Maturation of Anterior Cruciate Ligament Graft—Possibilities of Surgical Enhancement: What Do We Know So Far?
Abstract
:1. Introduction
2. Materials and Methods
- Preservation of remnants of a native ACL;
- Preservation of hamstring tibial attachment during an ACL reconstruction with a hamstring autograft;
- Enveloping of a graft with a periosteum;
- Preservation of muscle tissue on a harvested graft;
- Augmentation of a graft with an amniotic tissue;
- The usage of an intra-articular synovial graft.
- (anterior cruciate ligament reconstruction) AND (remnant preservation);
- (anterior cruciate ligament reconstruction) AND (attached hamstring graft);
- (anterior cruciate ligament reconstruction) AND (periosteum);
- (anterior cruciate ligament reconstruction) AND (muscle remnant);
- (anterior cruciate ligament reconstruction) AND ((amnion) OR (amniotic tissue));
- (anterior cruciate ligament reconstruction) AND (synovialization).
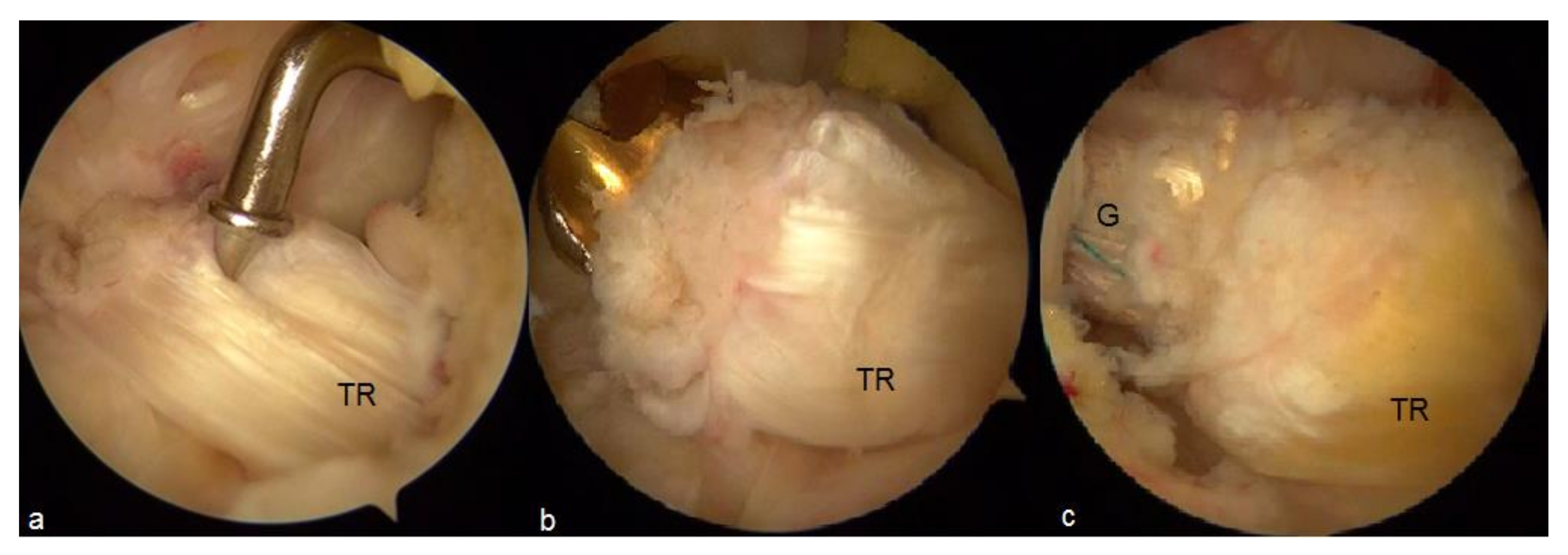
3. Remnant Preservation
4. Preservation of Hamstrings Tibial Insertion
5. Periosteum-Enveloped Graft
6. Preservation of Muscle Tissue on Tendon Graft
7. Amnion
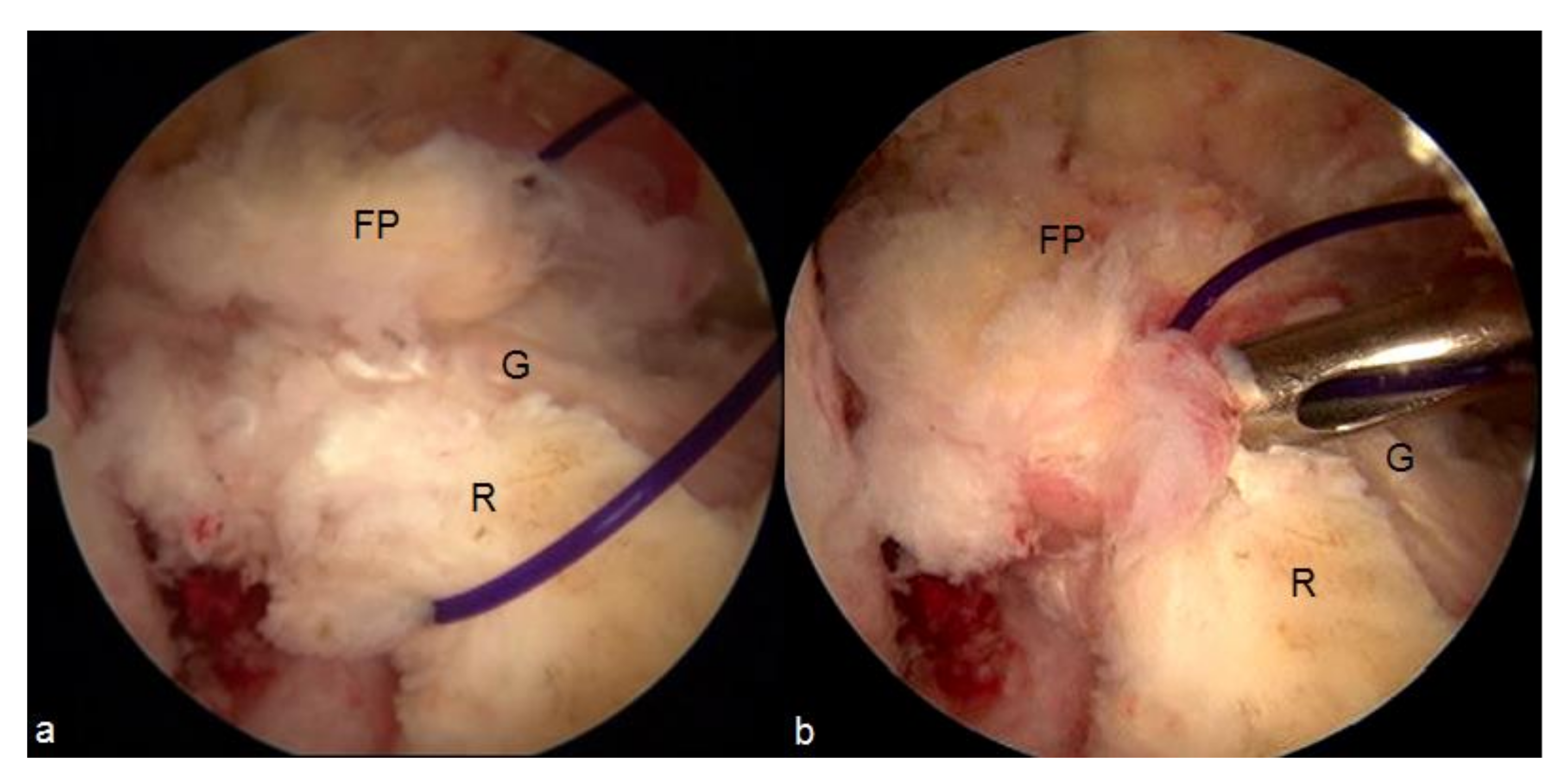
8. Intra-Articular Synovial Grafts
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanders, T.L.; Pareek, A.; Hewett, T.E.; Levy, B.A.; Dahm, D.L.; Stuart, M.J.; Krych, A.J. Long-term rate of graft failure after ACL reconstruction: A geographic population cohort analysis. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Muneta, T.; Koga, H. Anterior cruciate ligament remnant and its values for preservation. Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chahla, J.; Kennedy, M.I.; Aman, Z.S.; LaPrade, R.F. Ortho-Biologics for Ligament Repair and Reconstruction. Clin. Sports Med. 2019, 38, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zeng, C.; Pan, J.; Zhao, C.; Fang, H.; Cai, D. Remnant preservation in anterior cruciate ligament reconstruction versus standard techniques: A meta-analysis of randomized controlled trials. J. Sports Med. Phys. Fit. 2017, 57, 1014–1022. [Google Scholar]
- Tie, K.; Chen, L.; Hu, D.; Wang, H. The difference in clinical outcome of single-bundle anterior cruciate ligament reconstructions with and without remnant preservation: A meta-analysis. Knee 2016, 23, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-D.; Wang, F.-S.; Gao, S.-J.; Zhang, Y.-Z. Remnant preservation technique versus standard technique for anterior cruciate ligament reconstruction: A meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2018, 13, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Liu, Z.; Li, Y.; Peng, Y.; Xu, W.; Hu, N.; Huang, W. Is Remnant Preservation in Anterior Cruciate Ligament Reconstruction Superior to the Standard Technique? A Systematic Review and Meta-Analysis. BioMed Res. Int. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Li, X.; Zhang, H.; Liu, X.; Zhang, J.; Shen, J.W.; Feng, H. Anterior Cruciate Ligament Reconstruction with Remnant Preservation: A prospective, randomized controlled study. Am. J. Sports Med. 2012, 40, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Adachi, N.; Ochi, M.; Uchio, Y.; Sumen, Y. Anterior cruciate ligament augmentation under arthroscopy. A minimum 2-year follow-up in 40 patients. Arch. Orthop. Trauma Surg. 2000, 120, 128–133. [Google Scholar] [CrossRef]
