Optimization and Hydration Mechanism of Ecological Ternary Cements Containing Phosphogypsum
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Materials, Mix Composition and Specimen Preparation
2.1.1. Properties of Materials
2.1.2. Mix Composition
2.1.3. Specimen Preparation
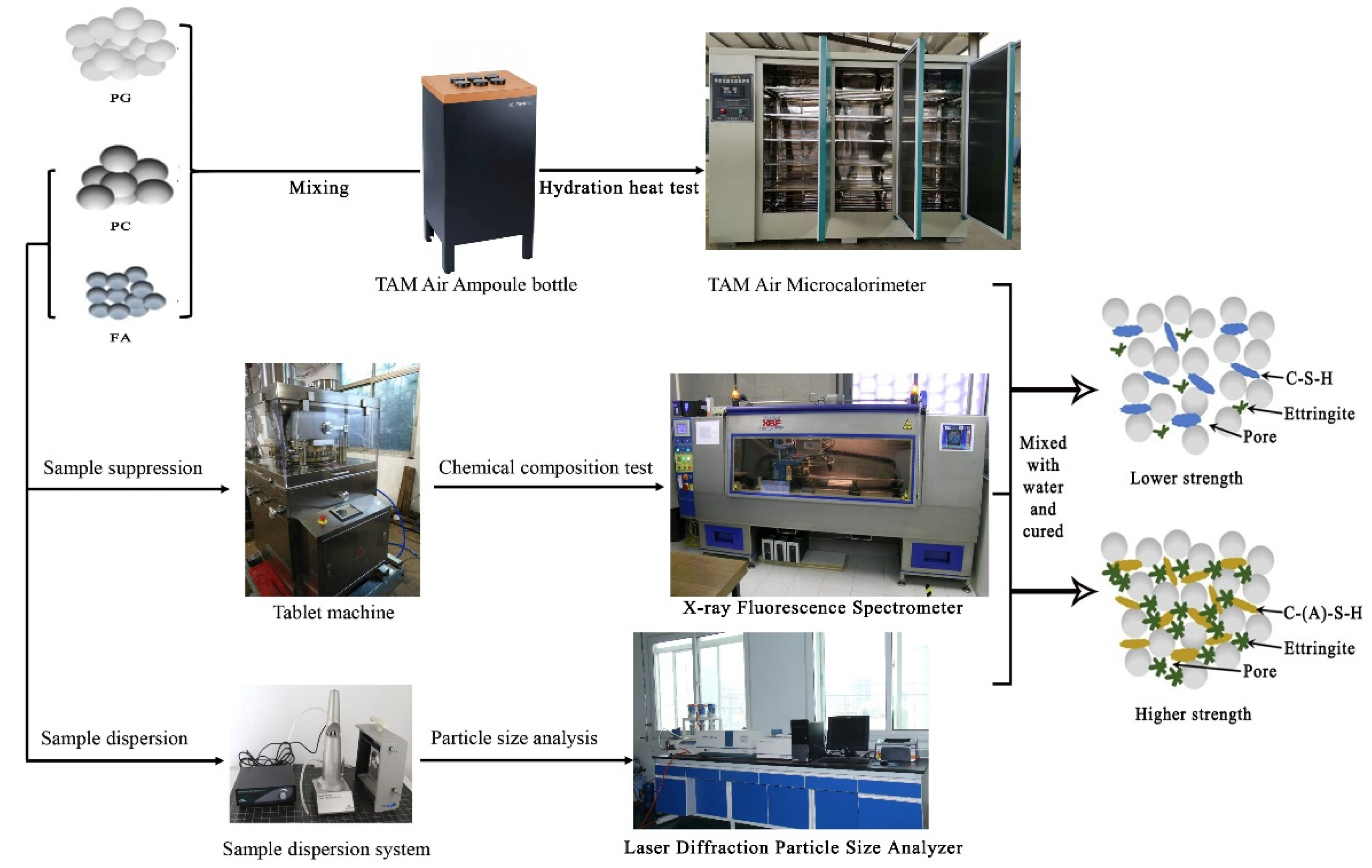
2.2. Methodology
2.2.1. Experimental Test Methods
2.2.2. Thermodynamic Modelling
3. Results and Discussion
3.1. Experimental Results
3.1.1. Uniaxial Compressive Strength
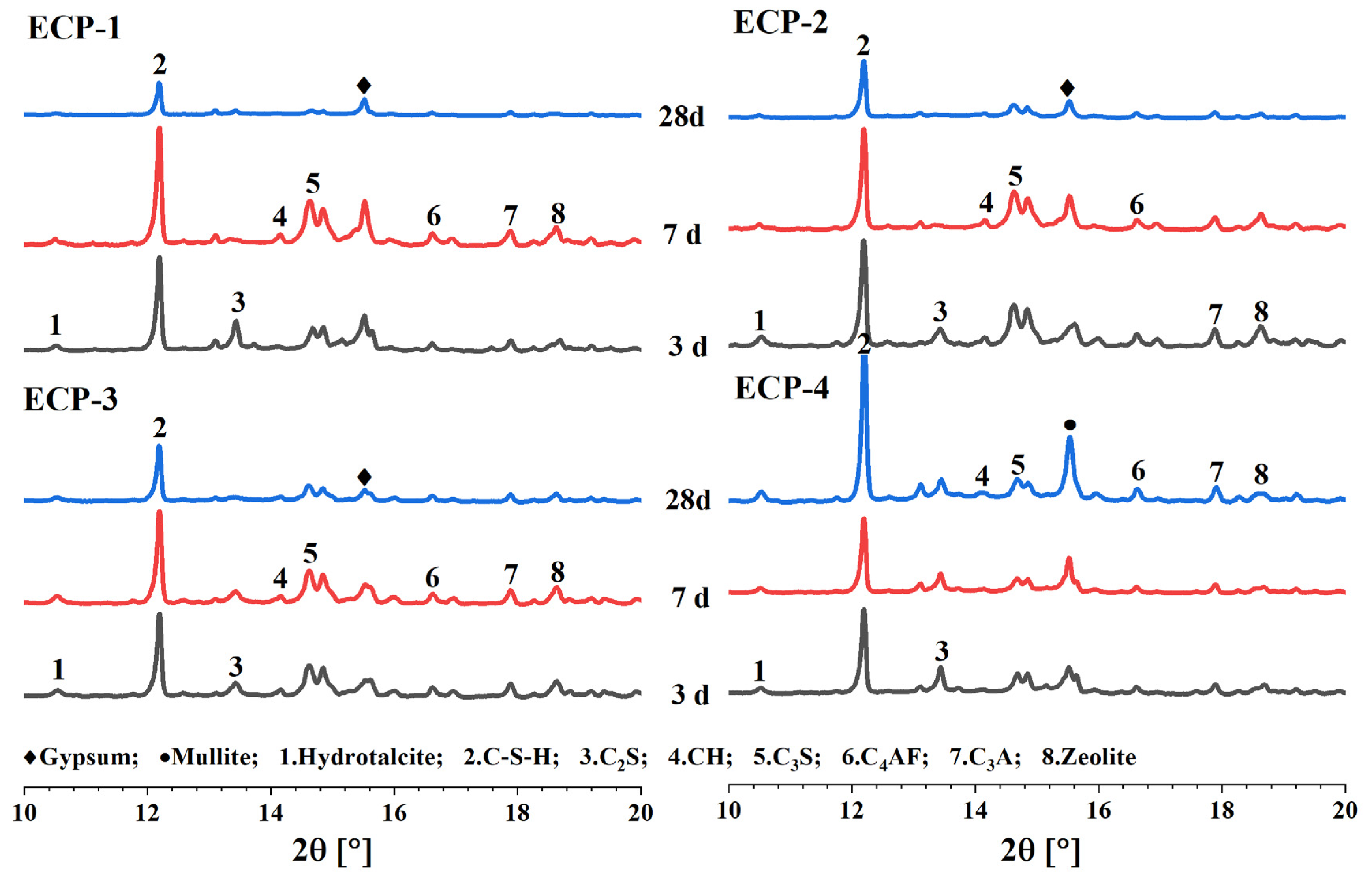
3.1.2. XRD Analysis
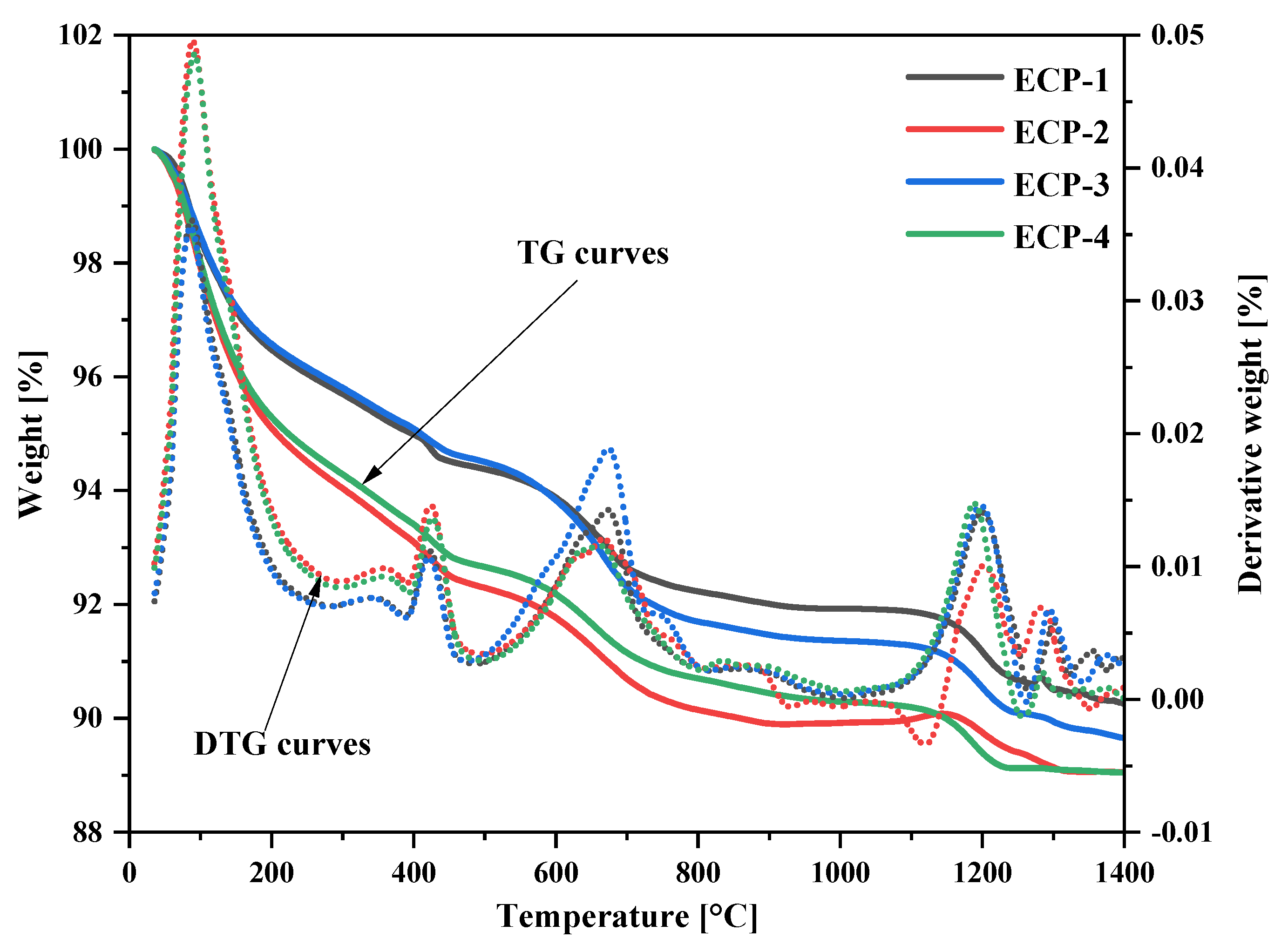
3.1.3. TG-DTG Analysis
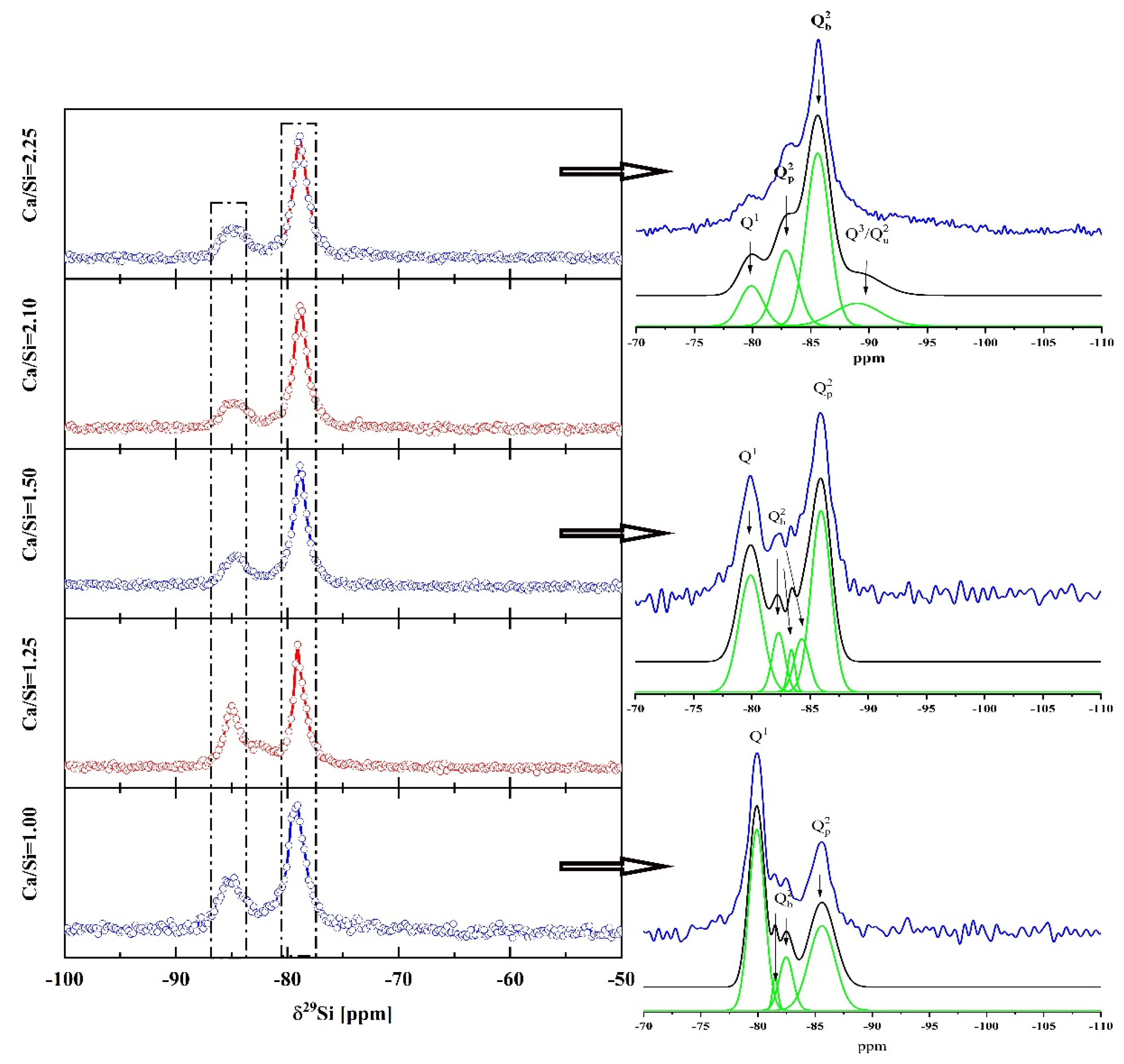
3.1.4. 29Si MAS-NMR Analysis
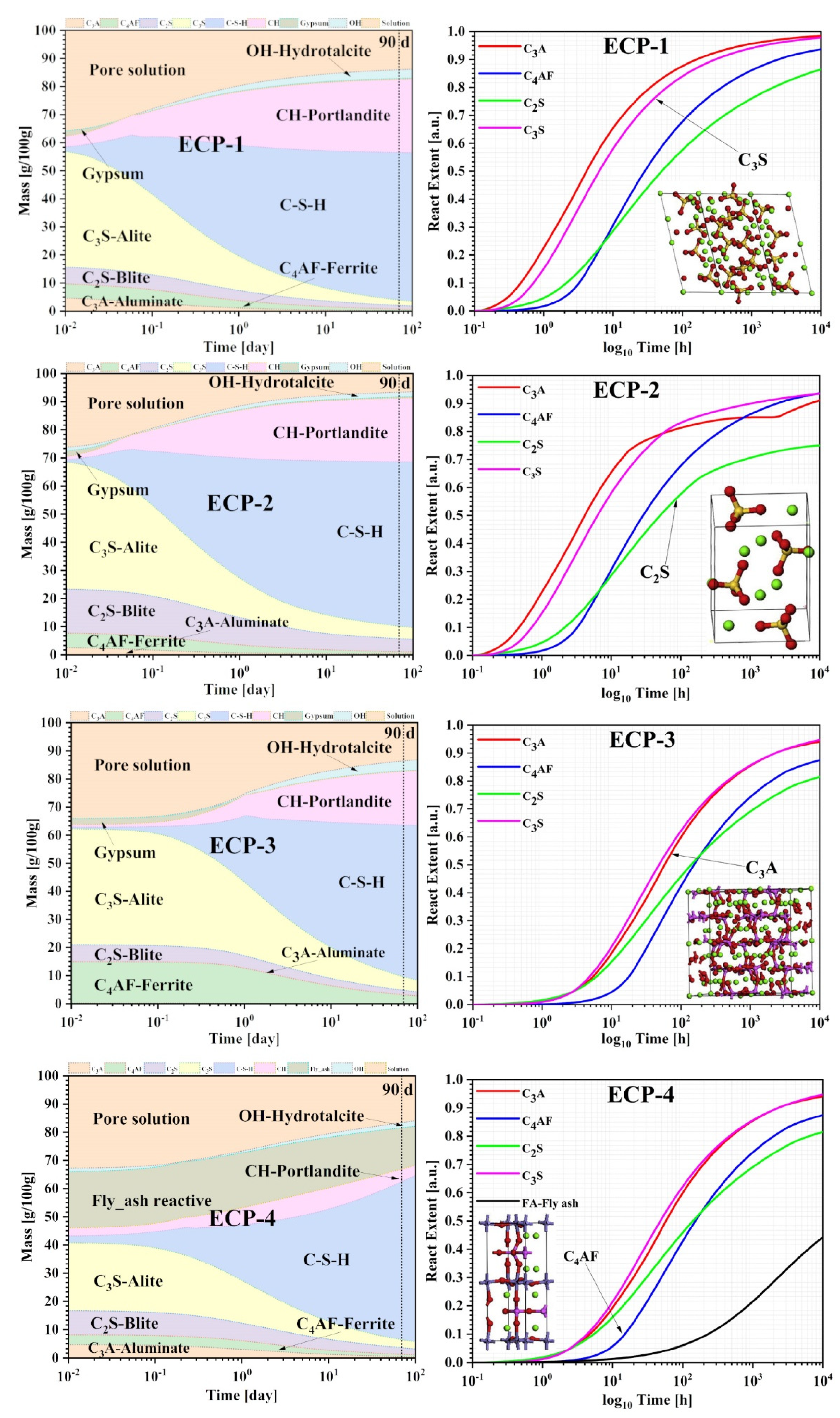
3.2. Thermodynamic Analysis
3.2.1. Thermodynamic Parameters Determination
3.2.2. Simulation and Calculation Base on GEMS
3.2.3. Thermodynamic Property Analysis
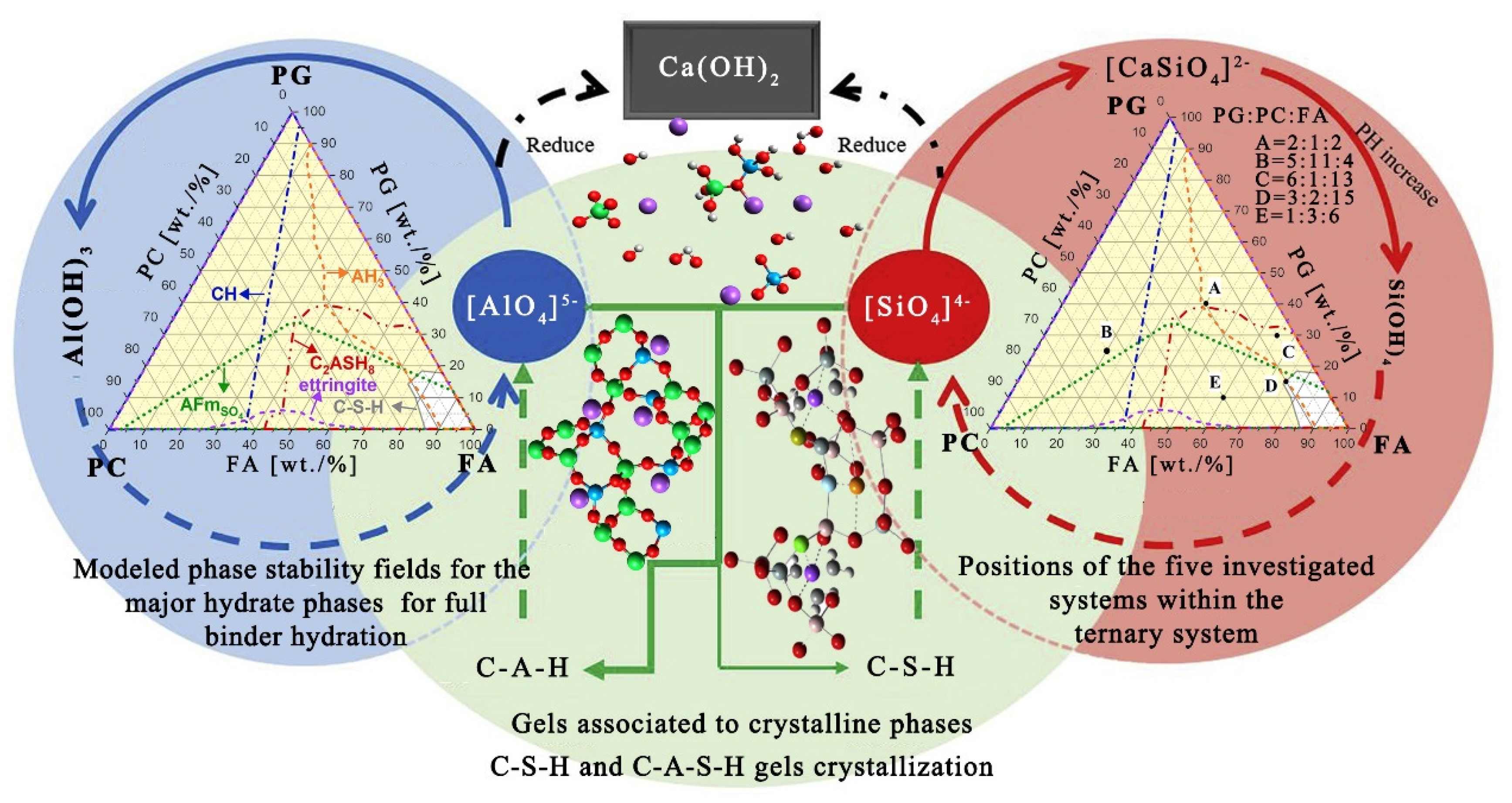
4. Evolution Mechanism of the Hydration Reaction
5. Conclusions
- (1)
- Macro and micro experimental analysis showed that the addition of PG at the early stage promoted the formation of AFt, dissolution of C3S, polymerization of C-S-H gels and morphological transformation. The amount of AFt crystals first increased, then decreased, and then increased again with the increase in the PG content, and the [SO4]2− generated by its dissolution could stimulate the activity of the FA. The synergistic promoting effect of PG and FA was confirmed. The hydration products were generated slowly in the early stage and increased rapidly in the later stage, during which AFt crystals were inserted into the C-S-H gels to make the structure of the whole hardened slurry more dense.
- (2)
- The thermodynamic model of ECP was established, and the thermodynamic properties were simply simulated by GEMS, which can quantitatively explain the dissolution of the C3S and the formation and evolution of the C-S-H gels between the PG-FA mineral admixture and hydration products of PC. The values of mineral single-phase CaO, SiO2H and H2Ohyd in hydration products gradually increased with the increase in the Ca/Si ratios. The values of CaOext and H2OOH were proportional, while the values of SiO2H and SiO2 were inversely proportional.
