Decoding of Processing Preferences from Language Paradigms by Means of EEG-ERP Methodology: Risk Markers of Cognitive Vulnerability for Depression and Protective Indicators of Well-Being? Cerebral Correlates and Mechanisms
Abstract
:1. Introduction
2. Decoding Processing Preferences by Means of EEG Methodology
3. Research Gaps
4. Exploring the Time Course of Stimulus-Driven, Self-Referential, and Emotional Processing by Means of EEG-ERPs
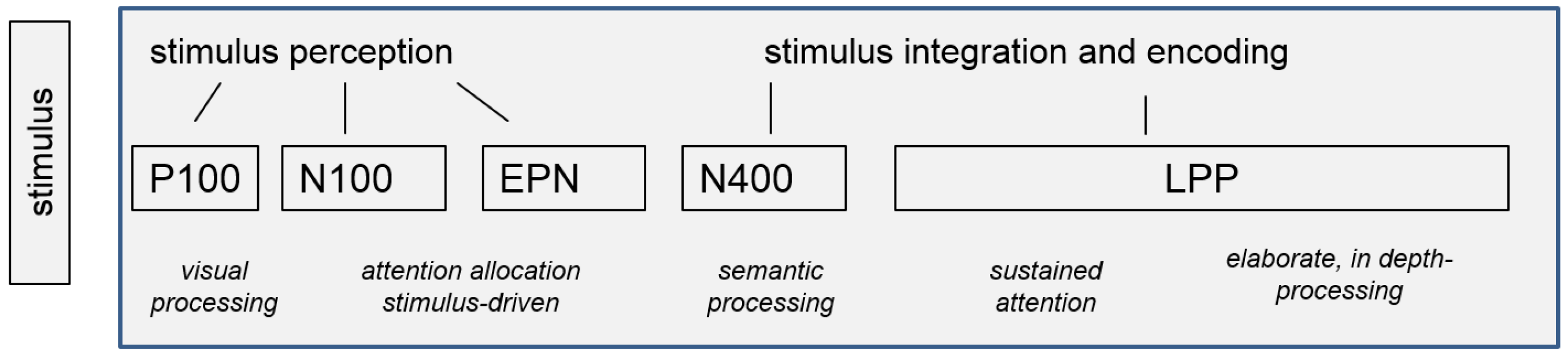
4.1. EEG Indicators of Healthy Self-Referential Emotional Processing
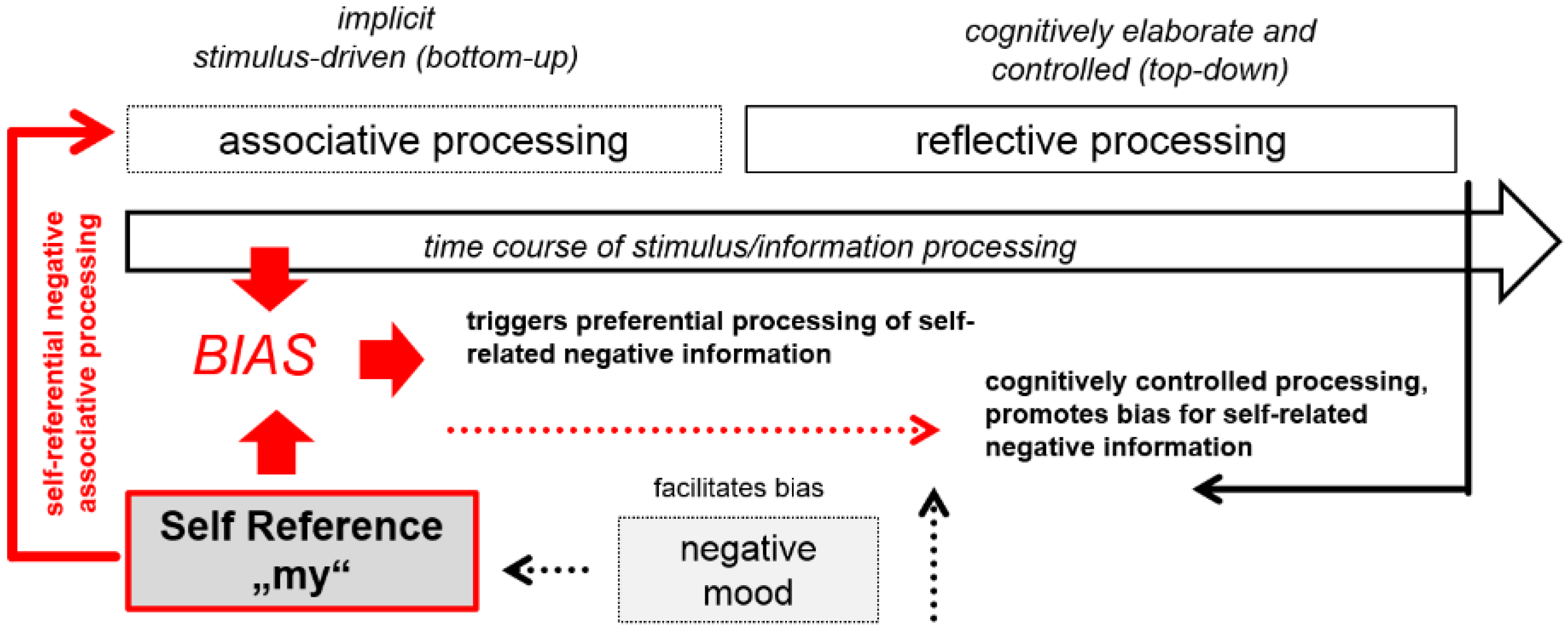
4.2. EEG Indicators of Cognitive Vulnerable Self-Referential Emotional Processing
4.3. Studying Self-Referential Emotional Processing Biases as Markers of Cognitive Vulnerability and Well-Being by Means of Language-Dependent EEG Paradigms: Potential Limitations and Advantages
5. Questions for the Future
- (1)
- At which stages of stimulus processing does an interaction between self-referential and emotional processing occur? Can the preliminary findings, illustrated in this manuscript, be replicated in larger cohorts of both, cognitively vulnerable and already depressed individuals vs. healthy controls?
- (2)
- To what degree can processing preferences for self-related negative and positive stimuli, respectively, be influenced by self-related attentive and cognitively controlled processing, and which of these influences are specific for depression and its risk?
- (3)
- Is self-negativity bias the only marker of cognitive vulnerability, or is a self-negativity bias accompanied by a reduced self-positivity bias as well (see Figure 4)?
- (4)
- Do the observed electrophysiological ERP correlates of the processing preferences for self-related negative or positive stimuli prove to be temporally stable markers of subjective well-being and cognitive vulnerability?
- (5)
- Do the results vary across languages, and do they also apply to a bilingual/multilingual context?
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.who.int/en/news-room/fact-sheets/detail/depression (accessed on 5 June 2022).
- Solmi, M.; Radua, J.; Olivola, M.; Croce, E.; Soardo, L.; Salazar de Pablo, G.; Il Shin, J.; Kirkbride, J.B.; Jones, P.; Kim, J.H.; et al. Age at onset of mental disorders worldwide: Large-scale meta-analysis of 192 epidemiological studies. Mol. Psychiatry 2022, 27, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Kalia, M. Neurobiological basis of depression: An update. Metabolism 2005, 54, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Foland-Ross, L.C.; Gotlib, I.H. Cognitive and neural aspects of information processing in major depressive disorder: An integrative perspective. Front. Psychol. 2012, 3, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotlib, I.H.; Joormann, J. Cognition and depression: Current status and future directions. Annu. Rev. Clin. Psychol. 2010, 6, 285–312. [Google Scholar] [CrossRef] [Green Version]
- Connolly, S.L.; Abramson, L.Y.; Alloy, L.B. Information processing biases concurrently and prospectively predict depressive symptoms in adolescents: Evidence from a self-referent encoding task. Cogn. Emot. 2016, 30, 550–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, E.; Barnett, K.J.; Cooper, N.J.; Tran, N.; Williams, L.M. An “integrative neuroscience” platform: Application to profiles of negativity and positivity bias. J. Integr. Neurosci. 2008, 7, 345–366. [Google Scholar] [CrossRef] [PubMed]