- Lee, B.-I.; Kwon, S.-W.; Kim, J.-B.; Choi, H.-S.; Min, K.-D. Comparison of Clinical Results According to Amount of Preserved Remnant in Arthroscopic Anterior Cruciate Ligament Reconstruction Using Quadrupled Hamstring Graft. Arthrosc. J. Arthrosc. Relat. Surg. 2008, 24, 560–568. [Google Scholar] [CrossRef]
- Gohil, S.; Annear, P.O.; Breidahl, W. Anterior cruciate ligament reconstruction using autologous double hamstrings: A comparison of standardversusminimal debridement techniques using MRI to assess revascularisation. A randomised prospective study with a one-year follow-up. J. Bone Jt. Surg. Br. Vol. 2007, 89, 1165–1171. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.I.; Kim, B.M.; Kho, D.H.; Kwon, S.W.; Kim, H.J.; Hwang, H.R. Does the tibial remnant of the anterior cruciate ligament promote ligamentization? Knee 2016, 23, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Lee, S.H.; Choi, S.H.; Lim, T.K. Magnetic Resonance Imaging Evaluation of Anterior Cruciate Ligament Reconstruction Using Quadrupled Hamstring Tendon Autografts: Comparison of remnant bundle preservation and standard technique. Am. J. Sports Med. 2010, 38, 1768–1777. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Cao, X.; Liu, L.; Liu, Y.; Li, R. The effect of remnant preservation on tibial tunnel enlargement in ACL reconstruction with hamstring autograft: A prospective randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Demirağ, B.; Ermutlu, C.; Aydemir, F.; Durak, K. A comparison of clinical outcome of augmentation and standard reconstruction techniques for partial anterior cruciate ligament tears. Jt. Dis. Relat. Surg. 2012, 23, 140–144. [Google Scholar]
- Naraoka, T.; Kimura, Y.; Tsuda, E.; Yamamoto, Y.; Ishibashi, Y. Does Remnant Preservation Influence Tibial Tunnel Enlargement or Graft-to-Bone Integration After Double-Bundle Anterior Cruciate Ligament Reconstruction Using Hamstring Autografts and Suspensory Fixation? A Computed Tomography and Magnetic Resonance Imaging Evaluation. Orthop. J. Sports Med. 2018, 6, 2325967118790238. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, S.; Kimura, M.; Hagiwara, K.; Ogoshi, A.; Nakagawa, T.; Shiozawa, H.; Ohsawa, T.; Chikuda, H. The remnant preservation technique reduces the amount of bone tunnel enlargement following anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 491–499. [Google Scholar] [CrossRef]
- Masuda, T.; Kondo, E.; Onodera, J.; Kitamura, N.; Inoue, M.; Nakamura, E.; Yagi, T.; Iwasaki, N.; Yasuda, K. Effects of Remnant Tissue Preservation on Tunnel Enlargement After Anatomic Double-Bundle Anterior Cruciate Ligament Reconstruction Using the Hamstring Tendon. Orthop. J. Sports Med. 2018, 6, 2325967118811293. [Google Scholar] [CrossRef] [Green Version]
