Thermophysical Properties of Inorganic Phase-Change Materials Based on MnCl2·4H2O
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Materials
2.2. Measurement
3. Results and Discussion
3.1. Thermal Property of As-Received PCMs
3.2. Thermal Property of Treated PCM
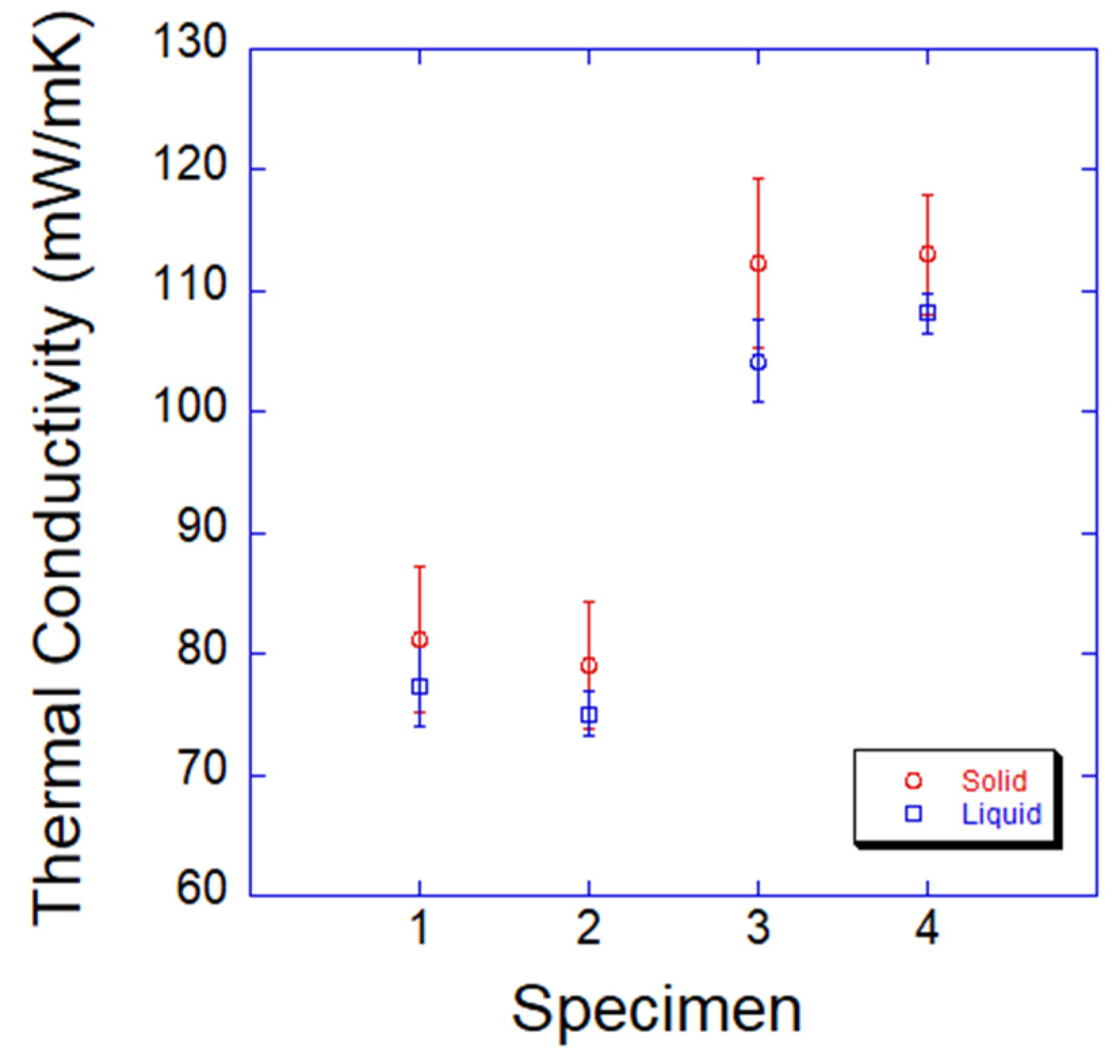
3.3. Thermal Conductivity and Thermal Diffusivity
4. Conclusions
- The melting/solidification temperature, latent heat, and thermal diffusivity were measured for MnCl2·4H2O PCMs, and the properties were provided. The melting temperatures of the PCMs under single phase were about 28 to 33 °C. Specimens 1 and 2, having a dual or slurry phase, led to superheating issues. The latent heat of the PCMs under the endothermic process was 146 to 176 J/g with a temperature range of 23 to 45 °C. The latent heats of as-received PCMs under the endothermic process were similar or higher than those of the commercial products with operating temperatures of 20 to 50 °C.
