Advances in Purpurin 18 Research: On Cancer Therapy
Abstract
:1. Introduction
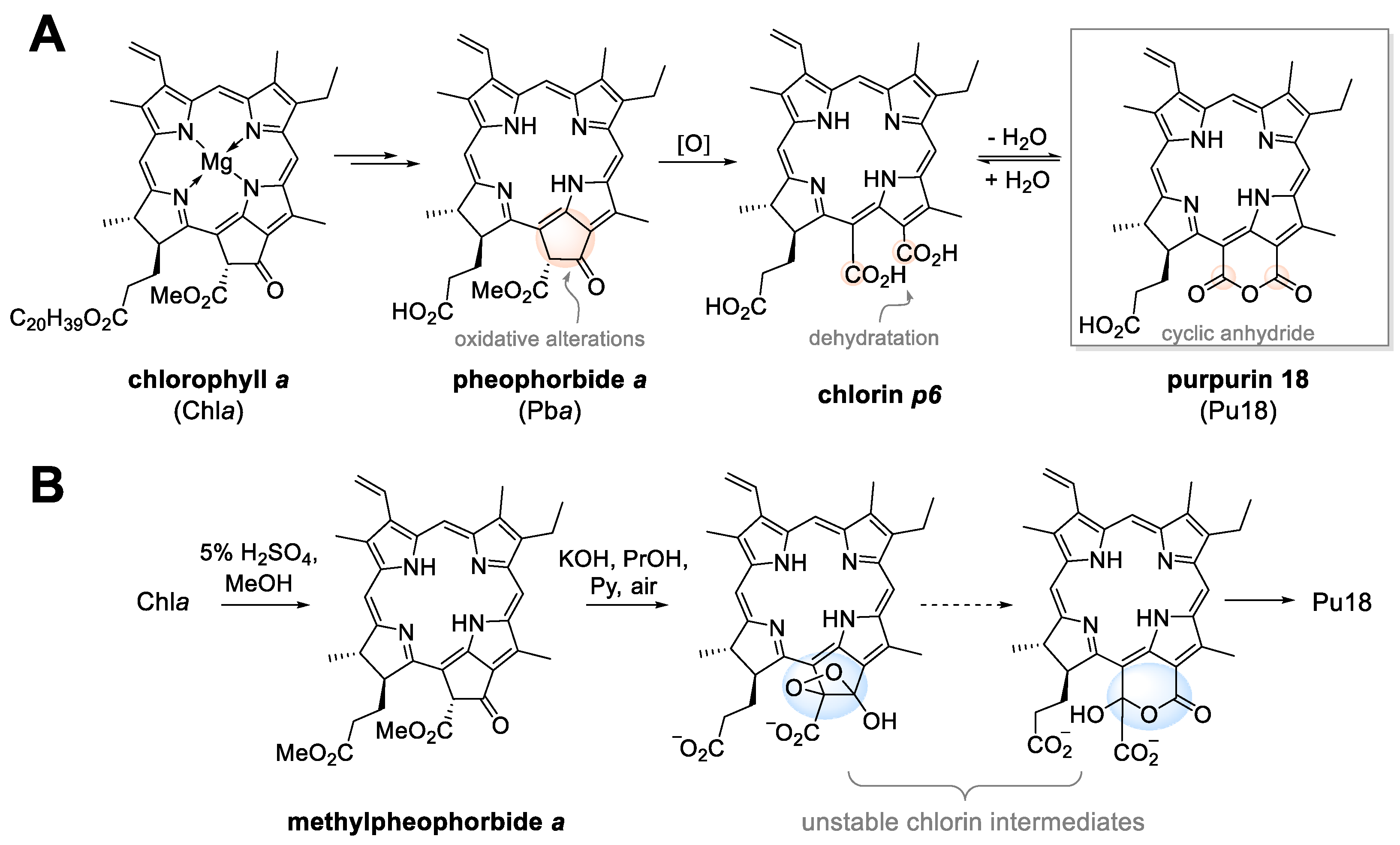
2. From Plant Dyes to Medicines
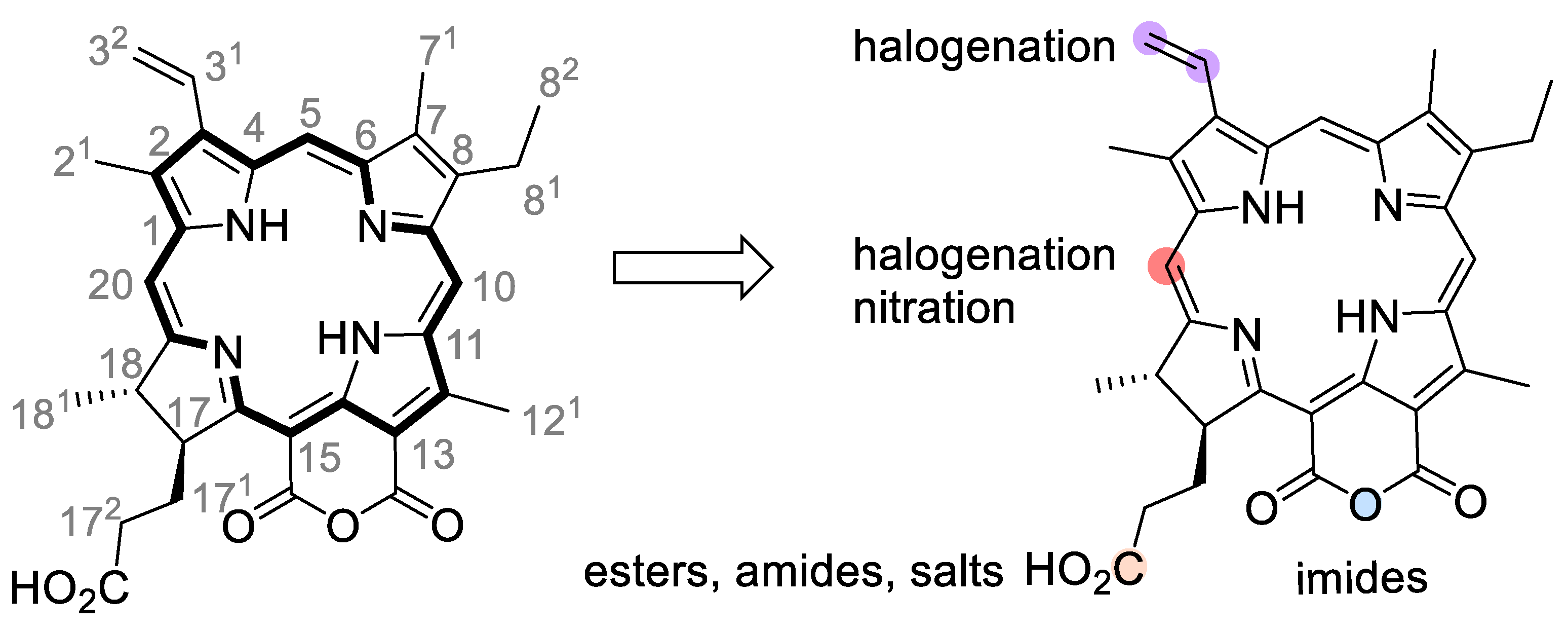
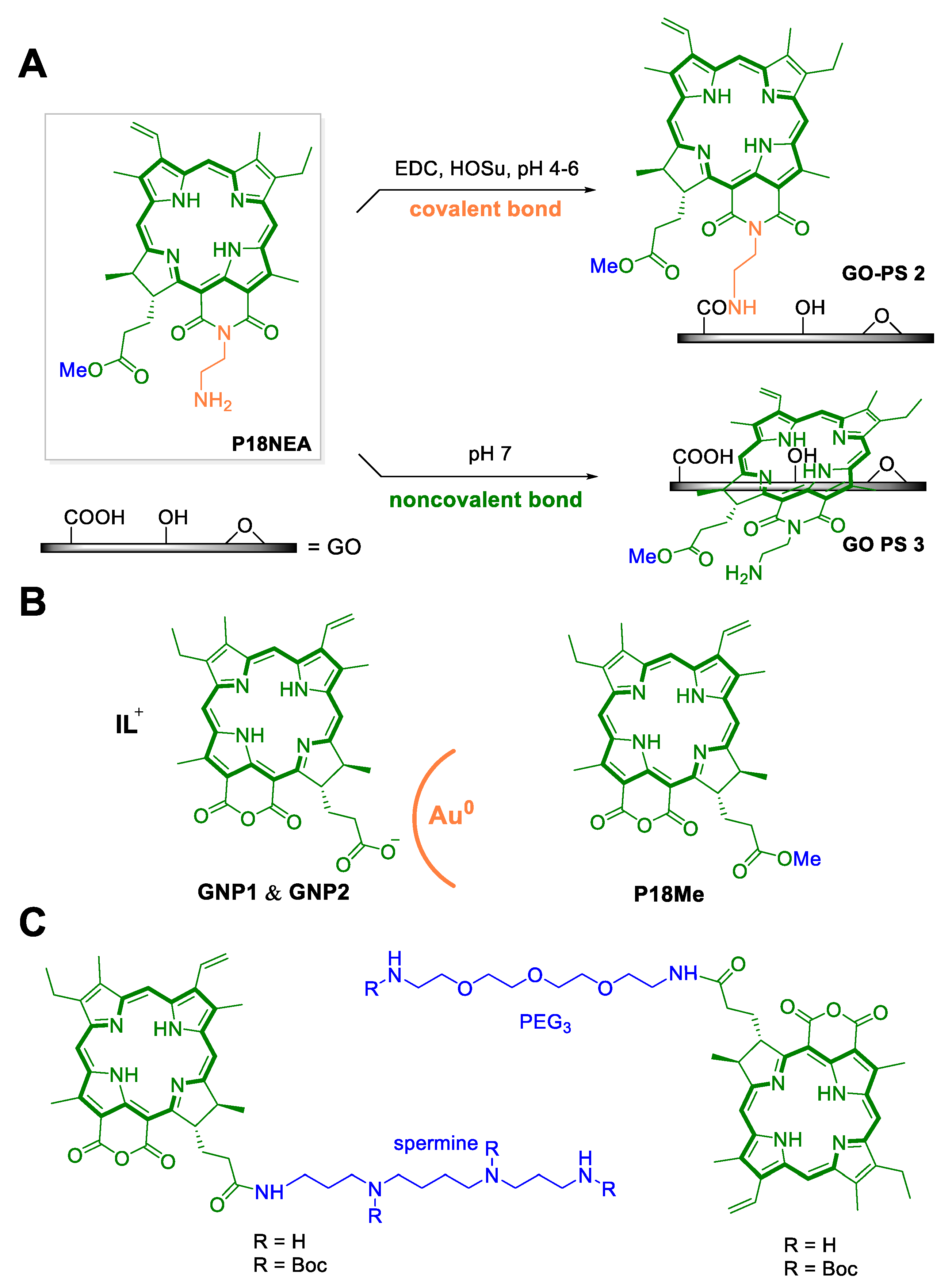
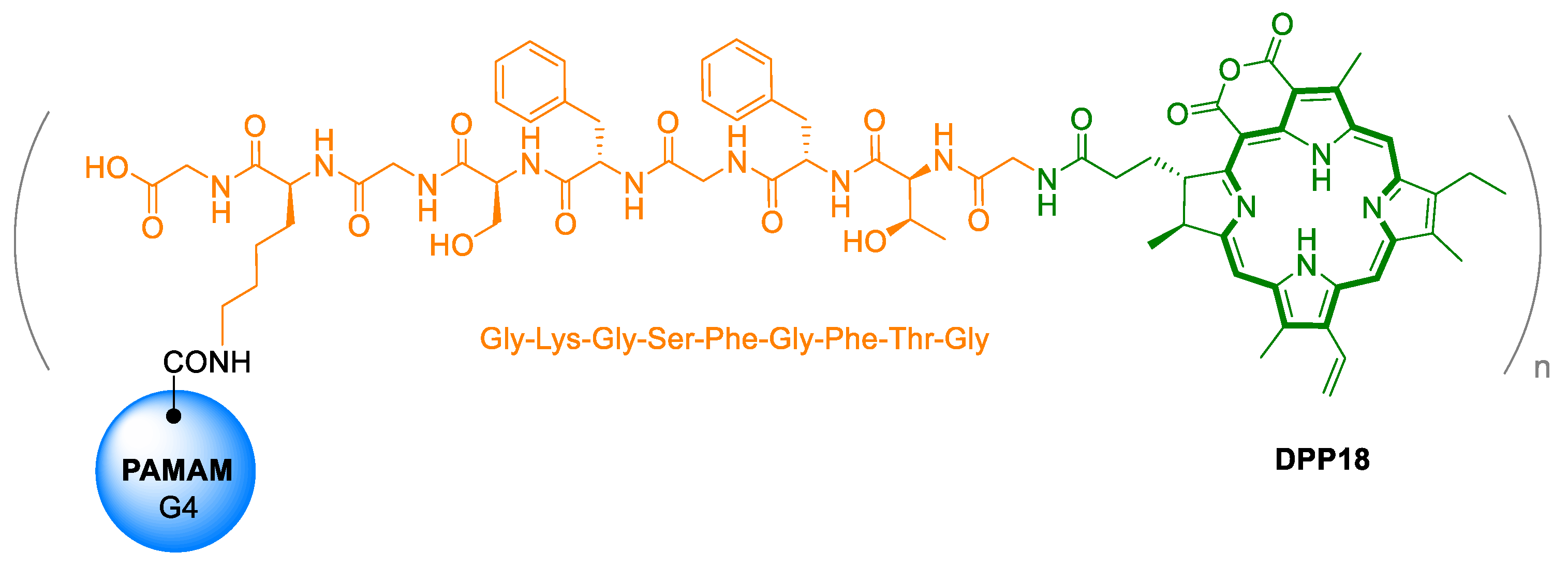
3. Purpurin 18 and Cancer Treatment
4. Photodynamic Therapy
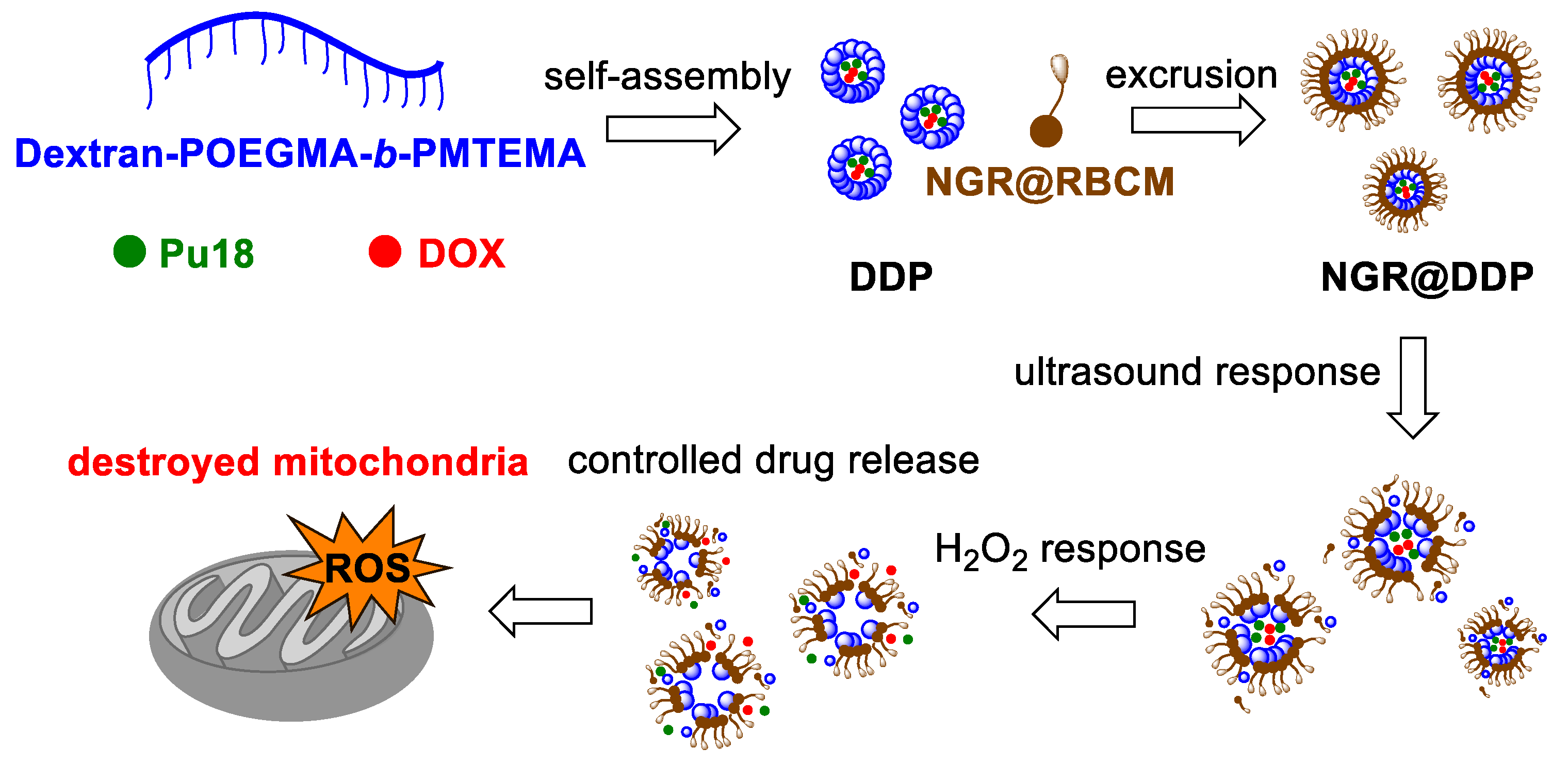
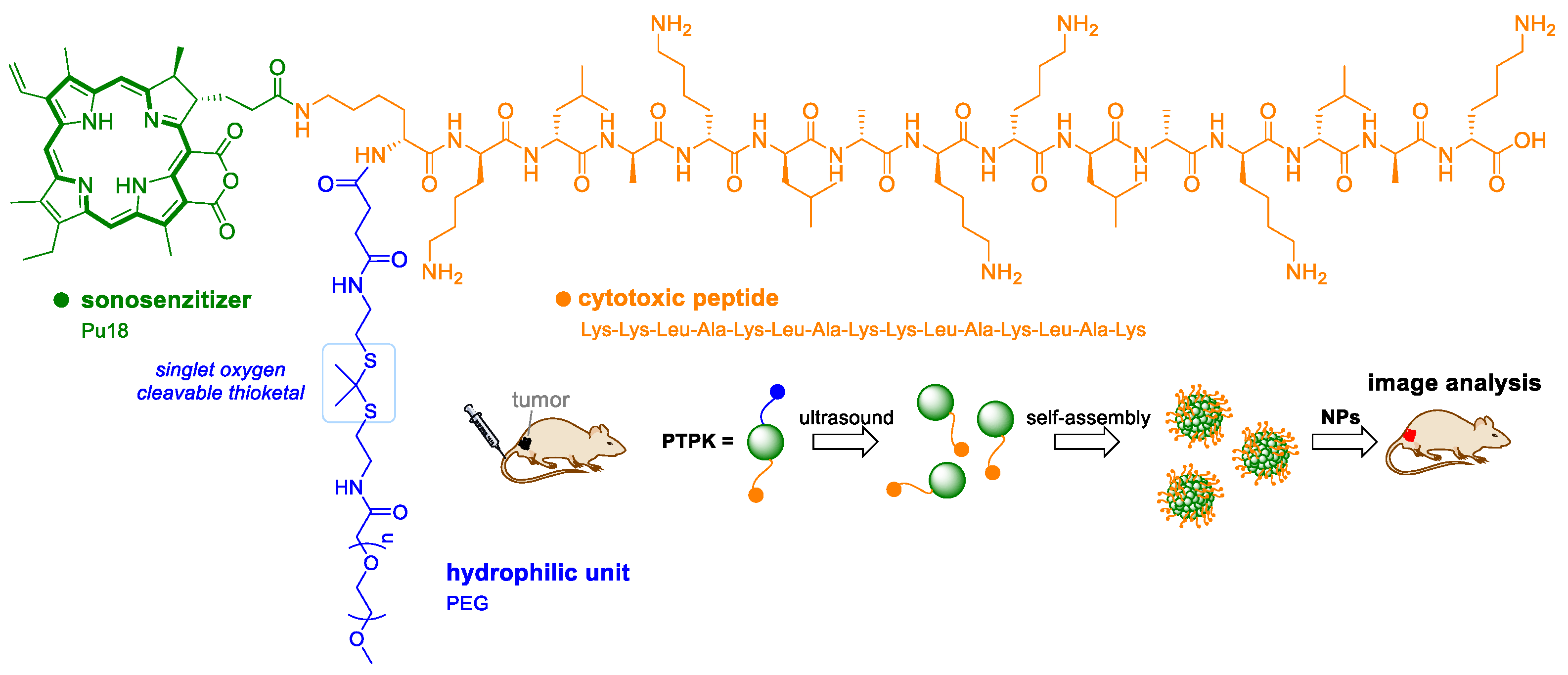
5. Sonodynamic Therapy and Sonophotodynamic Therapy
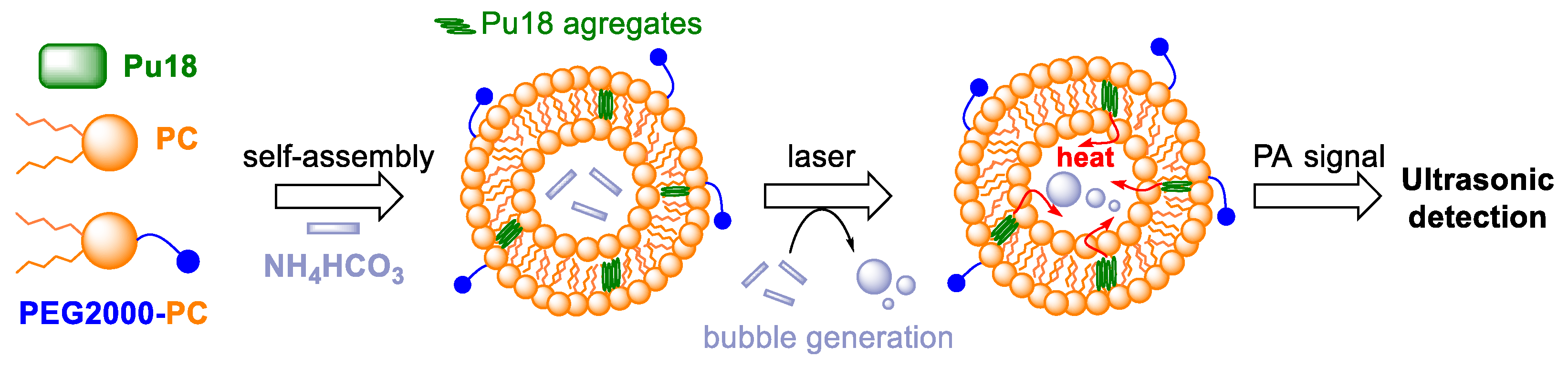
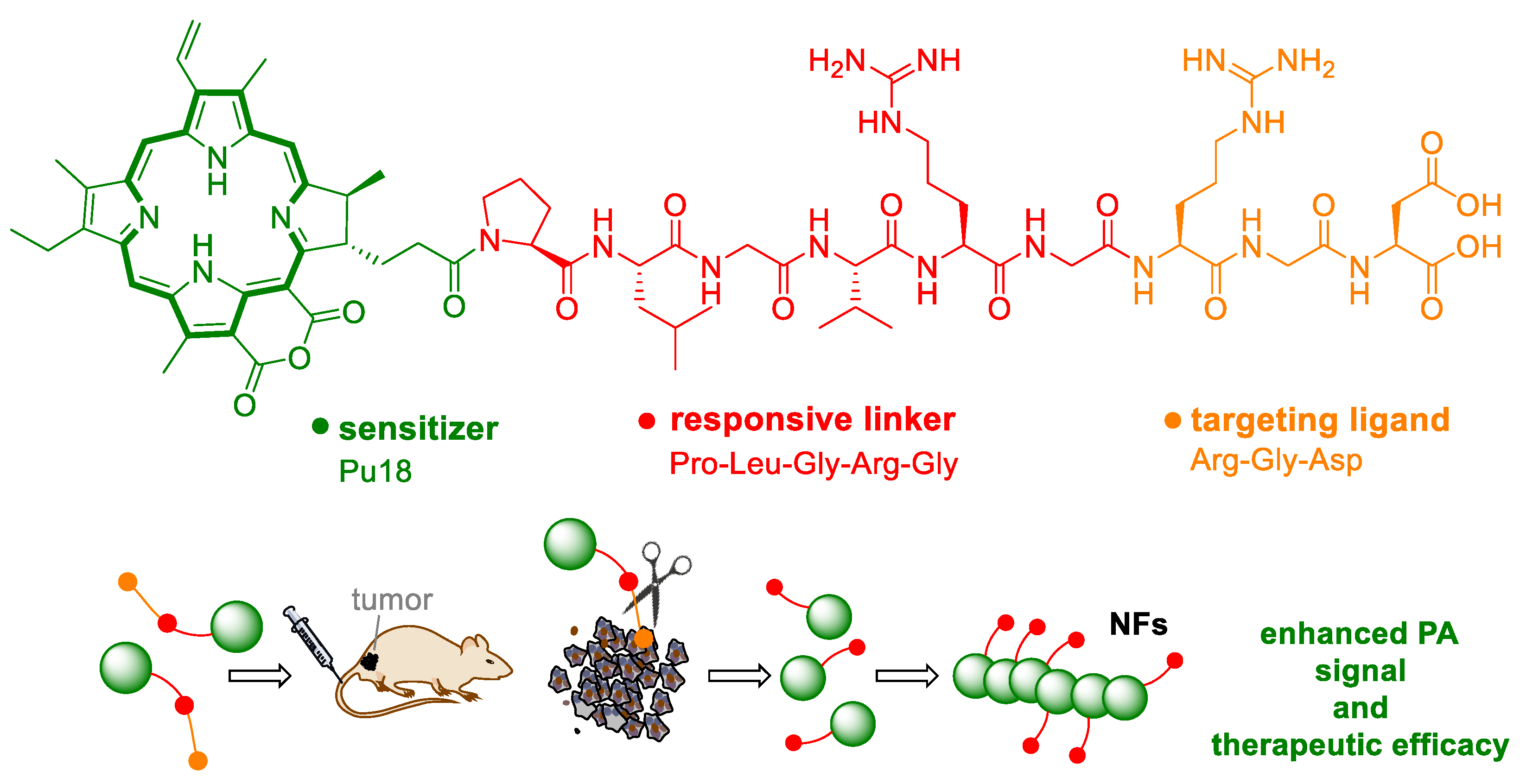
6. Photoacoustic Imaging and Therapy
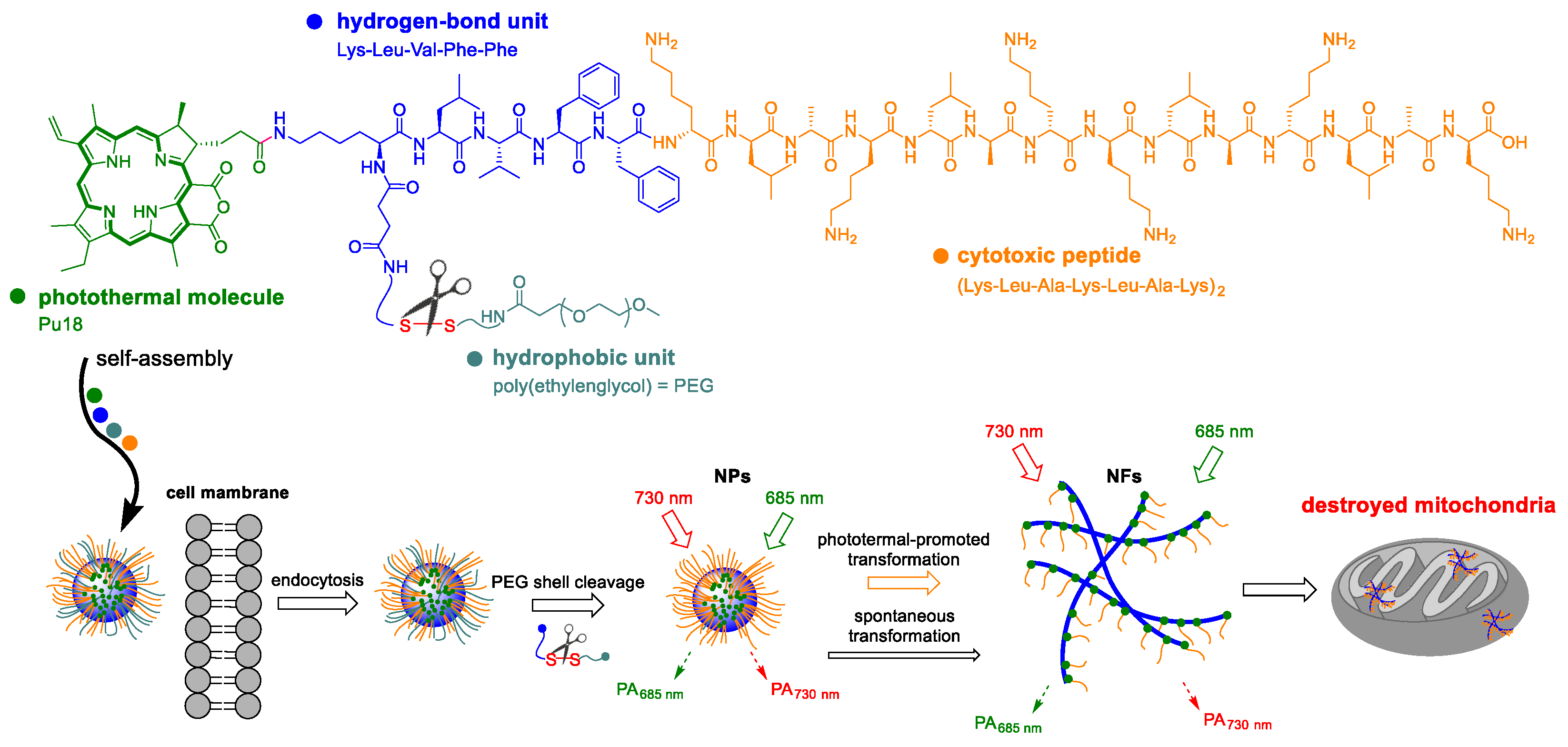
7. Photothermal Therapy
8. Phototeranostic
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | two-dimensional materials |
| 3D | three-dimensional materials |
| AHC⊂L | ammonium hydrogen carbonate encapsulated in liposomes |
| Chl | chlorophyll |
| Chla | chlorophyll a |
| Chlb | chlorophyll b |
| Cyt c | cytochrome c |
| DNA | deoxyribonucleic acid |
| DOX | doxorubicin |
| DPH NGs | well-designed prodrug nanogels |
| ER | endoplasmic reticulum |
| GNPs | gold nanoparticles |
| GNR | gold nanorods |
| GO | graphene oxide |
| HCPT | 10-hydroxycamptothecin |
| IC50 | half maximal inhibitory concentration |
| IL | ionic liquid |
| LED | light-emitting diode |
| MPPa | methyl pyropheophorbide |
| MSC | mesenchymal stem cells |
| NFs | nanofibers |
| NIR | near-infrared region |
| PAI | photoacoustic imaging |
| Pba | pheophorbide a |
| PDT | photodynamic therapy |
| PEG | polyethylene glycol |
| PMTEMA | poly[2-(methylthio)ethyl methacrylate] |
| POEGMA | poly(oligoethylene glycol) methacrylate |
| PS | photosensitizer |
| PTT | photothermal therapy |
| Pu18 | purpurin 18 |
| Pu18ME | purpurin 18 methyl ester |
| RBCM | red blood cell memembrane |
| RGD-Dex | peptide RGD with dextran |
| ROS | reactive oxygen species |
| SDT | sonodynamic therapy |
| ST | sonosensitizer |
| WSONs | water-soluble organic nanoparticles |
| ZnMPPa | zinc metal-introduced methyl pyropheophorbide |
References
- World Health Organization. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 28 December 2020).
- Hopper, C. Photodynamic therapy: A clinical reality in the treatment of cancer. Lancet Oncol. 2000, 1, 212–219. [Google Scholar] [CrossRef]