- Zaffagnini, S.; Golanò, P.; Farinas, O.; de Pasquale, V.; Strocchi, R.; Cortecchia, S.; Marcacci, M.; Visani, A. Vascularity and neuroreceptors of the pes anserinus: Anatomic Study. Clin. Anat. 2002, 16, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Papachristou, G.; Nikolaou, V.; Efstathopoulos, N.; Sourlas, J.; Lazarettos, J.; Frangia, K.; Papalois, A. ACL reconstruction with semitendinosus tendon autograft without detachment of its tibial insertion: A histologic study in a rabbit model. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 1175–1180. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.; Wan, F.; Ding, Z.; Chen, S.; Chen, J. Advantages of an Attached Semitendinosus Tendon Graft in Anterior Cruciate Ligament Reconstruction in a Rabbit Model. Am. J. Sports Med. 2018, 46, 3227–3236. [Google Scholar] [CrossRef] [PubMed]
- Bahlau, D.; Clavert, P.; Favreau, H.; Ollivier, M.; Lustig, S.; Bonnomet, F.; Ehlinger, M. Mechanical advantage of preserving the hamstring tibial insertion for anterior cruciate ligament reconstruction—A cadaver study. Orthop. Traumatol. Surg. Res. 2019, 105, 89–93. [Google Scholar] [CrossRef]
- Ruffilli, A.; Traina, F.; Evangelisti, G.; Borghi, R.; Perna, F.; Faldini, C. Preservation of hamstring tibial insertion in anterior cruciate ligament reconstruction: A review of the current literature. Musculoskelet. Surg. 2015, 99, 87–92. [Google Scholar] [CrossRef]
- Gupta, R.; Bahadur, R.; Malhotra, A.; Masih, G.D.; Sood, M.; Gupta, P.; Mathur, V.K. Outcome of Hamstring Autograft With Preserved Insertions Compared With Free Hamstring Autograft in Anterior Cruciate Ligament Surgery at 2-Year Follow-up. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, 2208–2216. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.; Li, J.; Zhang, H.; Fu, Y.; Wang, Y. Functional double-bundle anterior cruciate ligament reconstruction using hamstring tendon autografts with preserved insertions is an effective treatment for tibiofemoral instability. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 3471–3480. [Google Scholar] [CrossRef] [PubMed]
- Bahlau, D.; Favreau, H.; Eichler, D.; Lustig, S.; Bonnomet, F.; Ehlinger, M. Clinical, functional, and isokinetic study of a prospective series of anterior cruciate ligament ligamentoplasty with pedicular hamstrings. Int. Orthop. 2019, 43, 2557–2562. [Google Scholar] [CrossRef] [PubMed]
- Ruffilli, A.; Pagliazzi, G.; Ferranti, E.; Busacca, M.; Capannelli, D.; Buda, R. Hamstring graft tibial insertion preservation versus detachment in anterior cruciate ligament reconstruction: A prospective randomized comparative study. Eur. J. Orthop. Surg. Traumatol. 2016, 26, 657–664. [Google Scholar] [CrossRef]