- In the endothermic process, the PCMs with a liquid or dual phase exhibited superheating because of the insufficient holding time in the initial stage until the PCMs were fully solidified. Another cause of this was the low thermal diffusivity of the PCMs based on the thermal diffusivity measurement in this study.
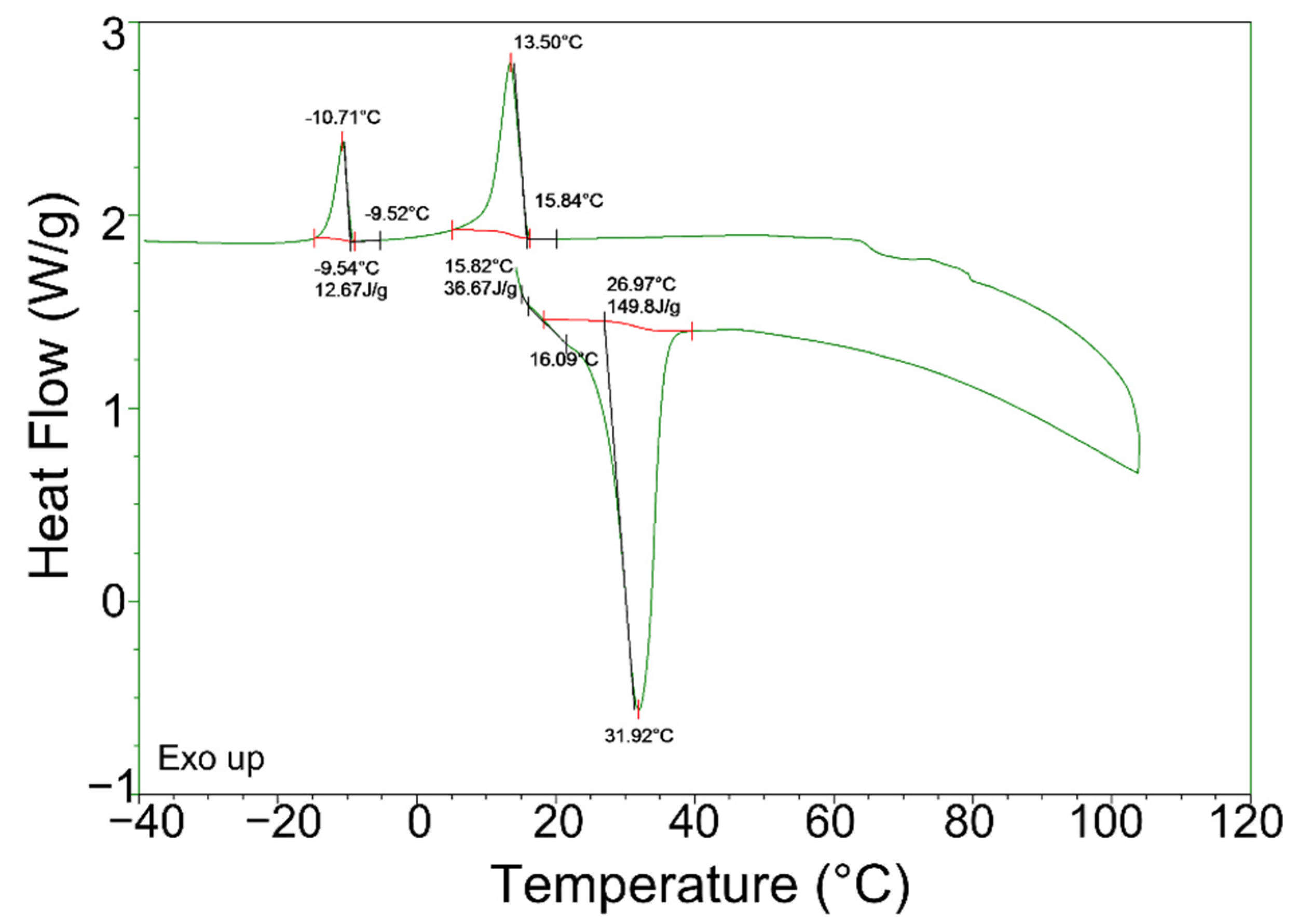
- In the exothermic process, more severe supercooling was observed in the DSC measurement. The high cooling rate and low thermal diffusivity of the PCMs used in this study caused inhomogeneous solidification, phase segregation, and, consequently, supercooling. Homogenization treatment prior to DSC could mitigate the supercooling issue, such as an increase in solidification temperature of about 15 °C compared with what was used for the as-received and treated PCMs. However, the treatment could not fully eliminate the supercooling issue.
