by pushing forward all the anterior teeth, especially in cases of the impaction of the third molar, which is a highly incident problem occurring in up to 68% of young adults in Europe [2]. However, Sidlauskas et al. found that the crowding in the lower incisor region was not higher in the dental arches with third molars than without them. The authors suggested that there was not enough evidence to incriminate wisdom teeth as being the most important etiologic factor in the anterior dental arch crowding [8]. In addition, another study by Stanaityte et al. found that even after the extraction of the lower third molar, the crowding in the anterior region remained the same and there was not any obvious relationship between the third molar and late teeth crowding [6]. Thus, the prophylactic removal of these teeth without any pathology in light of possible teeth crowding would not make sense.
The only agreement between modern scientists is that impacted third molars may affect adjacent second molars, but not the anterior teeth. This was confirmed by the study of Smailiene and al., where the authors found that the incidence of the external root resorption of the adjacent second molars was significantly associated with a mesial inclination and with a deep position of the impacted third molar [9]. In these cases, the extraction of the third molar was recommended and already in 1979, the American National Institute of Health established indications of LM3 removal: recurrent pericoronitis, untreated caries, cysts, periodontal disease and the resorption of the adjacent tooth root [10].
Nowadays, clinicians more frequently choose not to extract third molars that are well-positioned. Usually, these teeth are considered as an additional tool for the treating of the problems of the dentofacial system and can be used, for example, for auto-transplantation, which is very sensitive to the formation of the third molar roots, or mesialization of the third molar in the case of the extracted second molar due to caries complications.
Finally, all these innovative technologies offer us new treatment options, but for all of them, it is important to know the specificity of the development and position of third molars, and how far we are able to change these characteristics. This evaluation, for distinguishing the factors responsible for the formation and position of the lower third molars, can be conducted by the use of classical twin studies, which have not been performed to date. Previous twin studies played a huge role in evaluating the importance of genetic factors on tooth size, morphology and development [11,12,13]. The main consideration in the earlier twin studies was the reliability of the twin zygosity determination [14,15]. The precise determination at the level of 99.99% required the analysis of at least 15 highly polymorphic regions of DNA derived from blood or buccal cells of twins [16].
The structural equation modeling applied to twin studies can provide estimates of the proportions of four total phenotypic variations attributable to additive genetic effects, the common environmental and unique environmental components [17,18]. Therefore, the genetic environmental interplay for the lower third molar positional polymorphism is still not clear.
Thus, the present study aimed to determine the genetic influence on M3s impaction by comparison of monozygotic and dizygotic twins with the chronological age range from late puberty till adulthood, using the statistics of path analysis with the best fitting model and DNA zygosity determination based on 15 highly polymorphic DNA regions and Amel fragment of amelogenin gene.
This research hypothesized that genetic factors had a considerable proportion of the total variability in the lower third molar impaction, including the M3 eruption space and its angulations.
2. Materials and Methods
The Bioethical approval was obtained from the Regional Bioethical Committee of Kaunas (No. BE 2–12). The study was carried out in accordance with relevant guidelines and regulations.
2.1. Subjects
The study samples for this retrospective study were randomly selected from the data of the Twin Center of Lithuanian University of Health Sciences. The inclusion criteria were as follows: same-sex twins older than 15 years, the skeletal vertebral maturity stage (CVM) according to the Baccetti method 5–6, no missing teeth, no congenital syndromes or other anomalies, no previous orthodontic treatment, good quality digital panoramic radiographs and lateral cephalograms performed with the same machine and good quality patient plaster models. These plaster models had to be produced from the diagnostic impressions, which were taken using “Prestige” (Vannini Dental Industry, Grassina, Italy) silicon material and dental casts produced from stone “Marmorock N” class IV (Siladent, Goslar, Germany). The exclusion criteria were the following: younger than 15 years twins, CVM less than 5, missing teeth, previous orthodontic treatment, bad quality radiographs, or radiographs made with a different machine. The following formula was used for the sample size calculation:
where n—the minimum sample size for each sample; z1–α/2 = 1.96 and z1–β=0.84 if α = 0.05 and β = 0.2; s1, s2—the standard deviations of pilot samples; Δ—the smallest clinically important difference. The minimal calculated sample size for this study was 160 twins (80 DZ, 80 MZ).