- (3)
- The evolution law of the hydration product structure entails that PG generates [SiO4]2− ions by ionization and Ca-O-Ca and Si-O-Si covalent bonds break when they encounter alkali conditions combined with Si-O-Si, Al-O-Al, Al-O and Si-O covalent bonds broken by the PC. At the same time, it reunites with [SiO4]2− and [AlO4]5− ions ionized by the FA and is polymerized to form stable C-S-H and C-A-S-H hexahedral network structures.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ΔƒGo | The free energy of formation from the constituent oxides, kJ/mol |
| ΔGO | The electronegativity of the cation i in the crystalline environment of the mineral, kJ/mol; |
| ΔƒHo | Enthalpy of reaction, kJ/mol; |
| So | Heat capacity, J/mol·K; |
| M | Molar mass, kg·mol−1; |
| Cp | Standard molar heat capacity of species at T, P, (J·K−1·mol−1); |
| Vo | Standard molar volume (cm3·mol−1); |
| Vm | Molar volume, m3/mol. |
References
- National Development and Reform Commission. China Resources Comprehensive Utilization Annual Report (2014); Recyclable Resources and Circular Economy; National Development and Reform Commission: Beijing, China, 2014. [Google Scholar]
- Shi, C.; Jiménez, A.F.; Palomo, A. New Cements for the 21st Century: The Pursuit of an Alternative to Portland Cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Wang, B.; Yang, L.; Luo, T.; Cao, J. Study on the Kinetics of Hydration Transformation from Hemihydrate Phosphogypsum to Dihydrate Phosphogypsum in Simulated Wet Process Phosphoric Acid. ACS Omega 2021, 6, 7342–7350. [Google Scholar] [CrossRef]
- Amrani, M.; Taha, Y.; Kchikach, A.; Benzaazoua, M.; Hakkou, R. Phosphogypsum recycling: New horizons for a more sustainable road material application. J. Build. Eng. 2020, 30, 101267. [Google Scholar] [CrossRef]
