Mechanical and Durability Properties of Mortars Incorporating Red Mud, Ground Granulated Blast Furnace Slag, and Electric Arc Furnace Dust
Abstract
1. Introduction
2. Research Significance
3. Experimental Work
3.1. Materials and Mortar Mixes
3.2. Fresh and Hardened State Tests
4. Results and Discussion
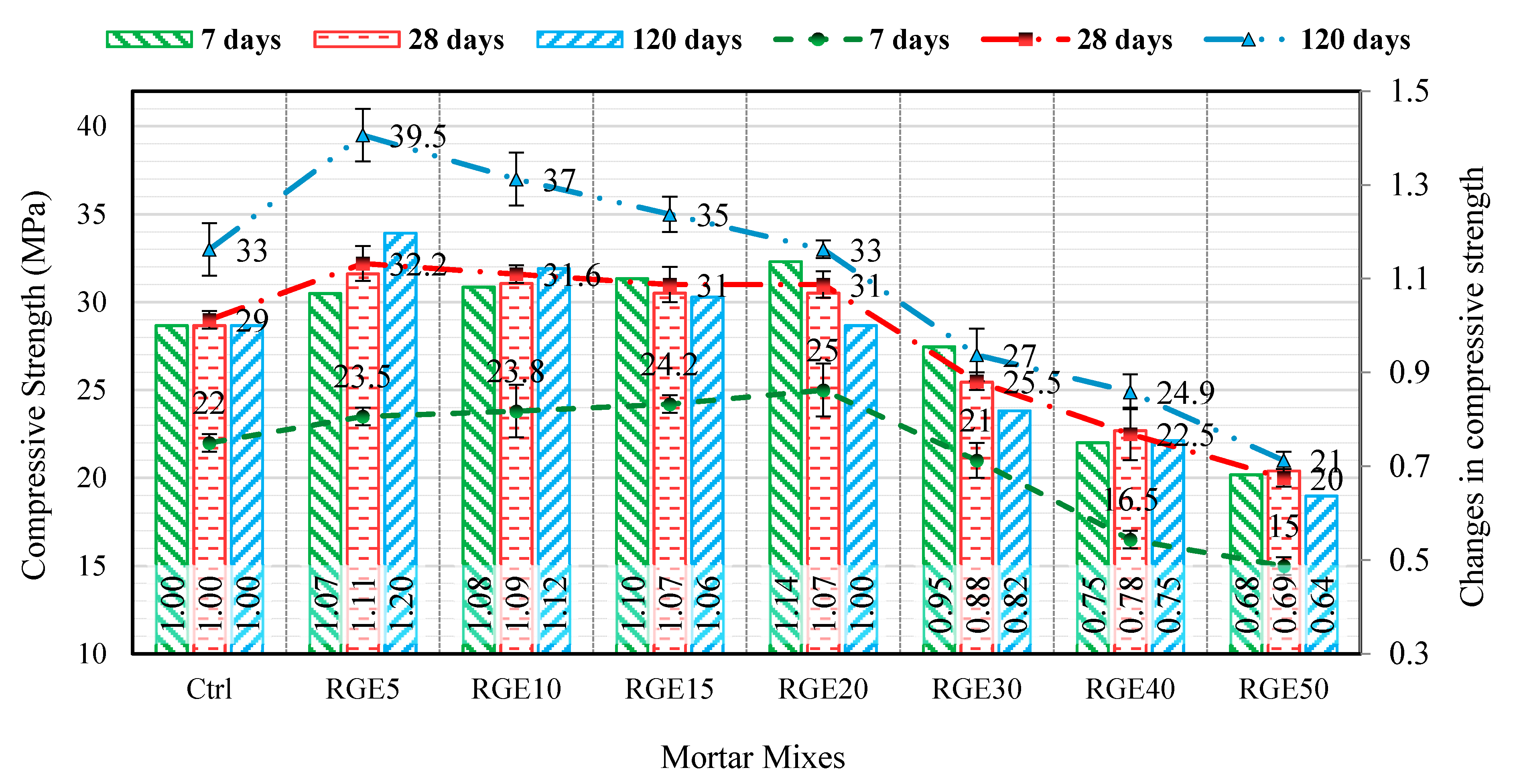
4.1. Compressive Strength
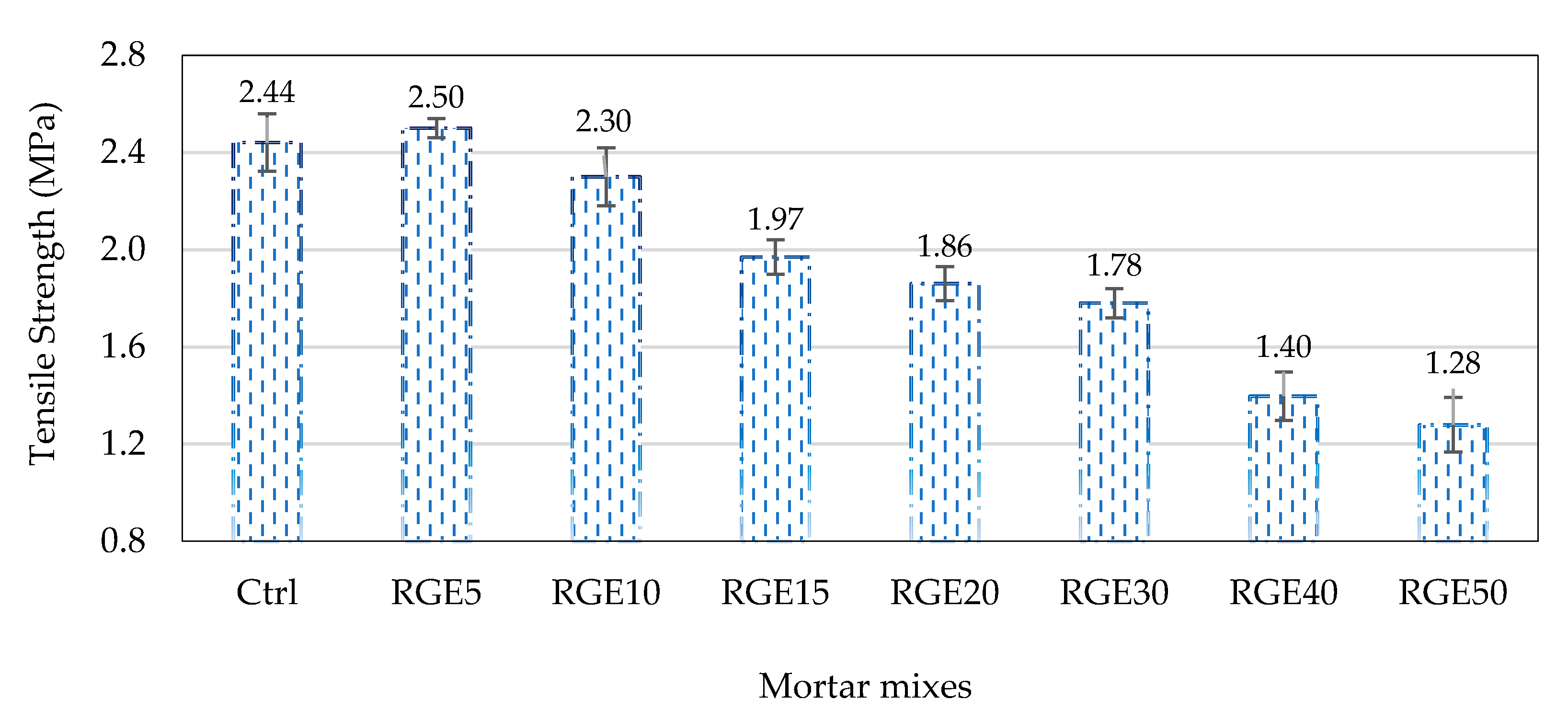
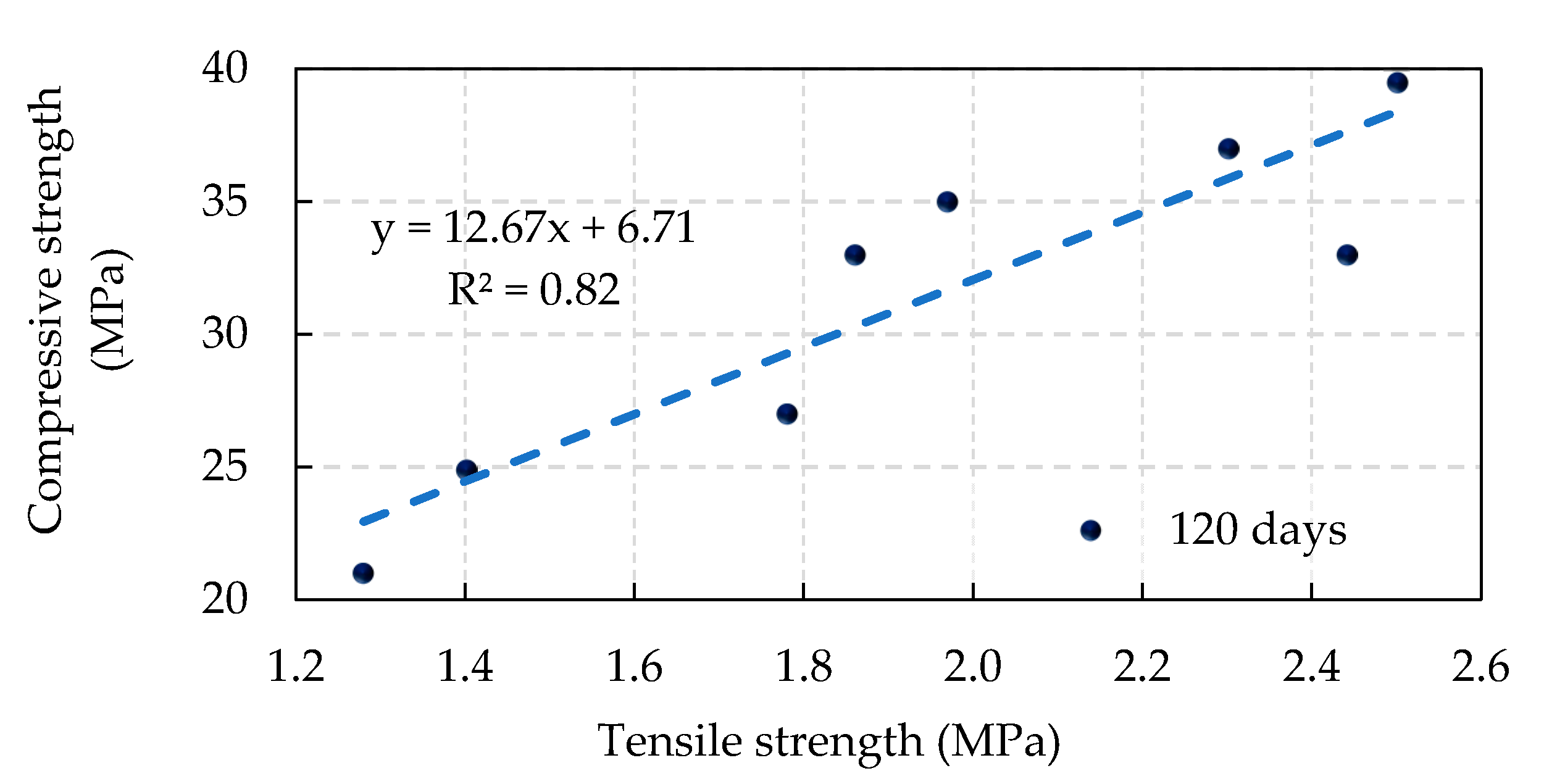
4.2. Splitting Tensile Strength
4.3. Freeze–Thaw Resistance
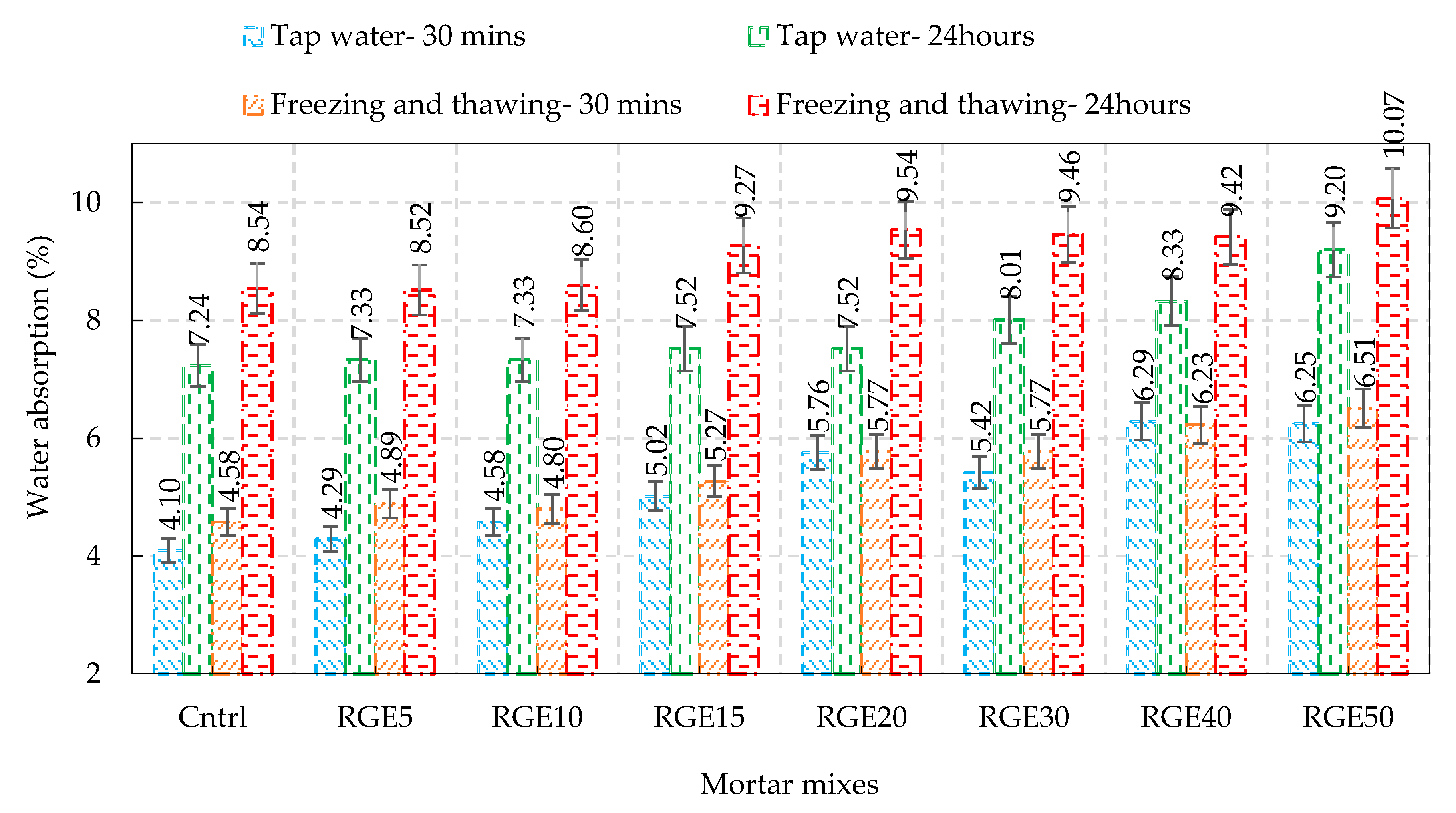
4.4. Permeability and Water Absorption
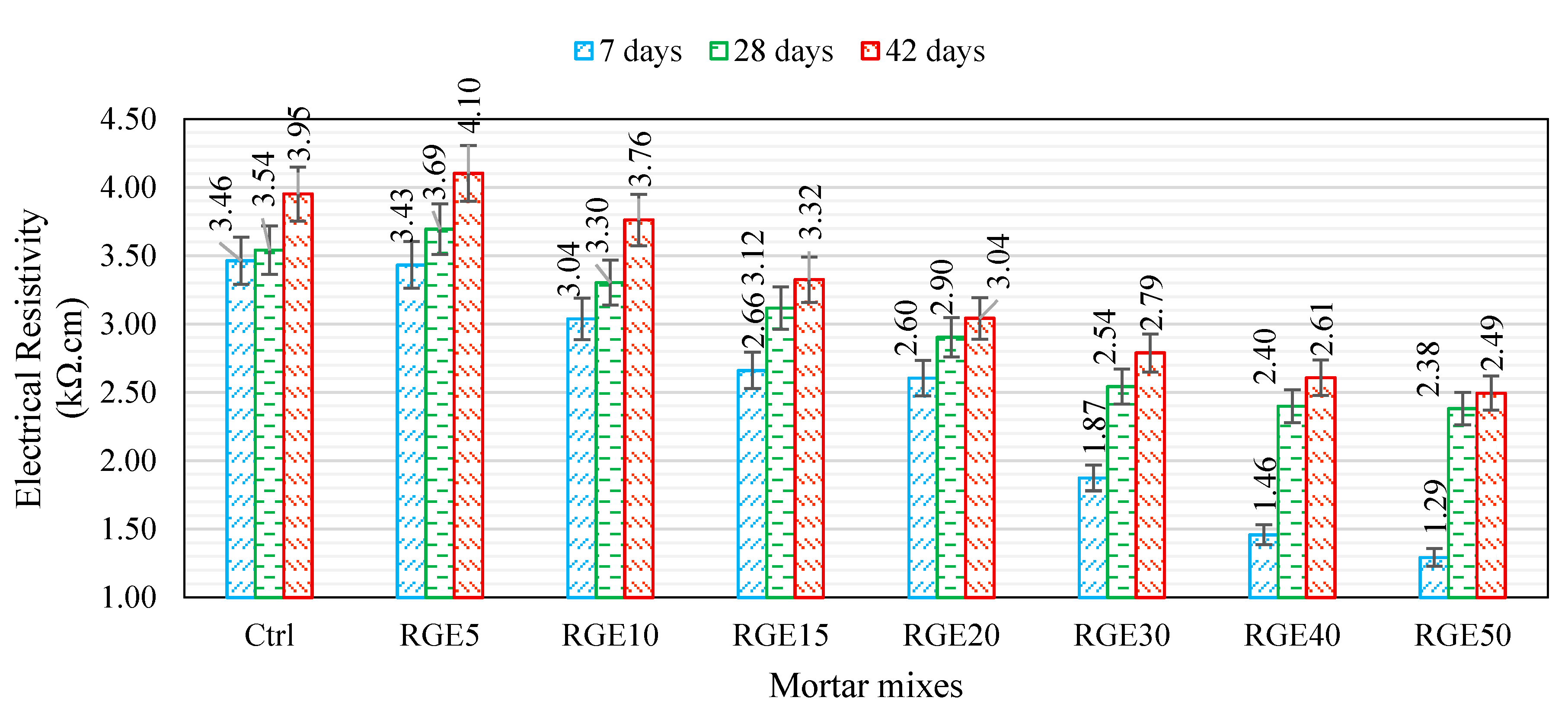
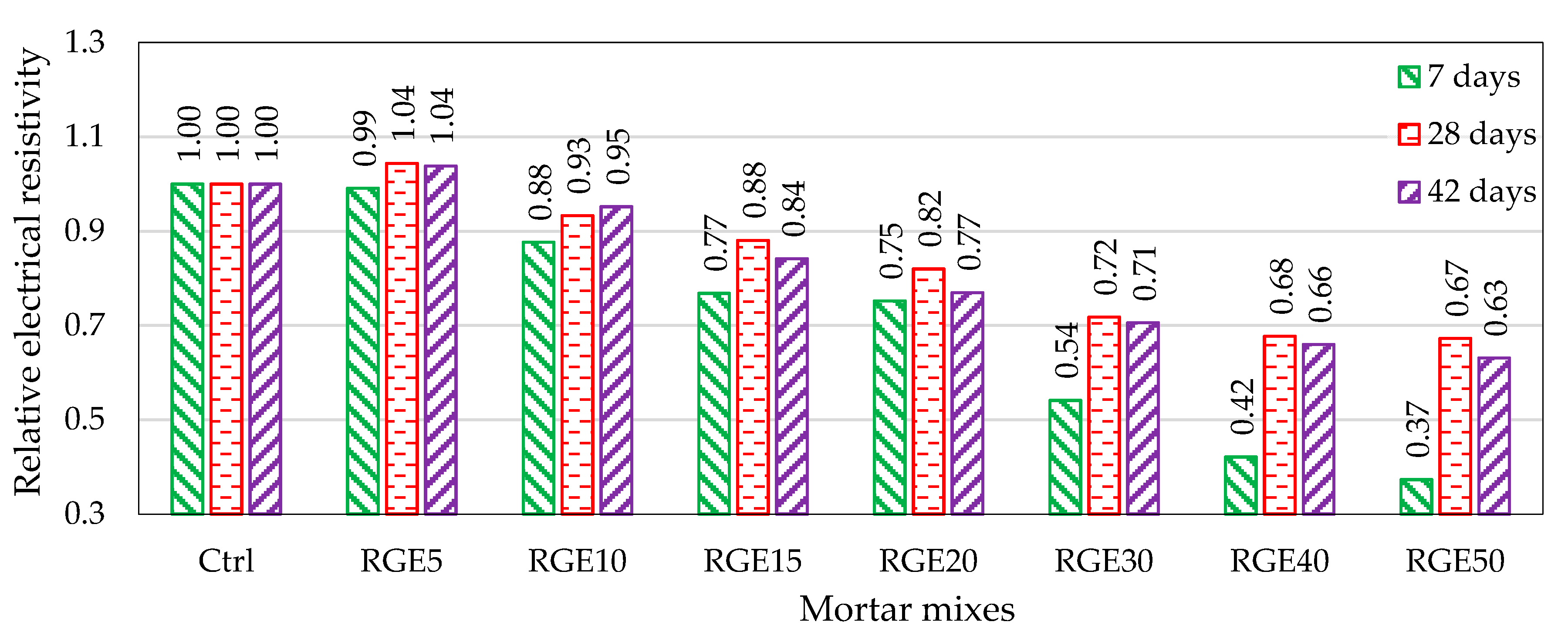
4.5. Electrical Resistivity
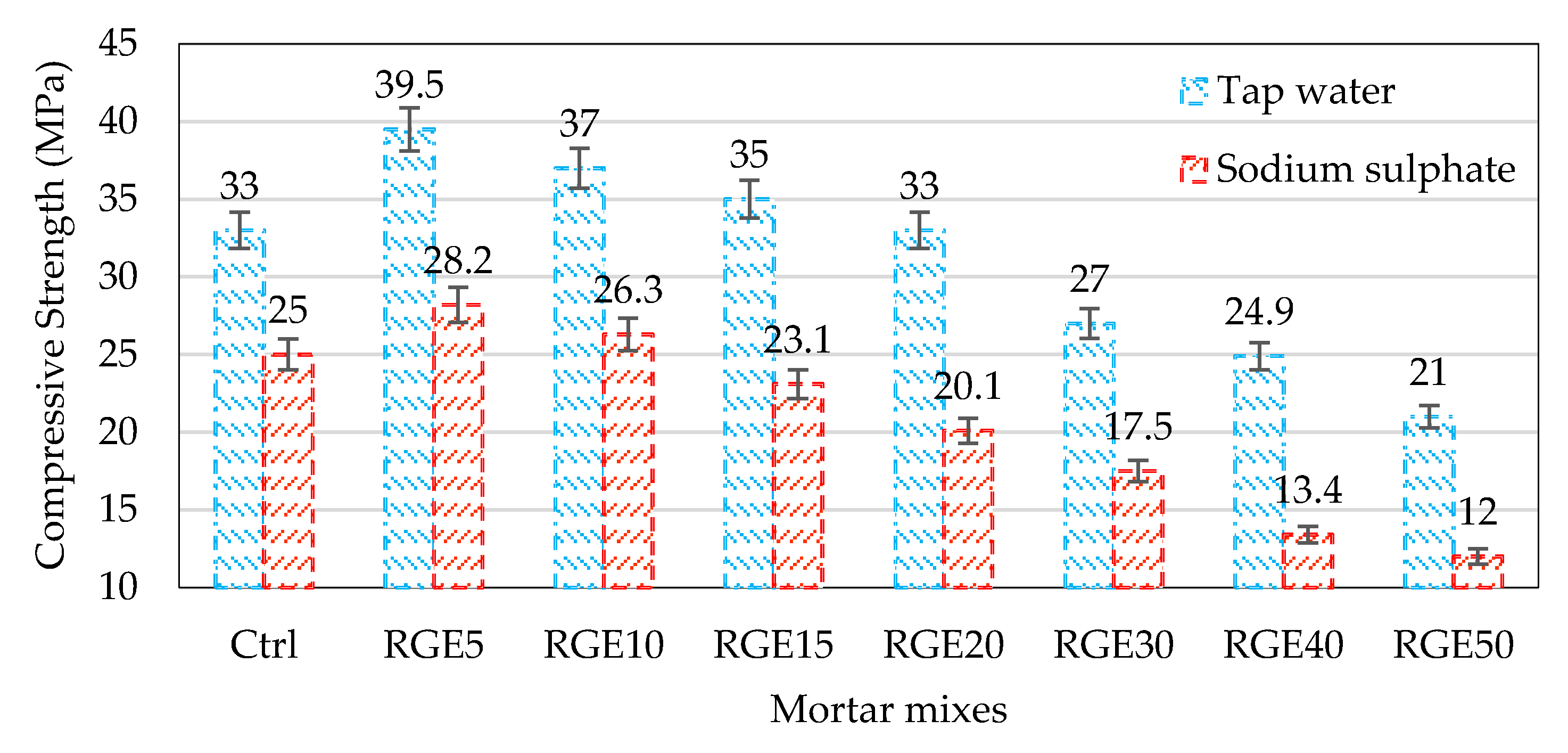
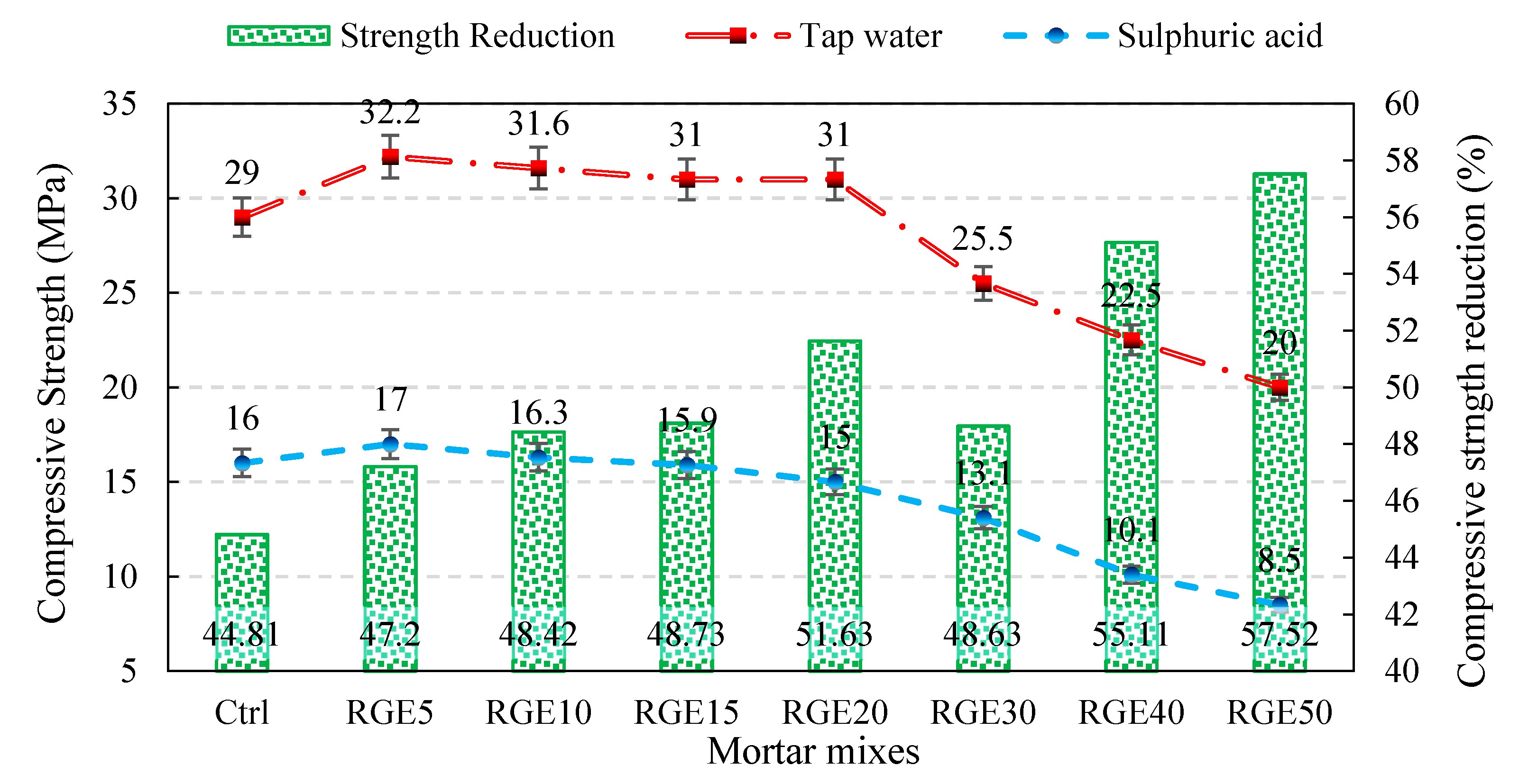
4.6. Durability against Sulphate Attack
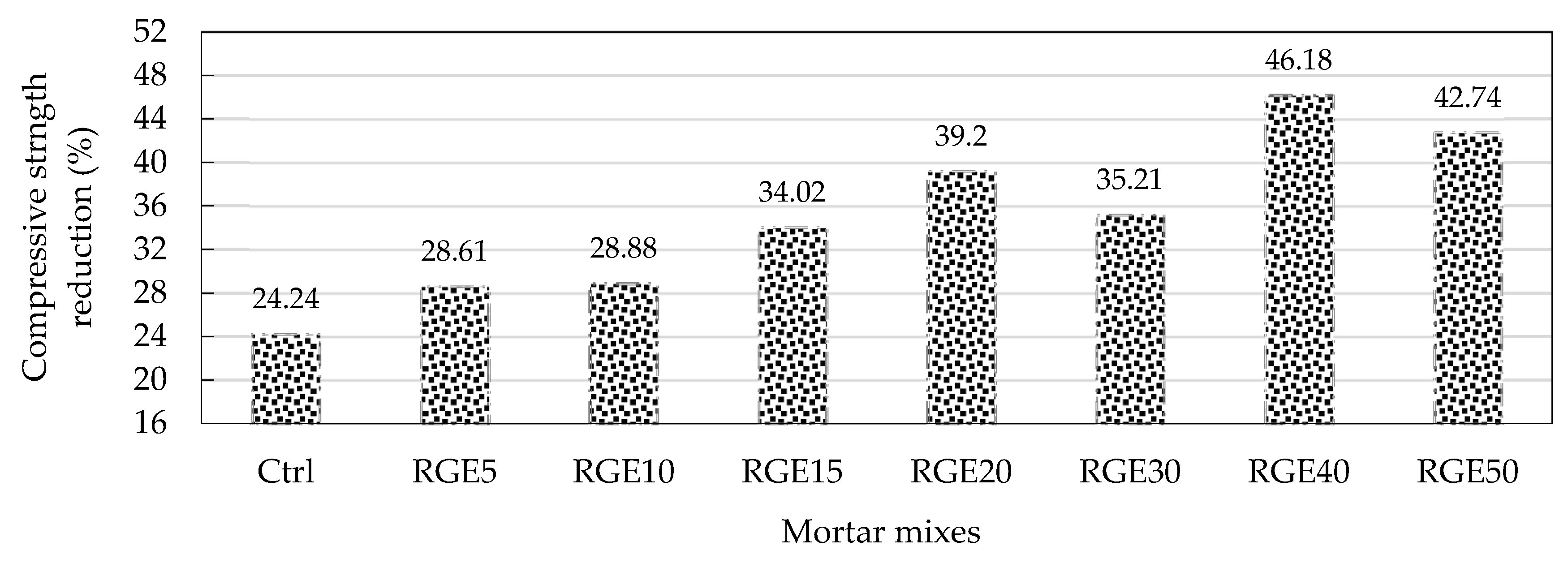
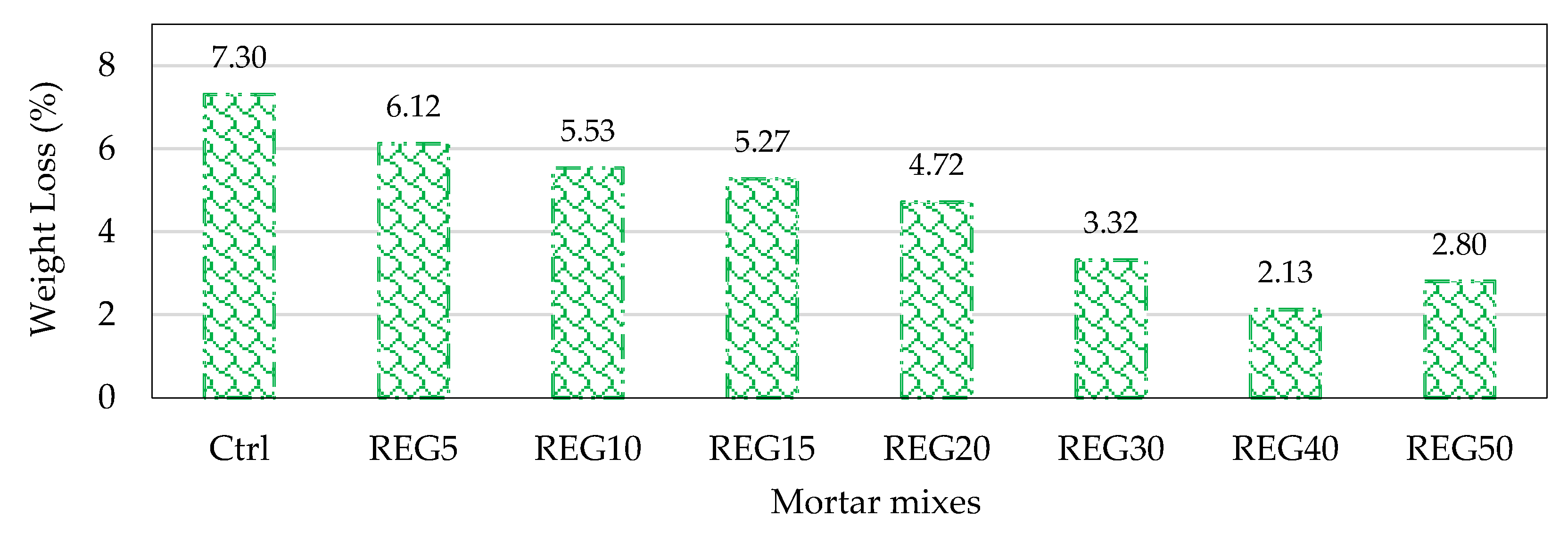
4.7. Durability against Sulphuric Acid
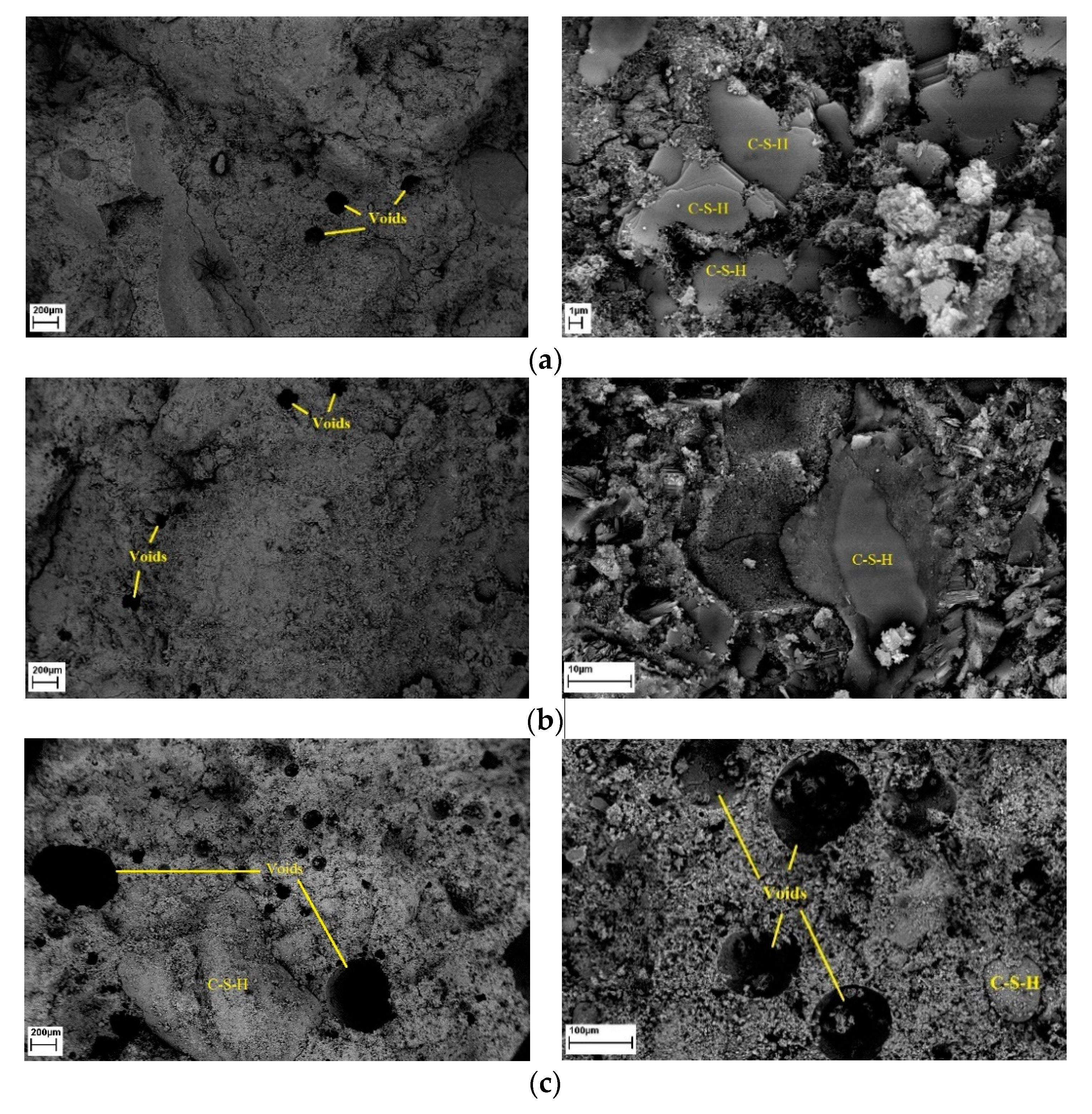
4.8. Microstructural Properties
5. Conclusions
- The slump of all the tested mixes ranged from 105 to 150 mm. In the specimens with 5% replacement ratio, slump was the same as that of the control specimen. However, increasing the replacement ratio over 5% resulted in the reduction in the slump;
- Replacing cement by RM, GGBFS, and EAFD up to 20% led to an increase in compressive strength resulting from the filler effect and latent pozzolanic reactivity of the waste powders. However, specimens with waste content higher than 20% experienced lower compressive strength compared to the control specimen;
- At 120 days of curing, the 30 min and 24 h water absorption test of specimens with 5 and 10% replacement showed the same results as that of control specimens. However, after passing the 10% replacement ratio, as the waste content increased, the water absorption followed the same trend, and the number of interconnected pores and water-saturated cavities rapidly multiplied, resulting in a higher water absorption capacity;
- The electrical resistivity of the mix with 5% waste content is the same as that of the control mix, and by increasing the replacement ratio, it decreases. This can be attributed to the presence of high contents of heavy metals in the waste, which can considerably boost the conductivity;
- By increasing the waste replacement ratio, the reduction in compressive strength as a result of sulphate attack escalated, while mixes with 10% replacement ratio had a higher compressive strength compared to that of the control mix. The higher reduction in compressive strength of specimens containing waste powders relative to the control specimen can be attributed to the slow pozzolanic reactivity of the waste powders;
- As the replacement ratio increased, the reduction in compressive strength and weight of specimens as a result of sulphuric acid attack increased and reduced, respectively. Specimens made with 15% replacement ratio had the same compressive strength as the control specimens. The specimens were exposed to a sulphuric acid solution at an early age, meaning that waste powders did not have enough time to develop pozzolanic reactions. This can be the main reason for the higher compressive strength loss of all mixes made with waste powders;
