Electrofenton with Reticular Vitreous Carbon and Iron Oxide Nanoparticles for Dye Removal: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Magnetic Materials on RVC Substrate
2.2. Characterization of the Electrodes
2.3. Degradation Processes
3. Results and Discussion
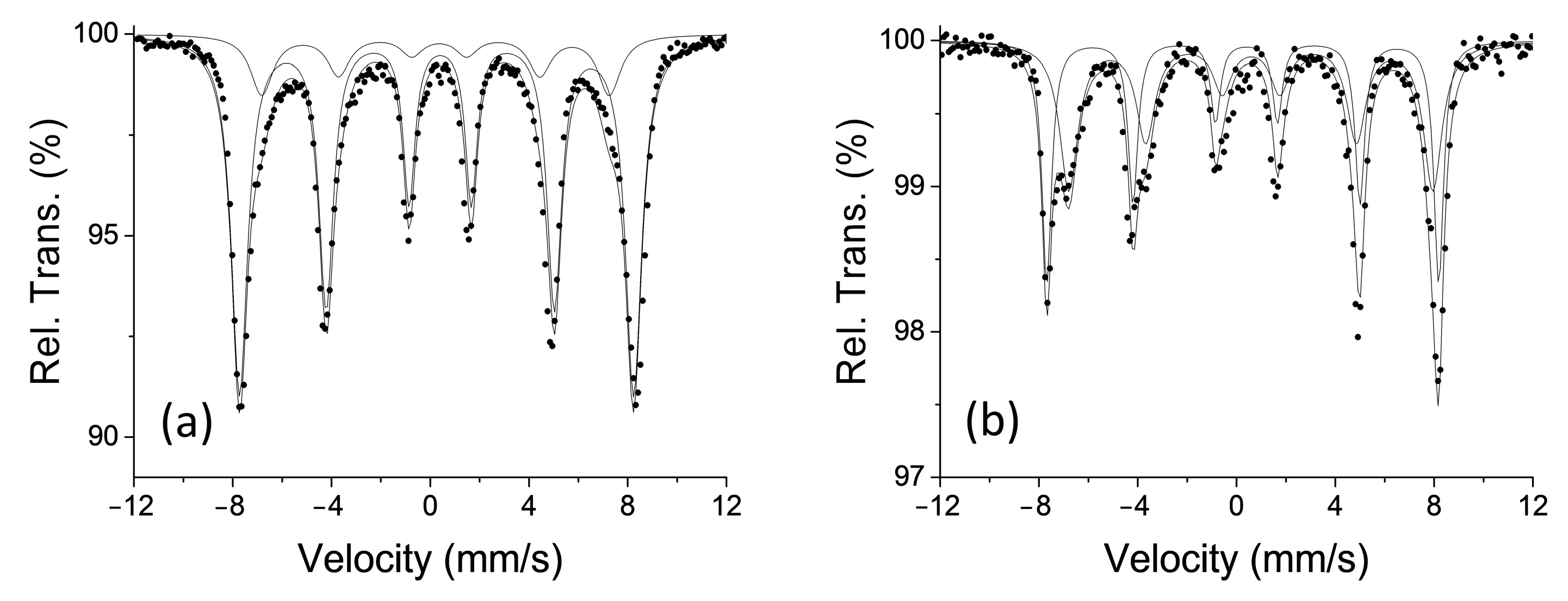
3.1. Characterization of the Electrodes
3.2. Degradation of Methylene Blue
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asghar, A.; Abdul Raman, A.A.; Wan Daud, W.M.A. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Sudoh, M.; Kodera, T.; Sakai, K.; Zhang, J.Q.; Koide, K. Oxidative Degradation of Aqueous Phenol Effluent with Electrogenerated Fenton’s Reagent. J. Chem. Eng. Jpn. 1986, 19, 513–518. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton’s Reaction Chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef] [PubMed]
- Oturan, M.A. An ecologically effective water treatment technique using electrochemically generated hydroxyl radicals for in situ destruction of organic pollutants: Application to herbicide 2,4-D. J. Appl. Electrochem. 2000, 30, 475–482. [Google Scholar] [CrossRef]
- Xia, G.; Lu, Y.; Xu, H. An energy-saving production of hydrogen peroxide via oxygen reduction for electro-Fenton using electrochemically modified polyacrylonitrile-based carbon fiber brush cathode. Sep. Purif. Technol. 2015, 156, 553–560. [Google Scholar] [CrossRef]
- Scialdone, O.; Galia, A.; Gattuso, C.; Sabatino, S.; Schiavo, B. Effect of air pressure on the electro-generation of H2O2 and the abatement of organic pollutants in water by electro-Fenton process. Electrochim. Acta 2015, 182, 775–780. [Google Scholar] [CrossRef]
- Pérez, J.F.; Sáez, C.; Llanos, J.; Cañizares, P.; López, C.; Rodrigo, M.A. Improving the Efficiency of Carbon Cloth for the Electrogeneration of H2O2: Role of Polytetrafluoroethylene and Carbon Black Loading. Ind. Eng. Chem. Res. 2017, 56, 12588–12595. [Google Scholar] [CrossRef]
- Pérez, J.F.; Galia, A.; Rodrigo, M.A.; Llanos, J.; Sabatino, S.; Sáez, C.; Schiavo, B.; Scialdone, O. Effect of pressure on the electrochemical generation of hydrogen peroxide in undivided cells on carbon felt electrodes. Electrochim. Acta 2017, 248, 169–177. [Google Scholar] [CrossRef]
- Li, Q.; Batchelor-McAuley, C.; Lawrence, N.S.; Hartshorne, R.S.; Jones, C.J.V.; Compton, R.G. A flow system for hydrogen peroxide production at reticulated vitreous carbon via electroreduction of oxygen. J. Solid State Electrochem. 2014, 18, 1215–1221. [Google Scholar] [CrossRef]
