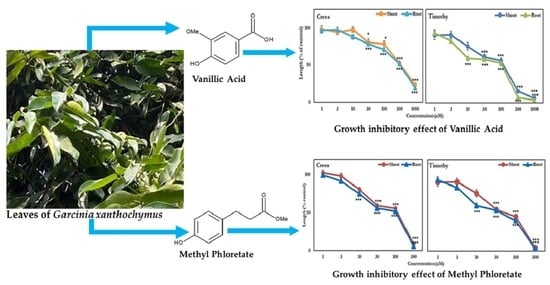
Identification and Application of Bioactive Compounds from Garcinia xanthochymus Hook. for Weed Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Sample
2.2. Model Test Species
2.3. Extraction and Bioassay
2.4. Purification of The Active Substances
2.5. Bioassay of the Isolated Compounds
2.6. Statistical Analysis
3. Results
3.1. Phytotoxic Activity of the G. Xanthochymus Extract
3.2. Bioativity of Different Fractions in The Separtion Steps
3.3. Characterization of The Compounds
3.4. Biological Activity of the Isolated Substances
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Colbach, N.; Darmency, H.; Fernier, A.; Granger, S.; Corre, V.L.; Messéan, A. Simulating changes in cropping practices in conventional and glyphosate-resistant maize. II. Weed impacts on crop production and biodiversity. Environ. Sci. Pollut. Res. 2017, 24, 13121–13135. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Chen, M.; Ye, H.C.; Zhang, Z.K.; Li, H.; Chen, L.L.; Zhang, J. Herbicidal activities of compounds isolated from the medicinal plant Piper sarmentosum. Ind. Crops Prod. 2019, 132, 41–47. [Google Scholar] [CrossRef]
- Magnoli, K.; Carranza, C.S.; Aluffi, M.E.; Magnoli, C.E.; Barberis, C.L. Herbicides based on 2, 4-D: Its behavior in agricultural environments and microbial biodegradation aspects. A review. Environ. Sci. Pollut. Res. Int. 2020, 27, 38501–38512. [Google Scholar] [CrossRef]
- Moss, S.; Ulber, L.; Hoed, I.D. A herbicide resistance risk matrix. Crop Prot. 2019, 115, 13–19. [Google Scholar] [CrossRef]
- Hossain, M.M. Recent perspective of herbicide: Review of demand and adoption in world agriculture. J. Bangladesh Agric. Univ. 2015, 13, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, A.I.; Pueyo, Y.; Pellissier, F.; Ramos, J.; Espinosa-Ruiz, A.; Millery, A.; Alados, C.L. Phytotoxic effects of volatile and water-soluble chemicals of Artemisia herba-alba. J. Arid Environ. 2018, 151, 1–8. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Duke, S.O.; Dayan, F.E.; Romagni, J.G.; Rimando, A.M. Natural products as sources of herbicides: Current status and future trends. Weed Res. 2000, 40, 99–111. [Google Scholar] [CrossRef]