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine microorganisms as a promising and sustainable source of bioactive molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Oo, Y.Y.N.; Su, M.C.; Kyaw, K.T. Extraction and determination of chlorophyll content from microalgae. Int. J. Adv. Res. Public 2017, 1, 298–301. [Google Scholar]
- Shahidi, F.; Janak Kamil, Y.V.A. Enzymes from fish and aquatic invertebrates and their application in the food industry. Trends Food Sci. Technol. 2001, 12, 435–464. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Andrade, P.B. Marine natural pigments: Chemistry, distribution and analysis. Dyes Pigm. 2014, 111, 124–134. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Khan, S.B.; Kong, C.S.; Kim, J.A.; Kim, S.K. Protective effect of Amphiroa dilatata on ROS induced oxidative damage and MMP expressions in HT1080 cells. Biotechnol. Bioproc. Eng. 2010, 15, 191–198. [Google Scholar] [CrossRef]
- Hosikian, A.; Lim, S.; Halim, R.; Danquah, M.K. Chlorophyll extraction from microalgae: A review on the process engineering aspects. Int. J. Chem. Eng. 2010, 2010, 391632. [Google Scholar] [CrossRef] [Green Version]
- Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Mondal, S.; Seo, H.; Lee, K.D.; Oh, J. Marine natural pigments as potential sources for therapeutic applications. Crit. Rev. Biotechnol. 2018, 38, 745–761. [Google Scholar] [CrossRef]
- Larkum, A.W.D.; Kühl, M. Chlorophyll d: The puzzle resolved. Trends Plant. Sci. 2005, 10, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Rüdiger, W. Biosynthesis of chlorophyll b and the chlorophyll cycle. Photosynth. Res. 2002, 74, 187–193. [Google Scholar] [CrossRef]
- Tanaka, A.; Tanaka, R. Chlorophyll metabolism. Curr. Opin. Plant. Biol. 2006, 9, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kuai, B.; Chen, J.; Hörtensteiner, S. The biochemistry and molecular biology of chlorophyll breakdown. J. Exp. Bot. 2018, 69, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Zvezdanović, J.; Marković, D. Bleaching of chlorophylls by UV irradiation in vitro: The effects on chlorophyll organization in acetone and n-hexane. Serb. Chem. Soc. 2008, 73, 271–282. [Google Scholar] [CrossRef]
- Koca, N.; Karadeniz, F.; Burdurlu, S.H. Effect of pH on chlorophyll degradation and colour loss in blanched green peas. Food Chem. 2007, 100, 609–615. [Google Scholar] [CrossRef]
- Hoober, J.K.; Sery, T.W.; Yamamoto, N. Photodynamic sensitizers from chlorophyll: Purpurin-18 and chlorin p6. Photochem. Photobiol. 1988, 48, 579–582. [Google Scholar] [CrossRef]