- Puertas, F.; Martínez-Ramírez, S.; Alonso, S.; Vázquez, T. Alkali-activated fly ash/slag cements: Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Villalón Fornés, I.; Doroševas, V.; Vaičiukynienė, D.; Nizevičienė, D. The Investigation of Phosphogypsum Specimens Processed by Press-Forming Method. Waste Biomass Valorization 2021, 12, 1539–1551. [Google Scholar] [CrossRef]
- Carriço, A.; Bogas, J.A.; Guedes, M. Thermoactivated Cementitious Materials—A Review. Constr. Build. Mater. 2020, 250, 118873. [Google Scholar] [CrossRef]
- Kulik, D.A.; Miron, G.D.; Lothenbach, B. A Structurally-Consistent CASH+ Sublattice Solid Solution Model for Fully Hydrated C-S-H Phases: Thermodynamic Basis, Methods, and Ca-Si-H2O Core Sub-Model. Cem. Concr. Res. 2022, 151, 106585. [Google Scholar]
- Mohamed, R.; Abd Razak, R.; Abdullah, M.M.; Abd Abd Rahim, S.Z.; Yuan-Li, L.; Sandu, A.V.; Wysłocki, J.J. Heat evolution of alkali-activated materials: A review on influence factors. Constr. Build. Mater. 2022, 314, 125651. [Google Scholar] [CrossRef]
- Machner, A.; Zajac, M.; Haha, M.B.; Kjellsen, K.O.; Geiker, M.R.; de Weerdt, K. Stability of the hydrate phase assemblage in Portland composite cements containing dolomite and metakaolin after leaching, carbonation, and chloride exposure. Cem. Concr. Compos. 2018, 89, 89–106. [Google Scholar] [CrossRef]
- Lothenbach, B. Thermodynamic equilibrium calculations in cementitious systems. Mater. Struct. 2010, 43, 1413–1433. [Google Scholar] [CrossRef]
- Gad, E.A.M.; Habib, A.O.; Mousa, M.M. Understanding the mechanism of decomposition reactions of neat and superplasticized ordinary Portland cement pastes using thermal analysis. J. Silic. Based Compos. Mater. 2018, 70, 98–103. [Google Scholar] [CrossRef]
- Kulik, D.A.; Wagner, T.; Dmytrieva, S.V.; Kosakowski, G.; Hingerl, F.F.; Chudnenko, K.V.; Berner, U.R. GEM-Selektor geochemical modeling package: Revised algorithm and GEMS3K numerical kernel for coupled simulation codes. Comput. Geosci. 2013, 17, 1–24. [Google Scholar] [CrossRef]