- Due to the filler effect of waste grains, improvement in the density of mortar mixes with low amounts of waste powders was observed in SEM images. In addition, 5% cement replaced with waste powders could improve the ITZ between aggregates and paste. However, increasing the replacement ratio degraded concrete properties as the size of pores increased, and the volume of needle-like products (most probably ettringite, which is mainly responsible for concrete strength) decreased.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crossin, E. The greenhouse gas implications of using ground granulated blast furnace slag as a cement substitute. J. Clean. Prod. 2015, 95, 101–108. [Google Scholar] [CrossRef]
- Asadi Shamsabadi, E.; Ghalehnovi, M.; de Brito, J.; Khodabakhshian, A. Performance of concrete with waste granite powder: The effect of superplasticizers. Appl. Sci. 2018, 8, 1808. [Google Scholar] [CrossRef]
- Shahmansouri, A.A.; Bengar, H.A.; AzariJafari, H. Life cycle assessment of eco-friendly concrete mixtures incorporating natural zeolite in sulfate-aggressive environment. Constr. Build. Mater. 2021, 268, 121136. [Google Scholar] [CrossRef]
- Rehan, R.; Nehdi, M. Carbon dioxide emissions and climate change: Policy implications for the cement industry. Environ. Sci. Policy 2005, 8, 105–114. [Google Scholar] [CrossRef]
- Shahmansouri, A.A.; Nematzadeh, M.; Behnood, A. Mechanical properties of GGBFS-based geopolymer concrete incorporating natural zeolite and silica fume with an optimum design using response surface method. J. Build. Eng. 2021, 36, 102138. [Google Scholar] [CrossRef]
- Muzenski, S.; Flores-Vivian, I.; Farahi, B.; Sobolev, K. Towards Ultrahigh Performance Concrete Produced with Aluminum Oxide Nanofibers and Reduced Quantities of Silica Fume. Nanomaterials 2020, 10, 2291. [Google Scholar] [CrossRef]
- Karimipour, A.; Ghalehnovi, M.; de Brito, J. Mechanical and durability properties of steel fibre-reinforced rubberised concrete. Constr. Build. Mater. 2020, 257, 119463. [Google Scholar] [CrossRef]
- De Domenico, D.; Faleschini, F.; Pellegrino, C.; Ricciardi, G. Structural behavior of RC beams containing EAF slag as recycled aggregate: Numerical versus experimental results. Constr. Build. Mater. 2018, 171, 321–337. [Google Scholar] [CrossRef]
- Menéndez, G.; Bonavetti, V.; Irassar, E. Strength development of ternary blended cement with limestone filler and blast-furnace slag. Cem. Concr. Compos. 2003, 25, 61–67. [Google Scholar] [CrossRef]
- Jun, Y.; Bae, Y.H.; Shin, T.Y.; Kim, J.H.; Yim, H.J. Alkali-Activated Slag Paste with Different Mixing Water: A Comparison Study of Early-Age Paste Using Electrical Resistivity. Materials 2020, 13, 2447. [Google Scholar] [CrossRef]
- Luo, S.; Liu, M.; Yang, L.; Chang, J.; Yang, W.; Yan, X.; Yu, H.; Shen, Y. Utilization of waste from alumina industry to produce sustainable cement-based materials. Constr. Build. Mater. 2019, 229, 116795. [Google Scholar] [CrossRef]
- Dodoo-Arhin, D.; Nuamah, R.A.; Agyei-Tuffour, B.; Obada, D.O.; Yaya, A. Awaso bauxite red mud-cement based composites: Characterisation for pavement applications. Case Stud. Constr. Mater. 2017, 7, 45–55. [Google Scholar] [CrossRef]
