Physical Hazards in Aepyceros melampus Carcasses Killed for Meat Purposes by Aerial and Thoracic Shots
Abstract
:1. Introduction
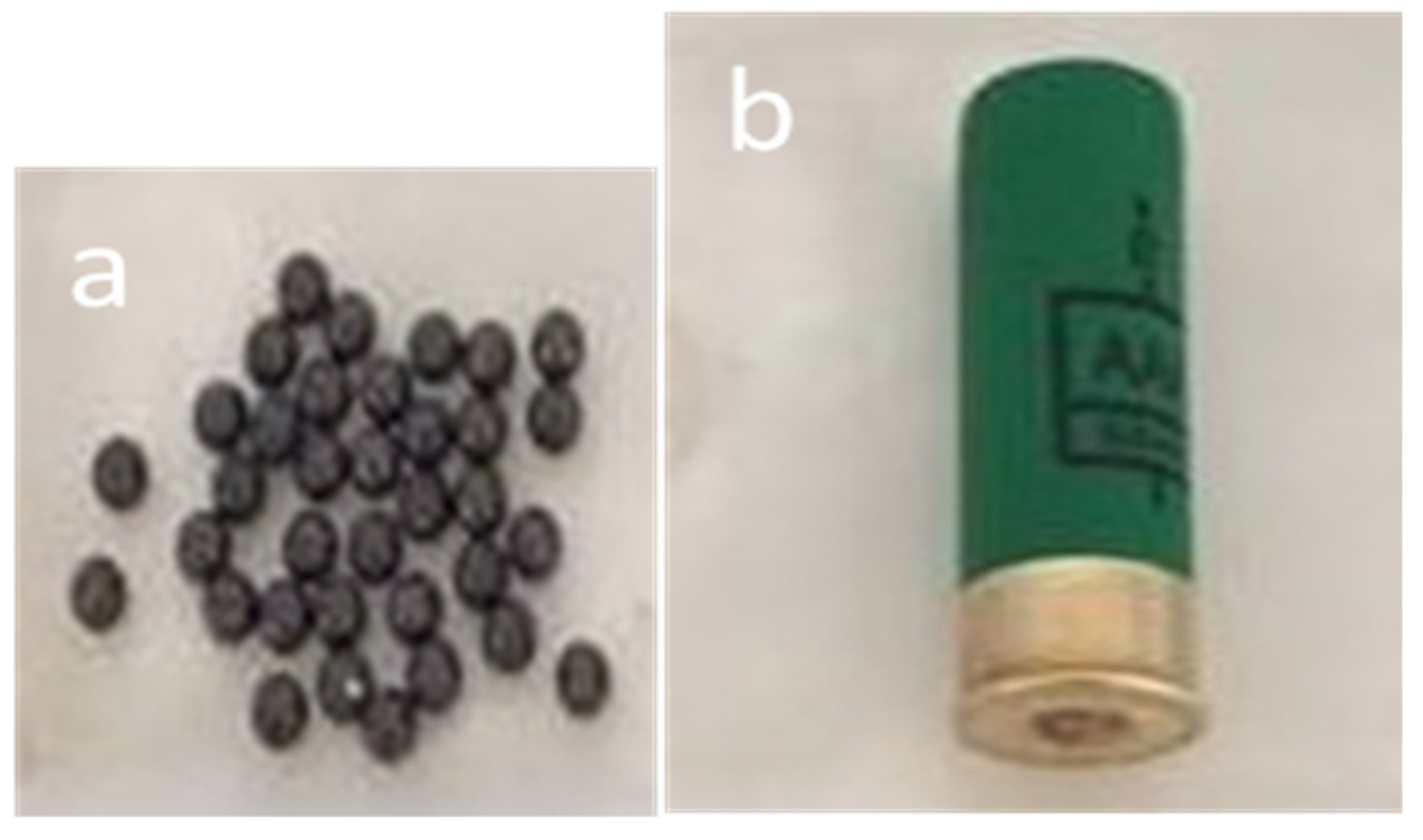
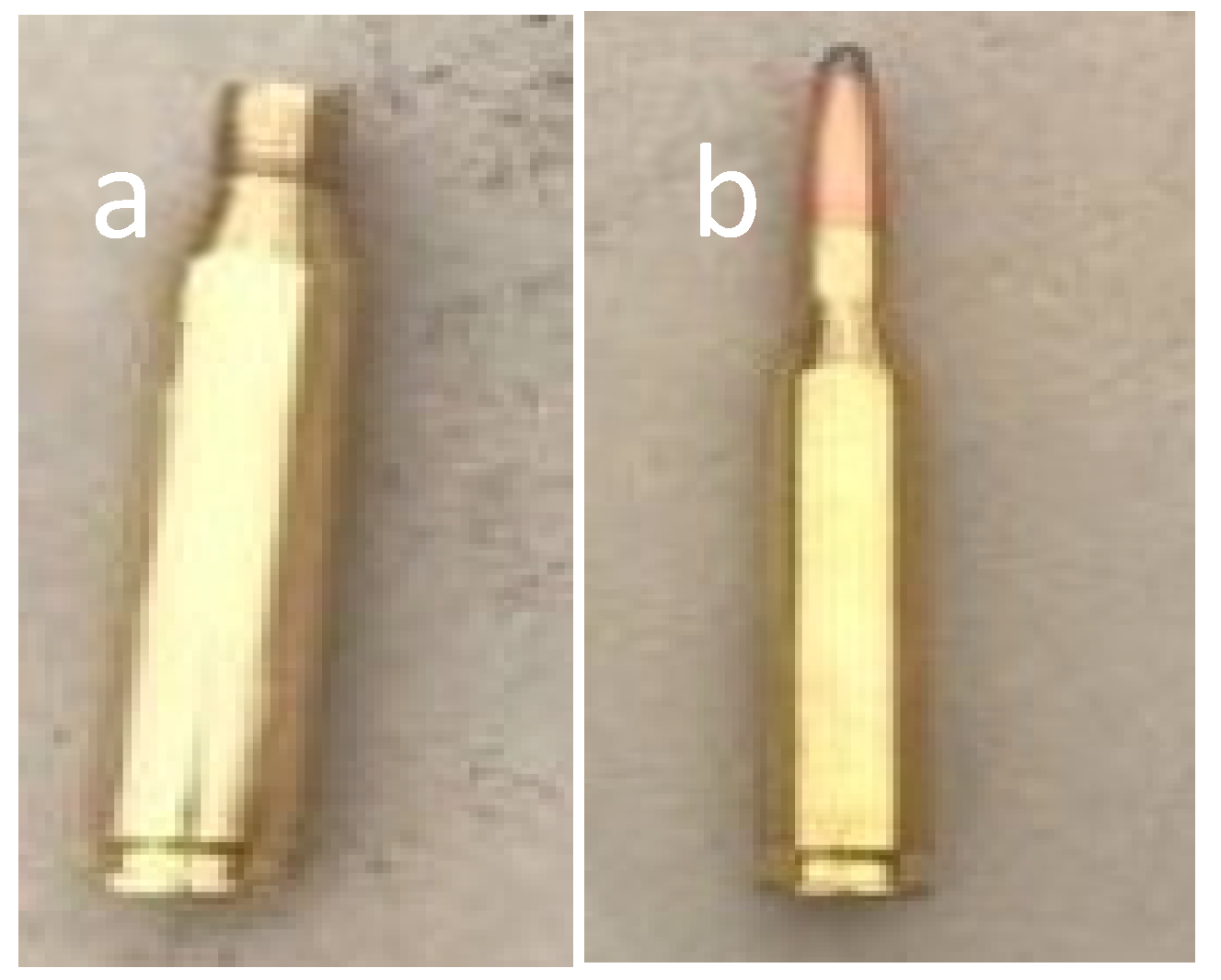
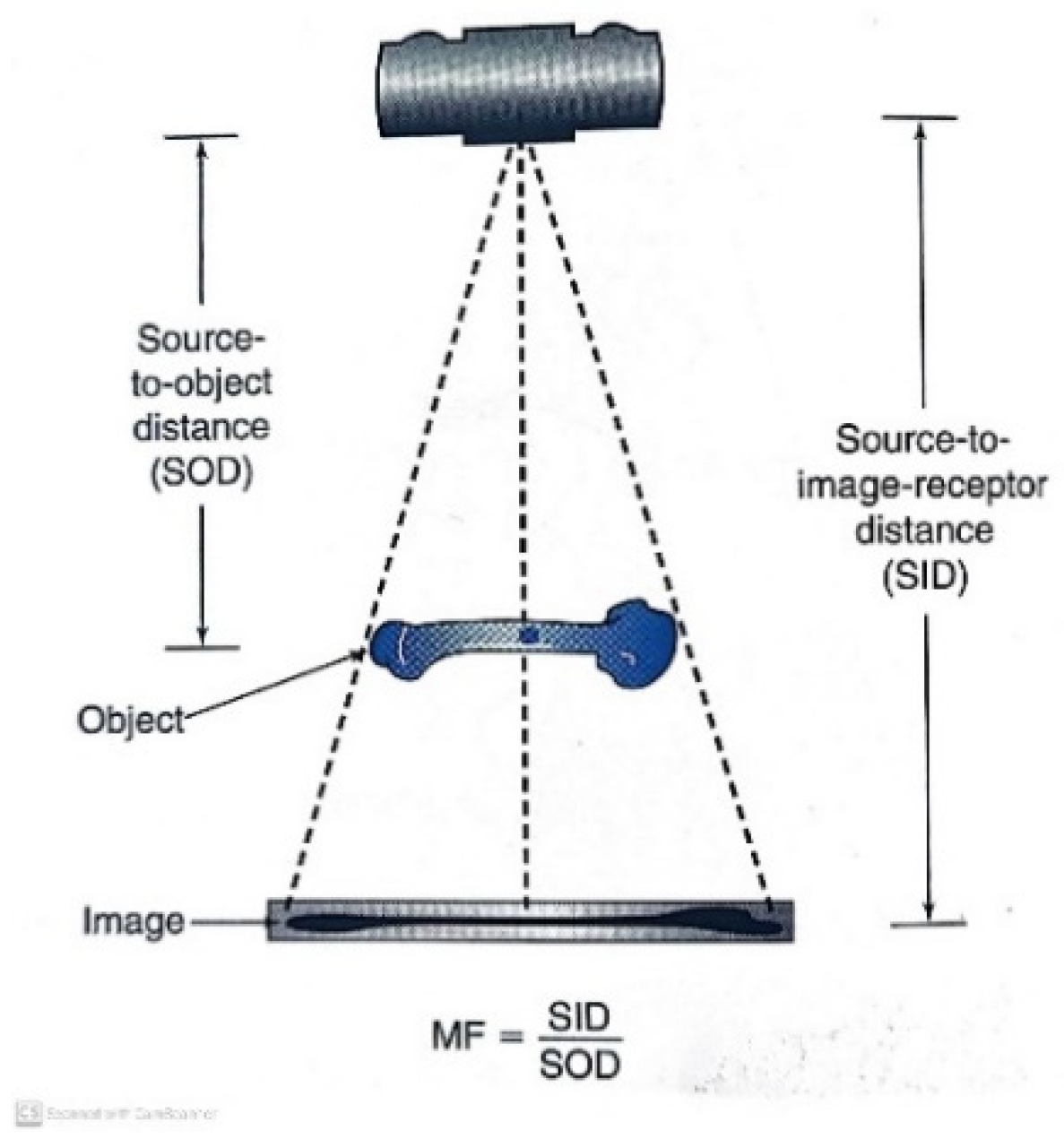
2. Materials and Methods
- IS = Image size
- OS = Object size
- SID = Source to image distance
- SOD = Source to object distance