- Lothenbach, B.; Kulik, D.A.; Matschei, T.; Balonis, M.; Baquerizo, L.; Dilnesa, B.; Miron, G.D.; Myers, R.J. Cemdata18: A Chemical Thermodynamic Database for Hydrated Portland Cements and Alkali-Activated Materials. Cem. Concr. Res. 2019, 115, 472–506. [Google Scholar] [CrossRef]
- Gartner, E.; Maruyama, I.; Chen, J. A new model for the C-S-H phase formed during the hydration of Portland cements. Cem. Concr. Res. 2017, 97, 95–106. [Google Scholar] [CrossRef]
- Accorinti, H.; Labarca, M. Commentary on the Models of Electronegativity. J. Chem. Educ. 2020, 97, 3474–3477. [Google Scholar] [CrossRef]
- Feil, D.; Jeffrey, G.A. The Polyhedral Clathrate Hydrates, Part 2. Structure of the Hydrate of Tetra Iso-Amyl Ammonium Fluoride. J. Chem. Phys. 1961, 35, 1863–1873. [Google Scholar] [CrossRef]
- Lee, H.X.; Wong, H.S.; Buenfeld, N.R. Effect of alkalinity and calcium concentration of pore solution on the swelling and ionic exchange of superabsorbent polymers in cement paste. Cem. Concr. Compos. 2018, 88, 150–164. [Google Scholar] [CrossRef]
- Pajares, I.; Martínez-Ramírez, S.; Blanco-Varela, M.T. Evolution of ettringite in presence of carbonate, and silicate ions. Cem. Concr. Compos. 2003, 25, 861–865. [Google Scholar] [CrossRef]
- Pang, X.; Meyer, C. Modeling Cement Hydration by Connecting a Nucleation and Growth Mechanism with a Diffusion Mechanism. Part II: Portland Cement Paste Hydration. Sci. Eng. Compos. Mater. 2016, 23, 605–615. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Lu, X.; Wang, S.; Ye, Z.; Li, C.; Cheng, X. Study on the hydration product of ettringite in cement paste with ethanol-diisopropanolamine. J. Therm. Anal. Calorim. 2020, 139, 1007–1016. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Monteiro, P.J.M. Preferred orientation of calcium aluminosilicate hydrate compacts: Implications for creep and indentation. Cem. Concr. Res. 2021, 143, 106371. [Google Scholar] [CrossRef]
- Walker, C.S.; Sutou, S.; Oda, C.; Mihara, M.; Honda, A. Calcium silicate hydrate (C-S-H) gel solubility data and a discrete solid phase model at 25 °C based on two binary non-ideal solid solutions. Cem. Concr. Res. 2016, 79, 1–30. [Google Scholar] [CrossRef]