- The diffusivities of PCMs were measured as between 9.76 × 10−6 and 2.35 × 10−5 mm2/s. The diffusivities of PCMs in the solid phase were higher than those in the liquid phase. The Fourier numbers of the PCMs were much lower than those in the references. The insufficient time for heat transfer resulted in high heating and cooling rates, and a very low level of thermal diffusivity was responsible for supercooling. Specimen 3 was the best PCM due to its high conductivity and high thermal diffusivity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Nomenclature | |
| Specific heat capacity, J/g °C | |
| T | Temperature, °C |
| Acronym | |
| Phase change material | PCM |
| Differential scanning calorimetry | DSC |
| Thermal energy storage | TES |
| Greek Symbols | |
| α | Thermal diffusivity, mm2/s |
| k | Thermal conductivity, mW/mK |
| Density | |
References
- US Energy Information Administrations. Washington, DC, USA. 2021. Available online: https://www.eia.gov/consumption/ (accessed on 15 June 2022).
- Abedin, A.H.; Rosen, M.A. A critical review of thermochemical energy storage systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Zalba, B.; Marın, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Banu, D.; Feldman, D.; Haghighat, F.; Paris, J.; Hawes, D. Energy-storing wallboard: Flammability tests. J. Mater. Civ. Eng. 1998, 10, 98–105. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Buddhi, D. PCM thermal storage in buildings: A state of art. Renew. Sustain. Energy Rev. 2007, 11, 1146–1166. [Google Scholar] [CrossRef]
- Furbo, S. Heat Storage Units Using a Salt Hydrate as Storage Medium Based on the Extra Water Principle; Commission of the European Communities: Luxembourg, 1983.
- Xie, N.; Huang, Z.; Luo, Z.; Gao, X.; Fang, Y.; Zhang, Z. Inorganic Salt Hydrate for Thermal Energy Storage. Appl. Sci. 2017, 7, 1317. [Google Scholar] [CrossRef] [Green Version]
- Palittin, I.D.; Kurniati, N.; Sutjahja, I.; Kurnia, D. Sonocrystallization Technique to Optimizing the Crystallization Process of PCM CaCl2. 6H2O. In Advanced Materials Research; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2015; pp. 559–562. [Google Scholar]
- Jin, X.; Xu, X.; Zhang, X.; Yin, Y. Determination of the PCM melting temperature range using DSC. Thermochim. Acta 2014, 595, 17–21. [Google Scholar] [CrossRef]
- Drissi, S.; Eddhahak, A.; Caré, S.; Neji, J. Thermal analysis by DSC of Phase Change Materials, study of the damage effect. J. Build. Eng. 2015, 1, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Castellón, C.; Günther, E.; Mehling, H.; Hiebler, S.; Cabeza, L.F. Determination of the enthalpy of PCM as a function of temperature using a heat-flux DSC-A study of different measurement procedures and their accuracy. Int. J. Energy Res. 2008, 32, 1258–1265. [Google Scholar] [CrossRef]
- Prakash, R.; Meenakshipriya, B.; Kumaravelan, R.; Vijayan, S. Preparation and study on thermophysical properties of inorganic salt hydrate as energy storage materials. Mater. Today Proc. 2021, 42, 450–456. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Niu, X.; Ling, X. Preparation, characterization, and thermal properties of microencapsulated phase change material for low-temperature thermal energy storage. Energy Fuels 2019, 33, 1631–1636. [Google Scholar] [CrossRef]
- Gschwander, S.; Haussmann, T.; Hagelstein, G.; Barreneche, C.; Ferrer, G.; Cabeza, L.; Hennemann, P. Standardization of PCM characterization via DSC. In Proceedings of the SHC 2015 International Conference on Solar Heating and Cooling for Buildings and Industry, Istanbul, Turkey, 2–4 December 2015; pp. 2–4. [Google Scholar]
- Morîntale, E.; Harabor, A.; Constantinescu, C.; Rotaru, P. Use of heat flows from DSC curve for calculation of specific heat of the solid materials. Phys. AUC 2013, 23, 89–94. [Google Scholar]
- Liu, L.; Zhang, X.; Xu, X.; Lin, X.; Zhao, Y.; Zou, L.; Wu, Y.; Zheng, H. Development of low-temperature eutectic phase change material with expanded graphite for vaccine cold chain logistics. Renew. Energy 2021, 179, 2348–2358. [Google Scholar] [CrossRef]