- Rude, S.S.; Durham-Fowler, J.A.; Baum, E.S.; Rooney, S.B.; Maestas, K.L. Self-report and Cognitive Processing Measures of Depressive Thinking Predict Subsequent Major Depressive Disorder. Cogn. Ther. Res. 2010, 34, 107–115. [Google Scholar] [CrossRef]
- Watters, A.J.; Williams, L.M. Negative biases and risk for depression; integrating self-report and emotion task markers. Depress. Anxiety 2011, 28, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Beevers, C.G. Cognitive vulnerability to depression: A dual process model. Clin. Psychol. Rev. 2005, 25, 975–1002. [Google Scholar] [CrossRef]
- Fossati, P. Is major depression a cognitive disorder? Rev. Neurol. 2018, 174, 212–215. [Google Scholar] [CrossRef]
- Beck, A.T. Depression: Causes and Treatment; University of Pennsylvania Press: Philadelphia, PA, USA, 1967. [Google Scholar]
- Beck, A.T.; Epstein, N.; Harrison, R. Cognitions, attitudes and personality dimensions in depression. Br. J. Cogn. Psychother. 1983, 1, 1–16. [Google Scholar]
- Luck, S.J. An Introduction to the Event-Related Potential Technique; MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Schupp, H.T.; Junghöfer, M.; Weike, A.I.; Hamm, A.O. Attention and emotion: An ERP analysis of facilitated emotional stimulus processing. Neuroreport 2003, 14, 1107–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kissler, J.; Herbert, C.; Peyk, P.; Junghofer, M. Buzzwords: Early cortical responses to emotional words during reading. Psychol. Sci. 2007, 18, 475–480. [Google Scholar] [CrossRef]
- Herbert, C.; Junghofer, M.; Kissler, J. Event related potentials to emotional adjectives during reading. Psychophysiology 2008, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.F.; Phillips, C.; Poeppel, D. A cortical network for semantics: (de)constructing the N400. Nat. Rev. Neurosci. 2008, 9, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Kutas, M.; Federmeier, K.D. Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn. Sci. 2000, 4, 463–470. [Google Scholar] [CrossRef]
- Kutas, M.; Hillyard, S.A. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science 1980, 207, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, M.; Schuch, S.; Schenck, W.; Fiedler, K. Mood states modulate activity in semantic brain areas during emotional word encoding. Cereb. Cortex 2007, 17, 1516–1530. [Google Scholar] [CrossRef] [Green Version]
- Herbert, C.; Kissler, J.; Junghöfer, M.; Peyk, P.; Rockstroh, B. Processing of emotional adjectives: Evidence from startle EMG and ERPs. Psychophysiology 2006, 43, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, V.; Codispoti, M.; Cardinale, R.; Bradley, M.M. Directed and motivated attention during processing of natural scenes. J. Cogn. Neurosci. 2008, 20, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Hajcak, G.; Dunning, J.P.; Foti, D. Motivated and controlled attention to emotion: Time-course of the late positive potential. Clin. Neurophysiol. 2009, 120, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Herbert, C.; Sfaerlea, A.; Blumenthal, T. Your emotion or mine: Labeling feelings alters emotional face perception—An ERP study on automatic and intentional affect labeling. Front. Hum. Neurosci. 2013, 7, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuiper, N.A.; Derry, P.A. Depressed and nondepressed content self-reference in mild depressives. J. Pers. 1982, 50, 67–80. [Google Scholar] [CrossRef]