- OID = Object to image distance
- O%MF = Object % of magnification
3. Results
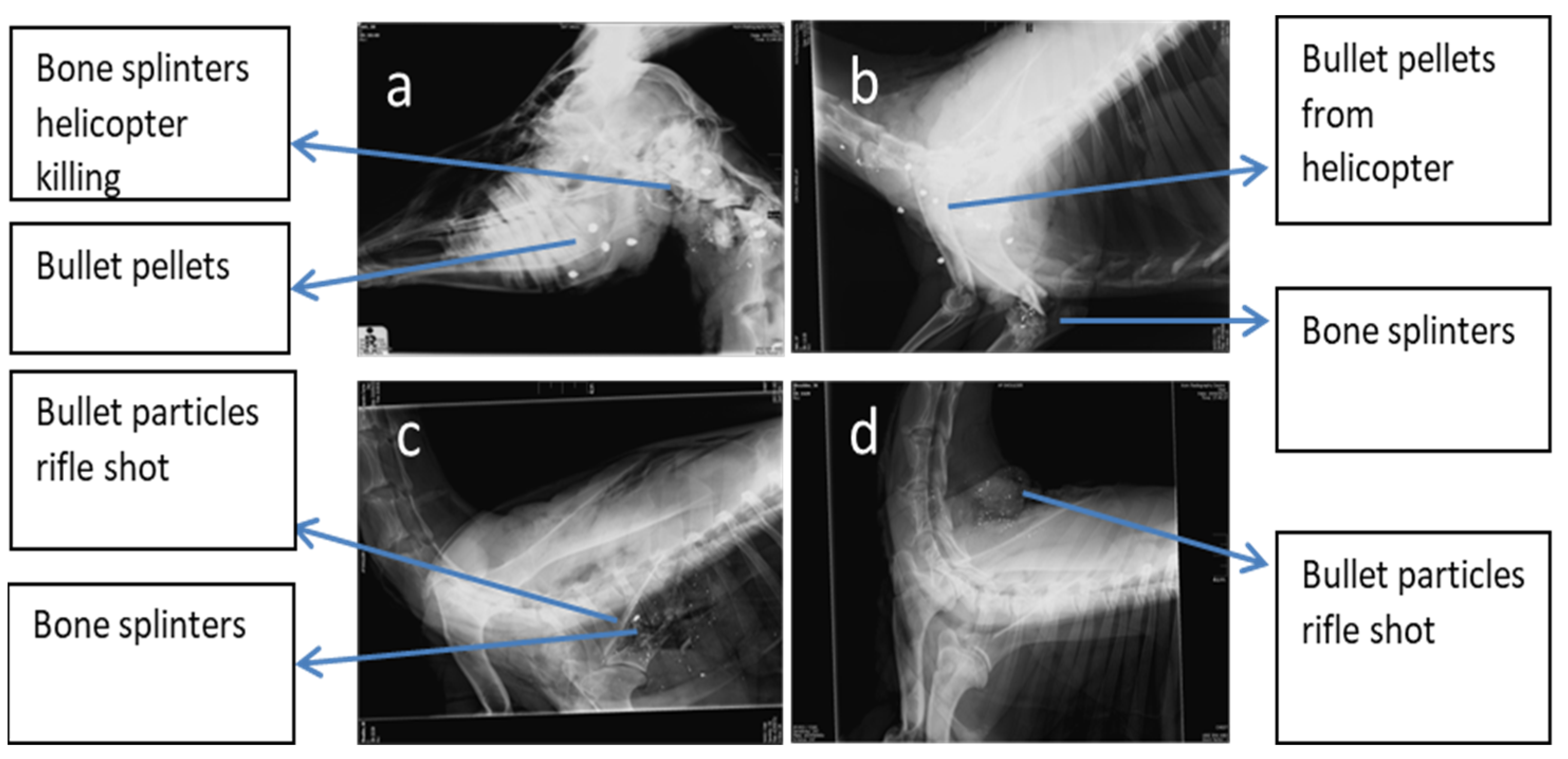
3.1. Impact and Dispersion of Physical Hazards
3.2. Particle Sizes of Bone Splinters and Bullet Fragments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavalheiro, C.P.; da Silva, M.C.A.; Leite, J.S.F.; da Silva, F.S.K.R.; Herrero, A.M.; Ruiz-Capillas, C. Physical hazards in meat products: Consumers’ complaints found on a Brazilian website. Food Control 2020, 108, 106892. [Google Scholar] [CrossRef]
- Demaurex, G.; Sallé, L. Detection of Physical Hazards. In Food Safety Management; Elsevier: Amsterdam, The Netherlands, 2014; pp. 511–533. [Google Scholar]
- Olsen, A.R. Regulatory action criteria for filth and other extraneous materials: I. Review of hard or sharp foreign objects as physical hazards in food. Regul. Toxicol. Pharmacol. 1998, 28, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keener, L. Chemical and Physical Hazards: The “Other” Food Safety Risk. Reproduced From Food Testing & Analysis: The Target Group. 2001. Available online: https://www.foodsafetyprofessionals.com/ (accessed on 23 February 2022).