- Drogat, N.; Barrière, M.; Granet, R.; Sol, V.; Krausz, P. High yield preparation of purpurin-18 from Spirulina maxima. Dyes Pigm. 2011, 88, 125–127. [Google Scholar] [CrossRef]
- Watanabe, N.; Yamamoto, K.; Ihshikawa, H.; Yagi, A.; Sakata, K.; Brinen, L.S.; Clardy, J. New chlorophyll-a-related compounds isolated as antioxidants from marine bivalves. J. Nat. Prod. 1993, 56, 305–317. [Google Scholar] [CrossRef]
- Louda, J.W.; Neto, R.R.; Magalhaes, A.R.M.; Schneider, V.F. Pigment alterations in the brown mussel Perna perna. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 150, 385–394. [Google Scholar] [CrossRef]
- Chen, K.; Ríos, J.J.; Pérez-Gálvez, A.; Roca, M. Comprehensive chlorophyll composition in the main edible seaweeds. Food Chem. 2017, 228, 625–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocampo, R.; Repeta, D.J. Structural determination of purpurin-18 (as methyl ester) from sedimentary organic matter. Org. Geochem. 1999, 30, 189–193. [Google Scholar] [CrossRef]
- Zenkevich, E.; Sagun, E.; Knyukshto, V.; Shulga, A.; Mironov, A.; Efremova, O.; Bonnett, R.; Songca, S.P.; Kassem, M. Photophysical and photochemical properties of potential porphyrin and chlorin photosensitizers for PDT. J. Photochem. Photobiol. B Biol. 1996, 33, 171–180. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, Z.J.; Cai, J.Q.; Li, L.L.; Wang, H. Synthesis and self-assembly behavior of chlorophyll derivatives for ratiometric photoacoustic signal optimization. Langmuir 2020, 36, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Qi, G.B.; Zhao, Y.X.; Qiao, S.L.; Yang, C.; Wang, H. In situ formation of nanofibers from purpurin18-peptide conjugates and the assembly induced retention effect in tumor sites. Adv. Mater. 2015, 27, 6125–6130. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Chandel, N.S. The two faces of reactive oxygen species in cancer. Ann. Rev. Cancer Biol. 2017, 1, 79–98. [Google Scholar] [CrossRef]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, K. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef]
- Richards-Kortum, R.; Sevick-Muraca, E. Quantitative optical spectroscopy for tissue diagnosis. Annu. Rev. Phys. Chem. 1996, 47, 555–606. [Google Scholar] [CrossRef] [Green Version]