2.2. Assessment of Skeletal Maturity
The skeletal maturity stage (CVM) was estimated by two expert operators from the lateral cephalograms twice with a 2 week interval using the Baccetti cervical vertebral maturation method [19].
2.3. Selection and Evaluation of Digital Panoramic Radiographs
All the panoramic radiograms were performed by the same machine (Carestream Kodak 9300) for all study participants using standard protocols of the patient positioning to avoid distortion on panoramic radiographs. Patients were oriented according to their mid-sagittal line, Frankfort plane and the canine-corner base of the nose alignment line. In addition, each panoramic radiogram was evaluated for the detection of geometric distortion. Trakinienė et al. found that the magnification in the lower first molar region was the most stable; thus, this tooth was used for the control of geometric distortions [20].
Consequently, in the recent research, all panoramic radiograms were checked by the same two expert operators comparing the magnification coefficients in the lower first molar region on the right and left sides. If this coefficient was the same on both sides, then the panoramic radiogram was accepted without geometric distortion, as a good quality panoramic radiogram. Other radiograms were excluded from the study. The analysis of panoramic radiograms was performed using Dolphin Imaging program 11.8. The measurements on the plaster casts were performed by the use of a digital caliper with the tips sharpened to a point and 0.01 mm accuracy (Dentaurum, Ispringen, Germany).
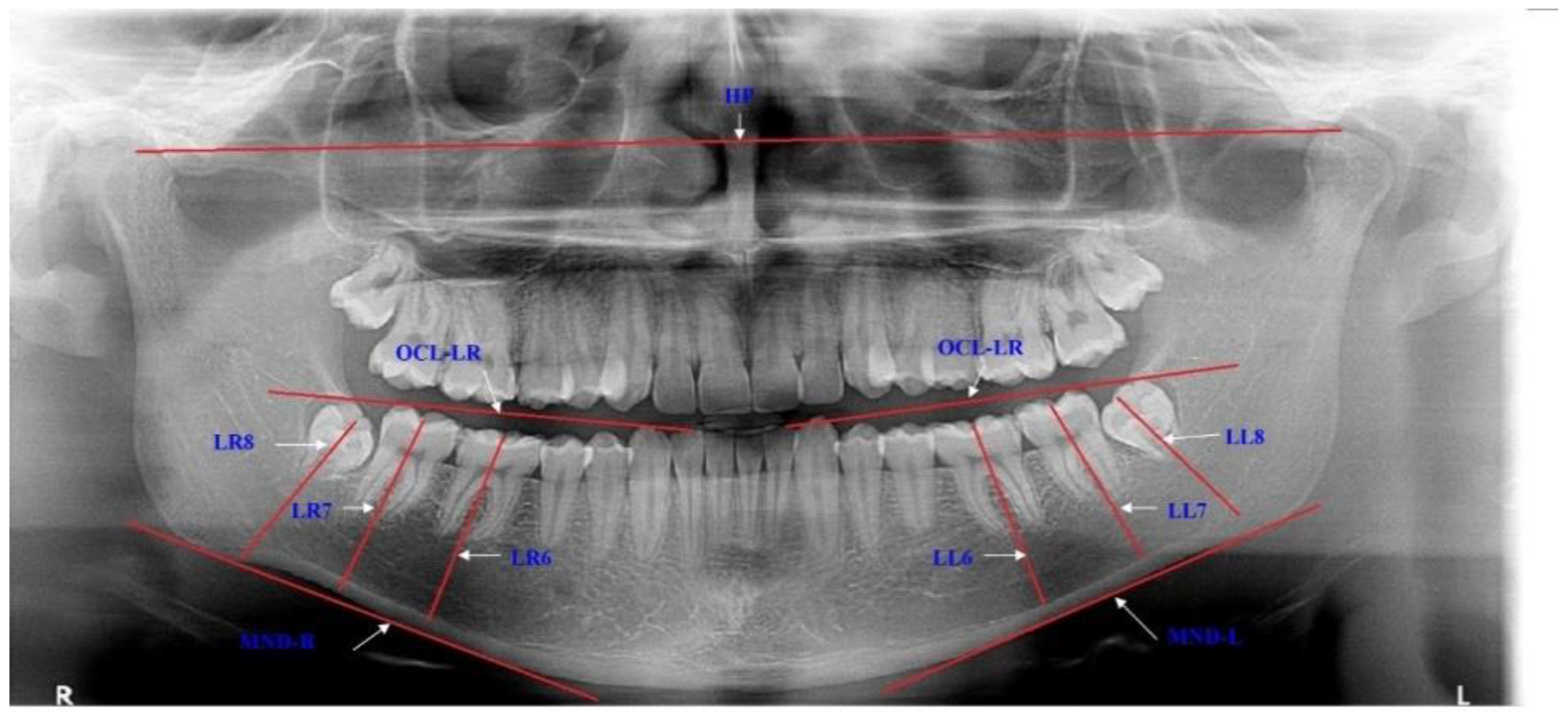
The impaction of the lower third molar was determined by the evaluation of each panoramic radiogram for the angulations and eruption levels of the lower molar and for the eruption space for the LM3. The positions of these teeth were determined using mandibular plane (MND), occlusal plane (OKL) and horizontal plane (HP) (Figure 1).