- Hooker, J.D. The Flora of British India; L. Reeve and Co.: London, UK, 1874. [Google Scholar]
- Verheij, E.W.M.; Coronel, R.E. Edible fruits and nuts. In Plant Resources of South-East Asia (PROSEA); Backhuys Publishers: Kerkwerve, The Netherlands, 1991; p. 175. [Google Scholar]
- Zhong, F.F.; Chen, Y.; Song, F.J.; Yang, G.Z. Three new xanthones from Garcinia xanthochymus. Acta Pharm. Sinica. 2008, 43, 938–941. [Google Scholar]
- Joseph, K.S.; Dandin, V.S.; Hosakatte, N.M. Chemistry and biological activity of Garcinia xanthochymus: A review. J. Biol. Act. Prod. Nat. 2016, 6, 173–194. [Google Scholar] [CrossRef]
- Perry, L.M.; Metzger, J. Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses; MIT Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Trisuwan, K.; Boonyaketgoson, S.; Rukachaisirikul, V.; Phongpaichit, S. Oxygenated xanthones and bioflavonoids from the twigs of Garcinia xanthochymus. Tetrahedron Lett. 2014, 55, 3600–3602. [Google Scholar] [CrossRef]
- Hassan, N.K.N.C.; Taher, M.; Susanti, D. Phytochemical constituents and pharmacological properties of Garcinia xanthochymus—A review. Biomed. Pharmacother. 2018, 106, 1378–1389. [Google Scholar] [CrossRef]
- Rob, M.M.; Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity and identification of phytotoxic substances from Schumannianthus dichotomus. Plants 2020, 9, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IBM, Corp. IBM SPSS Statistics for Windows; Version 16.0; IBM Corp: Armonk, NY, USA, 2016. [Google Scholar]
- Stalin, T.; Rajendiran, N. A study on the spectroscopy and photophysics of 4-hydroxy-3-methoxybenzoic acid in different solvents, pH and β-cyclodextrin. J. Mol. Struct. 2006, 794, 35–45. [Google Scholar] [CrossRef]
- Clough, J.M.; Jones, R.V.; McCann, H.; Morris, D.J.; Wills, M. Synthesis and hydrolysis studies of a peptide containing the reactive triad of serine proteases with an associated linker to a dye on a solid phase support. Org. Biomol. Chem. 2003, 1, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- Bari, I.N.; Kato-Noguchi, H. Phytotoxic effect of Filicium decipiens leaf extract. Am. Eurasian J. Agric. Environ. Sci. 2017, 17, 288–292. [Google Scholar] [CrossRef]