- Paneliya, S.; Khanna, S.; Utsav; Singh, A.P.; Patel, Y.K.; Vanpariya, A.; Makani, N.H.; Banerjee, R.; Mukhopadhyay, I. Core shell paraffin/silica nanocomposite: A promising phase change material for thermal energy storage. Renew. Energy 2021, 167, 591–599. [Google Scholar] [CrossRef]
- Bao, J.; Zou, D.; Zhu, S.; Ma, Q.; Wang, Y.; Hu, Y. A medium-temperature, metal-based, microencapsulated phase change material with a void for thermal expansion. Chem. Eng. J. 2021, 415, 128965. [Google Scholar] [CrossRef]
- Wen, R.; Liu, Y.; Yang, C.; Zhu, X.; Huang, Z.; Zhang, X.; Gao, W. Enhanced thermal properties of stearic acid/carbonized maize straw composite phase change material for thermal energy storage in buildings. J. Energy Storage 2021, 36, 102420. [Google Scholar] [CrossRef]
- Jin, X.; Xiao, Q.; Xu, T.; Huang, G.; Wu, H.; Wang, D.; Liu, Y.; Zhang, H.; Lai, A.C.K. Thermal conductivity enhancement of a sodium acetate trihydrate–potassium chloride–urea/expanded graphite composite phase–change material for latent heat thermal energy storage. Energy Build. 2021, 231, 110615. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Yun, B.Y.; Kang, Y.; Wi, S.; Kim, S. Three-dimensional hybrid carbon nanocomposite-based intelligent composite phase change material with leakage resistance, low electrical resistivity, and high latent heat. J. Ind. Eng. Chem. 2021, 98, 435–443. [Google Scholar] [CrossRef]
- Jin, W.; Jiang, L.; Chen, L.; Gu, Y.; Guo, M.; Han, L.; Ben, X.; Yuan, H.; Lin, Z. Preparation and characterization of capric-stearic acid/montmorillonite/graphene composite phase change material for thermal energy storage in buildings. Constr. Build. Mater. 2021, 301, 124102. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Wi, S.; Yun, B.Y.; Kim, S. Engineering biochar with multiwalled carbon nanotube for efficient phase change material encapsulation and thermal energy storage. Energy 2021, 216, 119294. [Google Scholar] [CrossRef]
- Chi, B.; Yao, Y.; Cui, S.; Jin, X. Preparation of graphene oxide coated tetradecanol/expanded graphite composite phase change material for thermal energy storage. Mater. Lett. 2021, 282, 128666. [Google Scholar] [CrossRef]
- Shin, H.K.; Park, M.; Kim, H.-Y.; Park, S.-J. Thermal property and latent heat energy storage behavior of sodium acetate trihydrate composites containing expanded graphite and carboxymethyl cellulose for phase change materials. Appl. Therm. Eng. 2015, 75, 978–983. [Google Scholar] [CrossRef]
- Sutjahja, I.M.; AU, S.R.; Kurniati, N.; Pallitine, I.D.; Kurnia, D. The role of chemical additives to the phase change process of CaCl2.6H2O to optimize its performance as latent heat energy storage system. J. Phys. Conf. Ser. 2016, 739, 012064. [Google Scholar] [CrossRef]
- Ryu, H.W.; Woo, S.W.; Shin, B.C.; Kim, S.D. Prevention of supercooling and stabilization of inorganic salt hydrates as latent heat storage materials. Sol. Energy Mater. Sol. Cells 1992, 27, 161–172. [Google Scholar] [CrossRef]
- Gutierrez, A.; Ushak, S.; Galleguillos, H.; Fernandez, A.; Cabeza, L.F.; Grágeda, M. Use of polyethylene glycol for the improvement of the cycling stability of bischofite as thermal energy storage material. Appl. Energy 2015, 154, 616–621. [Google Scholar] [CrossRef]
- Kazemi, Z.; Mortazavi, S.M. A new method of application of hydrated salts on textiles to achieve thermoregulating properties. Thermochim. Acta 2014, 589, 56–62. [Google Scholar] [CrossRef]
- Ramirez, B.G.; Glorieux, C.; Martinez, E.S.M.; Cuautle, J.F. Tuning of thermal properties of sodium acetate trihydrate by blending with polymer and silver nanoparticles. Appl. Therm. Eng. 2014, 62, 838–844. [Google Scholar] [CrossRef]
- Hawes, D.; Feldman, D.; Banu, D. Latent heat storage in building materials. Energy Build. 1993, 20, 77–86. [Google Scholar] [CrossRef]
- SpectraBase. Available online: https://spectrabase.com/spectrum/ (accessed on 9 March 2022).