Figure 1.
Description of the variables for estimating the positions of the lower third molars. LR8—longitudinal axis of the lower right third molar is traced from the midocclusal point to the midpoint of the root bifurcation, and the same on the opposite side with LL8; LR7—longitudinal axis of the lower right second molar is traced from the midocclusal point to the midpoint of the root bifurcation, and the same with LL7; LR6—longitudinal axis of the lower right first molar is traced from the midocclusal point to the midpoint of the root bifurcation, and the same with LL6; MND-R—right mandibular plane is drawn through most inferior border of the mandible, and the same with MND-L; OCL-LR—lower right occlusal plane is drawn through the incisal edge of the lower right lateral incisor to the distal cusp of the lower right second molar, and the same with OCL-LL; HP—horizontal plane is traced through the most superior parts of the right and left condyles.
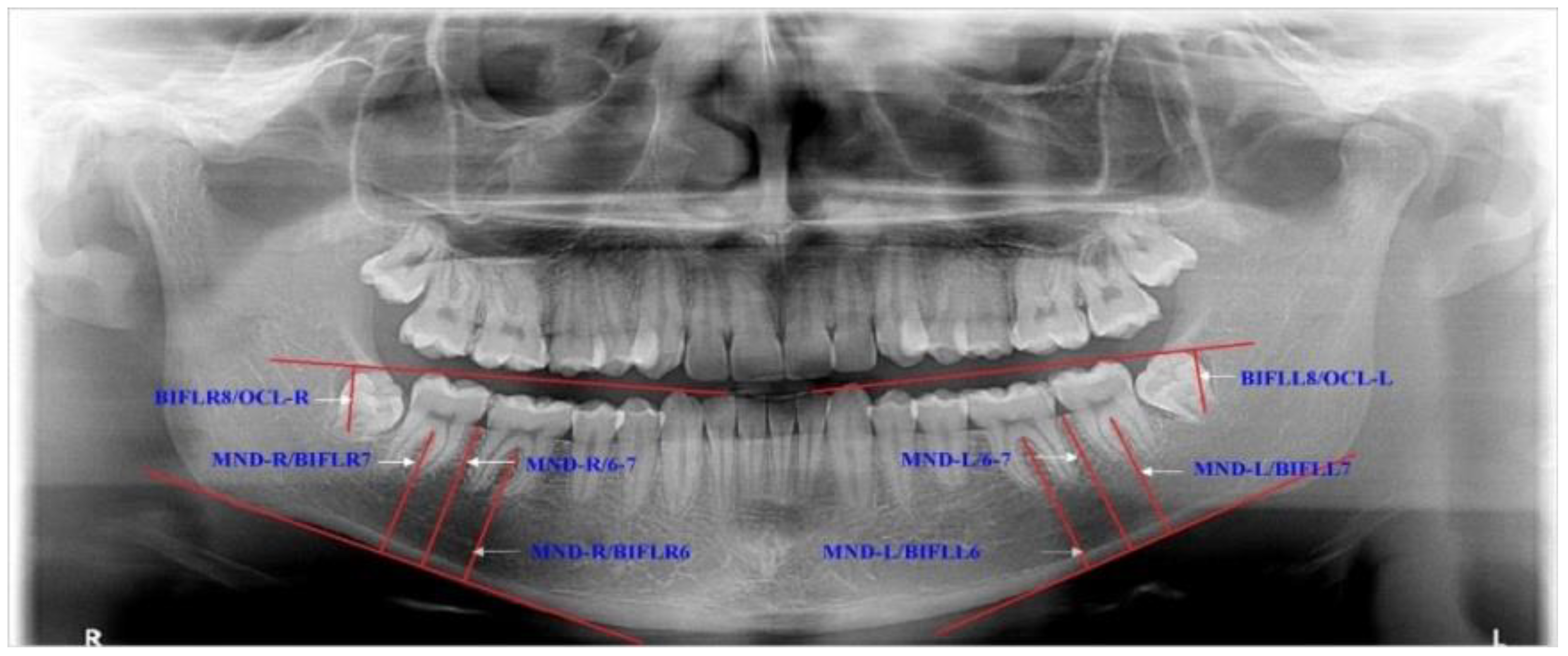
In addition, the eruption levels of lower molars were investigated according to the mandibular and occlusal planes (Figure 2).