- Tang, W.; Wang, Z.; Liu, Y.; Cui, H. Influence of red mud on fresh and hardened properties of self-compacting concrete. Constr. Build. Mater. 2018, 178, 288–300. [Google Scholar] [CrossRef]
- Liu, R.-X.; Poon, C.-S. Utilization of red mud derived from bauxite in self-compacting concrete. J. Clean. Prod. 2016, 112, 384–391. [Google Scholar] [CrossRef]
- Ghalehnovi, M.; Shamsabadi, E.A.; Khodabakhshian, A.; Sourmeh, F.; de Brito, J. Self-compacting architectural concrete production using red mud. Constr. Build. Mater. 2019, 226, 418–427. [Google Scholar] [CrossRef]
- Ghalehnovi, M.; Roshan, N.; Hakak, E.; Shamsabadi, E.A.; de Brito, J. Effect of red mud (bauxite residue) as cement replacement on the properties of self-compacting concrete incorporating various fillers. J. Clean. Prod. 2019, 240, 118213. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Z.; Chen, B. Experimental research on magnesium phosphate cements modified by red mud. Constr. Build. Mater. 2020, 231, 117131. [Google Scholar] [CrossRef]
- Gräfe, M.; Power, G.; Klauber, C. Bauxite residue issues: III. Alkalinity and associated chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]
- Miyake, M.; Akachi, T.; Matsuda, M. Preparation, structure and photocatalytic properties of cancrinite encapsulating lead and sulfide ions. J. Mater. Chem. 2005, 15, 791–797. [Google Scholar] [CrossRef]
- Senff, L.; Hotza, D.; Labrincha, J. Effect of red mud addition on the rheological behaviour and on hardened state characteristics of cement mortars. Constr. Build. Mater. 2011, 25, 163–170. [Google Scholar] [CrossRef]
- Da Silva Magalhães, M.; Faleschini, F.; Pellegrino, C.; Brunelli, K. Cementing efficiency of electric arc furnace dust in mortars. Constr. Build. Mater. 2017, 157, 141–150. [Google Scholar] [CrossRef]
- Chen, Q.; Hills, C.; Tyrer, M.; Slipper, I.; Shen, H.; Brough, A. Characterisation of products of tricalcium silicate hydration in the presence of heavy metals. J. Hazard. Mater. 2007, 147, 817–825. [Google Scholar] [CrossRef]
- Da Silva Magalhães, M.; Faleschini, F.; Pellegrino, C.; Brunelli, K. Effects of electric arc furnace dust (EAFD) addition on setting and strength evolutions of cement pastes and mortars. Eur. J. Environ. Civ. Eng. 2017, 1–13. [Google Scholar] [CrossRef]
- De Vargas, A.S.; Masuero, Â.B.; Vilela, A.C. Investigations on the use of electric-arc furnace dust (EAFD) in Pozzolan-modified Portland cement I (MP) pastes. Cem. Concr. Res. 2006, 36, 1833–1841. [Google Scholar] [CrossRef]
- Hamilton, I.W.; Sammes, N.M. Encapsulation of steel foundry bag house dusts in cement mortar. Cem. Concr. Res. 1999, 29, 55–61. [Google Scholar] [CrossRef]
- Al-Zaid, R.Z.; Al-Sugair, F.H.; Al-Negheimish, A.I. Investigation of potential uses of electric-arc furnace dust (EAFD) in concrete. Cem. Concr. Res. 1997, 27, 267–278. [Google Scholar] [CrossRef]
- Fares, G.; Al-Zaid, R.Z.; Fauzi, A.; Alhozaimy, A.M.; Al-Negheimish, A.I.; Khan, M.I. Performance of optimized electric arc furnace dust-based cementitious matrix compared to conventional supplementary cementitious materials. Constr. Build. Mater. 2016, 112, 210–221. [Google Scholar] [CrossRef]