- Grassi, A.; Casali, M.; Macchiarola, L.; Lucidi, G.A.; Cucurnia, I.; Filardo, G.; Lopomo, N.F.; Zaffagnini, S. Hamstring grafts for anterior cruciate ligament reconstruction show better magnetic resonance features when tibial insertion is preserved. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, H.; Tao, H.; Sun, Y.; Chen, S.; Chen, J. A Randomized Clinical Trial to Evaluate Attached Hamstring Anterior Cruciate Ligament Graft Maturity with Magnetic Resonance Imaging. Am. J. Sports Med. 2018, 46, 1143–1149. [Google Scholar] [CrossRef]
- Goradia, V.K.; Rochat, M.C.; Grana, W.A.; Egle, D.M. Strength of ACL reconstructions using semitendinosus tendon grafts. J. Okla. State Med. Assoc. 1998, 91, 275–277. [Google Scholar]
- Benjamin, M.; Toumi, H.; Ralphs, J.R.; Bydder, G.; Best, T.M.; Milz, S. Where tendons and ligaments meet bone: Attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J. Anat. 2006, 208, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Pathria, M.; Tadros, A. Muscle-Tendon-Enthesis Unit. Semin. Musculoskelet. Radiol. 2018, 22, 263–274. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Chen, W.-J.; Shih, C.-H.; Yang, C.-Y.; Liu, S.-J.; Lin, P.-Y. Enveloping the tendon graft with periosteum to enhance tendon-bone healing in a bone tunnel: A biomechanical and histologic study in rabbits. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Kyung, H.-S.; Kim, S.-Y.; Oh, C.-W.; Kim, S.-J. Tendon-to-bone tunnel healing in a rabbit model: The effect of periosteum augmentation at the tendon-to-bone interface. Knee Surg. Sports Traumatol. Arthrosc. 2003, 11, 9–15. [Google Scholar] [CrossRef]
- Youn, I.; Jones, D.G.; Andrews, P.J.; Cook, M.P.; Suh, J.-K.F. Periosteal Augmentation of a Tendon Graft Improves Tendon Healing in the Bone Tunnel. Clin. Orthop. Relat. Res. 2004, 419, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, W.J.; Shih, C.H. Enveloping of periosteum on the Hamstring Tendon graft in anterior cruciate ligament reconstruction. Arthroscopy 2002, 18, 27E. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, W.-J.; Shih, C.-H.; Chou, S.-W. Arthroscopic anterior cruciate ligament reconstruction with periosteum-enveloping hamstring tendon graft. Knee Surg. Sports Traumatol. Arthrosc. 2004, 12, 398–405. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chang, C.-H.; Su, C.-I.; Wang, K.-C.; Liu, H.-T.; Yu, C.-M.; Wong, C.-B.; Wang, I.-C. Arthroscopic Single-Bundle Anterior Cruciate Ligament Reconstruction with Periosteum-Enveloping Hamstring Tendon Graft: Clinical Outcome at 2 to 7 Years. Arthrosc. J. Arthrosc. Relat. Surg. 2010, 26, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Robert, H.; Es-Sayeh, J. The role of periosteal flap in the prevention of femoral widening in anterior cruciate ligament reconstruction using hamstring tendons. Knee Surg. Sports Traumatol. Arthrosc. 2003, 12, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Ćuti, T.; Antunović, M.; Marijanović, I.; Ivković, A.; Vukasović, A.; Matić, I.; Pećina, M.; Hudetz, D. Capacity of muscle derived stem cells and pericytes to promote tendon graft integration and ligamentization following anterior cruciate ligament reconstruction. Int. Orthop. 2017, 41, 1189–1198. [Google Scholar] [CrossRef]