- Hemmer, E.; Benayas, A.; Légaréa, F.; Vetrone, F. Exploiting the biological windows: Current perspectives on fluorescent bioprobes emitting above 1000 nm. Nanoscale Horiz. 2016, 1, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; Ettorre, A.; Sbrana, S.; Giovani, C.; Neri, P. Purpurin-18 in combination with light leads to apoptosis or necrosis in HL60 leukemia cells. Photochem. Photobiol. 2001, 73, 290–296. [Google Scholar] [CrossRef]
- Magi, B.; Ettorre, A.; Liberatori, S.; Bini, L.; Andreassi, M.; Frosali, S.; Neri, P.; Pallini, V.; Di Stefano, A. Selectivity of protein carbonylation in the apoptotic response to oxidative stress associated with photodynamic therapy: A cell biochemical and proteomic investigation. Cell Death Differ. 2004, 11, 842–852. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Zhang, B.; Yuan, Q.; Zhang, X.; Leung, W.; Xu, C. Photodynamic treatment with purpurin 18 effectively inhibits triple negative breast cancer by inducing cell apoptosis. Lasers Med. Sci. 2021, 36, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Potter, W.R.; SumLin, A.; Dougherty, T.J.; Pandey, R.K. Photosensitizers related to purpurin-18-N-alkylimides: A comparative in vivo tumoricidal ability of ester versus amide functionalities. Bioorg. Med. Chem. Lett. 2000, 10, 123–127. [Google Scholar] [CrossRef]
- Wang, J.J.; Yin, Y.F.; Yang, Z. Synthesis of purpurin-18 imide derivatives from chlorophyll-a and-b by modifications and functionalizations along their peripheries. J. Iran. Chem. Soc. 2013, 10, 583–591. [Google Scholar] [CrossRef]
- Wipo IP Portal. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO1995032206 (accessed on 24 February 2021).
- Lens.org. Available online: https://www.lens.org/lens/patent/US_5591847_A/fulltext (accessed on 24 February 2021).
- Pavlíčková, V.; Rimpelová, S.; Jurášek, M.; Záruba, K.; Fähnrich, J.; Křížová, I.; Bejček, J.; Rottnerová, Z.; Spiwok, V.; Drašar, P.; et al. PEGylated purpurin 18 with improved solubility: Potent compounds for photodynamic therapy of cancer. Molecules 2019, 24, 4477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, H.; Wang, Z.; Jin, Y. pH-Sensitive graphene oxide conjugate purpurin-18 methyl ester photosensitizer nanocomplex in photodynamic therapy. New J. Chem. 2018, 42, 13272–13284. [Google Scholar] [CrossRef]