- Hwang, C.L.; Lin, C.Y. Strength development of blended blast-furnace slag-cement mortars. J. Chin. Inst. Eng. 1986, 9, 233–239. [Google Scholar] [CrossRef]
- Osborne, G. Durability of Portland blast-furnace slag cement concrete. Cem. Concr. Compos. 1999, 21, 11–21. [Google Scholar] [CrossRef]
- Samad, S.; Shah, A.; Limbachiya, M.C. Strength development characteristics of concrete produced with blended cement using ground granulated blast furnace slag (GGBS) under various curing conditions. Sādhanā 2017, 42, 1203–1213. [Google Scholar] [CrossRef]
- Maslehuddin, M.; Awan, F.; Shameem, M.; Ibrahim, M.; Ali, M. Effect of electric arc furnace dust on the properties of OPC and blended cement concretes. Constr. Build. Mater. 2011, 25, 308–312. [Google Scholar] [CrossRef]
- Özkan, Ö.; Yüksel, İ. Studies on mortars containing waste bottle glass and industrial by-products. Constr. Build. Mater. 2008, 22, 1288–1298. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Pugazhmani, G.; Sripragadeesh, R.; Muthu, D.; Venkatasubramanian, C. Experimental study on the mechanical and durability properties of concrete with waste glass powder and ground granulated blast furnace slag as supplementary cementitious materials. Constr. Build. Mater. 2017, 156, 739–749. [Google Scholar] [CrossRef]
- Lozano-Lunar, A.; da Silva, P.R.; de Brito, J.; Fernández, J.M.; Jiménez, J.R. Safe use of electric arc furnace dust as secondary raw material in self-compacting mortars production. J. Clean. Prod. 2019, 211, 1375–1388. [Google Scholar] [CrossRef]
- Kang, S.-P.; Kwon, S.-J. Effects of red mud and alkali-activated slag cement on efflorescence in cement mortar. Constr. Build. Mater. 2017, 133, 459–467. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Labrincha, J.A.; Morelli, M.R. Potential use of natural red mud as pozzolan for Portland cement. Mater. Res. 2011, 14, 60–66. [Google Scholar] [CrossRef]
- Shubbar, A.A.; Jafer, H.; Abdulredha, M.; Al-Khafaji, Z.S.; Nasr, M.S.; Al Masoodi, Z.; Sadique, M. Properties of cement mortar incorporated high volume fraction of GGBFS and CKD from 1 day to 550 days. J. Build. Eng. 2020, 30, 101327. [Google Scholar] [CrossRef]
- Aprianti, E.; Shafigh, P.; Zawawi, R.; Hassan, Z.F.A. Introducing an effective curing method for mortar containing high volume cementitious materials. Constr. Build. Mater. 2016, 107, 365–377. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Moeini, M.A. Mechanical and durability properties of alkali activated slag coating mortars containing nanosilica and silica fume. Constr. Build. Mater. 2018, 163, 611–621. [Google Scholar] [CrossRef]
- ACI 211.1. Standard practice for selecting proportions for normal, heavyweight and mass concrete; Reported by ACI Committee 211: Indianapolis, IN, USA, 1991. [Google Scholar]
