Photocatalytic Cementitious Material for Eco-Efficient Construction—A Systematic Literature Review
Abstract
:1. Introduction
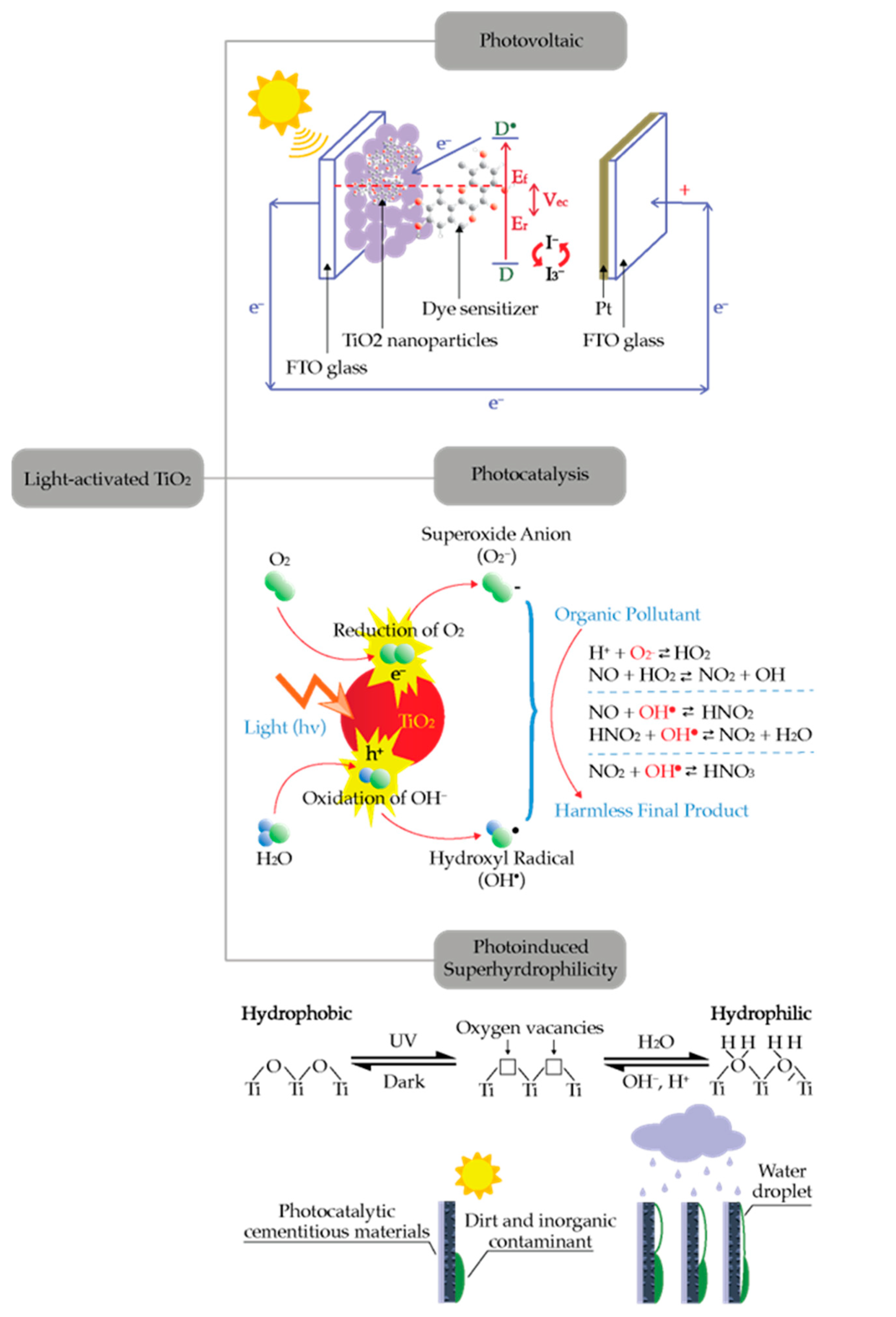
2. Principles of Photocatalysis
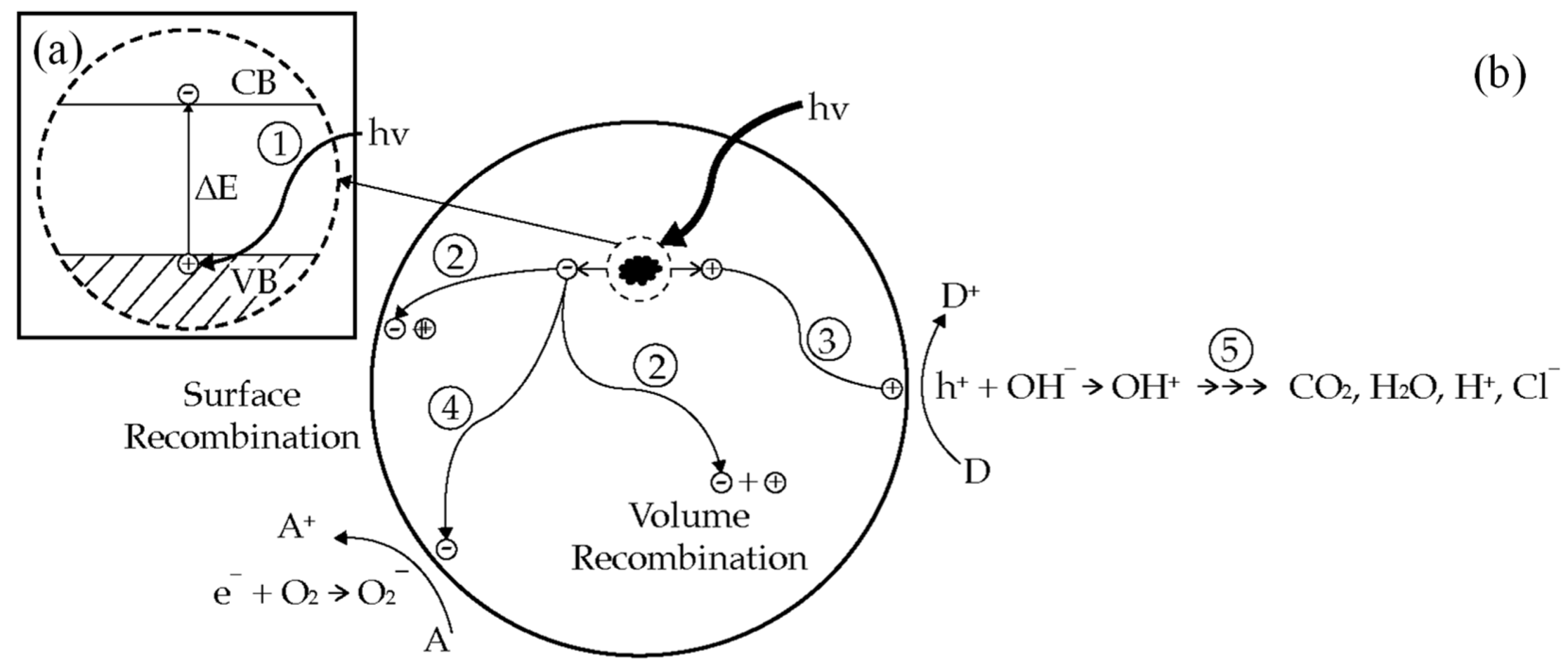