- Kang, E.S.; Lee, T.H.; Liu, Y.; Han, K.H.; Lee, W.K.; Yoon, I. Graphene oxide nanoparticles having long wavelength absorbing chlorins for highly-enhanced photodynamic therapy with reduced dark toxicity. Int. J. Mol. Sci. 2019, 20, 4344. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lee, T.H.; Lee, S.H.; Li, J.; Lee, W.K.; Yoon, I. Mitochondria-targeted water-soluble organic nanoparticles of chlorin derivatives for biocompatible photodynamic therapy. ChemNanoMat 2020, 6, 610–617. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, S.H.; Lee, W.K.; Yoon, I. Ionic liquid-dependent gold nanoparticles of purpurin-18 for cellular imaging and photodynamic therapy in vitro. Bull. Korean Chem. Soc. 2020, 41, 230–233. [Google Scholar] [CrossRef]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. Med. Chem. Commun. 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Darmostuk, M.; Jurášek, M.; Lengyel, K.; Zelenka, J.; RumLová, M.; Drašar, P.; RumL, T. Conjugation of chlorins with spermine enhances phototoxicity to cancer cells in vitro. J. Photochem. Photobiol. B Biol. 2017, 168, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Yang, M.; Zhu, Y.; Qu, X.; Mao, C. Stem cells loaded with nanoparticles as a drug carrier for in vivo breast cancer therapy. Adv. Mater. 2014, 26, 4627–4631. [Google Scholar] [CrossRef]
- Bechet, D.; Auger, F.; Couleaud, P.; Marty, E.; Ravasi, L.; Durieux, N.; Bonnet, C.; Plénat, F.; Frochot, C.; Mordon, S.; et al. Multifunctional ultrasmall nanoplatforms for vascular-targeted interstitial photodynamic therapy of brain tumors guided by real-time MRI. Nanomedicine 2015, 11, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Mallidi, S.; Zheng, X.; Rahmanzadeh, R.; Mir, Y.; Elrington, S.; Khurshid, A.; Hasan, T. Development and applications of photo-triggered theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1094–1124. [Google Scholar] [CrossRef] [Green Version]
- Lustig, R.A.; Vogl, T.J.; Fromm, D.; Cuenca, R.; Hsi, A.; D’Cruz, A.K.; Krajina, Z.; Turić, M.; Singhal, A.; Chen, J.C. A multicenter phase I safety study of intratumoral photoactivation of talaporfin sodium in patients with refractory solid tumors. Cancer 2003, 98, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Keltner, L.; Christophersen, J.; Zheng, F.; Krouse, M.; Singhal, A.; Wang, S. New technology for deep light distribution in tissue for phototherapy. Cancer J. 2002, 8, 154–163. [Google Scholar] [CrossRef]