- ASTM C267. Standard Test Methods for Chemical Resistance of Mortars, Grouts and Monolithic Surfacings and Polymer Concretes; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- ASTM C 143/143M-10. Standard Test Method for Slump of Hydraulic-Cement Concrete; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- ASTM C109/C109M-16a. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2 in. or [50-mm] Cube Specimens); ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM C496/C496M-11. Standard Test Method for Splitting Tensile Strength of Cylindrical Concrete Specimens; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM C666/C666M-03. Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing; ASTM International: West Conshohocken, PA, USA, 2003. [Google Scholar]
- ASTM C1012/C1012M-12. Standard Test Method for Length Change of Hydraulic-Cement Mortars Exposed to a Sulphate Solution; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- ASTM C642-13. Standard Test Method for Density, Absorption, and Voids in Hardened Concrete; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Khodabakhshian, A.; Ghalehnovi, M.; de Brito, J.; Shamsabadi, E.A. Durability performance of structural concrete containing silica fume and marble industry waste powder. J. Clean. Prod. 2018, 170, 42–60. [Google Scholar] [CrossRef]
- Zabihi, S.M.; Tavakoli, H.R. Evaluation of monomer ratio on performance of GGBFS-RHA alkali-activated concretes. Constr. Build. Mater. 2019, 208, 326–332. [Google Scholar] [CrossRef]
- Nedunuri, S.S.S.A.; Sertse, S.G.; Muhammad, S. Microstructural study of Portland cement partially replaced with fly ash, ground granulated blast furnace slag and silica fume as determined by pozzolanic activity. Constr. Build. Mater. 2020, 238, 117561. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Waste glass powder as cement replacement in concrete. J. Adv. Concr. Technol. 2014, 12, 468–477. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Elmoaty, A.E.M.A.; Auda, E.M. Re-use of waste marble dust in the production of cement and concrete. Constr. Build. Mater. 2014, 50, 28–41. [Google Scholar] [CrossRef]
- Wang, R.; Meyer, C. Performance of cement mortar made with recycled high impact polystyrene. Cem. Concr. Compos. 2012, 34, 975–981. [Google Scholar] [CrossRef]
- Toutanji, H.; Delatte, N.; Aggoun, S.; Duval, R.; Danson, A. Effect of supplementary cementitious materials on the compressive strength and durability of short-term cured concrete. Cem. Concr. Res. 2004, 34, 311–319. [Google Scholar] [CrossRef]
- Stark, J.; Ludwig, H.-M. Freeze-thaw and freeze-deicing salt resistance of concretes containing cement rich in granulated blast furnace slag. Mater. J. 1997, 94, 47–55. [Google Scholar]
- Vejmelková, E.; Keppert, M.; Grzeszczyk, S.; Skaliński, B.; Černý, R. Properties of self-compacting concrete mixtures containing metakaolin and blast furnace slag. Constr. Build. Mater. 2011, 25, 1325–1331. [Google Scholar] [CrossRef]
- Coussy, O.; Monteiro, P.J. Poroelastic model for concrete exposed to freezing temperatures. Cem. Concr. Res. 2008, 38, 40–48. [Google Scholar] [CrossRef]
- Lee, B.; Kim, G.; Nam, J.; Cho, B.; Hama, Y.; Kim, R. Compressive strength, resistance to chloride-ion penetration and freezing/thawing of slag-replaced concrete and cementless slag concrete containing desulfurization slag activator. Constr. Build. Mater. 2016, 128, 341–348. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J. Concrete Microstructure, Properties and Materials; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Tavasoli, S.; Nili, M.; Serpoush, B. Effect of GGBS on the frost resistance of self-consolidating concrete. Constr. Build. Mater. 2018, 165, 717–722. [Google Scholar] [CrossRef]