- Alloy, L.B.; Abramson, L.Y.; Murray, L.A.; Whitehouse, W.G.; Hogan, M.E. Self-referent information-processing in individuals at high and low cognitive risk for depression. Cogn. Emot. 1997, 11, 539–568. [Google Scholar] [CrossRef]
- Dozois, D.J.; Dobson, K.S. Information processing and cognitive organization in unipolar depression: Specificity and comorbidity issues. J. Abnorm. Psychol. 2001, 110, 236. [Google Scholar] [CrossRef] [PubMed]
- Dobson, K.S.; Shaw, B.F. Specificity and stability of self-referent encoding in clinical depression. J. Abnorm. Psychol. 1987, 96, 34–40. [Google Scholar] [CrossRef]
- Matt, G.E.; Vázquez, C.; Campbell, W.K. Mood-congruent recall of affectively toned stimuli: A meta-analytic review. Clin. Psychol. Rev. 1992, 12, 227–255. [Google Scholar] [CrossRef]
- Watson, L.A.; Dritschel, B.; Obonsawin, M.C.; Jentzsch, I. Seeing yourself in a positive light: Brain correlates of the self-positivity bias. Brain Res. 2007, 1152, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Allison, G.O.; Kamath, R.A.; Carrillo, V.; Alqueza, K.L.; Pagliaccio, D.; Slavich, G.M.; Shankman, S.A.; Auerbach, R.P. Self-referential Processing in Remitted Depression: An Event-Related Potential Study. Biol. Psychiatry Glob. Open Sci. 2021. [Google Scholar] [CrossRef]
- Shestyuk, A.Y.; Deldin, P.J. Automatic and strategic representation of the self in major depression: Trait and state abnormalities. Am. J. Psychiatry 2010, 167, 536–544. [Google Scholar] [CrossRef]
- Dainer-Best, J.; Trujillo, L.T.; Schnyer, D.M.; Beevers, C.G. Sustained engagement of attention is associated with increased negative self-referent processing in major depressive disorder. Biol. Psychol. 2017, 129, 231–241. [Google Scholar] [CrossRef]
- Blume, C.; Herbert, C. The HisMine-Paradigm: A new paradigm to investigate self-awareness employing pronouns. Soc. Neurosci. 2014, 9, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Herbert, C.; Blume, C.; Northoff, G. Can we distinguish an “I” and “ME” during listening?—An event-related EEG study on the processing of first and second person personal and possessive pronouns. Self Identity 2016, 15, 120–138. [Google Scholar] [CrossRef]
- Herbert, C.; Northoff, G.; Hautzinger, M. Depressive Symptome, kardiale Regulation und kortikale Verarbeitung bei Leistungssportlern. Dtsch. Z. Sportmed. 2016, 2016, 293–300. [Google Scholar] [CrossRef]
- Herbert, C.; Pauli, P.; Herbert, B.M. Self-reference modulates the processing of emotional stimuli in the absence of explicit self-referential appraisal instructions. Soc. Cogn. Affect. Neurosci. 2011, 6, 653–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbert, C.; Herbert, B.M.; Ethofer, T.; Pauli, P. His or mine? The time course of self-other discrimination in emotion processing. Soc. Neurosci. 2011, 6, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Fields, E.C.; Kuperberg, G.R. Dynamic Effects of Self-Relevance and Task on the Neural Processing of Emotional Words in Context. Front. Psychol. 2015, 6, 2003. [Google Scholar] [CrossRef] [Green Version]