- Kidd, S.; Spaeth, E.; Dembinski, J.L.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, V.L.; Weil, M.; Andreeff, M.; Marini, F.C. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 2009, 27, 2614–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.J.; Zhang, H.; Huang, H.; Wang, X.M.; Zhou, Z.Y.; Cui, F.Z.; An, Y.H. In vitro behavior of neural stem cells in response to different chemical functional groups. Biomaterials 2009, 30, 1036–1044. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, T.; Qiu, W.; Liang, M.; Gao, Y.; Xue, P.; Kang, Y.; Xu, Z. Bioresponsive prodrug nanogel-based polycondensate strategy deepens tumor penetration and potentiates oxidative stress. Chem. Eng. J. 2020, 127657. [Google Scholar] [CrossRef]
- Jia, D.; Ma, X.; Lu, Y.; Li, X.; Hou, S.; Gao, Y.; Xue, P.; Kang, Y.; Xu, Z. ROS-responsive cyclodextrin nanoplatform for combined photodynamic therapy and chemotherapy of cancer. Chin. Chem. Lett. 2020. [Google Scholar] [CrossRef]
- Yumita, N.; Nishigaki, R.; Umemura, K.; Umemura, S. Hematoporphyrin as a sensitizer of cell-damaging effect of ultrasound. Jpn. J. Cancer Res. 1989, 80, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Yumita, N.; Nishigaki, R.; Umemura, S. Sonodynamically induced antitumor effect of photofrin II on colon 26 carcinoma. J. Cancer Res. Clin. Oncol. 2000, 126, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Kudo, N.; Yamaguchi, S.; Sumiyoshi, K.; Motegi, H.; Kobayashi, H.; Terasaka, S.; Houkin, K. Porphyrin derivatives-mediated sonodynamic therapy for malignant gliomas in vitro. Ultrasound Med. Biol. 2015, 41, 2458–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.H.; Miyoshi, N.; Ishiguro, K.; Umemura, S.; Kawabata, K.; Yumita, N.; Sakata, I.; Takaoka, K.; Udagawa, T.; Nakajima, S.; et al. Combination effect of photodynamic and sonodynamic therapy on experimental skin squamous cell carcinoma in C3H/HeN mice. J. Dermatol. 2000, 27, 294–306. [Google Scholar] [CrossRef]
- Umemura, K.; Yumita, N.; Nishigaki, R.; Umemura, S. Sonodynamically induced antitumor effect of pheophorbide a. Cancer Lett. 1996, 102, 151–157. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Rosenthal, I.; Sostaric, J.Z.; Riesz, P. Sonodynamic therapy-a review of synergistic effects of drugs and ultrasound. Ultrason Snochem. 2004, 11, 349–363. [Google Scholar] [CrossRef]
- Canavese, G.; Ancona, A.; Racca, L.; Canta, M.; Dumontel, B.; Barbaresco, F.; Limongi, T.; Cauda, V. Nanoparticle-assisted ultrasound: A special focus on sonodynamic therapy against cancer. Chem. Eng. J. 2018, 340, 155–172. [Google Scholar] [CrossRef]
- Mišík, V.; Riesz, P. Free radical intermediates in sonodynamic therapy. Ann. N. Y. Acad. Sci. 2000, 899, 335–348. [Google Scholar] [CrossRef]