- Bustos-Terrones, Y.A.; Rojas-Valencia, M.; Alvarez-Gallegos, A.; Vargas-Estrada, L.; García, P. Removal of Basic Blue 9 Dye by Hydrogen Peroxide Activated by Electrogenerated Fe2+/Fe3+ and Simultaneous Production of Hydrogen. J. Environ. Prot. 2015, 6, 781–791. [Google Scholar] [CrossRef]
- Fockedey, E.; Van Lierde, A. Coupling of anodic and cathodic reactions for phenol electro-oxidation using three-dimensional electrodes. Water Res. 2002, 36, 4169–4175. [Google Scholar] [CrossRef]
- Xie, Y.B.; Li, X.Z. Interactive oxidation of photoelectrocatalysis and electro-Fenton for azo dye degradation using TiO2–Ti mesh and reticulated vitreous carbon electrodes. Mater. Chem. Phys. 2006, 95, 39–50. [Google Scholar] [CrossRef]
- El-Desoky, H.S.; Ghoneim, M.M.; El-Sheikh, R.; Zidan, N.M. Oxidation of Levafix CA reactive azo-dyes in industrial wastewater of textile dyeing by electro-generated Fenton’s reagent. J. Hazard. Mater. 2010, 175, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, M.M.; El-Desoky, H.S.; Zidan, N.M. Electro-Fenton oxidation of Sunset Yellow FCF azo-dye in aqueous solutions. Desalination 2011, 274, 22–30. [Google Scholar] [CrossRef]
- Vasconcelos, V.M.; Ponce-de-León, C.; Nava, J.L.; Lanza, M.R.V. Electrochemical degradation of RB-5 dye by anodic oxidation, electro-Fenton and by combining anodic oxidation–electro-Fenton in a filter-press flow cell. J. Electroanal. Chem. 2016, 765, 179–187. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R.; Velmathi, S.; Sanjini, N.S. Magnetite as a heterogeneous electro Fenton catalyst for the removal of Rhodamine B from aqueous solution. RSC Adv. 2014, 4, 5698–5708. [Google Scholar] [CrossRef]
- Choe, Y.J.; Kim, J.; Byun, J.Y.; Kim, S.H. An electro-Fenton system with magnetite coated stainless steel mesh as cathode. Catal. Today 2021, 359, 16–22. [Google Scholar] [CrossRef]
- Muzenda, C.; Arotiba, O.A. Improved Magnetite Nanoparticle Immobilization on a Carbon Felt Cathode in the Heterogeneous Electro-Fenton Degradation of Aspirin in Wastewater. ACS Omega 2022, 7, 19261–19269. [Google Scholar] [CrossRef]
- Gholizadeh, A.M.; Zarei, M.; Ebratkhahan, M.; Hasanzadeh, A. Phenazopyridine degradation by electro-Fenton process with magnetite nanoparticles-activated carbon cathode, artificial neural networks modeling. J. Environ. Chem. Eng. 2021, 9, 104999. [Google Scholar] [CrossRef]