- Tang, Y.; Chen, T.; Yu, S.; Qiao, Y.; Mu, S.; Hu, J.; Gao, F. Synthesis of graphene oxide anchored porous manganese sulfide nanocrystals via the nanoscale Kirkendall effect for supercapacitors. J. Mater. Chem. A 2015, 3, 12913–12919. [Google Scholar] [CrossRef]
- Bahtiar, S.; Taufiq, A.; Sunaryono; Hidayat, A.; Hidayat, N.; Diantoro, M.; Mufti, N.; Mujamilah. Synthesis, Investigation on Structural and Magnetic Behaviors of Spinel M-Ferrite [M = Fe; Zn; Mn] Nanoparticles from Iron Sand. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012052. [Google Scholar] [CrossRef] [Green Version]
- Min, K.-E.; Lee, J.-S.; Yoo, S.-H.; Kim, M.-S.; Kim, J.-K. Effects of Catalysts on the adhesive properties for flip chip bonding. Korean J. Mater. Res. 2010, 20, 681–685. [Google Scholar] [CrossRef] [Green Version]
- Min, K.-E.; Kim, H.-Y.; Bang, J.-H.; Kim, J.-H.; Kim, J.-K. Effects of Hardeners and Catalysts on the Reliability of Copper to Copper Adhesive Joint. Korean J. Mater. Res. 2011, 21, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Metselaar, H.S.C.; Silakhori, M. Shape-stabilized phase change materials with high thermal conductivity based on paraffin/graphene oxide composite. Energy Convers. Manag. 2013, 67, 275–282. [Google Scholar] [CrossRef]
- Testing, A.S.f. Materials. In Standard Test Method for Steady-state Thermal Transmission Properties by Means of the Heat Flow Meter Apparatus; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Liu, Z.; Chung, D. Calorimetric evaluation of phase change materials for use as thermal interface materials. Thermochim. Acta 2001, 366, 135–147. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; de Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Bland, A.; Khzouz, M.; Statheros, T.; Gkanas, E. PCMs for Residential Building Applications: A Short Review Focused on Disadvantages and Proposals for Future Development. Buildings 2017, 7, 78. [Google Scholar] [CrossRef] [Green Version]
- Koláček, M.; Charvátová, H.; Sehnálek, S. Experimental and Numerical Research of the Thermal Properties of a PCM Window Panel. Sustainability 2017, 9, 1222. [Google Scholar] [CrossRef] [Green Version]
- Dincer, I.; Rosen, M.A. Thermal Energy Storage Systems and Applications; John Wiley & Sons: New York, NY, USA, 2021. [Google Scholar]
- Pronk, P.; Infante Ferreira, C.A.; Witkamp, G.J. Superheating of ice slurry in melting heat exchangers. Int. J. Refrig. 2008, 31, 911–920. [Google Scholar] [CrossRef]
- Tip, T. Interpreting DSC Curves Part 1: Dynamic Measurements. Available online: https://www.eng.uc.edu/~beaucag/Classes/Characterization/DSCParts/Artifacts%20in%20DSC%20Usercom_11.pdf (accessed on 10 June 2022).
- Kuznik, F.; Virgone, J. Experimental assessment of a phase change material for wall building use. Appl. Energy 2009, 86, 2038–2046. [Google Scholar] [CrossRef] [Green Version]
- Climator. Available online: www.Climator.com (accessed on 9 March 2022).
- Insolcorp. Available online: www.insolcorp.com (accessed on 9 March 2022).
- PCM Manufacturers. Available online: www.teappcm.com (accessed on 9 March 2022).
- PLUSS. Available online: https://pluss.co.in (accessed on 9 March 2022).