- Fields, E.C.; Kuperberg, G.R. Loving yourself more than your neighbor: ERPs reveal online effects of a self-positivity bias. Soc. Cogn. Affect. Neurosci. 2015, 10, 1202–1209. [Google Scholar] [CrossRef] [Green Version]
- Fields, E.C.; Weber, K.; Stillerman, B.; Delaney-Busch, N.; Kuperberg, G.R. Functional MRI reveals evidence of a self-positivity bias in the medial prefrontal cortex during the comprehension of social vignettes. Soc. Cogn. Affect. Neurosci. 2019, 14, 613–621. [Google Scholar] [CrossRef]
- Northoff, G.; Heinzel, A.; de Greck, M.; Bermpohl, F.; Dobrowolny, H.; Panksepp, J. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. NeuroImage 2006, 31, 440–457. [Google Scholar] [CrossRef]
- Lemogne, C.; Delaveau, P.; Freton, M.; Guionnet, S.; Fossati, P. Medial prefrontal cortex and the self in major depression. J. Affect. Disord. 2012, 136, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Herbert, C.; Herbert, B.M.; Pauli, P. Emotional self-reference: Brain structures involved in the processing of words describing one’s own emotions. Neuropsychologia 2011, 49, 2947–2956. [Google Scholar] [CrossRef] [PubMed]
- Northoff, G.; Bermpohl, F. Cortical midline structures and the self. Trends Cogn. Sci. 2004, 8, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Northoff, G. How is our self related to midline regions and the default-mode network? NeuroImage 2011, 57, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Weis, P.P.; Herbert, C. Bodily Reactions to Emotional Words Referring to Own versus Other People’s Emotions. Front. Psychol. 2017, 8, 1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meixner, F.; Herbert, C. Whose emotion is it? Measuring self-other discrimination in romantic relationships during an emotional evaluation paradigm. PLoS ONE 2018, 13, e0204106. [Google Scholar] [CrossRef]
- Herbert, C.; Hesse, K.; Wildgruber, D. Emotion and self in psychotic disorders: Behavioral evidence from an emotional evaluation task using verbal stimuli varying in emotional valence and self-reference. J. Behav. Ther. Exp. Psychiatry 2018, 58, 86–96. [Google Scholar] [CrossRef]
- Diener, E.; Napa-Scollon, C.K.; Oishi, S.; Dzokoto, V.; Suh, E.M. Positivity and the Construction of Life Satisfaction Judgments: Global Happiness is not the Sum of its Parts. J. Happiness Stud. 2000, 1, 159–176. [Google Scholar] [CrossRef]
- Caprara, G.V.; Eisenberg, N.; Alessandri, G. Positivity: The Dispositional Basis of Happiness. J. Happiness Stud. 2017, 18, 353–371. [Google Scholar] [CrossRef]
- Uddin, L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015, 16, 55–61. [Google Scholar] [CrossRef]
- Sander, D.; Grafman, J.; Zalla, T. The human amygdala: An evolved system for relevance detection. Rev. Neurosci. 2003, 14, 303–316. [Google Scholar] [CrossRef] [PubMed]
- BDI-II—Beck-Depressions-Inventar Revision—Hogrefe Verlag. Available online: https://www.testzentrale.de/shop/beck-depressions-inventar.html (accessed on 5 June 2022).