- Yamanaka, I.; Onizawa, T.; Takenaka, S.; Otsuka, K. Direct and continuous production of hydrogen peroxide with 93% selectivity using a fuel-cell system. Angew. Chem. 2003, 42, 3653–3655. [Google Scholar] [CrossRef]
- Do, T.M.; Byun, J.Y.; Kim, S.H. An electro-Fenton system using magnetite coated metallic foams as cathode for dye degradation. Catal. Today 2017, 295, 48–55. [Google Scholar] [CrossRef]
- Lozano, I.; López, C.; Menendez, N.; Casillas, N.; Herrasti, P. Design, Construction and Evaluation of a 3D Printed Electrochemical Flow Cell for the Synthesis of Magnetite Nanoparticles. J. Electrochem. Soc. 2018, 165, H688–H697. [Google Scholar] [CrossRef]
- Abe, M.; Tamaura, Y. Ferrite Plating in Aqueous-Solution—New Technique for Preparing Magnetic Thin-Film. J. Appl. Phys. 1984, 55, 2614–2616. [Google Scholar] [CrossRef]
- Fock, J.; Bogart, L.K.; Gonzalez-Alonso, D.; Espeso, J.I.; Hansen, M.F.; Varon, M.; Frandsen, C.; Pankhurst, Q.A. On the “centre of gravity” method for measuring the composition of magnetite/maghemite mixtures, or the stoichiometry of magnetite-maghemite solid solutions, via Fe-57 Mossbauer spectroscopy. J. Phys. D-Appl. Phys. 2017, 50, 265005. [Google Scholar] [CrossRef]
- Brand, R.A. Improving the validity of hyperfine field distributions from magnetic alloys. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1987, 28, 398–416. [Google Scholar] [CrossRef]
- Malato, S.; Fernandez-Ibanez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, F.L.; Menendez, N.; Mazarío, E.; Herrasti, P. Electrofenton with Reticular Vitreous Carbon and Iron Oxide Nanoparticles for Dye Removal: A Preliminary Study. Appl. Sci. 2022, 12, 8293. https://doi.org/10.3390/app12168293
Rivera FL, Menendez N, Mazarío E, Herrasti P. Electrofenton with Reticular Vitreous Carbon and Iron Oxide Nanoparticles for Dye Removal: A Preliminary Study. Applied Sciences. 2022; 12(16):8293. https://doi.org/10.3390/app12168293
Chicago/Turabian StyleRivera, Fernanda L., Nieves Menendez, Eva Mazarío, and Pilar Herrasti. 2022. "Electrofenton with Reticular Vitreous Carbon and Iron Oxide Nanoparticles for Dye Removal: A Preliminary Study" Applied Sciences 12, no. 16: 8293. https://doi.org/10.3390/app12168293
APA StyleRivera, F. L., Menendez, N., Mazarío, E., & Herrasti, P. (2022). Electrofenton with Reticular Vitreous Carbon and Iron Oxide Nanoparticles for Dye Removal: A Preliminary Study. Applied Sciences, 12(16), 8293. https://doi.org/10.3390/app12168293