- Chen, H.; Liu, S.; Chen, Y.; Chen, C.; Yang, H.; Chen, Y. Food safety management systems based on ISO 22000: 2018 methodology of hazard analysis compared to ISO 22000: 2005. Accredit. Qual. Assur. 2019, 25, 23–37. [Google Scholar] [CrossRef]
- Verba, S.D.; Jensen, B.T.; Lynn, J.S. Electrocardiographic Responses to Deer Hunting in Men and Women. Wilderness Environ. Med. 2016, 27, 364–370. [Google Scholar] [CrossRef] [Green Version]
- Blackmore, A.C.; Trouwborst, A. Who owns and is responsible for the elephant in the room? Management plans for free-roaming elephant in South Africa. Bothalia-Afr. Biodivers. Conserv. 2018, 48, 1–6. [Google Scholar] [CrossRef]
- Needham, T.; Engels, R.A.; Bureš, D.; Kotrba, R.; Van Rensburg, B.J.; Hoffman, L.C. Carcass yields and physiochemical meat quality of semi-extensive and intensively farmed impala (Aepyceros melampus). Foods 2020, 9, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekker, J.L.; Hoffman, L.C.; Jooste, P.J. Essential food safety management points in the supply chain of game meat in South Africa. In Game Meat Hygiene in Focus; Springer: Berlin/Heidelberg, Germany, 2011; pp. 39–65. [Google Scholar]
- Cooper, S.M.; Van der Merwe, M. Game ranching for meat production in marginal African agricultural lands. J. Arid Land Stud. 2014, 24, 249–252. [Google Scholar]
- Nkosi, D.V.; Bekker, J.L.; Hoffman, L.C. Toxic Metals in Wild Ungulates and Domestic Meat Animals Slaughtered for Food Purposes: A Systemic Review. Foods 2021, 10, 2853. [Google Scholar] [CrossRef]
- Takamatsu, T.; Murata, T.; Koshikawa, M.K.; Watanabe, M. Weathering and dissolution rates among Pb shot pellets of differing elemental compositions exposed to various aqueous and soil conditions. Arch. Environ. Contam. Toxicol. 2010, 59, 91–99. [Google Scholar] [CrossRef]
- Mera, M.F.; Rubio, M.; Pérez, C.A.; Galván, V.; Germanier, A. SR μXRF and XRD study of the spatial distribution and mineralogical composition of Pb and Sb species in weathering crust of corroded bullets of hunting fields. Microchem. J. 2015, 119, 114–122. [Google Scholar] [CrossRef]
- Fountain, A.J.; Corey, A.; Malko, J.A.; Strozier, D.; Allen, J.W. Imaging appearance of ballistic wounds predicts bullet composition: Implications for MRI safety. AJR Am. J. Roentgenol. 2021, 216, 542–551. [Google Scholar] [CrossRef]
- Trinogga, A.L.; Courtiol, A.; Krone, O. Fragmentation of lead-free and lead-based hunting rifle bullets under real life hunting conditions in Germany. Ambio 2019, 48, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Hampton, J.O.; Eccles, G.; Hunt, R.; Bengsen, A.J.; Perry, A.L.; Parker, S.; Miller, C.J.; Joslyn, S.K.; Stokke, S.; Arnemo, J.M.; et al. A comparison of fragmenting lead-based and lead-free bullets for aerial shooting of wild pigs. PLoS ONE 2021, 16, e0247785. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Food Safety. 1999. Available online: http://www.who.int (accessed on 12 December 2021).