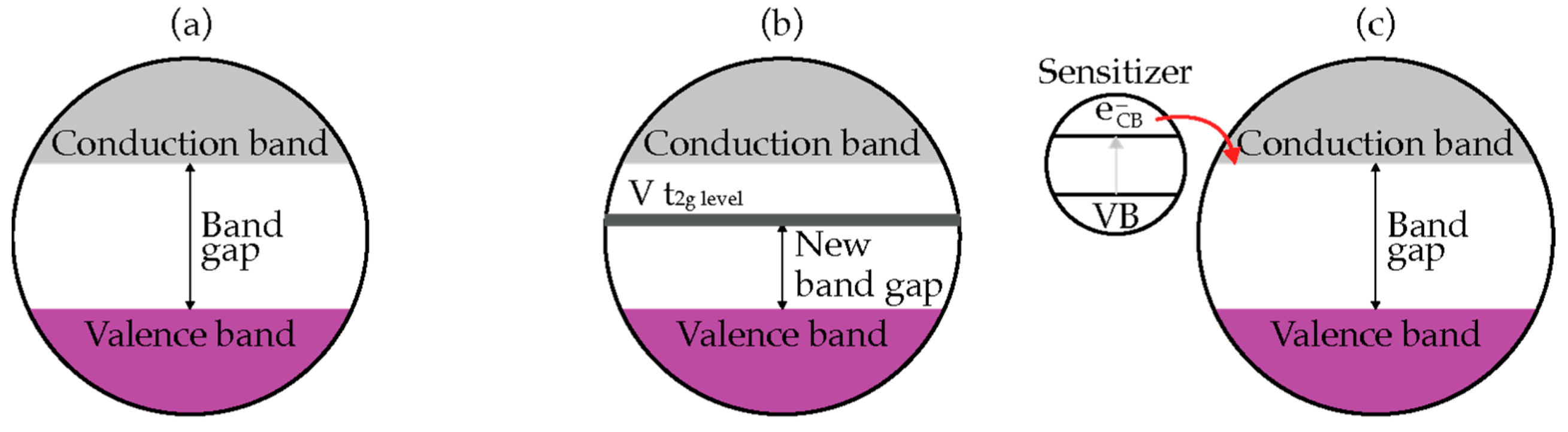
2.1. Semiconductor Photocatalysis
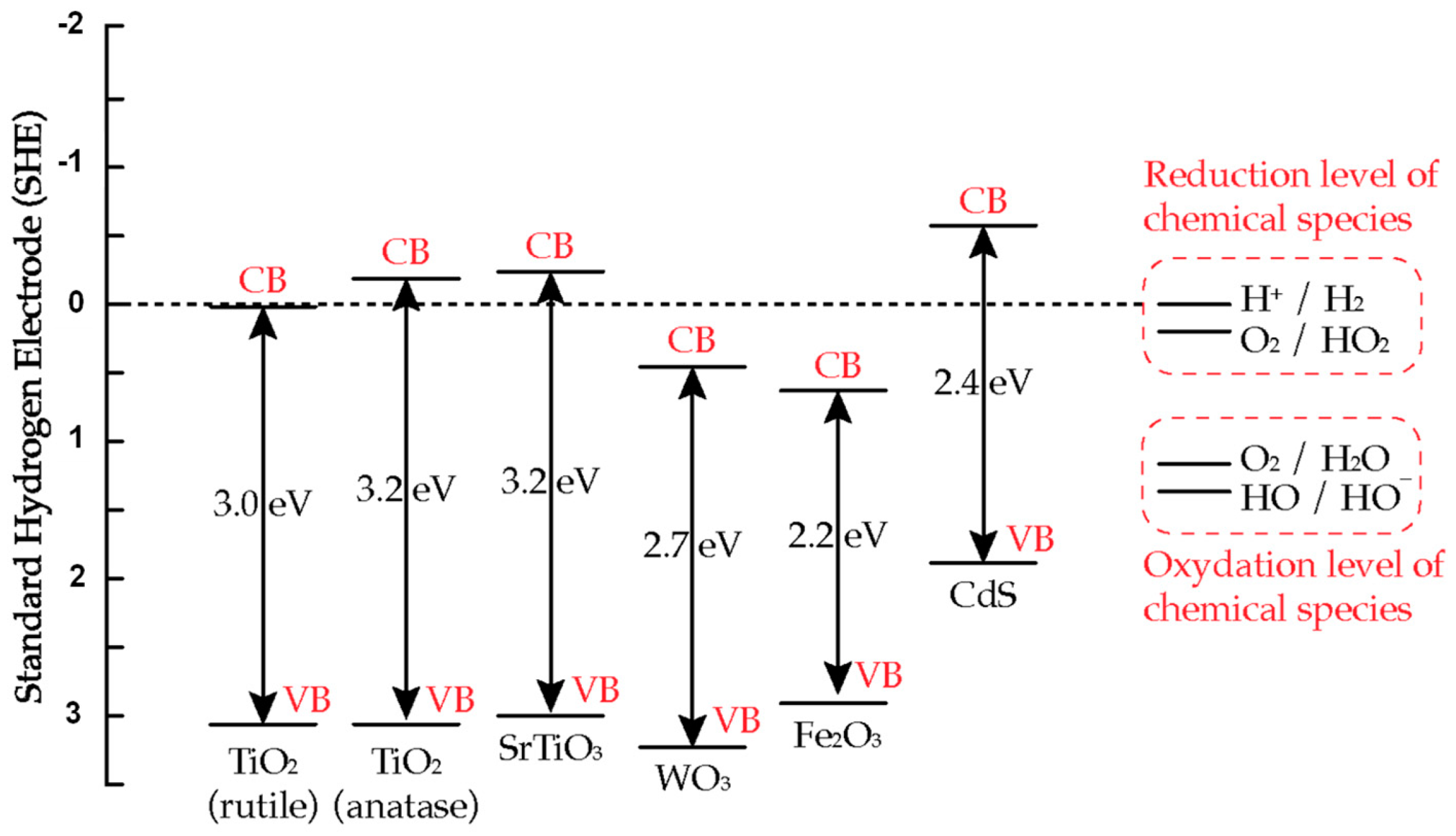
2.2. Bandgap and Band Edge Position
2.3. TiO2 Based Photocatalyst
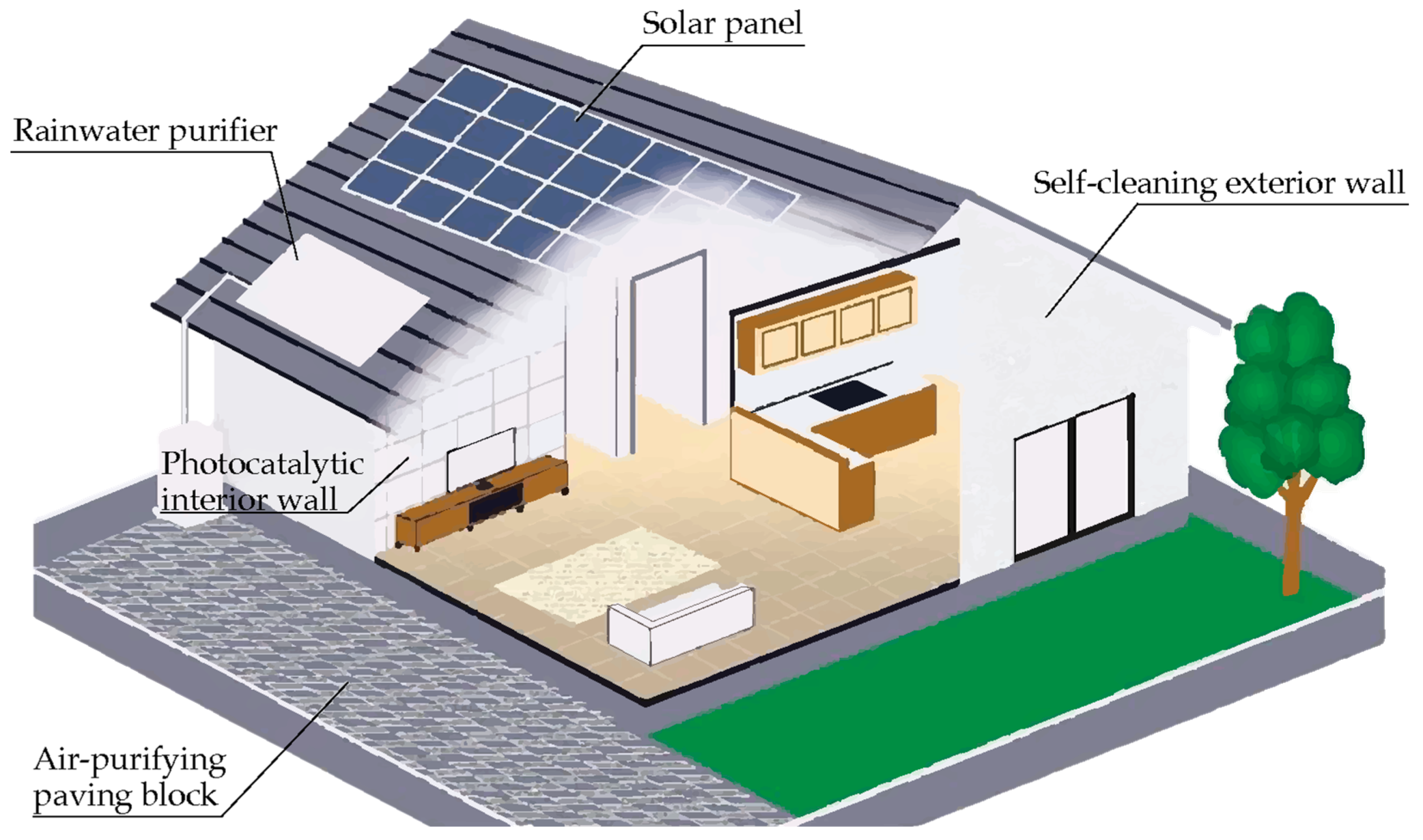
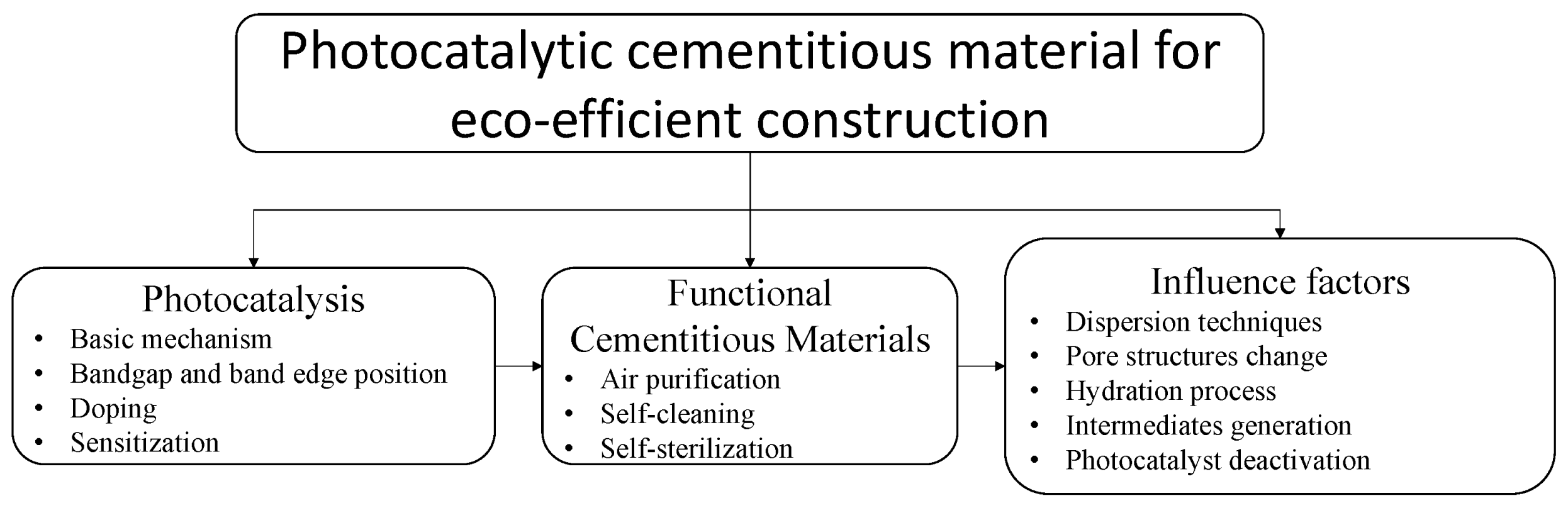