- Islam, M.S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. 2-Methoxystypandrone, a potent phytotoxic substance in Rumex maritimus L. Theor. Exp. Plant Physiol. 2017, 29, 195–202. [Google Scholar] [CrossRef]
- Islam, M.S.; Zaman, F.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic potential of Chrysopogon aciculatus (Retz.) Trin. (Poaceae). Weed Biol. Manag. 2019, 19, 51–58. [Google Scholar] [CrossRef]
- Hossen, K.; Das, K.R.; Okada, S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathic potential and active substances from Wedelia chinensis (Osbeck). Foods 2020, 9, 1591. [Google Scholar] [CrossRef]
- Hossen, K.; Kato-Noguchi, H. Determination of allelopathic properties of Acacia catechu (L.f.) Willd. Not. Bot. Horti Agrobot. Cluj Napoca 2020, 48, 2050–2059. [Google Scholar] [CrossRef]
- Zaman, F.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathic potential and identification of two allelopathic substances in Eleocharis atropurpurea. Plant Biosys. 2020. [Google Scholar] [CrossRef]
- Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic Activity and Growth Inhibitory Substances from Albizia richardiana (Voigt.) King & Prain. Appl. Sci. 2021, 11, 1455. [Google Scholar] [CrossRef]
- Rob, M.M.; Kato-Noguchi, H. Study of the allelopathic activity of Garcinia pedunculata Roxb. Plant Omics. 2019, 12, 31–36. [Google Scholar] [CrossRef]
- Tuyen, P.T.; Xuan, T.D.; Anh, T.T.T.; Van, T.M.; Ahmad, A.; Elzaawely, A.A.; Khanh, T.D. Weed suppressing potential and isolation of potent plant growth inhibitors from Castanea crenata Sieb. et Zucc. Molecules 2018, 23, 345. [Google Scholar] [CrossRef] [Green Version]
- Khadem, S.; Marles, R.J. Monocyclic phenolic acids; hydroxy-and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef]