- Valenza II, J.J.; Scherer, G.W. A review of salt scaling: II. Mechanisms. Cem. Concr. Res. 2007, 37, 1022–1034. [Google Scholar] [CrossRef]
- Valenza, J.J.; Scherer, G.W. Mechanism for salt scaling. J. Am. Ceram. Soc. 2006, 89, 1161–1179. [Google Scholar] [CrossRef]
- Shang, H.; Song, Y.; Ou, J. Behavior of air-entrained concrete after freeze-thaw cycles. Acta Mech. Solida Sin. 2009, 22, 261–266. [Google Scholar] [CrossRef]
- Cai, L.; Wang, H.; Fu, Y. Freeze–thaw resistance of alkali–slag concrete based on response surface methodology. Constr. Build. Mater. 2013, 49, 70–76. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Mirza, J.; Tahir, M.M. Evaluation of alkali-activated mortars containing high volume waste ceramic powder and fly ash replacing GBFS. Constr. Build. Mater. 2019, 210, 78–92. [Google Scholar] [CrossRef]
- Allahverdi, A.; Abadi, M.M.B.R.; Hossain, K.M.A.; Lachemi, M. Resistance of chemically-activated high phosphorous slag content cement against freeze–thaw cycles. Cold Reg. Sci. Technol. 2014, 103, 107–114. [Google Scholar] [CrossRef]
- Li, J.; Yao, Y. A study on creep and drying shrinkage of high performance concrete. Cem. Concr. Res. 2001, 31, 1203–1206. [Google Scholar] [CrossRef]
- Yang, C.; Cho, S. An electrochemical method for accelerated chloride migration test of diffusion coefficient in cement-based materials. Mater. Chem. Phys. 2003, 81, 116–125. [Google Scholar] [CrossRef]
- Cui, L.; Cahyadi, J.H. Permeability and pore structure of OPC paste. Cem. Concr. Res. 2001, 31, 277–282. [Google Scholar] [CrossRef]
- Lozano-Lunar, A.; da Silva, P.R.; de Brito, J.; Alvarez, J.I.; Fernández, J.; Jimenez, J.R. Performance and durability properties of self-compacting mortars with electric arc furnace dust as filler. J. Clean. Prod. 2019, 219, 818–832. [Google Scholar] [CrossRef]
- Madandoust, R.; Mohseni, E.; Mousavi, S.Y.; Namnevis, M. An experimental investigation on the durability of self-compacting mortar containing nano-SiO2, nano-Fe2O3 and nano-CuO. Constr. Build. Mater. 2015, 86, 44–50. [Google Scholar] [CrossRef]
- Koushkbaghi, M.; Kazemi, M.J.; Mosavi, H.; Mohseni, E. Acid resistance and durability properties of steel fiber-reinforced concrete incorporating rice husk ash and recycled aggregate. Constr. Build. Mater. 2019, 202, 266–275. [Google Scholar] [CrossRef]
- Sturm, P.; Gluth, G.; Jäger, C.; Brouwers, H.; Kühne, H.-C. Sulfuric acid resistance of one-part alkali-activated mortars. Cem. Concr. Res. 2018, 109, 54–63. [Google Scholar] [CrossRef]
- Hadigheh, S.A.; Gravina, R.; Smith, S.T. Effect of acid attack on FRP-to-concrete bonded interfaces. Constr. Build. Mater. 2017, 152, 285–303. [Google Scholar] [CrossRef]
- Grengg, C.; Mittermayr, F.; Koraimann, G.; Konrad, F.; Szabó, M.; Demeny, A.; Dietzel, M. The decisive role of acidophilic bacteria in concrete sewer networks: A new model for fast progressing microbial concrete corrosion. Cem. Concr. Res. 2017, 101, 93–101. [Google Scholar] [CrossRef]