- Vigo, T.L.; Frost, C. Temperature-sensitive hollow fibers containing phase change salts. Text. Res. J. 1982, 52, 633–637. [Google Scholar] [CrossRef]
- Nagano, K.; Mochida, T.; Takeda, S.; Domański, R.; Rebow, M. Thermal characteristics of manganese (II) nitrate hexahydrate as a phase change material for cooling systems. Appl. Therm. Eng. 2003, 23, 229–241. [Google Scholar] [CrossRef]
- Kośny, J.; Biswas, K.; Miller, W.; Kriner, S. Field thermal performance of naturally ventilated solar roof with PCM heat sink. Sol. Energy 2012, 86, 2504–2514. [Google Scholar] [CrossRef]
- Yüksel, N. The Review of Some Commonly Used Methods and Techniques to Measure the Thermal Conductivity of Insulation Materials. In Insulation Materials in Context of Sustainability; IntechOpen: London, UK, 2016. [Google Scholar]
- Wang, J.; Xie, H.; Xin, Z.; Li, Y.; Chen, L. Enhancing thermal conductivity of palmitic acid based phase change materials with carbon nanotubes as fillers. Sol. Energy 2010, 84, 339–344. [Google Scholar] [CrossRef]
- Saeed, R.M.R. Thermal Characterization of Phase Change Materials for Thermal Energy Storage; Missouri University of Science and Technology: Rolla, MI, USA, 2016. [Google Scholar]
- Sarı, A.; Kaygusuz, K. Thermal and heat transfer characteristics in a latent heat storage system using lauric acid. Energy Convers. Manag. 2002, 43, 2493–2507. [Google Scholar] [CrossRef]