Figure 2.
Description of the variables for estimating eruption levels of the lower third molars. MND-R/BIFLR6—the distance from the MND-R plane to the bifurcation of the right lower first molar, and the same with MND-L/BIFLL6; MND-R/BIFLR7—the distance from the MND-R plane to the bifurcation of the right lower second molar, and the same with MND-L/BIFLL7; MND-R/6-7—the distance from the MND-R plane to the edge of the alveolus ridge between the lower right second and first molars, and the same with MND-L/6-7; BIFLR8/OCL-R—the distance from the OCL-R plane to the bifurcation of the right lower third molar, and the same with BIFLL8/OCL-L.
The distances between occlusal surfaces of the third and second molars (DLR8/LR7, DLL8/LL7) were measured from the mesial cusps of the lower third molars tangentially to the lines, which extended over the occlusal surfaces of the second molars in the right and left side. All measurements were performed using Dolphin Imaging program 11.8.
2.4. Genetic Assessment and Determination
The genetic analysis for the determination of the zygosity was performed using a DNA test with the polymerase chain reaction set for the amplification of the short tandem repeats and 15 specific DNA markers (D8S1179, D21S11, D7S820, CSF1PO, D3S1358, TH01, D13S317, D16S539, D2S1338, D19S433, vWA, TROX, D18S51, D5S818 and FGA) and Amel fragment of the amelogenin gene for the comparison of the genetic profiles from the venous blood of twins. The genetic analysis by model fitting was conducted with the OpenMx package) and was performed using maximum likelihood genetic structural equation modeling [21]. This analysis allowed the determination of the significance of 4 different components of variance: the additive genetic factor (A), the shared environment (C), the non-additive genetic factor (D) and the unique environment (E). The Akaike information criterion (AIC) statistics and the difference in the chi-square (χ2) value relative to the change in degrees of freedom indicated the best fitting model, which was chosen with the lowest AIC value. [22].
2.5. Statistical Methods and Method Error
Statistical analysis was performed using SPSS 17 version. The statistics included mean and standard deviation; hypotheses of the interrelations between the characteristics were tested using the Student criterion and Pearson correlation coefficients (r). A p-value of 0.05 was considered significant. The agreement of the interobserver measurements was investigated by interclass correlation coefficient (ICC) statistics. The study design power was 0.8.
3. Results
The study group consisted of 212 twins and was divided into the dizygotic and monozygotic twins groups. The dizygotic twins group included 80 twins with the mean age 18 ± 0.6, 42 (52%) males and 38 (48%) females with CVM5 stage 42% and CVM6 stage 58%. The monozygotic twins group consisted of 132 subjects with the mean age 19 ± 0.7, 78 (59%) males and 54 (41%) females with CVM5 stage 40% and CVM6 stage 60%. The differences between the groups of age, sex and CVM were not statistically significant; p values ranged from 0.47 to 0.89. The ICC for all the measurements fluctuated from 0.79 to 0.89 and showed almost perfect agreement. The method error by Bland–Altman for repeatability of the angular measurements ranged from 0.56 to 1.07° and for the linear measurements varied from 0.25 to 0.57 mm with no significant differences. The Kolmogorov–Smirnov tests proved the normal distribution for all the studied variables; thus, the use of the parametric tests was established.
