Application of Hydrogen Peroxide to Improve the Microbiological Stability of Food Ice Produced in Industrial Facilities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Food Ice Production
2.2. Determination of the Concentrations of H2O2 in Food Ice
2.3. Inactivation Assays
2.4. Statistical Analysis
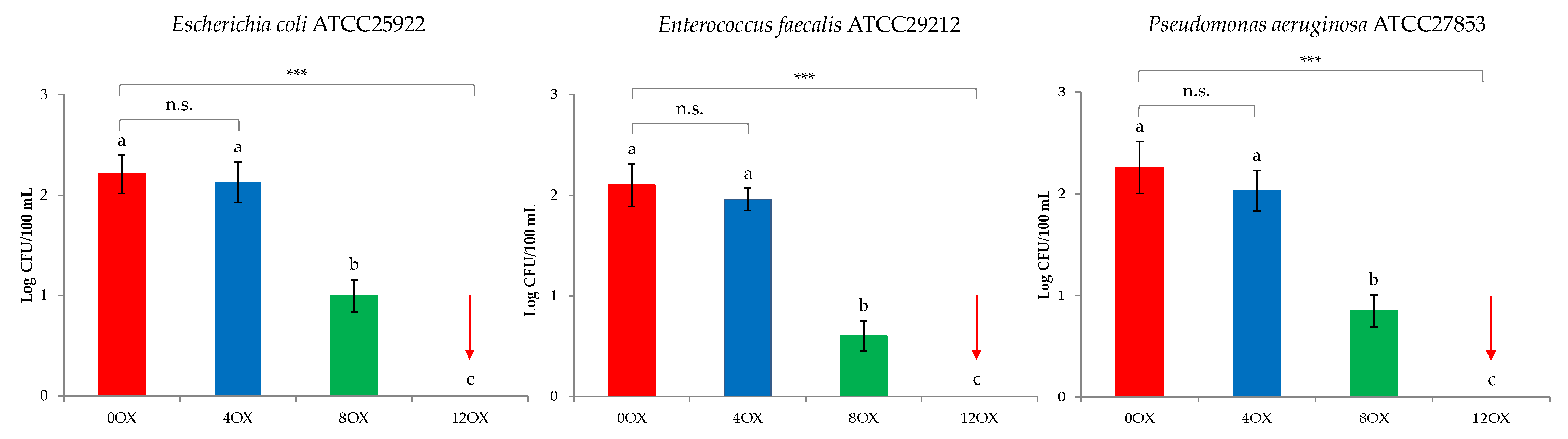
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrell, R.A.; Hunter, P.R.; Nichols, G. Microbiological standards for water and their relationship to health risk. Commun. Dis. Public Health 2000, 3, 8–13. [Google Scholar] [PubMed]
- Lautenschlager, K.; Hwang, C.; Liu, W.; Boon, N.; Koester, O.; Vrouwenvelder, H.; Egli, T.; Hammes, F. A microbiology-based multi-parametric approach towards assessing biological stability in drinking water distribution networks. Water Res. 2013, 47, 3015–3025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, F.; Cao, S.; Jin, W.; Zhou, X.; Ding, W.; Tu, R.; Han, F.S.; Wang, C.; Jang, Q.; Huang, H.; et al. Inactivation of chlorine-resistant bacterial spores in drinking water using UV irradiation, UV/Hydrogen peroxide and UV/Peroxymonosulfate: Efficiency and mechanism. J. Clean. Prod. 2020, 243, 118666. [Google Scholar] [CrossRef]
- Settanni, L.; Gaglio, R.; Stucchi, C.; De Martino, S.; Francesca, N.; Moschetti, G. Presence of pathogenic bacteria in ice cubes and evaluation of their survival in different systems. Ann. Microbiol. 2017, 67, 827–835. [Google Scholar] [CrossRef] [Green Version]
- Gaglio, R.; Francesca, N.; Di Gerlando, R.; Mahony, J.; De Martino, S.; Stucchi, C.; Moschetti, G.; Settanni, L. Enteric bacteria of food ice and their survival in alcoholic beverages and soft drinks. Food Microbiol. 2017, 67, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Falcao, J.P.; Dias, A.M.G.; Correa, E.F.; Falcao, D.P. Microbiological quality of ice used to refrigerate foods. Food Microbiol. 2002, 19, 269–276. [Google Scholar] [CrossRef]
- Regulation (EC) No 1882/2003 of the European Parliament and of the Council of 29 September 2003 Adapting to Council Decision 1999/468/EC the Provisions Relating to Committees Which Assist the Commission in the Exercise of Its Implementing Powers Laid down in Instruments Subject to the Procedure Referred to in Article 251 of the EC Treaty. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/PDF/?uri=CELEX:32003R1882&from=EN (accessed on 25 November 2020).