- Ghebes, C.A.; Groen, N.; Cheuk, Y.C.; Fu, S.C.; Fernandes, H.M.; Saris, D.B. Muscle-Secreted Factors Improve Anterior Cruciate Ligament Graft Healing: An In Vitro and In Vivo Analysis. Tissue Eng. Part A 2018, 24, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hou, C.; Wu, B.; Tian, M.; Zhou, X. Effect of muscle preserved on tendon graft on intra-articular healing in anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2012, 21, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Riboh, J.C.; Saltzman, B.M.; Yanke, A.B.; Cole, B.J. Human Amniotic Membrane-Derived Products in Sports Medicine: Basic Science, Early Results, and Potential Clinical Applications. Am. J. Sports Med. 2016, 44, 2425–2434. [Google Scholar] [CrossRef]
- Heckmann, N.; Auran, R.; Mirzayan, R. Application of Amniotic Tissue in Orthopedic Surgery. Am. J. Orthop. 2016, 45, e421–e425. [Google Scholar]
- Li, Y.; Liu, Z.; Jin, Y.; Zhu, X.; Wang, S.; Yang, J.; Ren, Y.; Fu, Q.; Xiong, H.; Zou, G.; et al. Differentiation of Human Amniotic Mesenchymal Stem Cells into Human Anterior Cruciate Ligament Fibroblast Cells by In Vitro Coculture. BioMed Res. Int. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodall, B.M.; Elena, N.; Gamboa, J.T.; Shin, E.C.; Pathare, N.; McGahan, P.J.; Chen, J.L. Anterior Cruciate Ligament Reconstruction with Amnion Biological Augmentation. Arthrosc. Tech. 2018, 7, e355–e360. [Google Scholar] [CrossRef] [Green Version]
- Lavender, C.; Bishop, C. The Fertilized Anterior Cruciate Ligament: An All-Inside Anterior Cruciate Ligament Reconstruction Augmented with Amnion, Bone Marrow Concentrate, and a Suture Tape. Arthrosc. Tech. 2019, 8, e555–e559. [Google Scholar] [CrossRef] [Green Version]
- Scapinelli, R. Vascular anatomy of the human cruciate ligaments and surrounding structures. Clin. Anat. 1997, 10, 151–162. [Google Scholar] [CrossRef]
- Unterhauser, F.N.; Bail, H.J.; Höher, J.; Haas, N.P.; Weiler, A. Endoligamentous Revascularization of an Anterior Cruciate Ligament Graft. Clin. Orthop. Relat. Res. 2003, 414, 276–288. [Google Scholar] [CrossRef]
- Ateschrang, A.; Schreiner, A.J.; Ahmad, S.S.; Schröter, S.; Hirschmann, M.T.; Körner, D.; Kohl, S.; Stöckle, U.; Ahrend, M.-D. Improved results of ACL primary repair in one-part tears with intact synovial coverage. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 37–43. [Google Scholar] [CrossRef]
- Noh, J.H.; Yang, B.G.; Roh, Y.H.; Lee, J.S. Synovialization on second-look arthroscopy after anterior cruciate ligament reconstruction using Achilles allograft in active young men. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Kondo, E.; Yasuda, K. Second-Look Arthroscopic Evaluations of Anatomic Double-Bundle Anterior Cruciate Ligament Reconstruction: Relation with Postoperative Knee Stability. Arthrosc. J. Arthrosc. Relat. Surg. 2007, 23, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, K.; Ebisz, M.; Góralczyk, A.; Laprade, R.F.; Hermanowicz, K. Synovialization and Revascularization Enhancement in Repaired and Reconstructed ACL: PCL Fat Pad Transfer Technique. Arthrosc. Tech. 2020, 9, e1559–e1563. [Google Scholar] [CrossRef] [PubMed]