- McHale, A.P.; Callan, J.F.; Nomikou, N.; Fowley, C.; Callan, B. Sonodynamic therapy: Concept, mechanism and application to cancer treatment. In Therapeutic Ultrasound. Advances in Experimental Medicine and Biology; Escoffre, J.M., Bouakaz, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 880, pp. 429–450. ISBN 978-3-319-22536-4. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Zhang, Y.; Chitgupi, U.; Geng, J.; Wang, Y.; Zhang, Y.; Cook, T.R.; Xia, J.; Lovell, J.F. A phosphorus phthalocyanine formulation with intense absorbance at 1000 nm for deep optical imaging. Theranostics 2016, 6, 688–697. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Tian, Y.; Xu, S.; Ren, E.; Bai, S.; Chen, X.; Chu, C.; Xu, Z.; Liu, G. Multi-responsive bottlebrush-like unimolecules self-assembled nano-riceball for synergistic sono-chemotherapy. Small Methods 2020, 2000416. [Google Scholar] [CrossRef]
- Enyedi, K.N.; Tóth, S.; Szakács, G.; Mező, G. NGR-peptide−drug conjugates with dual targeting properties. PLoS ONE 2017, 12, e0178632. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Wu, J.; Jin, L.; Hong, L.; Wang, F.; Mao, Z.; Wu, M. Cancer cell membrane-coated gold nanorods for photothermal therapy and radiotherapy on oral squamous cancer. J. Mater. Chem. B 2020, 8, 7253–7263. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.B.; Zhang, X.H.; Chen, Y.; Chen, H.; Qiao, Z.Y.; Wang, H. Ultrasound-activated cascade effect for synergistic orthotopic pancreatic cancer therapy. iScience 2020, 23, 101144. [Google Scholar] [CrossRef]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.S.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef]
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT03897270 (accessed on 28 December 2020).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT04428528?term=photoacoustic&cond=Cancer&draw=2&rank=8 (accessed on 28 December 2020).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT04110249?term=NCT04110249&draw=2&rank=1 (accessed on 28 December 2020).
- Available online: https://clinicaltrials.gov/ct2/show/NCT04437030?term=photoacoustic&cond=Cancer&draw=2&rank=9 (accessed on 28 December 2020).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT04248166?term=photoacoustic&cond=Cancer&draw=2&rank=1 (accessed on 28 December 2020).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT04339374?term=photoacoustic&cond=Cancer&draw=2&rank=6 (accessed on 28 December 2020).