- Lee, K.H.; Ab Samad, L.S.; Lwin, P.M.; Riedel, S.F.; Magin, A.; Bashir, M.; Vaishampayan, P.A.; Lin, W.J. On the Rocks: Microbiological Quality and Microbial Diversity of Packaged Ice in Southern California. J. Food Prot. 2017, 80, 1041–1049. [Google Scholar] [CrossRef]
- Noor Izani, N.J.; Zulaikha, A.R.; Mohamad Noor, M.R.; Amri, M.A.; Mahat, N.A. Contamination of faecal coliforms in ice cubes sampled from food outlets in Kubang Kerian, Kelantan. Trop. Biomed. 2012, 29, 71–76. [Google Scholar]
- Chavasit, V.; Sirilaksanamanon, K.; Phithaksantayothin, P.; Norapoompipat, Y.; Parinyasiri, T. Measures for controlling safety of crushed ice and tube ice in developing country. Food Control 2011, 22, 118–123. [Google Scholar] [CrossRef]
- Lateef, A.; Oloke, J.K.; Kana, E.B.G.; Pacheco, E. The microbiological quality of ice used to cool drinks and foods in Ogbomoso Metropolis, Southwest, Nigeria. Int. J. Food Saf. 2006, 8, 39–43. [Google Scholar]
- Ukwo, S.P.; Ndaeyo, N.U.; Udoh, E.J. Microbiological quality and safety evaluation of fresh juices and edible ice sold in Uyo Metropolis, South-South, Nigeria. Int. J. Food Saf. 2011, 13, 374–378. [Google Scholar]
- Francesca, N.; Gaglio, R.; Stucchi, C.; De Martino, S.; Moschetti, G.; Settanni, L. Yeasts and moulds contaminants of food ice cubes and their survival in different drinks. J. Appl. Microbiol. 2018, 124, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickens, D.L.; DuPont, H.L.; Johnson, P.C. Survival of bacterial enteropathogens in the ice of popular drinks. JAMA 1985, 253, 3141–3143. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Ohnishi, S.; Ito, T. Osmoelastic coupling in biological structures: Decrease in membrane fluidity and osmophobic association of phospholipid vesicles in response to osmotic stress. Biochemistry 1989, 28, 3710–3715. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 2004, 1666, 142–157. [Google Scholar] [CrossRef] [Green Version]
- Kamada, Y.; Jung, U.S.; Piotrowski, J.; Levin, D.E. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a nove1 aspect of the heat shock response. Genes Dev. 1995, 9, 1559–1571. [Google Scholar] [CrossRef] [Green Version]
- de Jonge, H.W.; Dekkers, D.H.; Bastiaanse, E.M.; Bezstarosti, K.; van der Laarse, A.; Lamers, J.M. Eicosapentaenoic acid incorporation in membrane phospholipids modulates receptor-mediated phospholipase C and membrane fluidity in rat ventricular myocytes in culture. J. Mol. Cell. Cardiol. 1996, 28, 1097–1108. [Google Scholar] [CrossRef] [Green Version]
- Los, D.A.; Murata, N. Regulation of enzymatic activity and gene expression by membrane fluidity. Sci. Signal Transduct. Knowl. Environ. 2000, 62, 1. [Google Scholar] [CrossRef]
- Wagner, M.; Brumelis, D.; Gehr, R. Disinfection of wastewater by hydrogen peroxide or peracetic acid: Development of procedures for measurement of residual disinfectant and application to a physicochemically treated municipal effluent. Water Environ. Res. 2002, 74, 33–50. [Google Scholar] [CrossRef]
- Veschetti, E.; Cutilli, D.; Bonadonna, L.; Briancesco, R.; Martini, C.; Cecchini, G.; Anastasi, P.; Ottaviani, M. Pilot-plant comparative study of peracetic acid and sodium hypochlorite wastewater disinfection. Water Res. 2003, 37, 78–94. [Google Scholar] [CrossRef]
- Finnegan, M.; Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Mode of action of hydrogen peroxide and other oxidizing agents: Differences between liquid and gas forms. J. Antimicrob. Chemother. 2010, 65, 2108–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, R.J.; Teel, A.L. Hydroxyl radical and non-hydroxyl radical pathways for trichloroethylene and perchloroethylene degradation in catalyzed H2O2 propagation systems. Water Res. 2019, 159, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Labas, M.D.; Zalazar, C.S.; Brandi, R.J.; Cassano, A.E. Reaction kinetics of bacteria disinfection employing hydrogen peroxide. Biochem. Eng. J. 2008, 38, 78–87. [Google Scholar] [CrossRef]
- Li, W.; Bonakdarpour, A.; Gyenge, E.; Wilkinson, D.P. Production of Hydrogen Peroxide for Drinking Water Treatment in a Proton Exchange Membrane Electrolyzer at Near-Neutral pH. J. Electrochem. Soc. 2020, 167, 044502. [Google Scholar] [CrossRef]
- Pedahzur, R.; Lev, O.; Fattal, B.; Shuval, H.I. The interaction of silver ions and hydrogen peroxide in the inactivation of E. coli: A preliminary evaluation of a new long acting residual drinking water disinfectant. Water Sci. Technol. 1995, 31, 123–129. [Google Scholar] [CrossRef]
- Italian Standards Institution. Chemicals Used for Treatment of Water Intended for Human Consumption. In Hydrogen Peroxide; Italian Standards Institution: Milan, Italy, 2016. [Google Scholar]
- Istituto Nazionale Ghiaccio Alimentare. Manuale di Corretta Prassi Operativa per la Produzione di Ghiaccio Alimentare. 2015. Available online: https://www.salute.gov.it/imgs/C_17_pagineAree_1187_listaFile_itemName_0_file.pdf (accessed on 25 November 2021).