- Sanchez, M.C. Food Law and Regulation for Non-Lawyers: A US Perspective; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Nauman, K.; Paulsen, P. Food Safety and Food Security Implications of Game. In Trends in Food Safety and Protection; CRC Press: Boca Raton, FL, USA, 2017; pp. 187–204. [Google Scholar]
- South Africa. Standard for the Registration of Hunter for Harvesting Wild Game Intended for Export of Game Meat. Pretoria Government Gazette. VPN/08/2010-01. 2010. Available online: https://www.dalrrd.gov.za/ (accessed on 16 April 2022).
- Van Schalkwyk, D.L.; Hoffman, L.C. Guidelines for the Harvesting & Processing of Wild Game in Namibia 2016; Ministry of Environment & Tourism: Windhoek, Namibia, 2016.
- South Africa. Standard for Registration or Re-Registration of a Game Farm for Export Status. Pretoria Government Gazzett. VPN/05/2017/01. 2008. Available online: https://www.dalrrd.gov.za/ (accessed on 17 April 2022).
- South Africa. Standard for the Ante and Post Mortem Meat Inspection and Hygiene Control at Point of Game Harvest. Pretoria Government Gazette. VPN/09/2010-01. 2008. Available online: https://www.nda.agric.za (accessed on 18 April 2022).
- Food Standard Australia New Zealand. Contaminants and Natural Toxicants Federal Register of Legislative Instruments. 2015. Available online: https://www.legislation.gov.au/ (accessed on 16 April 2022).
- Canadian Food Inspection Agency. Reference Data Base for Hazard Identification. 2008. Available online: https://active.inspection.gc.ca/ (accessed on 16 April 2022).
- Codex Alimentarius Commission. Recommended International Code of Practice General Principles of Food Hygiene; CAC/RCP 1-1969, Revision 4 (2003); Codex Alimentarius Commission: Rome, Italy, 2010; p. 10. Available online: https://www.fao.org/ (accessed on 8 November 2021).
- EU Regulation No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safety. Official Journal of the European Communities. 2002, pp. 1–24. Available online: https://eur-lex.europa.eu/ (accessed on 21 April 2022).
- GB 2762-2012; National Food Safety Standard: Maximum Levels of Contaminants in Food. Ministry of Health of the People’s Republic of China: Beijing, China, 2012. Available online: https://www.dgav.pt/wp (accessed on 12 February 2022).
- FDA. Potential Hazards Associated with the Manufacturing, Processing, Packing, and Holding of Human Food. Food Safety in the Center for Food Safety and Applied Nutrition at the U.S. Food and Drug Administration. 2018. Available online: https://www.fda.gov/ (accessed on 20 April 2022).
- ISO 22000:2018; Food Safety Management Systems—Requirements for Any Organization in the Food Chain. International Organization for Standardization: Geneva, Switzerland, 2018.