- Batish, D.R.; Lavanya, K.; Pal, H.S.; Kohli, R.K. Root-mediated allelopathic interference of nettle-leaved goosefoot (Chenopodium murale) on wheat (Triticum aestivum). J. Agron. Crop. Sci. 2007, 193, 37–44. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bai, F.; Fang, L.; Hu, H.; Yang, Y.; Feng, X.; Sun, D. Vanillic acid mitigates the ovalbumin (OVA)-induced asthma in rat model through prevention of airway inflammation. Biosci. Biotechnol. Biochem. 2019, 83, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-T.; Zheng, C.-Y.; Hu, W.; Xu, W.-W.; Wang, H.-F. The allelopathy and allelopathic mechanism of phenolic acids on toxic Microcystis aeruginosa. J. Appl. Phycol. 2010, 22, 71–77. [Google Scholar] [CrossRef]
- Comi, M.; Lligadas, G.; Ronda, J.C.; Galia, M.; Cadiz, V. Renewable benzoxazine monomers from ″lignin-like″ naturally occurring phenolic derivatives. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4894–4903. [Google Scholar] [CrossRef]
- Picinelli, A.; Dapena, E.; Mangas, J.J. Polyphenolic pattern in apple tree leaves in relation to scab resistance. A preliminary study. J. Agric. Food Chem. 1995, 43, 2273–2278. [Google Scholar] [CrossRef] [Green Version]
- Trejo-Machin, A.; Verge, P.; Puchot, L.; Quintana, R. Phloretic acid as an alternative to the phenolation of aliphatic hydroxyls for the elaboration of polybenzoxazine. Green Chem. 2017, 19, 5065–5073. [Google Scholar] [CrossRef]
- Sasaki, S.; Kitamura, S.; Negoro, N.; Suzuki, M.; Tsujihata, Y.; Suzuki, N.; Kobayashi, M. Design, synthesis, and biological activity of potent and orally available G protein-coupled receptor 40 agonists. J. Med. Chem. 2011, 54, 1365. [Google Scholar] [CrossRef]
- Suzuki, M.; Chozin, M.A.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity of Chinese violet (Asystasia gangetica (L.) T. Anderson) and two phytotoxic substances. Weed Biol. Manag. 2019, 19, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Piyatida, P.; Suenaga, K.; Kato-Noguchi, H. Allelopathic potential and chemical composition of Rhinacanthus nasutus extracts. Allelopath. J. 2010, 26, 207–216. [Google Scholar]
- Macias, F.A.; Galindo, J.C.G.; Massanet, G.M. Potentials allelopathic activity of several sesquiterpene lactone models. Phytochemistry 1992, 31, 1969–1977. [Google Scholar] [CrossRef]
- Pèrez, F.J. Allelopathic effect of hydroxamic acids from cereals on Avena sativa and A. fatua. Phytochemistry 1990, 29, 773–776. [Google Scholar] [CrossRef]