- Kim, C.; Song, K.H.; Gao, F.; Wang, L.V. Sentinel lymph nodes and lymphatic vessels: Noninvasive dual-modality in vivo mapping by using indocyanine green in rats—volumetric spectroscopic photoacoustic imaging and planar fluorescence imaging. Radiology 2010, 255, 442–450. [Google Scholar] [CrossRef]
- Song, K.H.; Stein, E.W.; Margenthaler, J.A.; Wang, L.V. Noninvasive photoacoustic identification of sentinel lymph nodes containing methylene blue in vivo in a rat model. J. Biomed. Opt. 2008, 13, 054033. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wang, Z.; Wang, L.; Wang, Z.; Wang, H.; Li, G.; Qiao, Z.Y.; Xu, W.; Wang, H. High-performance identification of human bladder cancer using a signal self-amplifiable photoacoustic nanoprobe. ACS Appl. Mater. Interfaces 2018, 10, 28331–28339. [Google Scholar] [CrossRef]
- Zhao, L.H.; Lin, Q.L.; Wei, J.; Huai, Y.L.; Wang, K.J.; Yan, H.Y. CD44v6 expression in patients with stage II or stage III sporadic colorectal cancer is superior to CD44 expression for predicting progression. Int. J. Clin. Exp. Pathol. 2015, 8, 692–701. [Google Scholar] [PubMed]
- Ma, L.; Dong, L.; Chang, P. CD44v6 engages in colorectal cancer progression. Cell Death Dis. 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Cao, M.; He, Y.; Zhang, G.; Liu, Y.; Du, Y.; Yang, C.; Gao, F. CD44v6 targeted by miR-193b-5p in the coding region modulates the migration and invasion of breast cancer cells. J. Cancer 2020, 11, 260–271. [Google Scholar] [CrossRef]
- Lipponen, P.; Aaltoma, S.; Kosma, V.M.; Ala-Opas, M.; Eskelinen, M. Expression of CD44 standard and variant-v6 proteins in transitional cell bladder tumours and their relation to prognosis during a long-term follow-up. J. Pathol. 1998, 186, 157–164. [Google Scholar] [CrossRef]
- Zhang, X.H.; Cheng, D.B.; Ji, L.; An, H.W.; Wang, D.; Yang, Z.X.; Chen, H.; Qiao, Z.Y.; Wang, H. Photothermal-promoted morphology transformation in vivo monitored by photoacoustic imaging. Nano Lett. 2020, 20, 1286–1295. [Google Scholar] [CrossRef]
- Cheng, D.B.; Qi, G.B.; Wang, J.Q.; Cong, Y.; Liu, F.H.; Yu, H.; Qiao, Z.Y.; Wang, H. In situ monitoring intracellular structural change of nanovehicles through photoacoustic signals based on phenylboronate-linked RGD-dextran/purpurin 18 conjugates. Biomacromolecules 2017, 18, 1249–1258. [Google Scholar] [CrossRef]
- Xiao, B.; Hong, L.; Cai, X.; Mei, S.; Zhang, P.; Shao, L. The true colors of autophagy in doxorubicin-induced cardiotoxicity. Oncol. Lett. 2019, 18, 2165–2172. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Lu, L.; Yan, S.; Yi, H.; Yao, H.; Wu, D.; He, G.; Tao, X.; Deng, X. Autophagy and doxorubicin resistance in cancer. Anti-Cancer Drugs 2018, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy Levy, J.M.; Thorburn, A. Autophagy in cancer: Moving from understanding mechanism to improving therapy responses in patients. Cell Death Diff. 2020, 27, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.X.; Wang, Y.; Qiao, S.L.; An, H.W.; Wang, J.; Ma, Y.; Wang, L.; Wang, H. “In vivo self-assembled” nanoprobes for optimizing autophagy-mediated chemotherapy. Biomaterials 2017, 141, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Zhang, D.; Li, L.L.; Qiao, Z.Y.; Zhang, J.C.; Zhao, Y.X.; Qi, G.B.; Wan, D.; Pan, J.; Wang, H. In situ construction and characterization of chlorin-based supramolecular aggregates in tumor cells. ACS Appl. Mater. Interfaces 2016, 8, 22875–22883. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.B.; Lee, T.H.; Yoon, I.; Shim, Y.K.; Lee, W.K. Gold nanorod–photosensitizer complex obtained by layer-by-layer method for photodynamic/photothermal therapy in vitro. Chem. Asian J. 2015, 10, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.H. An introduction to the clinical practice of theranostics in oncology. Br. J. Radiol. 2018, 91, 20180440. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Gao, Y.; Wang, P.; He, G.; Blum, N.T.; Lin, J.; Liu, Q.; Wang, X.; Huang, P. Six birds with one stone: Versatile nanoporphyrin for single-laser-triggered synergistic phototheranostics and robust immune activation. Adv. Mater. 2020, 32, 2004481. [Google Scholar] [CrossRef] [PubMed]




| Drug | Cell Line | Light Dose | IC50 Light | IC50 Dark | Growth Inhibition Light | Ref. |
|---|---|---|---|---|---|---|
| Pu18 | Human fibroblast | 5 W·m−2 | - | - | 50% (from 10 µM) | [18] |
| HL-60 | 1 J·cm−2 | - | - | from 0.2 µM | [31] | |
| 4T1 | 0.3 J·cm−2 | - | >10 µM | >50% (from 0.5 µM) | [33] | |
| 0.74 J·cm−2 | - | >50% (from 0.25 µM) | ||||
| 1.59 J·cm−2 | - | |||||
| 3.7 J·cm−2 | - | |||||
| Pu18+PEG3 1 | LNCa PPC-3 U-2 OS MiaPaCa-2 MCF-7 HeLa | 4 J·cm−2 | 0.02 µM 0.65 µM 1.83 µM 0.45 µM 0.59 µM 0.02 | >10 µM | [38] | |
| GO6-Pu18 | HepG2 | 5 mW·cm−2 | 1.4 mg·mL−1 | >5 mg·mL−1 | [39] | |
| GO6-PS2 | A459 | 2 J·cm−2 | 0.31 µM | >14 µM | [40] | |
| GO6-PS3 | 0.20 µM | |||||
| Pu18ME-WSON 2 | HeLa | 0.83 µM | >10 µM | [41] | ||
| GNP1 3 | A549 | 0.38 μg·mL−1 | >20 μg·mL−1 | [42] | ||
| GNP2 3 | 4.34 μg·mL−1 | |||||
| DPH NGs 4 | 4T1 | 0.5 W·cm−2 | - | - | >50% (HCPT 8: 2 μg·mL−1, pu18: 5 μg·mL−1) | [53] |
| Pu18 | - | - | 38.72%(8.7 μg·mL−1) | [51] | ||
| PDP 5 | - | - | 82.83% (DOX 7: 5 μg·mL−1, p18: 8.7 μg·mL−1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlíčková, V.; Škubník, J.; Jurášek, M.; Rimpelová, S. Advances in Purpurin 18 Research: On Cancer Therapy. Appl. Sci. 2021, 11, 2254. https://doi.org/10.3390/app11052254
Pavlíčková V, Škubník J, Jurášek M, Rimpelová S. Advances in Purpurin 18 Research: On Cancer Therapy. Applied Sciences. 2021; 11(5):2254. https://doi.org/10.3390/app11052254
Chicago/Turabian StylePavlíčková, Vladimíra, Jan Škubník, Michal Jurášek, and Silvie Rimpelová. 2021. "Advances in Purpurin 18 Research: On Cancer Therapy" Applied Sciences 11, no. 5: 2254. https://doi.org/10.3390/app11052254