- Govender, R. A review of HACCP and the South African abattoir hygiene management system towards integration. Int. J. Food Saf. Nutr. Public Health 2016, 6, 65–84. [Google Scholar] [CrossRef]
- Brown, M.; Brown, L. Lavin’s Radiography for Veterinary Technicians-E-Book; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Whitley, A.S.; Jefferson, G.; Holmes, K.; Sloane, C.; Anderson, C.; Hoadley, G. Clark’s Positioning in Radiography 13E; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Fauber, T.L. Radiographic Imaging and Exposure-E-Book; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780323661393. [Google Scholar]
- Buker, K. Lavin’s Radiography for Veterinary Technicians. Can. Vet. J. 2019, 60, 1206. [Google Scholar]
- Ball, J.; Price, T. Chesney’s Radiographic Imaging; Blackwell Science Ltd.: Hoboken, NJ, USA, 1995. [Google Scholar]
- Viganò, R.; Demartini, E.; Riccardi, F.; Corradini, A.; Besozzi, M.; Lanfranchi, P.; Chiappini, P.L.; Cottini, A.; Gaviglio, A. Quality parameters of hunted game meat: Sensory analysis and pH monitoring. Ital. J. Food Saf. 2019, 8, 7724. [Google Scholar] [CrossRef] [Green Version]
- Kamieniarz, R.; Jankowiak, Ł.; Fratczak, M.; Panek, M.; Wojtczak, J.; Tryjanowski, P. The relationship between hunting methods and the sex, age and body mass of wild boar Sus scrofa. Animals 2020, 10, 2345. [Google Scholar] [CrossRef]
- Silberbauer, B.L.; Strydom, P.E.; Hoffman, L.C. An Exploratory Study into the Influence of Sex on Body Measurements, Carcass Weights and Meat Yields of Giraffe (Giraffa camelopardalis angolensis). Foods 2021, 10, 2245. [Google Scholar] [CrossRef]
- Winkelmayer, R. A note on game meat, animal welfare and ethics. In Trends in Game Meat Hygiene: From Forest to Fork; Wageningen Academic Publishers: Wageningen, The Netherlands, 2014; pp. 98–102. [Google Scholar]
- Wassenaar, A.; Kempen, E.; van Eeden, T. Exploring South African consumers’ attitudes towards game meat—Utilizing a multi-attribute attitude model. Int. J. Consum. Stud. 2019, 43, 437–445. [Google Scholar] [CrossRef]
- Irschik, I.; Bauer, F.; Sager, M.; Paulsen, P. Copper residues in meat from wild artiodactyls hunted with two types of rifle bullets manufactured from copper. Eur. J. Wildl. Res. 2013, 59, 129–136. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.; Das, A.; Biswas, S. Hazards and safety issues of meat and meat products. In Food Safety and Human Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 145–168. [Google Scholar]
- Dobson, A.D.; Milner-Gulland, E.; Ingram, D.J.; Keane, A. A framework for assessing impacts of wild meat hunting practices in the tropics. Hum. Ecol. 2019, 47, 449–464. [Google Scholar] [CrossRef] [Green Version]
- MacKinnon, M. Meat and other animal products. In The Routledge Handbook of Diet and Nutrition in the Roman World; Routledge: London, UK, 2018; pp. 150–162. [Google Scholar]
- Gaviglio, A.; Marescotti, M.E.; Demartini, E. The local value chain of hunted red deer meat: A scenario analysis based on a northern Italian case study. Resources 2018, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, D.M.; Epps, C.W.; Taylor, D.S. Estimating lead fragmentation from ammunition for muzzleloading and black powder cartridge rifles. J. Fish Wildl. Manag. 2016, 7, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Benini, J.C. Frangible Metal Bullets, Ammunition and Method of Making Such Articles. U.S. Patent 6090178A, 18 July 2000. [Google Scholar]
- Woodford, L.P.; Forsyth, D.M.; Hampton, J.O. Scavenging birds at risk of ingesting fragments of lead bullets from kangaroo and deer carcasses in south-eastern Australia. Aust. Field Ornithol. 2020, 37, 112–116. [Google Scholar] [CrossRef]
- Cromie, R.; Newth, J.; Strong, E. Transitioning to non-toxic ammunition: Making change happen. Ambio 2019, 48, 1079–1096. [Google Scholar] [CrossRef]