The angulations of the lower third, second and first molar did not have statistically significant differences in dizygotic twins. The correlations between siblings varied from 0.28 to 0.41 and were the highest in the region of the first and second molar. Whereas monozygotic twins had higher correlations which ranged from 0.69 to 0.98. The highest correlation values were for the first and second molars (Table 1).

Table 1.
The distribution of the teeth angulations among dizygotic and monozygotic twins (* p < 0.05).
The eruption space analysis for the lower third molar did not show significant differences between siblings in dizygotic and monozygotic twins. However, the correlations fluctuated from 0.53 to 0.91 and again, were higher in the monozygotic twins (Table 2).

Table 2.
The eruption space for the lower third molars. LES-R—the space for the lower right third molar; LES-L—the space for the lower left third molar (* p < 0.05).
The eruption level of the evaluated teeth in the dizygotic twins showed almost the same tendency among siblings. The correlations fluctuated from 0.13 to 0.50 and were the highest for the first and second molar eruption levels. Furthermore, the eruption values in the monozygotic twins among the siblings did not differ statistically significantly. The correlations ranged from 0.76 to 0.94 and were the highest for the first and second molars.
Finally, the genetic analysis showed that the most parsimonious fitting model for the angulation of the molars and their eruption space was AE ( additive genetic and specific environmental effect), although for the teeth eruption level the model ACE (additive genetic, common and specific environmental effect) showed higher significance. The genetic determinants were moderate to high, with a minimum of 60% reaching 88%. A specific environment affected up to 28%, and the common environment reached 32% (Table 3).

Table 3.
Path coefficients for the different variables. A—additive genetic factors; D—non-additive genetic factors; C—common or shared environment; E—unique environment factors.
4. Discussion
The hypothesis of this study that genetic factors played a key role in the lower third molar position and eruption space using a genetic model of twins was supported.
One of the considerations of such study designs is the estimation of the age of the study group. There was no doubt that during adolescence, the lower third molars were changing their position; thus, it was necessary to collect data with the patients after the growth spurt. It was found that the formation of the pulp chamber and radicular bifurcation of the mandibular third molar was an indicator of the deceleration of the growth spurt. Consequently, the twins with at least CVM stage 5 were chosen for the study sample [23].
First, this study showed that the space for the eruption of the lower third molar was highly affected by genetic factors, and this matched with the findings of Trakiniene et al. with regard to the eruption space for the upper third molars [24]. The differences of eruption space in dizygotic and monozygotic twins were not statistically significant (p values varied from 0.14 to 0.17 in dizygotic twins and from 0.19 to 0.23 in monozygotic twins). The correlations between siblings varied from 0.53 to 0.60 in dizygotic twins but were much higher in monozygotic twins (it ranged from 0.89 to 0.91). The best fitting model for space for the upper third molar eruption was AE, where the effect of additive genetic factors was a slightly lower and reached 61% (instead of 69% in the current study). However, the impact of the common environment for the space of upper third molars was higher by 8 percent and reached 39%. This difference might be due to the stronger genetic determination of the shape of the mandible. Previous studies suggested that the horizontal linear variables of the lower jaw seemed to be more influenced by genetic factors than the vertical ones [17]. The same tendency was seen even in the agenesis of M3 where additive genetic factors had an even up to 19% higher effect on the agenesis of lower third molars in comparison to the agenesis of upper third molars [25,26].
In addition, while analyzing the position of these teeth, the angulations of M3s did not have statistically significant differences in either dizygotic or monozygotic twins (p values varied from 0.1 to 0.99). The correlations between siblings varied from 0.28 to 0.98 and were higher in monozygotic twins. Richardson in the non-twin sample found that until the age of 15 years, the lower third molars had a tendency to incline mesial approximately 11 degrees without any clear reason [27]. According to the recent study, this may be most affected by genetic factors. The findings of our research showed that the additive genetic effect on the position of the lower third molars was almost the same as on the position of the upper third molars and varied from 74 to 76 percent. In addition, these findings are consistent with those of many others, indicating that most of the variation of dental development in humans, such as teeth crown size or mineralization of the germs of third molars, had a strong genetic component [2,11,12,28]. Considering the findings of our study with regard to the position of lower third molars and their eruption space, which were the most important factors for the impaction of lower third molars, it may be concluded that the retention of lower wisdom teeth was mostly affected by additive genetic factors with a small part of the effect from the common and specific environments. This is a very promising area in biotechnology, especially now, when we have a modern tool—DNA scissors.