- Sand, W.; Bock, E. Concrete corrosion in the Hamburg sewer system. Environ. Technol. 1984, 5, 517–528. [Google Scholar] [CrossRef]
- Hadigheh, S.A.; Ke, F.; Kashi, S. 3D acid diffusion model for FRP-strengthened reinforced concrete structures: Long-term durability prediction. Constr. Build. Mater. 2020, 261, 120548. [Google Scholar] [CrossRef]
- Ramezanianpour, A.; Zolfagharnasab, A.; Bahmanzadeh, F.; Ramezanianpour, A. Assessment of high performance concrete containing mineral admixtures under sulfuric acid attack. Amirkabir J. Civ. Eng. 2018, 50, 121–138. [Google Scholar]
- Deb, P.S.; Sarker, P.K.; Barbhuiya, S. Sorptivity and acid resistance of ambient-cured geopolymer mortars containing nano-silica. Cem. Concr. Compos. 2016, 72, 235–245. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S. Acid resistance of inorganic polymer binders. 1. Corrosion rate. Mater. Struct. 2012, 45, 1–14. [Google Scholar] [CrossRef]
- Fattuhi, N.; Hughes, B. The performance of cement paste and concrete subjected to sulphuric acid attack. Cem. Concr. Res. 1988, 18, 545–553. [Google Scholar] [CrossRef]
- Israel, D.; Macphee, D.E.; Lachowski, E. Acid attack on pore-reduced cements. J. Mater. Sci. 1997, 32, 4109–4116. [Google Scholar] [CrossRef]
- Luga, E.; Atis, C.D. Optimization of heat cured fly ash/slag blend geopolymer mortars designed by “Combined Design” method: Part 1. Constr. Build. Mater. 2018, 178, 393–404. [Google Scholar] [CrossRef]
- Sadique, M.; Al-Nageim, H. Hydration kinetics of a low carbon cementitious material produced by physico-chemical activation of high calcium fly ash. J. Adv. Concr. Technol. 2012, 10, 254–263. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Eatmon, T.D. A life-cycle assessment of Portland cement manufacturing: Comparing the traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Gemelli, E.; Lourenci, S.; Folgueras, M.; Camargo, N. Assessment of industrial wastes in mortar layers deposited on stainless steel sheets of sinks. Cerâmica 2004, 50, 336–344. [Google Scholar] [CrossRef]
- Saha, A.K.; Sarker, P.K. Effect of sulphate exposure on mortar consisting of ferronickel slag aggregate and supplementary cementitious materials. J. Build. Eng. 2020, 28, 101012. [Google Scholar] [CrossRef]
- Pera, J.; Boumaza, R.; Ambroise, J. Development of a pozzolanic pigment from red mud. Cem. Concr. Res. 1997, 27, 1513–1522. [Google Scholar] [CrossRef]
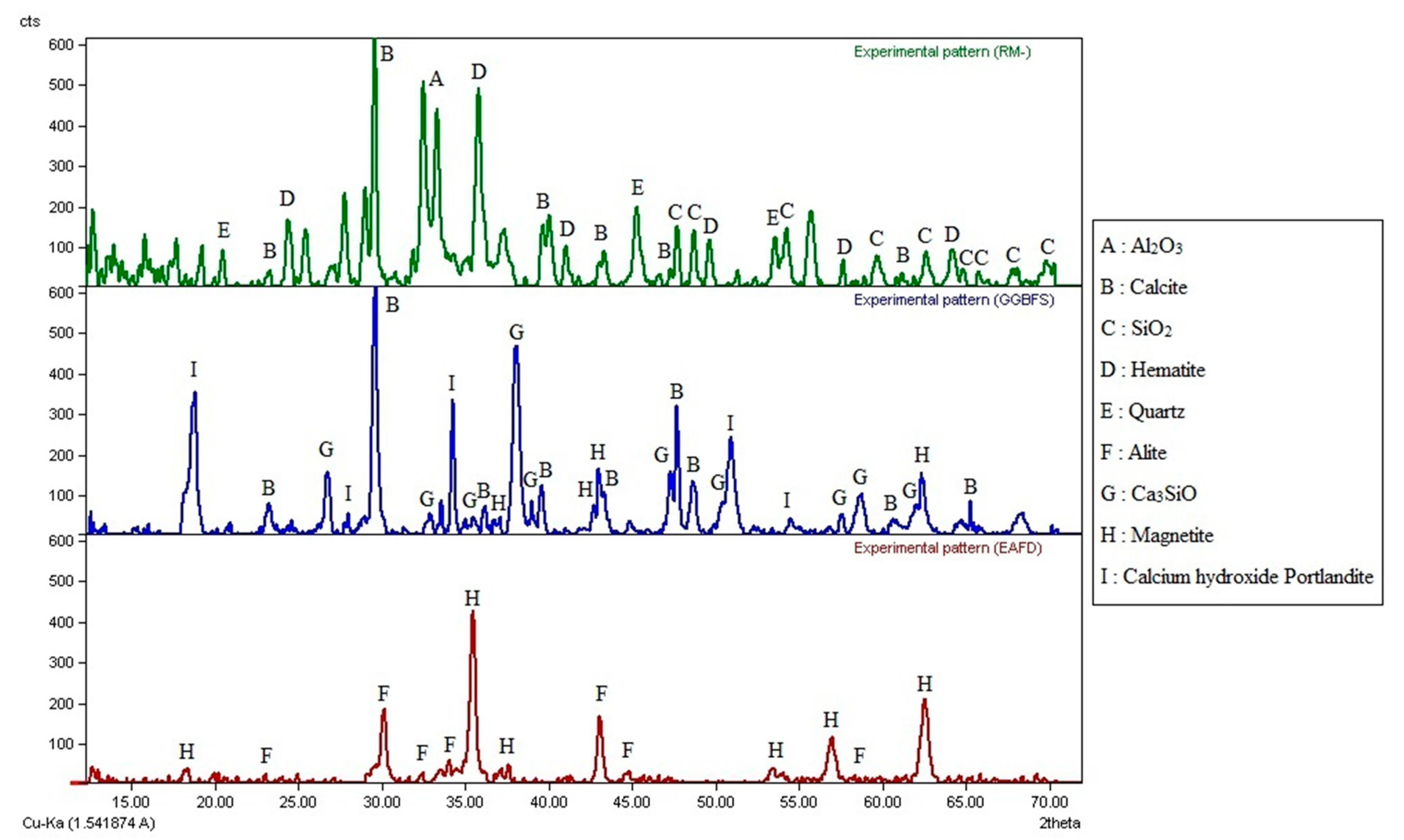
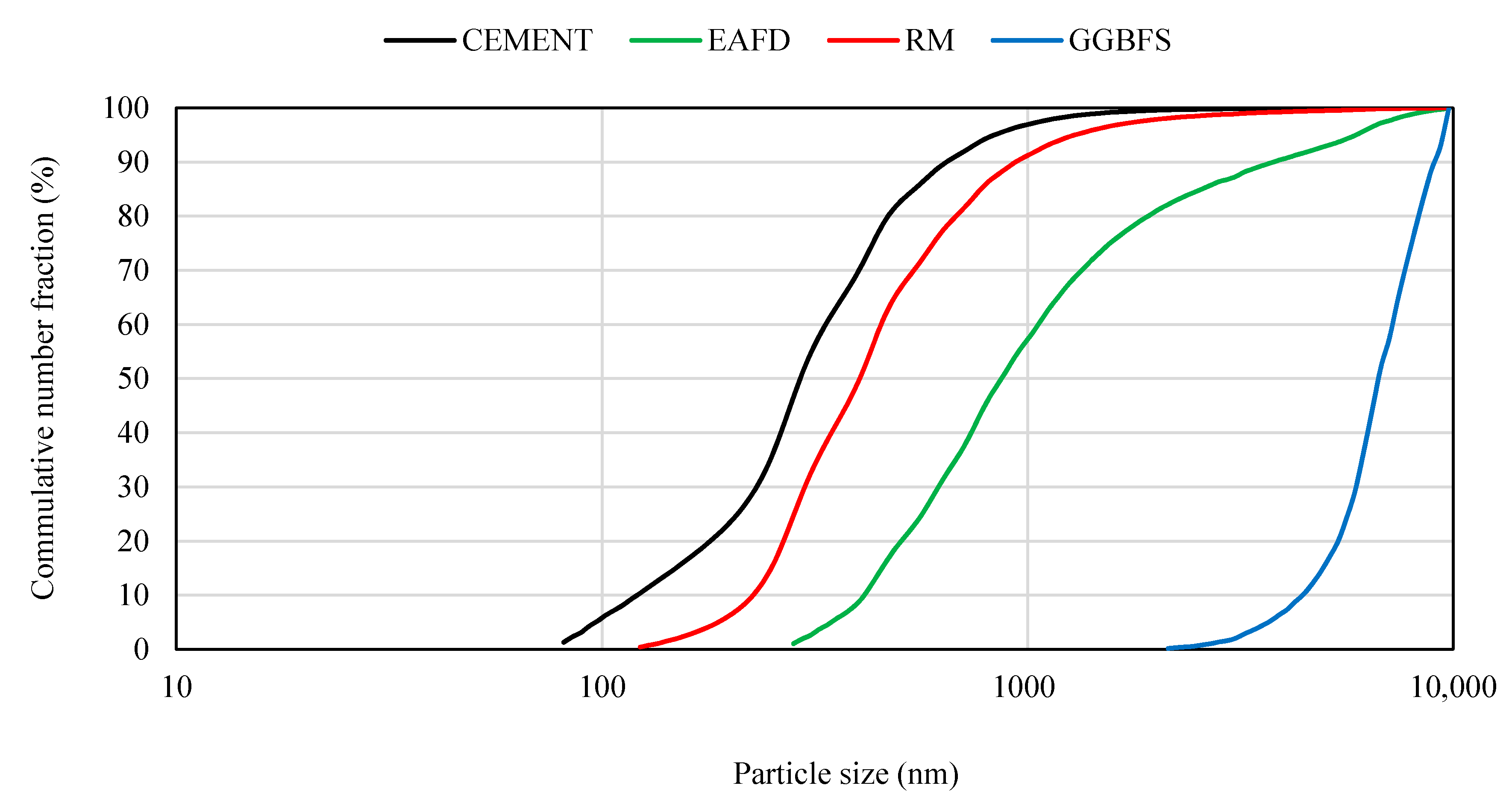
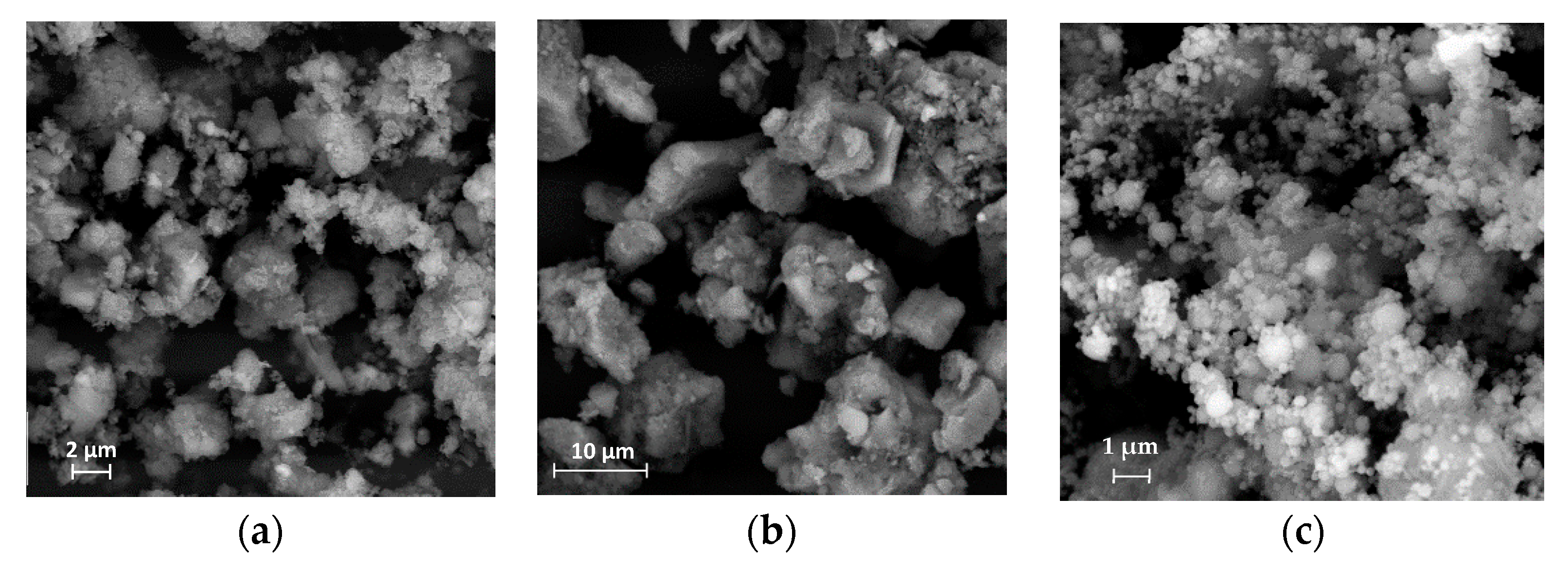

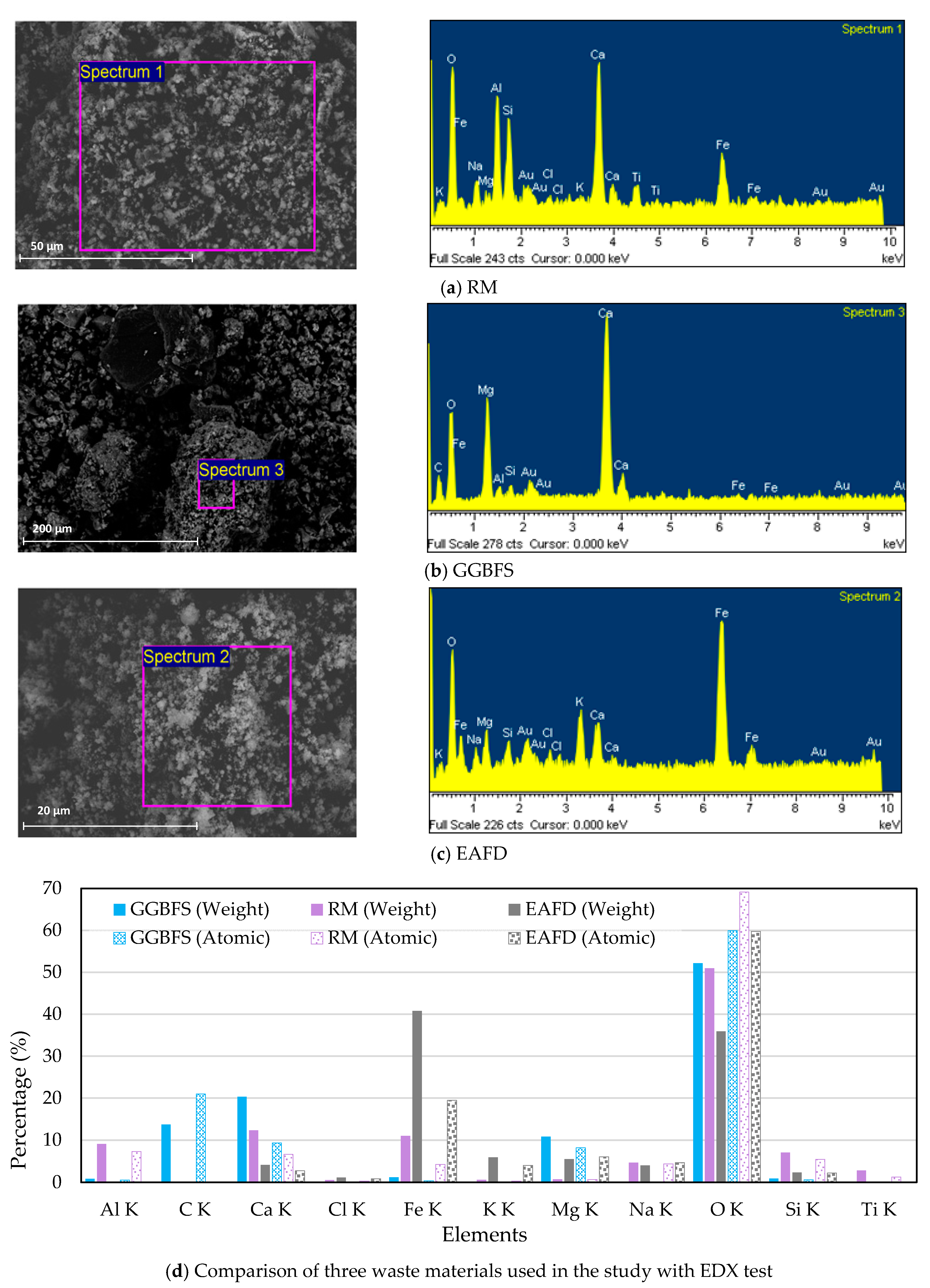



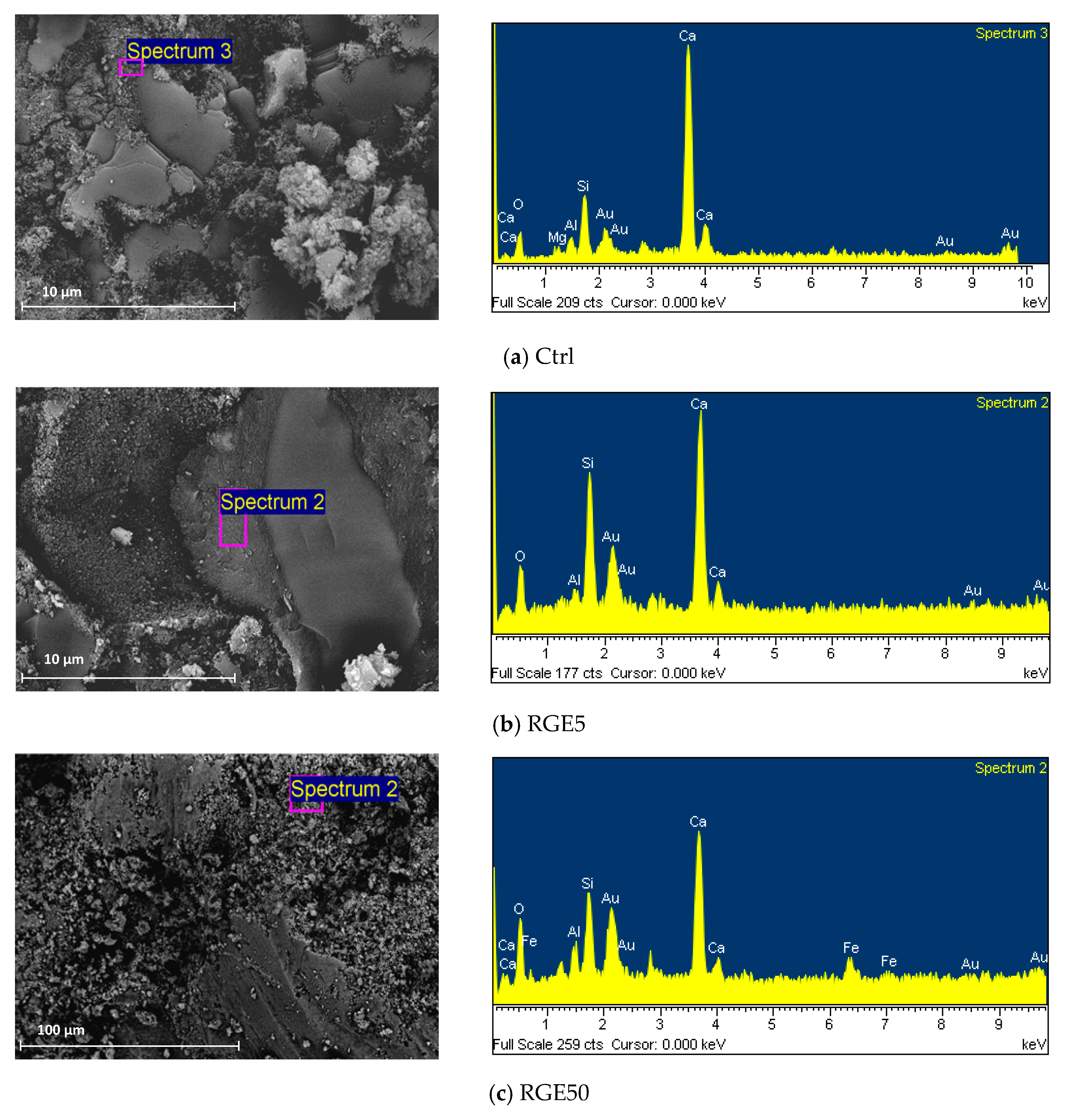
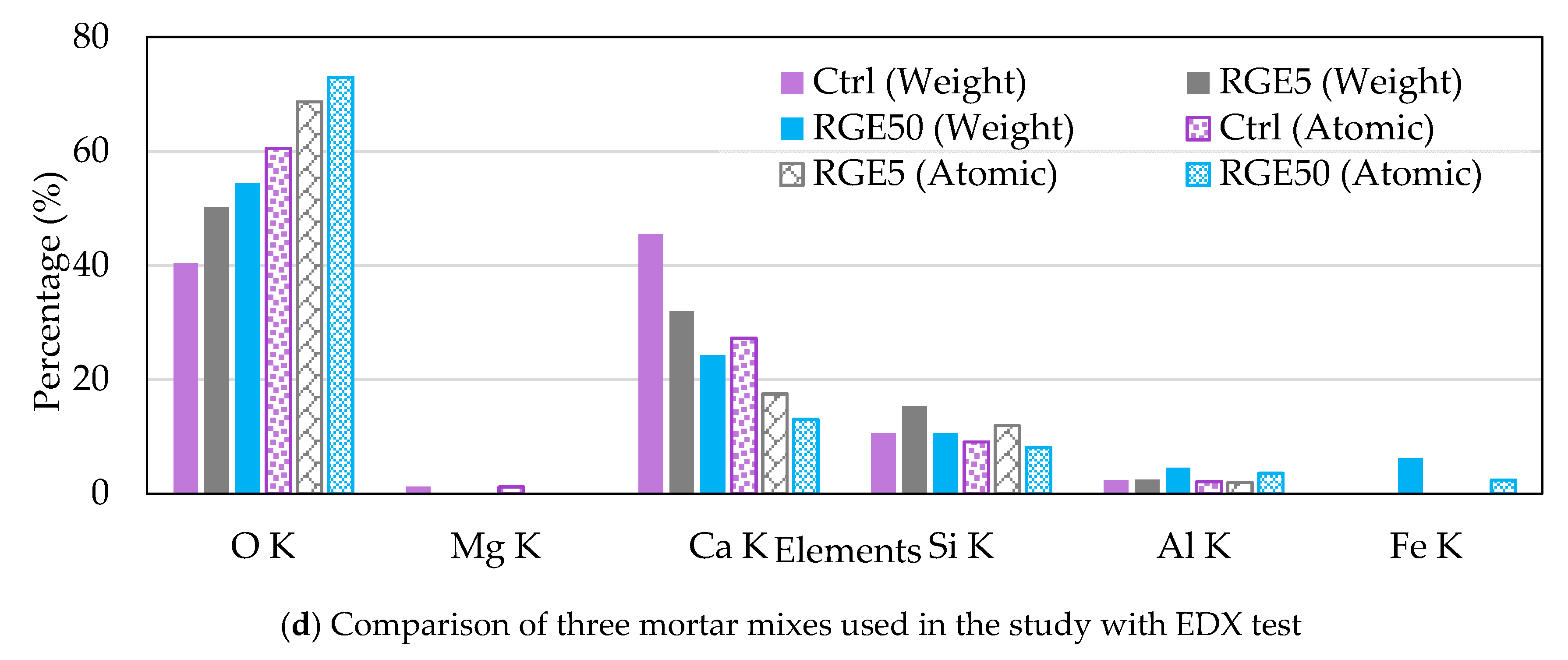
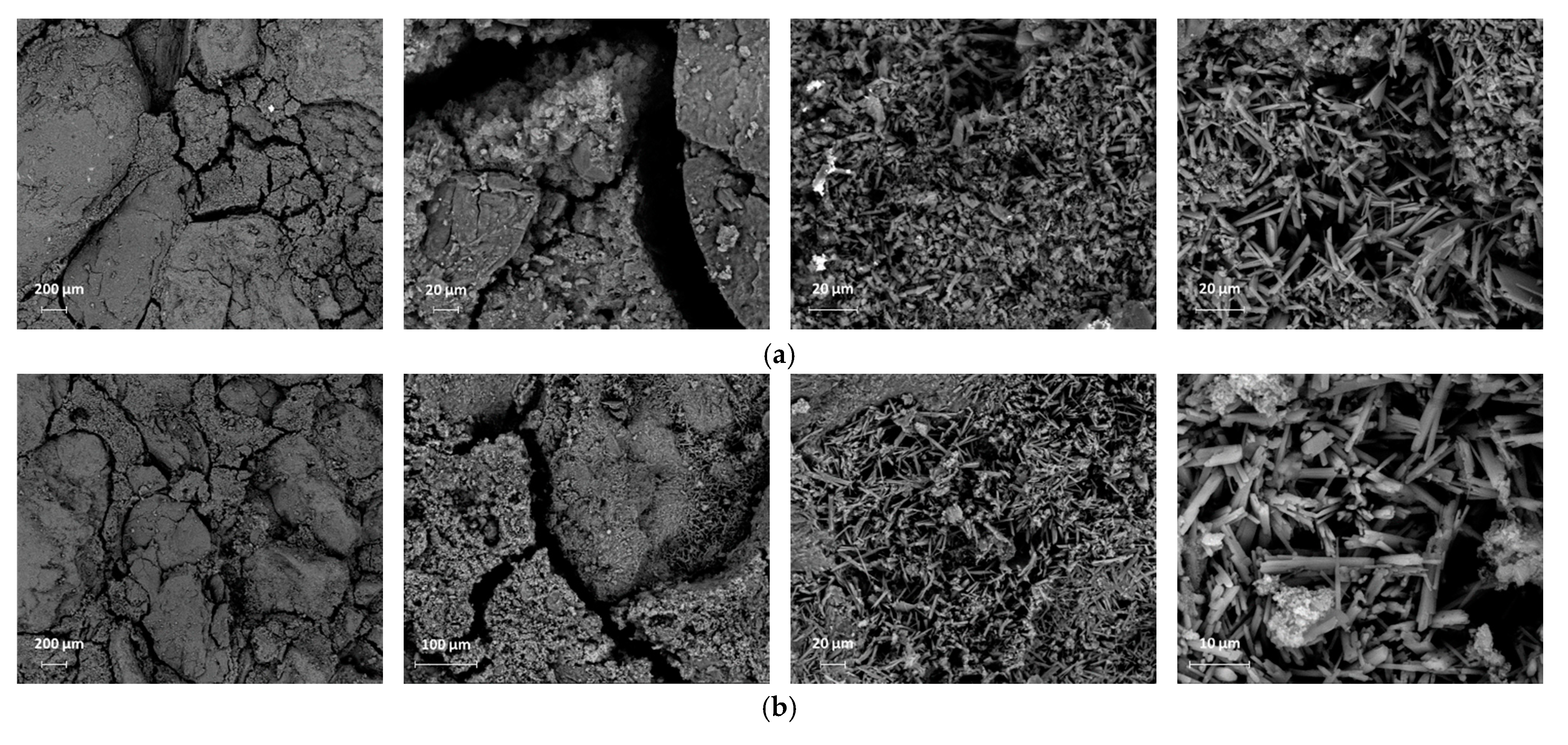
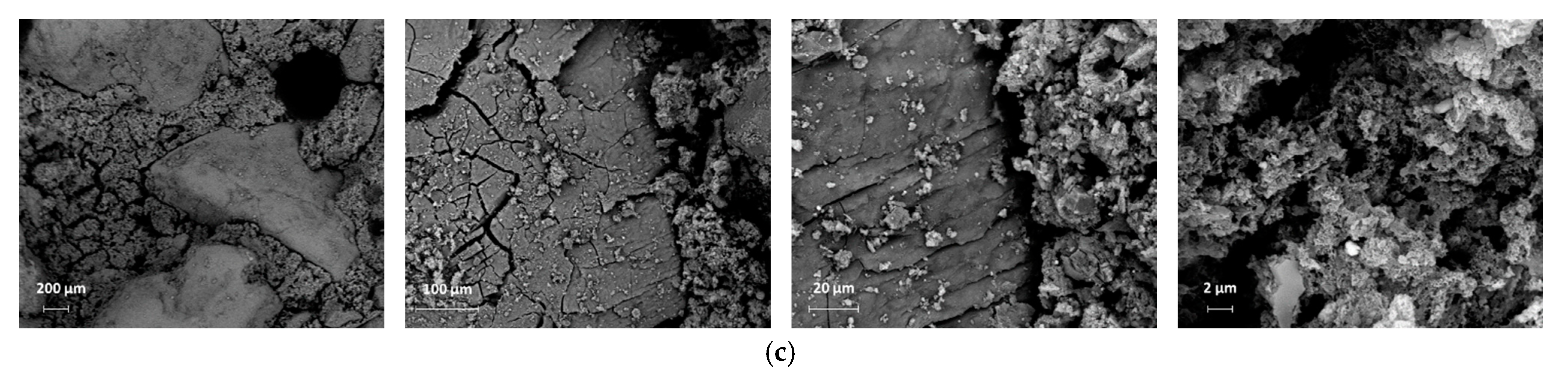
| Compounds | Cement (wt.%) | RM (wt%) | GGBFS (wt%) | EAFD (wt%) |
|---|---|---|---|---|
| SiO2 | 21.6 | 13.4 | 11.5 | 4.5 |
| Al2O3 | 4.3 | 16.9 | 2.8 | 0.3 |
| Fe2O3 | 3.1 | 23.8 | 7.1 | 53.3 |
| CaO | 62.1 | 18.3 | 34.5 | 10.5 |
| SO3 | 2.0 | 0.5 | 0.3 | 0.6 |
| MgO | 2.6 | 1.8 | 22.2 | 4.0 |
| K2O | --- | 0.5 | 0.4 | 5.7 |
| MnO2 | --- | 0.1 | 0.7 | 0.8 |
| Na2O | --- | 4.4 | 0.2 | 3.4 |
| ZnO | --- | --- | 0.2 | 1.1 |
| PbO2 | --- | --- | --- | --- |
| CdO | --- | --- | --- | --- |
| NiO | --- | --- | --- | --- |