| Author | Year of Publication | Study Design | Intervention | Mean Follow-Up | Outcomes |
|---|---|---|---|---|---|
| Ma et al. [4] | 2017 | Meta-analysis | - | - | Significantly higher Lysholm score, better arthrometer results and lower tibial tunnel enlargement in remnant-preserving reconstruction. No statistically significant difference regarding IKDC grade, IKDC score, Lachman test, Pivot-shift test, range of motion (ROM), and the incidence of the cyclops lesion. |
| Tie et al. [5] | 2016 | Meta-analysis | - | - | Significantly lower incidence of tibial tunnel enlargement in remnant-preserving reconstructions. No statistically significant difference regarding arthrometer results, Lachman test, Pivot-shift test, IKDC score and Lysholm score. |
| Wang et al. [6] | 2018 | Meta-analysis | - | - | Significantly higher Lysholm score and better arthrometer results in remnant-preserving reconstructions. No statistically significant difference regarding IKDC score, complications, Pivot-shift test and Lachman test. |
| Wang et al. [7] | 2019 | Meta-analysis | - | - | Significantly better arthrometer results and Lysholm score in remnant-preserving group. No statistically significant difference regarding graft maturation, complications, IKDC score, Lachman test and pivot-shift test. |
| Hong et al. [8] | 2012 | Randomized controlled trial | Single-bundle ACLR with a 4-strand allograft. | 25.7 months | No statistically significant difference regarding Lysholm score, IKDC grade, Lachman test, Pivot-shift test, arthrometer results, graft synovial coverage and passive angle reproduction test. |
| Adachi et al. [9] | 2000 | Retrospective cohort study | Single-bundle ACLR with a hamstring autograft or fascia lata allograft. | 3.2 years | Significantly better arthrometer results and passive angle reproduction test in favor of remnant-preserving group. No statistically significant difference regarding Lysholm score and Gillquist score. |
| Lee et al. [10] | 2008 | Case series Comparison of patients with over 20% of preserved remnant (I) and those with less than 20% of preserved remnant (II) | Single-bundle ACLR with a pedunculated hamstring autograft | 35.1 months | Significantly better threshold to detection of passive motion at 30° and reproduction of passive positioning at 15° and 30° in the I group. |
| Gohil et al. [11] | 2007 | Randomized controlled trial | Single-bundle ACLR with a hamstring autograft | 12 months | The remnant-preserving group had higher SNQ than the remnant-sacrificing group at 2 months of follow-up and lower SNQ at 6 months of follow-up. |
| Lee et al. [12] | 2016 | Retrospective cohort study | Single-bundle ACLR with a hamstring autograft | 18 months | The remnant-preserving group had higher SNQ than the remnant-sacrificing group at 2-4 months of follow-up and lower SNQ at 12-18 months of follow-up. |