- Herbert, C.; Ostermair, J.; Herbst, S.; Pauli, P.; Reif, A.; Fallgatter, A.; Herrman, M. It’s Yours! The Negativity Bias in Major Depressive Disorder is Self-Specific: Evidence from Event-Related Brain Potential Studies; DGPPN; German Society for Psychiatry and Psychotherapy, Psychosomatics and Neurology: Berlin, Germany, 2014. [Google Scholar]
- Auerbach, R.P.; Stanton, C.H.; Proudfit, G.H.; Pizzagalli, D.A. Self-referential processing in depressed adolescents: A high-density event-related potential study. J. Abnorm. Psychol. 2015, 124, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Herbert, C. Processing Preferences for Self-Related Emotional Words as Markers of Cognitive Vulnerability and Well-Being—Cerebral and Behavioral Correlates and Mechanisms. Project Funded by the German Research Foundation (DFG). 2019–2023. Available online: https://gepris.dfg.de/gepris/projekt/415209420 (accessed on 5 June 2022).
- Cosmides, L.; Tooby, J. Evolutionary psychology and the emotions. In Handbook of Emotions, 2nd ed.; Lewis, M., Haviland-Jones, J.M., Eds.; Guilford: New York, NY, USA, 2000; pp. 91–115. [Google Scholar]
- Lang, P.J. Emotion and motivation: Toward consensus definitions and a common research purpose. Emot. Rev. 2010, 2, 229–233. [Google Scholar] [CrossRef]
- Kissler, J.; Assadollahi, R.; Herbert, C. Emotional and semantic networks in visual word processing: Insights from ERP studies. In Understanding Emotions; Elsevier: Amsterdam, The Netherlands, 2006; pp. 147–183. ISBN 0079-6123. [Google Scholar]
- Herbert, C.; Ethofer, T.; Fallgatter, A.J.; Walla, P.; Northoff, G. The Janus face of language: Where are the emotions in words and where are the words in emotions? Front. Psychol. 2018, 9, 650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahanshahloo, H.R.; Shamsi, M.; Ghasemi, E.; Kouhi, A. Automated and ERP-Based Diagnosis of Attention-Deficit Hyperactivity Disorder in Children. J. Med. Signals Sens. 2017, 7, 26–32. [Google Scholar] [CrossRef]
- Chapman, R.M.; Nowlis, G.H.; McCrary, J.W.; Chapman, J.A.; Sandoval, T.C.; Guillily, M.D.; Gardner, M.N.; Reilly, L.A. Brain event-related potentials: Diagnosing early-stage Alzheimer’s disease. Neurobiol. Aging 2007, 28, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Mumtaz, W.; Malik, A.S.; Yasin, M.A.M.; Xia, L. Review on EEG and ERP predictive biomarkers for major depressive disorder. Biomed. Signal Process. Control 2015, 22, 85–98. [Google Scholar] [CrossRef]
- De Aguiar Neto, F.S.; Rosa, J.L.G. Depression biomarkers using non-invasive EEG: A review. Neurosci. Biobehav. Rev. 2019, 105, 83–93. [Google Scholar] [CrossRef]
- Wu, Y.J.; Thierry, G. How reading in a second language protects your heart. J. Neurosci. 2012, 32, 6485–6489. [Google Scholar] [CrossRef] [Green Version]
- Jończyk, R.; Korolczuk, I.; Balatsou, E.; Thierry, G. Erratum to: Keep calm and carry on: Electrophysiological evaluation of emotional anticipation in the second language. Soc. Cogn. Affect. Neurosci. 2021, 16, 642. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbert, C. Decoding of Processing Preferences from Language Paradigms by Means of EEG-ERP Methodology: Risk Markers of Cognitive Vulnerability for Depression and Protective Indicators of Well-Being? Cerebral Correlates and Mechanisms. Appl. Sci. 2022, 12, 7740. https://doi.org/10.3390/app12157740
Herbert C. Decoding of Processing Preferences from Language Paradigms by Means of EEG-ERP Methodology: Risk Markers of Cognitive Vulnerability for Depression and Protective Indicators of Well-Being? Cerebral Correlates and Mechanisms. Applied Sciences. 2022; 12(15):7740. https://doi.org/10.3390/app12157740
Chicago/Turabian StyleHerbert, Cornelia. 2022. "Decoding of Processing Preferences from Language Paradigms by Means of EEG-ERP Methodology: Risk Markers of Cognitive Vulnerability for Depression and Protective Indicators of Well-Being? Cerebral Correlates and Mechanisms" Applied Sciences 12, no. 15: 7740. https://doi.org/10.3390/app12157740