3. Photocatalytic Cementitious Materials
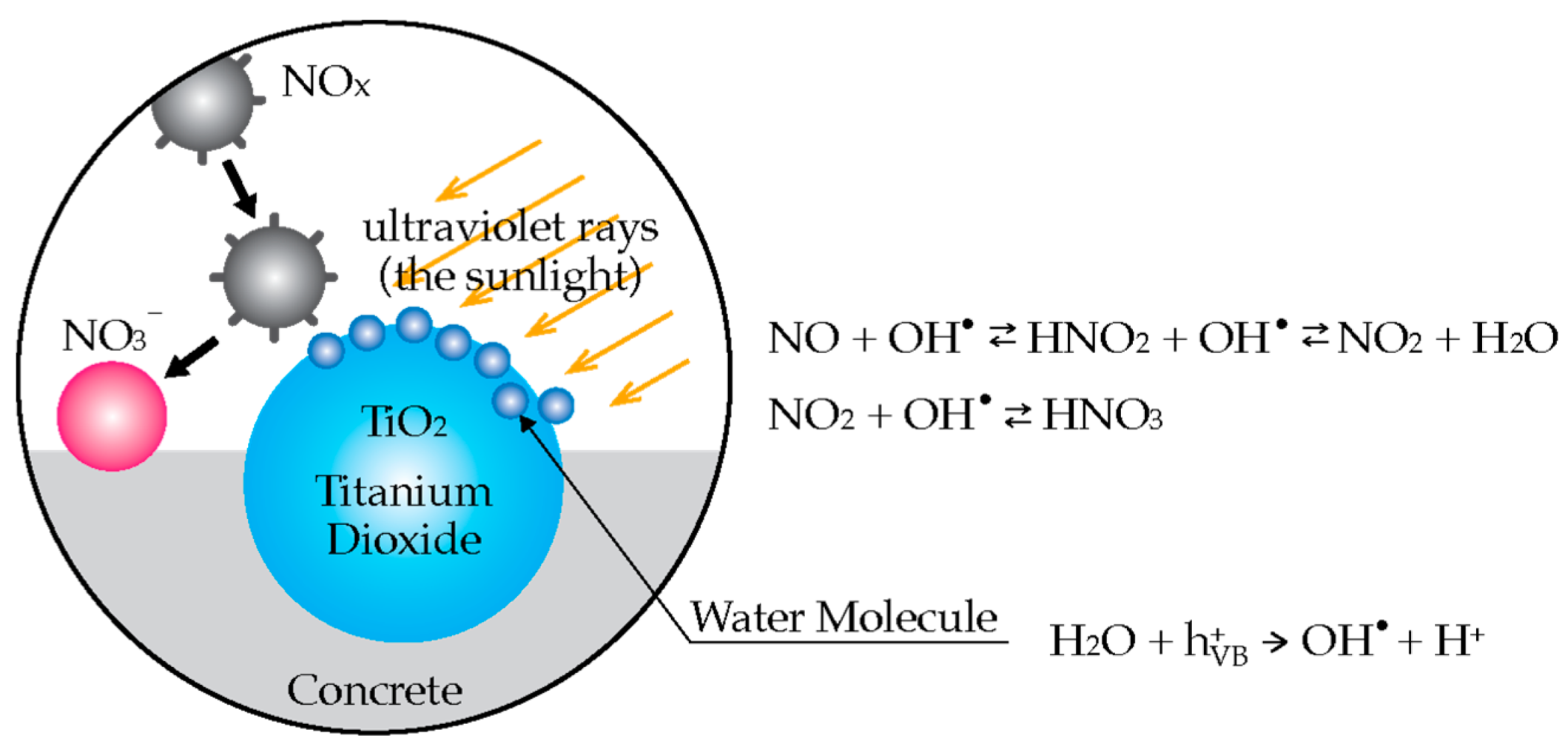
3.1. Air Purifying Cement-Based Materials
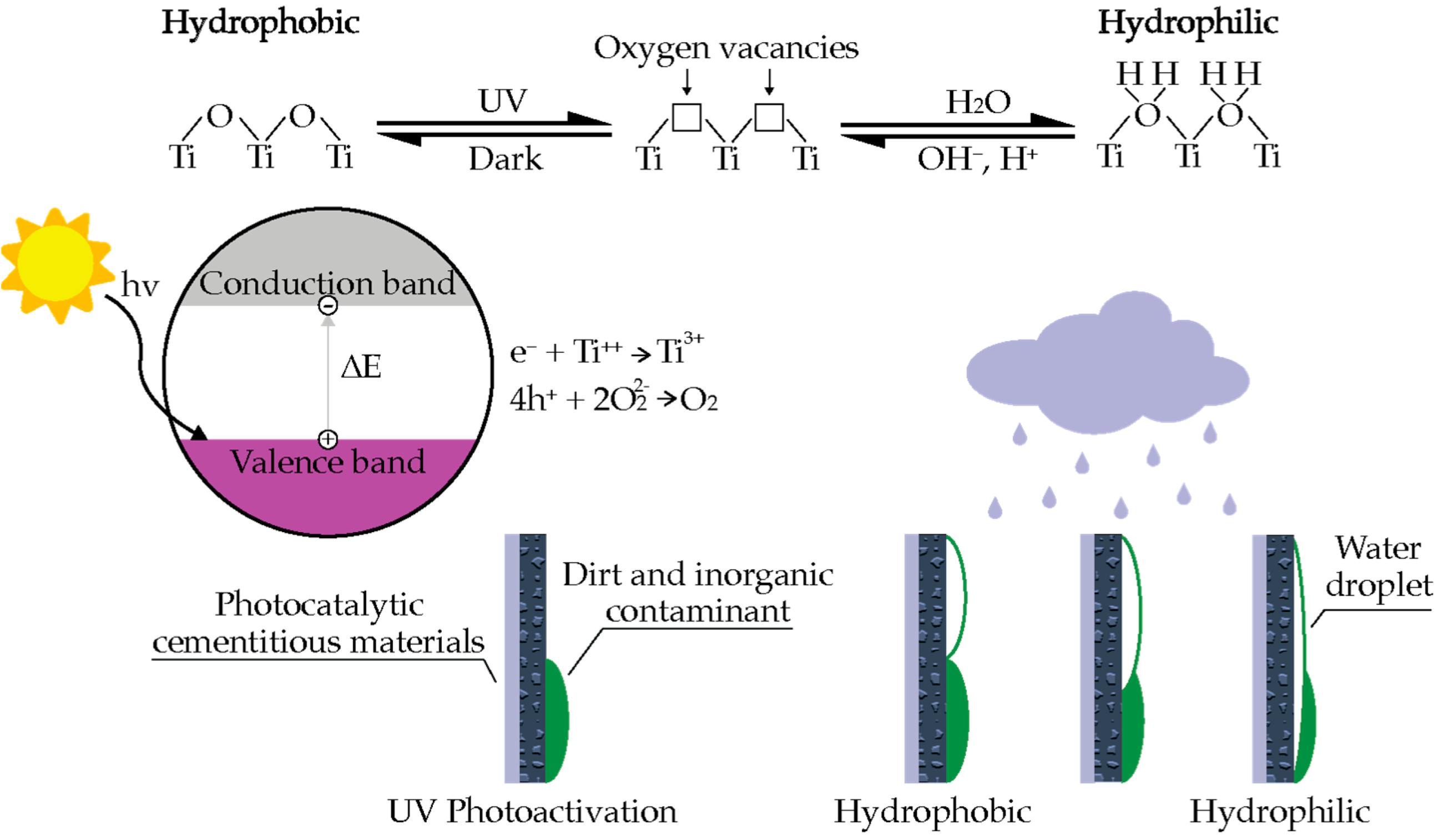
3.2. Self-Cleaning Cementitious Materials
3.3. Self-Sterilizing Cement-Based Coated Surfaces
4. Discussion
4.1. Influence of Using Cementitious Materials as Catalyst Supporting Media on Photocatalytic Activity
4.1.1. TiO2 Dispersion
4.1.2. Pore Structure
4.2. Effect of Incorporating TiO2 into Cement-Based Material
4.3. Photocatalyst Deactivation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. The Cost of a Polluted Environment: 1.7 Million Child Deaths a Year, Says WHO. 2017. Available online: https://www.who.int/news/item/06-03-2017-the-cost-of-a-polluted-environment-1-7-million-child-deaths-a-year-says-who#:~:text=year%2C%20says%20WHO-,The%20cost%20of%20a%20polluted%20environment%3A%201.7%20million,deaths%20a%20year%2C%20says%20WHO&text=More%20than%201%20in%204,are%20attributable%20to%20unhealthy%20environments (accessed on 5 February 2022).