- Stanisavljevic, R.; Ðjokic, D.; Milenkovic, J.; Ðukanovic, L.; Stevovic, V.; Simic, A.; Dodig, D. Seed germination and seedling vigour of Italian ryegrass, cocksfoot and timothy following harvest and storage. Cienc. Agrotec. 2011, 35, 1141–1148. [Google Scholar] [CrossRef]
- Shehzad, M.; Tanveer, A.; Ayub, M.; Mubeen, K.; Sarwar, N.; Ibrahim, M.; Qadir, I. Effect of weed-crop competition on growth and yield of garden cress (Lepidium sativum L.). J. Med. Plants Res. 2011, 5, 6169–6172. [Google Scholar] [CrossRef]
- Sanchez-Maldonado, A.F.; Schieber, A.; Ganzle, M.G. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Maffei, M.; Bertea, C.M.; Garneri, F.; Scannerini, S. Effect of benzoic acid hydroxy-and methoxy-ring substituents during cucumber (Cucumis sativus L.) germination. I. Isocitrate lyase and catalase activity. Plant Sci. 1999, 141, 139–147. [Google Scholar] [CrossRef]
- Levi-Minzi, R.; Saviozzi, A.; Riffaldi, R. Organic acids as seed germination inhibitors. J. Environ. Sci. Health 1994, 29, 2203–2217. [Google Scholar] [CrossRef]
- Pinho, I.A.; Lopes, D.V.; Martins, R.C.; Quina, M.J. Phytotoxicity assessment of olive mill solid wastes and the influence of phenolic compounds. Chemosphere 2017, 185, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, Y.; Xu, S.; Wang, L.; Han, S. Quantitative structure-activity relationships for the toxicity to the tadpole Rana japonica of selected phenols. Bull. Environ. Contam. Toxicol. 2000, 64, 859–865. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Wang, Y.; Wang, L. Mechanism-based quantitative structure–activity relationships for the inhibition of substituted phenols on germination rate of Cucumis sativus. Chemosphere 2002, 46, 241–250. [Google Scholar] [CrossRef]
- Jităreanu, A.; Tătărîngă, G.; Zbancioc, A.M.; Stănescu, U. Toxicity of some cinnamic acid derivatives to common bean (Phaseolus vulgaris). Not. Bot. Horti. Agrobo. 2011, 39, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Waśko, A.; Szwajgier, D.; Polak-Berecka, M. The role of ferulic acid esterase in the growth of Lactobacillus helveticus in the presence of phenolic acids and their derivatives. Eur. Food Res. Technol. 2014, 238, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, T. Comparative effects of aromatic compounds on inhibition of lettuce fruit germination. Ann. Bot. 1978, 42, 419–427. [Google Scholar] [CrossRef]