Finally, this study found that the effect of environmental factors on the impaction of lower third molars ranged from 12% to 34%; thus, this effect should not be negated. These findings correspond with the results of Dempsey, where the specific environment ranged from 8% to 29% for permanent teeth sizes (excluding third molars) [13]. Nevertheless, more studies should be conducted for the identification of the genetic and environmental predictors for the determination of the impaction of the lower third molars. When these approaches are identified at the DNA level, this would have a huge impact on the understanding of the epigenetic influences on a local tissue level and in the clearness of the interaction of genetic, epigenetic and environmental factors. This knowledge could help to develop new approaches to the prevention and treatment of many developmental problems affecting human dentition [15].
Limitations
The major limitation of twin studies is that they do not represent the whole society as a general unit. The cultural inheritance expressed as C in this study is a population concept and is irrelevant to the individual as well. Nevertheless, these studies are useful for the evaluation of the importance of genetic and environmental factors in the manifestation of the traits of the third molar variability and their impaction.
5. Conclusions
The conclusion was that a considerable proportion of third molar impaction could be attributed to additive genetic effects and the common environment, whereas the specific environment had a lower, but significant impact.
Author Contributions
Conceptualization and Methodology, G.T., D.S., K.L., T.T. and A.Š. Formal Analysis, Draft Preparation, Writing, Review and Editing, G.T., D.S., K.L., T.T. and A.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Lithuanian University of Health Sciences.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Lithuanian University of Health Sciences, Kaunas (No. BE 2–12).
Informed Consent Statement
Informed consent statement was obtained from all participants.
Data Availability Statement
Data may be available under reasonable request.
Acknowledgments
The authors express their thanks to the families of the twins who have participated in the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Janakiraman, E.N.; Alexander, M.; Sanjay, P. Prospective analysis of frequency and contributing factors of nerve injuries following third-molar surgery. J. Craniofac. Surg. 2010, 21, 784–786. [Google Scholar] [CrossRef]
- Elsey, M.J.; Rock, W.P. Influence of orthodontic treatment on development of third molars. Br. J. Oral Maxillofac. Surg. 2000, 38, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Juodzbalys, G.; Daugela, P. Mandibular third molar impaction: Review of literature and a proposal of a classification. J. Oral Maxillofac. Res. 2013, 4. [Google Scholar] [CrossRef]
- Stanaitytė, R.; Trakinienė, G.; Gervickas, A. Do wisdom teeth induce anterior teeth crowding? A systematic literature reviews. Stomatol. Balt. Dent Maxillofac. J. 2014, 16, 15–18. [Google Scholar]
- Pahkala, R.; Pahkala, A.; Laine, T. Eruption pattern of permanent teeth in a rural community in northeastern Finland. Acta Odontol. Scand. 1991, 49, 341–396. [Google Scholar] [CrossRef] [PubMed]
- Stanaitytė, R.; Trakinienė, G.; Gervickas, A. Lower dental arch changes after bilateral third molar removal. Stomatol. Balt. Dent Maxillofac. J. 2014, 16, 31–36. [Google Scholar]
- Ganss, C.; Hochban, W.; Kielbassa, A.M.; Umstadt, H.E. Prognosis of third molar eruption. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 1993, 76, 688–693. [Google Scholar] [CrossRef]
- Šidlauskas, A.; Trakinienė, G.; Damušienė, R. Effect of the lower third molars on the lower dental arch crowding. Stomatologija 2006, 8, 80–88. [Google Scholar] [PubMed]