- Cuesta, A.; Zea-Garcia, J.D.; Londono-Zuluaga, D.; de la Torre, A.G.; Santacruz, I.; Vallcorba, O.; Dapiaggi, M.; Sanfélix, S.G.; Aranda, M.A. Multiscale understanding of tricalcium silicate hydration reactions. Sci. Rep. 2018, 8, 8544. [Google Scholar] [CrossRef]
- Masoudi, R.; Hooton, R.D. Examining the hydration mechanism of supersulfated cements made with high and low-alumina slags. Cem. Concr. Compos. 2019, 103, 193–203. [Google Scholar] [CrossRef]
- John, E.; Stephan, D. Calcium Silicate Hydrate—in-Situ Development of the Silicate Structure Followed by Infrared Spectroscopy. J. Am. Ceram. Soc. 2021, 104, 6611–6624. [Google Scholar] [CrossRef]
- Roosz, C.; Vieillard, P.; Blanc, P.; Gaboreau, S.; Gailhanou, H.; Braithwaite, D.; Montouillout, V.; Denoyel, R.; Henocq, P.; Madé, B. Thermodynamic properties of C-S-H, C-A-S-H and M-S-H phases: Results from direct measurements and predictive modelling. Appl. Geochem. 2018, 92, 140–156. [Google Scholar] [CrossRef]
- Andersen, M.D.; Jakobsen, H.J.; Skibsted, J. Characterization of White Portland Cement Hydration and the C-S-H Structure in the Presence of Sodium Aluminate by 27Al and 29Si MAS NMR Spectroscopy. Cem. Concr. Res. 2004, 34, 857–868. [Google Scholar] [CrossRef]
- Wolf, J.J.; Jansen, D.; Goetz-Neunhoeffer, F.; Neubauer, J. Application of thermodynamic modeling to predict the stable hydrate phase assemblages in ternary CSA-OPC-anhydrite systems and quantitative verification by QXRD. Cem. Concr. Res. 2020, 128, 105956. [Google Scholar] [CrossRef]






| Sample | SO3 | CaO | SiO2 | P2O5 | Al2O3 | MgO | Fe2O3 | TiO2 | LOI | SSA 1 |
|---|---|---|---|---|---|---|---|---|---|---|
| PG 2 | 47.39 | 32.44 | 7.61 | 1.02 | 0.66 | 0.38 | 0.31 | 0.09 | 2.16 | 0.349 |
| FA | 0.1 | 2.45 | 54.15 | 0.33 | 32.25 | 2.79 | 4.26 | 1.02 | 0.06 | 0.737 |
| PC | 1.92 | 61.41 | 21.74 | 0.16 | 6.19 | 2.23 | 3.2 | 0.39 | 0.95 | 1.280 |
| Combination | Notation | PC | FA | HG | DG | PG | PES | W/S 1 | W/C 2 | Curing Conditions | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [%] | [%] | [%] | [%] | [%] | [%] | 1:3 | 1:1 | Dry 3 | Moist 4 | ||