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Simonsen, M.E. Chapter 4—Heterogeneous Photocatalysis. In Chemistry of Advanced Environmental Purification Processes of Water; Søgaard, E.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 135–170. [Google Scholar]
- Herrmann, J.-M.; Tahiri, H.; Ait-Ichou, Y.; Lassaletta, G.; González-Elipe, A.; Fernández, A. Characterization and photocatalytic activity in aqueous medium of TiO2 and Ag-TiO2 coatings on quartz. Appl. Catal. B Environ. 1997, 13, 219–228. [Google Scholar] [CrossRef]
- Pestana, C.J.; Edwards, C.; Prabhu, R.; Robertson, P.K.; Lawton, L. Photocatalytic degradation of eleven microcystin variants and nodularin by TiO2 coated glass microspheres. J. Hazard. Mater. 2015, 300, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Rachel, A.; Subrahmanyam, M.; Boule, P. Comparison of photocatalytic efficiencies of TiO2 in suspended and immobilised form for the photocatalytic degradation of nitrobenzenesulfonic acids. Appl. Catal. B Environ. 2002, 37, 301–308. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. Heterogeneous photocatalysis: From water photolysis to applications in environmental cleanup. Int. J. Hydrogen Energy 2007, 32, 2664–2672. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.-S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Bawono, A.A.; Tan, Z.H.; Hamdany, A.H.; NguyenDinh, N.; Qian, S.; Lechner, B.; Yang, E.-H. Bright and slip-proof engineered cementitious composites with visible light activated photo-catalysis property for pavement in tunnels. Cem. Concr. Compos. 2020, 114, 103788. [Google Scholar] [CrossRef]
- Hamdany, A.H.; Ding, Y.; Qian, S. Mechanical and antibacterial behavior of photocatalytic lightweight engineered cementitious composites. J. Mater. Civ. Eng. 2021, 33, 04021262. [Google Scholar] [CrossRef]
- Liu, J.; Jee, H.; Lim, M.; Kim, J.H.; Kwon, S.J.; Lee, K.M.; Nezhad, E.Z.; Bae, S. Photocatalytic performance evaluation of titanium dioxide nanotube-reinforced cement paste. Materials 2020, 13, 5423. [Google Scholar] [CrossRef]
- Strokova, V.; Gubareva, E.; Ogurtsova, Y.; Fediuk, R.; Zhao, P.; Vatin, N.; Vasilev, Y. Obtaining and properties of a photocatalytic composite material of the “SiO2–TiO2” system based on various types of silica raw materials. Nanomaterials 2021, 11, 866. [Google Scholar] [CrossRef]
- Cassar, L. Photocatalysis of cementitious materials: Clean buildings and clean air. MRS Bull. 2004, 29, 328–331. [Google Scholar] [CrossRef]
- Krishnan, P.; Zhang, M.-H.; Yu, L.; Feng, H. Photocatalytic degradation of particulate pollutants and self-cleaning performance of TiO2-containing silicate coating and mortar. Constr. Build. Mater. 2013, 44, 309–316. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Llorente, I.; Castellote, M. TiO2 cement-based materials: Understanding optical properties and electronic band structure of complex matrices. Catal. Today 2017, 287, 203–209. [Google Scholar] [CrossRef]
- Yang, L.; Wang, F.; Shu, C.; Liu, P.; Zhang, W.; Hu, S. TiO2/porous cementitious composites: Influences of porosities and TiO2 loading levels on photocatalytic degradation of gaseous benzene. Constr. Build. Mater. 2017, 150, 774–780. [Google Scholar] [CrossRef]
- Guo, M.Z.; Ling, T.C.; Poon, C.S. Nano-TiO2-based architectural mortar for NO removal and bacteria inactivation: Influence of coating and weathering conditions. Cem. Concr. Compos. 2013, 36, 102–109. [Google Scholar] [CrossRef]
- Singh, V.; Sandeep, K.; Kushwaha, H.; Powar, S.; Vaish, R. Photocatalytic, hydrophobic and antimicrobial characteristics of ZnO nano needle embedded cement composites. Constr. Build. Mater. 2018, 158, 285–294. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced electron transfer reactions for macromolecular syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef] [PubMed]
- Menzel, J.P.; De Groot, H.J.M.; Buda, F. Photoinduced electron transfer in donor–acceptor complexes: Isotope effect and dynamic symmetry breaking. J. Phys. Chem. Lett. 2019, 10, 6504–6511. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Kubacka, A.; Suarez-Diez, M.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Dos Santos, V.A.P.M.; Fernández-García, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [PubMed]
- Maury, A.; de Belie, N. State of the art of TiO2 containing cementitious materials: Self-cleaning properties. Mater. Constr. 2010, 60, 33–50. [Google Scholar] [CrossRef]
- Murata, Y. Air purifying pavement: Development of photocatalytic concrete blocks. J. Adv. Oxid. Technol. 1999, 4, 227–230. [Google Scholar]
- Miessler, G.L.; Fischer, P.J.; Tarr, D.A. Inorganic Chemistry, 5th ed.; Pearson: Boston, MA, USA, 2014. [Google Scholar]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Kamat, P.V. Photochemistry on nonreactive and reactive (semiconductor) surfaces. Chem. Rev. 1993, 93, 267–300. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Gratzel, M. Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 1995, 95, 49. [Google Scholar] [CrossRef]