- Hampton, J.O.; Laidlaw, M.; Buenz, E.; Arnemo, J.M. Heads in the sand: Public health and ecological risks of lead-based bullets for wildlife shooting in Australia. Wildl. Res. 2018, 45, 287–306. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Ying, Y. Food safety evaluation based on near infrared spectroscopy and imaging: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1913–1924. [Google Scholar] [CrossRef]
- Buncic, S.; Alban, L.; Blagojevic, B. From traditional meat inspection to development of meat safety assurance programs in pig abattoirs–the European situation. Food Control 2019, 106, 106705. [Google Scholar] [CrossRef]
- Menozzi, A.; Menotta, S.; Fedrizzi, G.; Lenti, A.; Cantoni, A.M.; Di Lecce, R.; Gnudi, G.; Pérez-López, M.; Bertini, S. Lead and copper in hunted wild boars and radiographic evaluation of bullet fragmentation between ammunitions. Food Addit. Contam. Part B 2019, 12, 182–190. [Google Scholar] [CrossRef]
- Woldehanna, S.; Zimicki, S. An expanded One Health model: Integrating social science and One Health to inform study of the human-animal interface. Soc. Sci. Med. 2015, 129, 87–95. [Google Scholar] [CrossRef]
- Irschik, I.; Wanek, C.; Bauer, F.; Sager, M.; Paulsen, P. Composition of bullets used for hunting and food safety considerations. In Trends in Game Meat Hygiene: From Forest to Fork; Wageningen Academic Publishers: Wageningen, The Netherlands, 2014; pp. 283–289. [Google Scholar]
- Stankevičiūtė, J.; Pėtelis, K.; Baranauskaitė, J.; Narauskaitė, G. Influence of lead shot pellets injury on meat yield of European hare (Lepus europaeus Pallas). Rural. Dev. 2013, 2013, 245. [Google Scholar]
- Broadway, M.S.; McCallen, E.B.; Caudell, J.; Stewart, C.M. Ammunition type and shot placement determine lead fragmentation in deer. J. Wildl. Manag. 2020, 84, 1406–1414. [Google Scholar] [CrossRef]
- Stärk, K.; Alonso, S.; Dadios, N.; Dupuy, C.; Ellerbroek, L.; Georgiev, M.; Hardstaff, J.; Huneau-Salaün, A.; Laugier, C.; Mateus, A.; et al. Strengths and weaknesses of meat inspection as a contribution to animal health and welfare surveillance. Food Control 2014, 39, 154–162. [Google Scholar] [CrossRef]
- Kautto, A.H.; Vågsholm, I.; Niskanen, R. Meat inspection of reindeer—A rich source of data for monitoring food safety and animal and environmental health in Sweden. Infect. Ecol. Epidemiol. 2017, 7, 1340695. [Google Scholar] [CrossRef] [Green Version]
- Czarniecka-Skubina, E.; Stasiak, D.M.; Latoch, A.; Owczarek, T.; Hamulka, J. Consumers’ Perception and Preference for the Consumption of Wild Game Meat among Adults in Poland. Foods 2022, 11, 830. [Google Scholar] [CrossRef]

| Size (mm) | Rifle (n = 18) | Shotgun (n = 12) | ||
|---|---|---|---|---|
| Bullet Fragments | Bone Splinters | Pellet Fragments | Bone Splinters | |
| 2–6 | 58 | 7 | 44 | 11 |
| >6–25 | 7 | 38 | 6 | 3 |
| >25 | 1 | 9 | 0 | 3 |
| Total | 65 | 54 | 51 | 17 |
| LSD (p = 0.00012) | LSD (p = 0.00059) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkosi, D.V.; Bekker, J.L.; Gower, L.A.; Van der Watt, M.; Hoffman, L.C. Physical Hazards in Aepyceros melampus Carcasses Killed for Meat Purposes by Aerial and Thoracic Shots. Appl. Sci. 2022, 12, 6861. https://doi.org/10.3390/app12146861
Nkosi DV, Bekker JL, Gower LA, Van der Watt M, Hoffman LC. Physical Hazards in Aepyceros melampus Carcasses Killed for Meat Purposes by Aerial and Thoracic Shots. Applied Sciences. 2022; 12(14):6861. https://doi.org/10.3390/app12146861
Chicago/Turabian StyleNkosi, Davies Veli, Johan Leon Bekker, Luzyl Amely Gower, Marie Van der Watt, and Louwrens Christiaan Hoffman. 2022. "Physical Hazards in Aepyceros melampus Carcasses Killed for Meat Purposes by Aerial and Thoracic Shots" Applied Sciences 12, no. 14: 6861. https://doi.org/10.3390/app12146861