| PC | 1st test | 100 | --- | --- | --- | --- | --- | --- | ✓ | --- | ✓ |
| PC+HG | PH | 80 | --- | 20 | --- | --- | --- | ✓ | ✓ | --- | ✓ |
| PC+DG | PD | 80 | --- | --- | 20 | --- | --- | ✓ | ✓ | --- | ✓ |
| PC+PG | PP | 80 | --- | --- | --- | 20 | --- | ✓ | ✓ | --- | ✓ |
| PC+FA | 2nd test | 61 | 19 | --- | --- | --- | --- | ✓ | ✓ | ✓ | ✓ |
| PC+FA+HG | PFH | 61 | 19 | 20 | --- | --- | --- | ✓ | ✓ | ✓ | ✓ |
| PC+FA+DG | PFD | 61 | 19 | --- | 20 | --- | --- | ✓ | ✓ | ✓ | ✓ |
| PC+FA+PG | PFP | 61 | 19 | --- | --- | 20 | --- | ✓ | ✓ | ✓ | ✓ |
| PC+PES | 3rd test | 73.49 | --- | --- | --- | --- | 0.90 | --- | ✓ | ✓ | --- |
| PC+FA+PG | ECP-1 | 73.49 | 8.25 | --- | --- | 18.26 | 1.03 | ✓ | ✓ | ✓ | --- |
| PC+FA+PG | ECP-2 | 73.49 | 11.66 | --- | --- | 14.85 | 0.98 | ✓ | ✓ | ✓ | --- |
| PC+FA+PG | ECP-3 | 73.49 | 14.85 | --- | --- | 11.66 | 0.95 | ✓ | ✓ | --- | ✓ |
| PC+FA+PG | ECP-4 | 73.49 | 18.26 | --- | --- | 8.25 | 0.91 | ✓ | ✓ | --- | ✓ |
| Experimental Method | Logogram | Types | Softwares |
|---|---|---|---|
| Particle Size Distribution | PSD | BT-9300HT, China | Origin 2022b OriginLab Corporation, in Northampton, Massachusetts, USA. |
| Uniaxial compressive strength | UCS | HY400X125 according to GB/T 17671-1999 | Materials Studio 2019 Accelrys Corporation, in San Diego, California, USA. |
| Mercury intrusion porosimetry | MIP | Micromeritics-9310 MIP device, maximum and minimum applied pressure of 100 MPa and 0.2 MPa | LAMMPS 19.0 US Deparment of Energy, in Washington, District of Columbia, USA. |
| Isothermal calorimetry | TA | An eight channel Thermal Activity Monitor (TAM) Air calorimeter | Visio 2021 Microsoft Corporation, in Redmond, Washington, District of Columbia, USA. |
| Scanning Electron Microscopy | SEM | Zeiss EVO18, Carl Zeiss AG, Oberkochen, Germany | MDI Jade 6 ICDD, in Delaware County, PA |
| X-ray fluorescence | XRF | PANalytical AXIOS spectrometer | VESTA 3 National Museum of Nature and Science, Kokuritsu Kagaku Hakubutsukan, Japan. |