- Charles, J.M. Performance and Stability of CaCl2·6H2O-Based Phase Change Materials. Ph.D. Thesis, Lehigh University, Bethlehem, PA, USA, 2019. [Google Scholar]
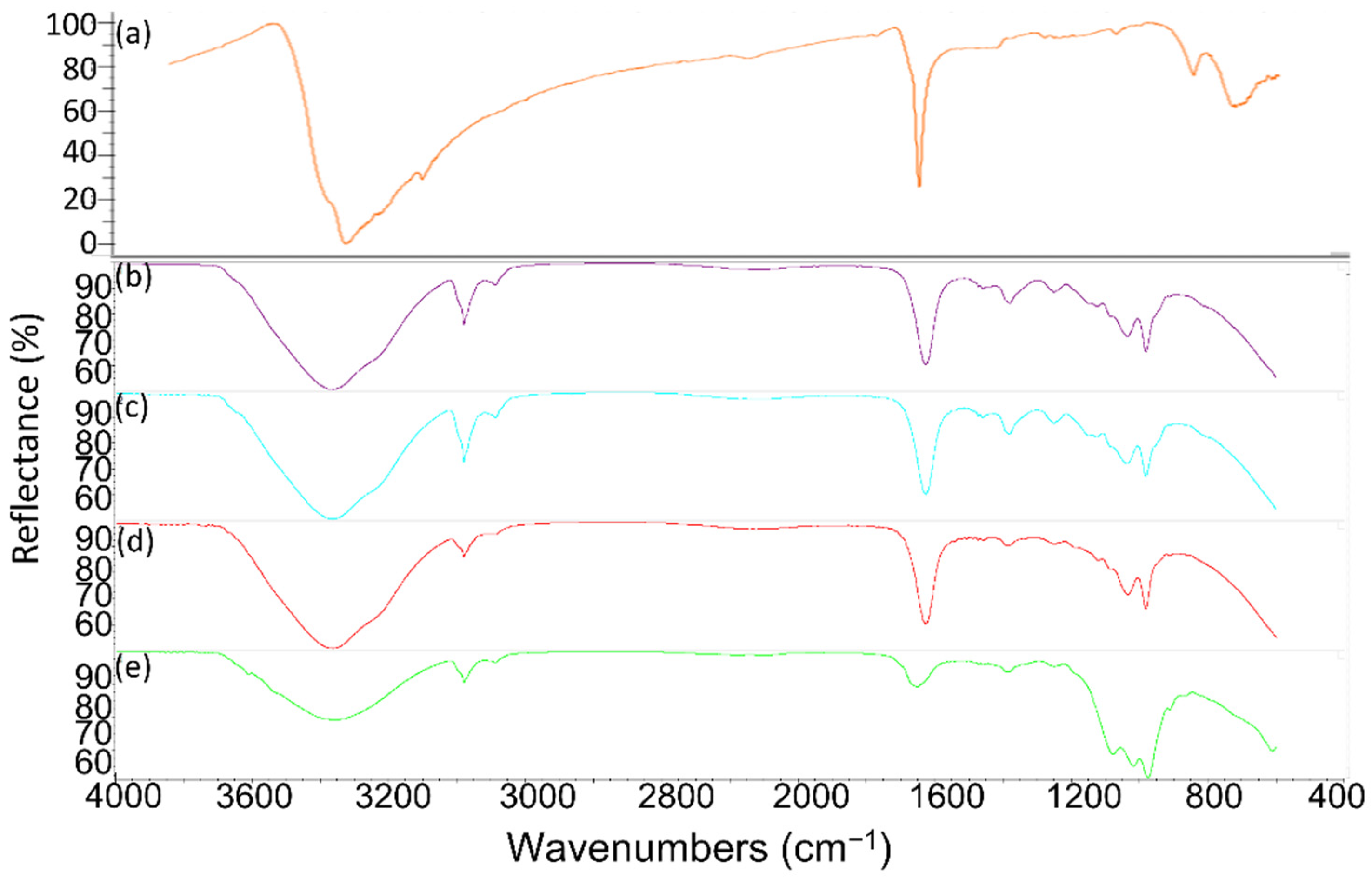
| PCM | Reference Melting Temperature | Phase | |
|---|---|---|---|
| As-Received | Treated | ||
| Specimen 1 | 21 °C | Liquid | - |
| Specimen 2 | 23 °C | Solid | - |
| Solid/Liquid | Liquid | ||
| Specimen 3 | 26 °C | Solid | - |
| Specimen 4 | 30 °C | Solid | - |
| PCMs | Temperature for Liquid Phase Conductivity (°C) | Temperature for Solid Phase Conductivity (°C) | ||
|---|---|---|---|---|
| Upper Plate | Lower Plate | Upper Plate | Lower Plate | |
| Specimen 1 | 21 | 35 | 14 | 26 |
| Specimen 2 | 21 | 35 | 14 | 26 |
| Specimen 3 | 33 | 43 | 21 | 31 |
| Specimen 4 | 39 | 49 | 31 | 41 |
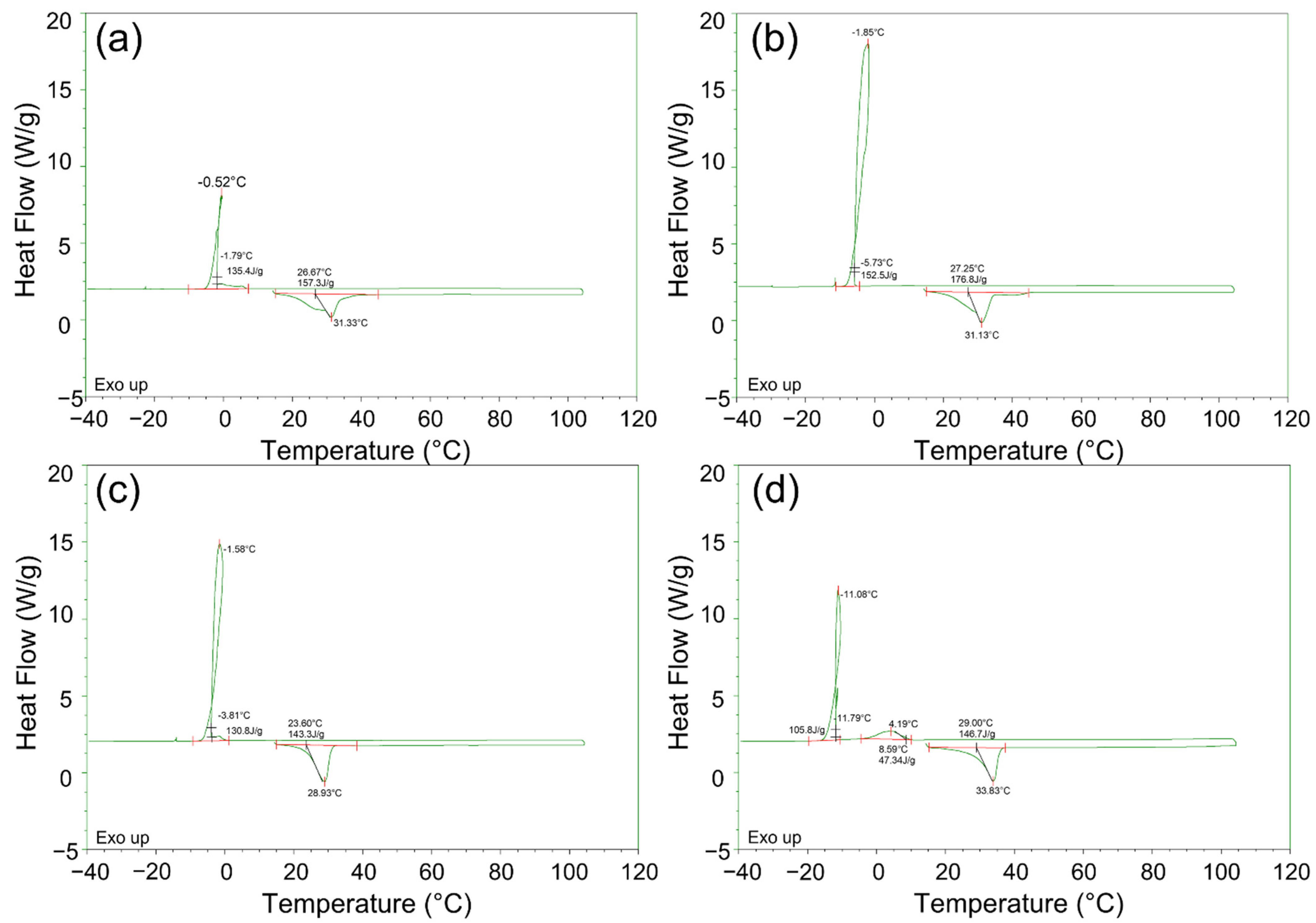
| PCMs | Process | Onset Temp. (°C) | Peak Temp. (°C) | End Temp. (°C) | Latent Heat (J/g) |
|---|---|---|---|---|---|
| Specimen 1 | Endothermic | 26.6 | 31.3 | 43 | 157.3 |
| Specimen 2 | 27.2 | 31.1 | 43 | 176.8 | |
| Specimen 3 | 23.6 | 28.9 | 44 | 146.0 | |
| Specimen 4 | 28.9 | 33.8 | 45 | 161.0 | |
| Specimen 1 | Exothermic | −1.8 | −0.5 | −7.6 | 135.4 |