- Smailiene, D.; Trakiniene, G.; Beinoriene, A. Relationship between the position of impacted third molars and external root resoption of adjacenet second molars: A retrospective CBCT study. Medicina 2019, 55, 305. [Google Scholar] [CrossRef] [PubMed]
- Bishara, S.E.; Treder, J.E.; Damon, P. Changes in the dental arches and dentition between 25 and 45 years of age. Angle Orthod. 1999, 66, 417–422. [Google Scholar]
- Eguchi, S.; Townsend, G.; Richards, L. Genetic contribution todental arch size variation in Australian twins. Arch. Oral Biol. 2004, 49, 1015–1102. [Google Scholar] [CrossRef]
- Hughes, T.G.; Demsey, P.; Richards, L. Genetic analysis of deciduous tooth size in Australian twins. Arch. Oral Biol. 2000, 45, 997–1004. [Google Scholar] [CrossRef]
- Hughes, T.; Bockmann, M.; Mihailidis, S. Genetic, epigenetic, and environmental influences on dentofacial structures and oral health: Ongoing studies of Australian twins and their families. Twin Res. Hum. Gen. 2013, 16, 43–51. [Google Scholar] [CrossRef]
- Vuollo, V.; Sidlauskas, M.; Sidlauskas, A. Comparing facial 3D analysis with DNA testing to determine zygosities of twins. Twin Res. Hum. Gen. 2015, 18, 306–313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Townsend, G.; Hughes, T.; Luciano, M. Genetic and environmental influences on human dental variation: A critical evaluation of studies involving twins. Arch. Oral Biol. 2009, 54, 45–51. [Google Scholar] [CrossRef]
- Nyholt, D. On the probability of dizygotic twins being concordant for two alleles at multiple polymorphic loci. Twin Res. Hum. Gen. 2006, 9, 194–197. [Google Scholar] [CrossRef][Green Version]
- Šidlauskas, M.; Šalomskienė, L.; Andriuškevičiūtė, I. Heritability of mandibular cephalometric variables in twins with completed craniofacial growth. Eur. J. Orthod. 2016, 38, 493–502. [Google Scholar] [CrossRef]
- Dempsey, P.; Townsend, G. Genetic and environmental contributions to variation in human tooth size. Hered 2001, 86, 685–693. [Google Scholar] [CrossRef]
- Baccetti, T.; Franchi, L.; McNamara, J.A. Method for the assessment of optimal treatment timing in dentofacial orthopedics. Semin. Orthod. 2005, 11, 119–129. [Google Scholar] [CrossRef]
- Trakinienė, G.; Šidlauskas, A.; Švalkauskienė, V. The magnification in the lower third and second molar region in the digital panoramic radiographs. J. Forensic Dent. Sci. 2017, 9, 91–95. [Google Scholar] [CrossRef]
- Neale, M.; Cardon, L. Methodology for Genetic Studies of Twins and Families; Springer Science Business Media: Dordrecht, The Netherlands, 1992. [Google Scholar]
- Akaike, H. Factor analysis and AIC. Psychometrika 1987, 52, 317–332. [Google Scholar] [CrossRef]
- Trakinienė, G.; Smailienė, D.; Kučiauskienė, A. Evaluation of skeletal maturity using maxillary canine, mandibular second and third molar calcification stages. Eur. J. Orthod. 2016, 38, 398–403. [Google Scholar] [CrossRef]
- Trakiniene, G.; Sidlauskas, A.; Trakinis, T. The impact of genetics and environmental factors on the position of the upper third molars. J. Oral Maxillofac. Surg. 2018, 76, 2271–2279. [Google Scholar] [CrossRef]
- Trakinienė, G.; Ryliškytė, M.; Kiaušaitė, A. Prevalence of teeth number anomalies in orthodontic patients. Stomatol. Balt. Dent Maxillofac. J. 2013, 15, 47–53. [Google Scholar]
- Trakiniene, G.; Sidlauskas, A.; Andriuskeviciute, I. Impact of genetics on third molar agenesis. Sci. Rep. 2018, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.E. The role of the third molar in the cause of late lower arch crowding: A review. Am. J. Orthod. Dentofac. Orthop. 1989, 95, 79–83. [Google Scholar] [CrossRef]
- Trakiniene, G.; Andriuskeviciute, I.; Salomskiene, L. Genetic and environmental influences on third molar root mineralization. Arch. Oral Biol. 2019, 98, 220–225. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).