| X-ray diffraction | XRD | X-Pert PRO DY2198 XRD, QXRD of Topas 4.2, Bruker AXS | Photoshop CS6 Adobe Systems Inc., in San Jose, California, USA. |
| Thermogravimetric-differential thermal analysis | TG-DTG | Mettler TGA/DSC 250 analyzer | GEM-Selektor v.3 Laboratory for Waste Management (LES) of the Paul Scherrer Institute, Forschungsstrasse, Villigen, Aargau, Switzerland. |
| Fourier Transform Infrared Spectroscopy | FTIR | PerkinElmer FTIR Spectrometer | |
| Nuclear magnetic resonance spectroscopy | NMR | 29Si and 27Al solid-state MAS NMR |
| Component Oxides | ΔfG0 | ΔGO | S0 | Cp | V0 |
|---|---|---|---|---|---|
| [kJ/mol] | [kJ/mol] | [J/mol·K] | [J/mol·K] | [cm3/mol] | |
| CaO | −603.30 | 303.60 | 43.80 | 47.99 | 16.52 |
| CaOext | −603.30 | 464.23 | --- | --- | --- |
| SiO2 | −856.28 | 221.31 | 56.41 | 62.16 | 30.28 |
| SiO2H | −856.28 | 323.81 | --- | --- | --- |
| H2OOH | −187.50 | 187.50 | 7.05 | −13.33 | −1.93 |
| H2Ohyd | −221.47 | 221.47 | 47.95 | 43.56 | 13.96 |
| Specimen | 3 Days | 7 Days | 28 Days | 90 Days | 180 Days |
|---|---|---|---|---|---|
| PH | 39.4 | 50.0 | 67.1 | --- | --- |
| PD | 36.6 | 45.9 | 61.0 | --- | --- |
| PP | 18.1 | 19.9 | 32.3 | --- | --- |
| PFA | 37.8 | 39.7 | 51.1 | --- | --- |
| PFD | 33.2 | 39.5 | 45.6 | --- | --- |
| PFP | 25.1 | 28.9 | 38.8 | --- | --- |
| ECP-1 | 11.5 | 20.8 | 29.3 | 42.7 | 58.6 |
| ECP-2 | 28.3 | 34.1 | 46.3 | 58.4 | 76.9 |
| ECP-3 | 37.2 | 47.8 | 60.4 | 73.6 | 85.7 |
| ECP-4 | 51.6 | 64.8 | 72.4 | 87.1 | 92.1 |
| Species Parameters | C3S | C-S-H-jen | Portlandite | H2O(I) | |
|---|---|---|---|---|---|
| ΔƒHo | kJ/mol | −2931 | −2723 | −985 | −286 |
| ΔƒGo | kJ/mol | −2784.33 | −2480.81 | −897.01 | −237.18 |
| Vm | 10−6 m3/mol | 72.4 | 106 * | 33.1 | 18.1 |
| M | kg/mol | 0.22833 | 0.19132 | 0.07409 | 0.01802 |
| Phases | Formula | log KS0 |
|---|---|---|
| (Al-) ettringite | Ca6Al2(SO4)3(OH)12•26H2O → 2Al(OH)4− + 6Ca2+ +26H2O + 4OH− + 3SO42− | −44.9 |
| C4AsH14 | Ca4Al2(SO4)(OH)12•6H2O → 2Al(OH)4− + 4Ca2+ + 6H2O + 4OH− + SO42− | −29.26 |
| Friedel’s salt | Ca4Al2Cl2(OH)12•4H2O → 2Al(OH)4− + 4Ca2+ + 4H2O + 4OH− + 2Cl−SO42− | −27.27 |