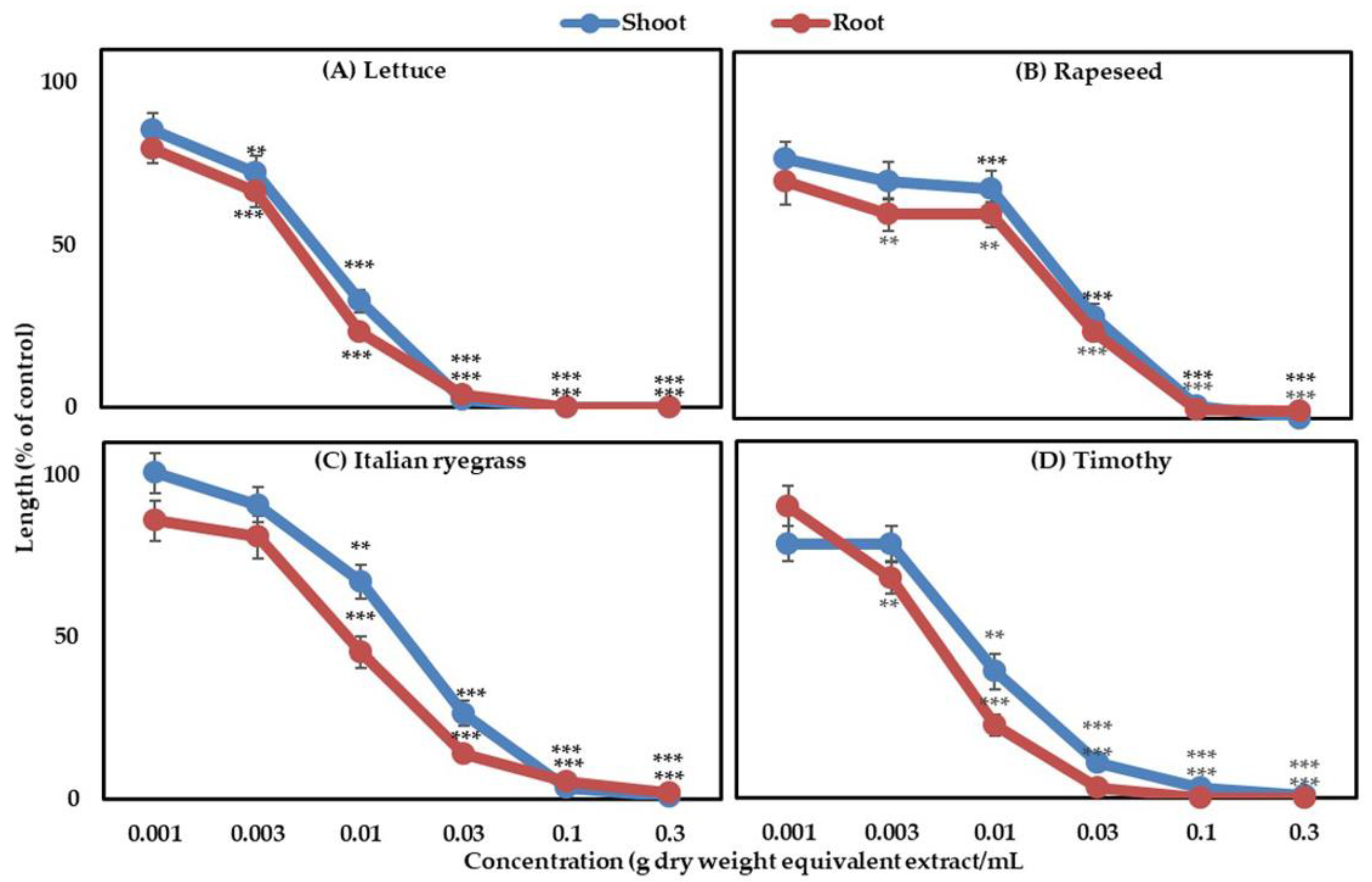

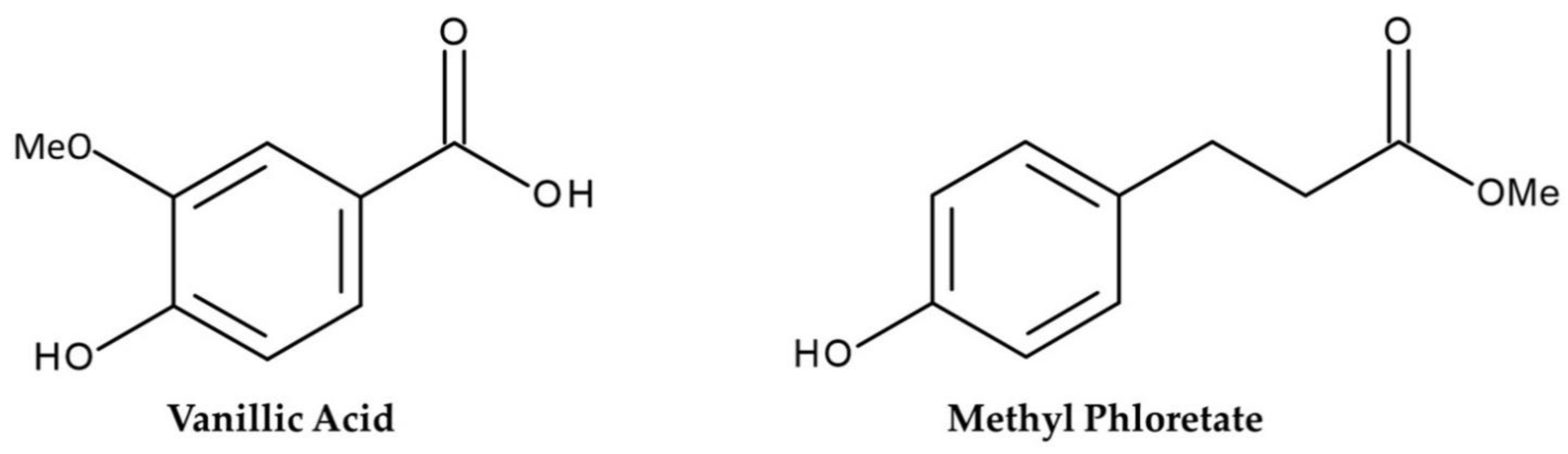
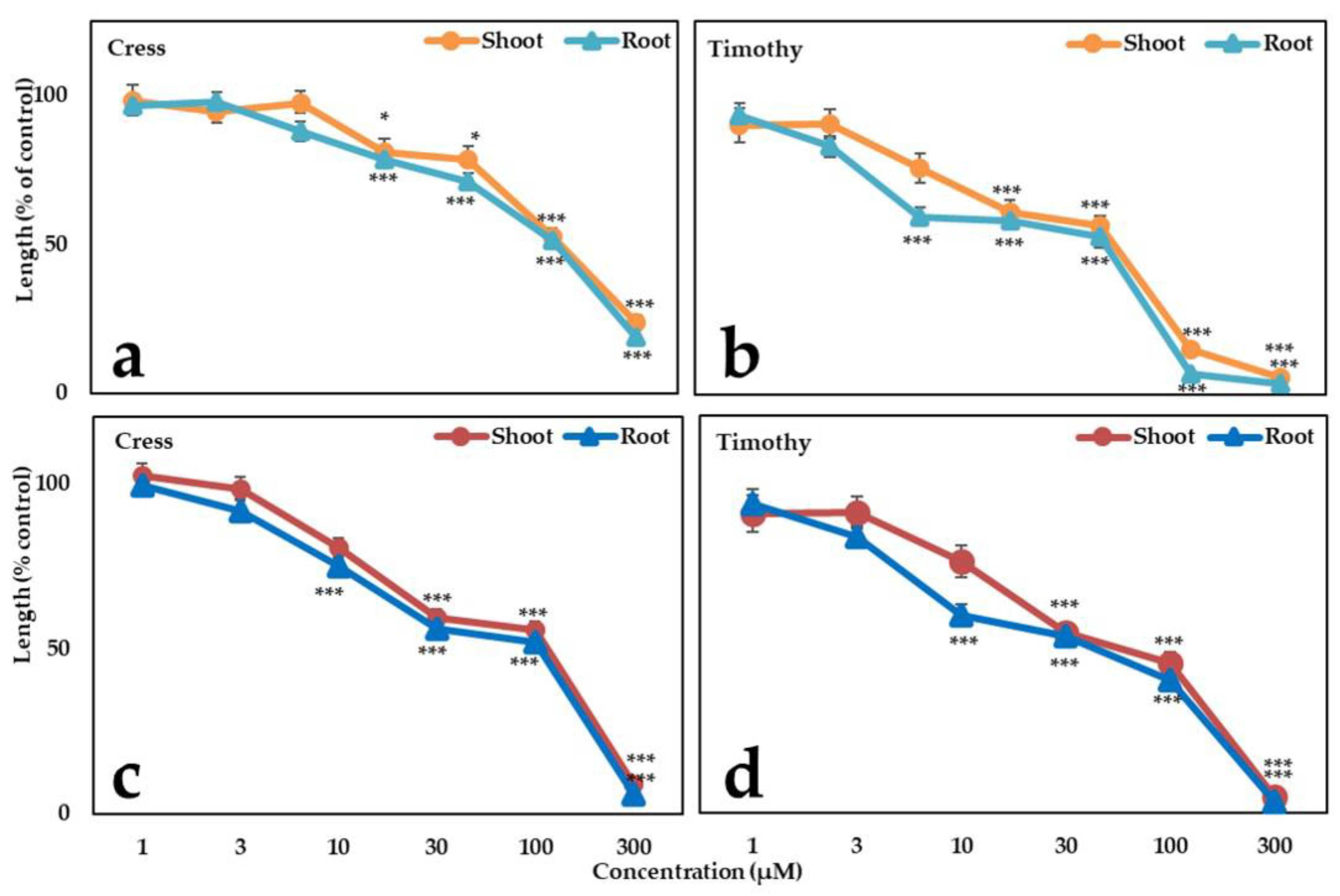
| Aqueous Methanol Extracts of G. xanthochymus (mg Dry Weight Equivalent Extract/mL) | ||
|---|---|---|
| Test plant Species | Shoot | Root |
| Lettuce | 7.13 ± 0.94 | 4.73 ± 0.61 |
| Rapeseed | 17.20 ± 0.68 | 14.23 ± 0.85 |
| Italian ryegrass | 15.84 ± 0.78 | 8.53 ± 0.99 |
| Timothy | 7.13 ± 0.72 | 4.81 ± 0.97 |
| Tested Species | Vanillic Acid | Methyl Phloretate | |||
|---|---|---|---|---|---|
| Shoot *** | Root *** | Shoot *** | Root *** | ||
| dicot | Cress | 331.7 ± 7.1 | 314.7 ± 8.3 | 113.7 ± 3.8 | 104.7 ± 2.9 |
| Monocot | Timothy | 118.8 ± 4.4 | 107.3 ± 4.1 | 53.4 ± 2.8 | 40.6 ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rob, M.M.; Hossen, K.; Khatun, M.R.; Iwasaki, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Identification and Application of Bioactive Compounds from Garcinia xanthochymus Hook. for Weed Management. Appl. Sci. 2021, 11, 2264. https://doi.org/10.3390/app11052264
Rob MM, Hossen K, Khatun MR, Iwasaki K, Iwasaki A, Suenaga K, Kato-Noguchi H. Identification and Application of Bioactive Compounds from Garcinia xanthochymus Hook. for Weed Management. Applied Sciences. 2021; 11(5):2264. https://doi.org/10.3390/app11052264
Chicago/Turabian StyleRob, Md. Mahfuzur, Kawsar Hossen, Mst. Rokeya Khatun, Keitaro Iwasaki, Arihiro Iwasaki, Kiyotake Suenaga, and Hisashi Kato-Noguchi. 2021. "Identification and Application of Bioactive Compounds from Garcinia xanthochymus Hook. for Weed Management" Applied Sciences 11, no. 5: 2264. https://doi.org/10.3390/app11052264
APA StyleRob, M. M., Hossen, K., Khatun, M. R., Iwasaki, K., Iwasaki, A., Suenaga, K., & Kato-Noguchi, H. (2021). Identification and Application of Bioactive Compounds from Garcinia xanthochymus Hook. for Weed Management. Applied Sciences, 11(5), 2264. https://doi.org/10.3390/app11052264