- Meng, F.; Li, G.; Zhang, B.; Guo, J. Chemical kinetics and particle size effects of activated carbon for free chlorine removal from drinking water. Water Pract. Technol. 2019, 14, 19–26. [Google Scholar] [CrossRef]
- Seo, S.J.; Jeon, H.; Lee, J.K.; Kim, G.Y.; Park, D.; Nojima, H.; Lee, J.; Moon, S.H. Investigation on removal of hardness ions by capacitive deionization (CDI) for water softening applications. Water Res. 2010, 44, 2267–2275. [Google Scholar] [CrossRef]
- Caggiano, G.; Marcotrigiano, V.; Trerotoli, P.; Diella, G.; Rutigliano, S.; Apollonio, F.; Marzella, A.; Triggiano, F.; Gramegna, M.; Lagravinese, D.; et al. Food hygiene surveillance in Italy: Is food ice a public health risk? Int. J. Environ. Res. 2020, 17, 2408. [Google Scholar]
- Decreto Legislativo 2 Febbraio 2001, n. 31. “Attuazione della Direttiva 98/83/CE Relativa alla Qualità delle Acque Destinate al Consumo Umano”. GU n. 52 del 3 Marzo 2001-Supplemento Ordinario n. 41. Available online: https://www.camera.it/parlam/leggi/deleghe/01031dl.htm (accessed on 24 November 2021).
- Moschetti, G.; Settanni, L. Acqua e bevande non alcoliche. In Microbiologia dei Prodotti Alimentari; Farris, A., Gobbetti, M., Neviani, E., Vincenzini, M., Eds.; Casa Editrice Ambrosiana: Milan, Italy, 2012; pp. 141–152. [Google Scholar]
- Ríos-Castillo, A.G.; González-Rivas, F.; Rodríguez-Jerez, J.J. Bactericidal efficacy of hydrogen peroxide-based disinfectants against gram-positive and gram-negative bacteria on stainless steel surfaces. J. Food Sci. 2017, 82, 2351–2356. [Google Scholar] [CrossRef]
- Lineback, C.B.; Nkemngong, C.A.; Wu, S.T.; Li, X.; Teska, P.J.; Oliver, H.F. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob. Resist. Infect. Control 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Alfa, M.J.; Jackson, M.A. New hydrogen peroxide–based medical-device detergent with germicidal properties: Comparison with enzymatic cleaners. Am. J. Infect. Control 2001, 29, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arefin, S.; Sarker, M.A.H.; Islam, M.A.; Harun-ur-Rashid, M.; Islam, M.N. Use of Hydrogen Peroxide (H2O2) in raw cow’s milk preservation. J. Adv. Vet. Anim. Res. 2017, 4, 371–377. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbaccia, P.; Lipocelli, L.; Moschetti, G.; Francesca, N.; De Martino, S.; Arrigo, V.; Gaglio, R.; Settanni, L. Application of Hydrogen Peroxide to Improve the Microbiological Stability of Food Ice Produced in Industrial Facilities. Appl. Sci. 2022, 12, 210. https://doi.org/10.3390/app12010210
Barbaccia P, Lipocelli L, Moschetti G, Francesca N, De Martino S, Arrigo V, Gaglio R, Settanni L. Application of Hydrogen Peroxide to Improve the Microbiological Stability of Food Ice Produced in Industrial Facilities. Applied Sciences. 2022; 12(1):210. https://doi.org/10.3390/app12010210
Chicago/Turabian StyleBarbaccia, Pietro, Leopoldo Lipocelli, Giancarlo Moschetti, Nicola Francesca, Simone De Martino, Vincenzo Arrigo, Raimondo Gaglio, and Luca Settanni. 2022. "Application of Hydrogen Peroxide to Improve the Microbiological Stability of Food Ice Produced in Industrial Facilities" Applied Sciences 12, no. 1: 210. https://doi.org/10.3390/app12010210
APA StyleBarbaccia, P., Lipocelli, L., Moschetti, G., Francesca, N., De Martino, S., Arrigo, V., Gaglio, R., & Settanni, L. (2022). Application of Hydrogen Peroxide to Improve the Microbiological Stability of Food Ice Produced in Industrial Facilities. Applied Sciences, 12(1), 210. https://doi.org/10.3390/app12010210