| Cr2O3 | --- | --- | 0.1 | --- |
| L.O.I | --- | 14.9 | 19.5 | 15.5 |
| TiO2 | --- | 5.2 | 0.3 | 0.1 |
| BaO | --- | --- | 0.2 | --- |
| V2O5 | --- | --- | --- | 0.1 |
| Cl | --- | --- | --- | 0.1 |
| P2O5 | --- | 0.2 | --- | --- |
| C3A | 4.3 | --- | --- | --- |
| No | Name | Water (g) | Cement (g) | RM (g) | GGBFS (g) | EAFD (g) | Sand (g) | W/b | Slump (mm) | Density (kg/m3) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ctrl | 1800 | 2820 | 0 | 0 | 0 | 10,563 | 0.64 | 150 | 2165 |
| 2 | RGE5 | 1800 | 2679 | 47 | 47 | 47 | 10,563 | 0.64 | 150 | 2168 |
| 3 | RGE10 | 1800 | 2538 | 94 | 94 | 94 | 10,563 | 0.64 | 145 | 2161 |
| 4 | RGE15 | 1800 | 2397 | 141 | 141 | 141 | 10,563 | 0.64 | 140 | 2158 |
| 5 | RGE20 | 1800 | 2256 | 188 | 188 | 188 | 10,563 | 0.64 | 135 | 2153 |
| 6 | RGE30 | 1800 | 1974 | 282 | 282 | 282 | 10,563 | 0.64 | 130 | 2147 |
| 7 | RGE40 | 1800 | 1692 | 376 | 376 | 376 | 10,563 | 0.64 | 115 | 2120 |
| 8 | RGE50 | 1800 | 1410 | 470 | 470 | 470 | 10,563 | 0.64 | 105 | 2106 |
| Test | Testing Age (Days) | Type of Specimens | Reference Test Method | Specific Information |
|---|---|---|---|---|
| Slump | Fresh mortar | ASTM C143 [42] | ||
| Compressive strength | 7, 28, 120 | Cube (50 mm) | ASTM C109 [43] | |
| Splitting tensile strength | 120 | Cylinder (50 × 100 mm) | ASTM C496 [44] | |
| Freeze–thaw cycle | 28 (start of cycles) | Cube (50 mm) | ASTM C666 [45] | After 30 cycles One cycle in a day Freezing: in 12 h Thawing:12 h |
| Sodium sulphate attack | 28 (start of cycles) | Cube (50 mm) | ASTM C1012 [46] | 20% sodium sulphate |
| Sulphuric acid attack | 28 (start of cycles) | Cube (50 mm) | ASTM C267 [41] | 25% sulphuric acid |
| Water absorption | 28, 120 | Cube (50 mm) | ASTM C642 [47] | |
| Electrical resistivity | 7, 28, 42 | Cylinder (50 × 100 mm) | Khodabakhshian et al. [48] | Constant voltage of 30 V |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabzi, J.; Asadi Shamsabadi, E.; Ghalehnovi, M.; Hadigheh, S.A.; Khodabakhshian, A.; Brito, J.d. Mechanical and Durability Properties of Mortars Incorporating Red Mud, Ground Granulated Blast Furnace Slag, and Electric Arc Furnace Dust. Appl. Sci. 2021, 11, 4110. https://doi.org/10.3390/app11094110
Sabzi J, Asadi Shamsabadi E, Ghalehnovi M, Hadigheh SA, Khodabakhshian A, Brito Jd. Mechanical and Durability Properties of Mortars Incorporating Red Mud, Ground Granulated Blast Furnace Slag, and Electric Arc Furnace Dust. Applied Sciences. 2021; 11(9):4110. https://doi.org/10.3390/app11094110
Chicago/Turabian StyleSabzi, Javad, Elyas Asadi Shamsabadi, Mansour Ghalehnovi, S. Ali Hadigheh, Ali Khodabakhshian, and Jorge de Brito. 2021. "Mechanical and Durability Properties of Mortars Incorporating Red Mud, Ground Granulated Blast Furnace Slag, and Electric Arc Furnace Dust" Applied Sciences 11, no. 9: 4110. https://doi.org/10.3390/app11094110
APA StyleSabzi, J., Asadi Shamsabadi, E., Ghalehnovi, M., Hadigheh, S. A., Khodabakhshian, A., & Brito, J. d. (2021). Mechanical and Durability Properties of Mortars Incorporating Red Mud, Ground Granulated Blast Furnace Slag, and Electric Arc Furnace Dust. Applied Sciences, 11(9), 4110. https://doi.org/10.3390/app11094110