| Specimen 2 | −5.7 | −1.8 | −10.3 | 152.5 | |
| Specimen 3 | −4.0 | −1.6 | −9.5 | 131.0 | |
| Specimen 4 | 8.6 | 3.7 | −6.4 | 50.7 | |
| −11.7 | −11.0 | −20 | 106.5 |
| PCM | αsolid (mm2/s) | αliquid (mm2/s) |
|---|---|---|
| Specimen 1 | 1.34 × 10−5 | 1.22 × 10−5 |
| Specimen 2 | 1.40 × 10−5 | 9.76 × 10−6 |
| Specimen 3 | 2.35 × 10−5 | 1.60 × 10−5 |
| Specimen 4 | 1.56 × 10−5 | 1.07 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, K.-E.; Jang, J.-W.; Kim, J.-K.; Wern, C.; Yi, S. Thermophysical Properties of Inorganic Phase-Change Materials Based on MnCl2·4H2O. Appl. Sci. 2022, 12, 6338. https://doi.org/10.3390/app12136338
Min K-E, Jang J-W, Kim J-K, Wern C, Yi S. Thermophysical Properties of Inorganic Phase-Change Materials Based on MnCl2·4H2O. Applied Sciences. 2022; 12(13):6338. https://doi.org/10.3390/app12136338
Chicago/Turabian StyleMin, Kyung-Eun, Jae-Won Jang, Jun-Ki Kim, Chien Wern, and Sung Yi. 2022. "Thermophysical Properties of Inorganic Phase-Change Materials Based on MnCl2·4H2O" Applied Sciences 12, no. 13: 6338. https://doi.org/10.3390/app12136338
APA StyleMin, K.-E., Jang, J.-W., Kim, J.-K., Wern, C., & Yi, S. (2022). Thermophysical Properties of Inorganic Phase-Change Materials Based on MnCl2·4H2O. Applied Sciences, 12(13), 6338. https://doi.org/10.3390/app12136338