| Ahn et al. [13] | 2010 | Retrospective cohort study | Single-bundle ACLR with a hamstring autograft | 6.3 months | The SNQ did not differ significantly between the both groups. |
| Zhang et al. [14] | 2014 | Randomized controlled trial Tunnel diameter evaluated with plain radiographs | Single-bundle ACLR with a hamstring autograft | 24.5 months | Significantly lower incidence of tibial tunnel enlargement in the remnant-preserving group. |
| Demirağ et al. [15] | 2012 | Randomized controlled trial Tunnel diameter evaluated with plain radiographs | Single-bundle ACLR with a hamstring autograft | 24.3 months | Significantly lower tibial and femoral tunnel widening in the remnant-preserving group. |
| Naraoka et al. [16] | 2018 | Prospective cohort study Tunnel diameter evaluated with computed tomography | Double-bundle ACLR with hamstring autograft | 1 year | Significantly greater posterolateral tibial tunnel enlargement in the remnant-preserved group 1 year after ACLR. |
| Yanagisawa et al. [17] | 2018 | Prospective cohort study Tunnel diameter evaluated with computed tomography | Double-bundle ACLR with hamstring autograft | 11.7-13.8 months | Significantly lower incidence of femoral and tibial anteromedial tunnel enlargement in the remnant-preserving group. |
| Masuda et al. [18] | 2018 | Prospective cohort study Tunnel diameter evaluated with computed tomography | Double-bundle ACLR with hamstring autograft | 1 year | Significantly lower incidence of femoral anteromedial tunnel enlargement in the remnant-preserving group. |
| Author. | Year of Publication | Study Design | Intervention | Mean Follow-Up | Key Findings |
|---|---|---|---|---|---|
| Papachristou et al. [20] | 2007 | Animal study | ACLR with pedunculated hamstring autograft | - | Preservation of hamstring tibial insertion allowed to bypass the stage of graft necrosis. |
| Liu et al. [21] | 2018 | Animal study | ACLR with pedunculated hamstring autograft | - | Preservation of hamstring tibial insertion allowed to bypass the stage of graft necrosis. Restoration of fibrocartilaginous bone-tendon interface in the pedunculated hamstring group. Significantly higher histologic scores of the tendon-bone interface, failure load and stiffness as well as smaller bone tunnel area and larger bone volume/total volume in the pedunculated hamstring group. |
| Bahlau et al. [22] | 2019 | Cadaver study | Group 1: Hamstring autograft with intact tibial insertion without interference screw; Group 2: Hamstring autograft with intact tibial insertion and interference screw; Group 3: free hamstring autograft fixed with an interference screw | - | The load at failure was 33% higher in group 1 than group 3. The load at failure of group 2 was 25% higher than group 1 and 65% higher than group 3. |