| Nitrate-AFm | Ca4Al2 (OH)12(NO3)2•4H2O → 2Al(OH)4− + 4Ca2+ + 4H2O + 4OH− + 2NO3− | −28.67 |
| CH | Ca(OH)2 → Ca2+ + 2OH− | −5.2 |
| C-S-H1 | Ca2+ + SiO32− → CaSiO3O | 2.9 |
| C-S-H2 | Ca2+ + HSiO32− → CaHSiO3+ | 0.5 |
| CsH2 | CaSO4•2H2O → Ca2+ +SO42− + 2H2O | −4.581 |
| CsH0.5 | CaSO4•0.5H2O → Ca2+ +SO42− + 0.5H2O | −3.59 |
| Cs | CaSO4 → Ca2+ + SO42− | −4.357 |
| SiO2 | SiO2(quartz) → SiO20 | −3.476 |
| CH [11] | C3S + 5.3H → C1.7SH4 + 1.3CH | |
| C2S + 4.3H → C1.7SH4 + 0.3CH | ||
| C4AF + xC SH2 + yH → zCmA SHn + CH+FH3 | ||
| C-S-H [14] | Calcium-silicates + H2O → C-S-H1 + Ca(OH)2 | |
| Ca(OH)2 + reactive SiO2 + H2O → C-S-H2 |
| Phases | Ca/Si | CaO | CaOext | SiO2 | SiO2H | H2OOH | H2Ohyd | ΔfGo | ΔGO | So | Cp | Vo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | --- | % | % | % | % | % | % | kJ/mol | kJ/mol | J/mol·K | J/mol·K | cm3/mol |
| C1.25S1.25H2.5 | 1.0 | 0.69 | 0.00 | 0.61 | 0.39 | 0.28 | 0.68 | −2650.4 | −2782.0 | 197.0 | 234.1 | 79 |
| C0.8S0.7H1.8 | 1.25 | 0.70 | 0.00 | 0.61 | 0.39 | 0.28 | 0.69 | −1656.7 | −1742.4 | 144.4 | 166.9 | 48 |
| C1.5SH2.5 | 1.5 | 0.83 | 0.23 | 0.33 | 0.67 | 0.34 | 0.89 | −2594.8 | −2722.4 | 202.0 | 237.0 | 81 |
| C1.67SH2.1 | 2.1 | 0.92 | 0.31 | 0.17 | 0.83 | 0.37 | 1.06 | −2601.9 | −2723.0 | 175.1 | 210.1 | 78 |
| C1.5S0.7H2.5 | 2.25 | 0.96 | 0.46 | 0.08 | 0.92 | 0.45 | 1.12 | −2285.2 | −2400.7 | 203.1 | 232.8 | 81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Ni, X.; Ma, B. Optimization and Hydration Mechanism of Ecological Ternary Cements Containing Phosphogypsum. Appl. Sci. 2022, 12, 8726. https://doi.org/10.3390/app12178726
Hou J, Ni X, Ma B. Optimization and Hydration Mechanism of Ecological Ternary Cements Containing Phosphogypsum. Applied Sciences. 2022; 12(17):8726. https://doi.org/10.3390/app12178726
Chicago/Turabian StyleHou, Jiaojiao, Xiaoyang Ni, and Baosong Ma. 2022. "Optimization and Hydration Mechanism of Ecological Ternary Cements Containing Phosphogypsum" Applied Sciences 12, no. 17: 8726. https://doi.org/10.3390/app12178726