| Gupta et al. [24] | 2017 | Randomized controlled trial | ACLR with pedunculated hamstring autograft | 24 months | Significantly better arthrometer results, higher Cincinnati knee score and lower difference between the preinjury and post-surgery Tegner level of sports activity in pedunculated hamstring group. |
| Zhang et al. [25] | 2019 | Randomized controlled trial | Functional double bundle ACL reconstruction with pedunculated hamstring graft vs. single-bundle ACL reconstruction with free hamstring autograft | 28.2 months | Significantly better Tegner Activity Scale score, IKDC, KOOS and Lysholm Knee Scoring Scale in the functional double bundle reconstruction group. Lower Pivot-shift grade in the functional double bundle reconstruction group. |
| Bahlau et al. [26] | 2019 | Case series | ACLR with pedunculated hamstring autograft | 30 months | Lysholm score 95/100 and IKDC score 91/100 at the final follow up. 2 mm difference of the anteroposterior laxity compared to the contralateral knee at the final follow up. Significant decrease of the quadriceps and flexor muscles strength deficit during the follow up. |
| Ruffilli et al. [27] | 2016 | Randomized controlled trial | ACLR with pedunculated hamstring autograft | 24 months | IKDC score above 90 in the both groups at the final follow-up. Better intra-articular morphology of the pedunculated graft at the final MRI. No differences in graft integration between the both groups. |
| Grassi et al. [28] | 2020 | Randomized controlled trial | Over-the-top ACLR with pedunculated hamstring graft | 18 months | Less liquid within the graft, smaller tunnel diameter and lower SNQ of the intra-tunnel graft in the pedunculated graft group in the follow up MRIs. |
| Liu et al. [29] | 2018 | Randomized Controlled Trial | ACLR with pedunculated hamstring autograft | 24 months | Lower and stable SNQ in the pedunculated hamstring group in the follow up MRIs. Significantly lower SNQ values in the study group versus the control group at 6 and 12 months of the follow up. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebisz, M.; Góralczyk, A.; Mostowy, M.; LaPrade, R.F.; Malinowski, K. Maturation of Anterior Cruciate Ligament Graft—Possibilities of Surgical Enhancement: What Do We Know So Far? Appl. Sci. 2021, 11, 3597. https://doi.org/10.3390/app11083597
Ebisz M, Góralczyk A, Mostowy M, LaPrade RF, Malinowski K. Maturation of Anterior Cruciate Ligament Graft—Possibilities of Surgical Enhancement: What Do We Know So Far? Applied Sciences. 2021; 11(8):3597. https://doi.org/10.3390/app11083597
Chicago/Turabian StyleEbisz, Michał, Adrian Góralczyk, Marcin Mostowy, Robert F. LaPrade, and Konrad Malinowski. 2021. "Maturation of Anterior Cruciate Ligament Graft—Possibilities of Surgical Enhancement: What Do We Know So Far?" Applied Sciences 11, no. 8: 3597. https://doi.org/10.3390/app11083597
APA StyleEbisz, M., Góralczyk, A., Mostowy, M., LaPrade, R. F., & Malinowski, K. (2021). Maturation of Anterior Cruciate Ligament Graft—Possibilities of Surgical Enhancement: What Do We Know So Far? Applied Sciences, 11(8), 3597. https://doi.org/10.3390/app11083597