- Evans, R.C.; Douglas, P.; Burrow, H.D. Applied Photochemistry [Electronic Resource]; Evans, R.C., Douglas, P., Burrow, H.D., Eds.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Kirk, R.E.; Othmer, D.F. Encyclopedia of Chemical Technology, 4th ed.; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Agrios, A.; Pichat, P. State of the art and perspectives on materials and applications of photocatalysis over TiO2. J. Appl. Electrochem. 2005, 35, 655–663. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Xiaobo, C.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891. [Google Scholar]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Analysis of electronic structures of 3d transition metal-doped TiO2 based on band calculations. J. Phys. Chem. Solids 2002, 63, 1909–1920. [Google Scholar] [CrossRef]
- Braslavsky, S.E. Glossary of terms used in photochemistry, 3rd edition (IUPAC Recommendations 2006). Pure Appl. Chem. 2007, 79, 293–465. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Hua, X.; Dahlgren, R.L.; Lappin, A.G.; Patterson, L.K.; Kamat, P.V. Photochemistry of Ru(bpy){sub 2}(dcbpy){sup 2+} on Al{sub 2}O{sub 3} and TiO{sub 2} surfaces. An insight into the mechanism of photosensitization. J. Phys. Chem. 1995, 99, 10883. [Google Scholar]
- Zhu, W.; Bartos, P.J.M.; Porro, A. Application of nanotechnology in construction. Mater. Struct. 2004, 37, 649–658. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Account/Revue: Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Ohama, Y.; van Gemert, D. Applications of Titanium Dioxide Photocatalysis to Construction Materials. [Electronic Resource]:State-of-the-Art Report of the RILEM Technical Committee 194-TDP. RILEM State of the Art Reports: 5; Springer Science+Business Media B.V.: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Guerrini, G.L. Photocatalytic performances in a city tunnel in Rome: NOx monitoring results. Constr. Build. Mater. 2012, 27, 165–175. [Google Scholar] [CrossRef]
- Strini, A.; Cassese, S.; Schiavi, L. Measurement of benzene, toluene, ethylbenzene and oxylene gas phase photodegradation by titanium dioxide dispersed in cementitious materials using a mixed flow reactor. Appl. Catal. B Environ. 2005, 61, 90–97. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Ling, T.-C.; Poon, C.S. Photocatalytic NOx degradation of concrete surface layers intermixed and spray-coated with nano-TiO2: Influence of experimental factors. Cem. Concr. Compos. 2017, 83, 279–289. [Google Scholar] [CrossRef]
- Lee, B.Y.; Jayapalan, A.R.; Bergin, M.H.; Kurtis, K.E. Photocatalytic cement exposed to nitrogen oxides: Effect of oxidation and binding. Cem. Concr. Res. 2014, 60, 30–36. [Google Scholar] [CrossRef]
- Martinez, T.; Bertron, A.; Escadeillas, G.; Ringot, E.; Simon, V. BTEX abatement by photocatalytic TiO2-bearing coatings applied to cement mortars. Build. Environ. 2014, 71, 186–192. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Balbuena, J.; Yusta, M.C.; Sanchez, L.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Photocatalytic NOx abatement by calcium aluminate cements modified with TiO2: Improved NO2 conversion. Cem. Concr. Res. 2015, 70, 67–76. [Google Scholar] [CrossRef]
- Poon, C.; Cheung, E. NO removal efficiency of photocatalytic paving blocks prepared with recycled materials. Constr. Build. Mater. 2007, 21, 1746–1753. [Google Scholar] [CrossRef]
- Ramirez, A.M.; Demeestere, K.; De Belie, N.; Mäntylä, T.; Levänen, E. Titanium dioxide coated cementitious materials for air purifying purposes: Preparation, characterization and toluene removal potential. Build. Environ. 2010, 45, 832–838. [Google Scholar] [CrossRef]
- Sugrañez, R.; Álvarez, J.; Cruz-Yusta, M.; Mármol, I.; Morales, J.; Vila, J.; Sánchez, L. Enhanced photocatalytic degradation of NOx gases by regulating the microstructure of mortar cement modified with titanium dioxide. Build. Environ. 2013, 69, 55–63. [Google Scholar] [CrossRef]
- Devahasdin, S.; Fan, C.; Li, K.; Chen, D.H. TiO2 photocatalytic oxidation of nitric oxide: Transient behavior and reaction kinetics. J. Photochem. Photobiol. A Chem. 2003, 156, 161–170. [Google Scholar] [CrossRef]
- Mills, A.; Hill, C.; Robertson, P.K. Overview of the current ISO tests for photocatalytic materials. J. Photochem. Photobiol. A Chem. 2012, 237, 7–23. [Google Scholar] [CrossRef]
- Jayapalan, A.R.; Lee, B.Y.; Land, E.M.; Bergin, M.H.; Kurtis, K.E. Photocatalytic efficiency of cement-based materials: Demonstration of proposed test method. ACI Mater. J. 2015, 112, 219. [Google Scholar] [CrossRef]
- Giannantonio, D.J.; Kurth, J.C.; Kurtis, K.E.; Sobecky, P.A. Effects of concrete properties and nutrients on fungal colonization and fouling. Int. Biodeterior. Biodegrad. 2009, 63, 252–259. [Google Scholar] [CrossRef]
- Gu, J.-D.; Ford, T.E.; Berke, N.S.; Mitchell, R. Biodeterioration of concrete by the fungus Fusarium. Int. Biodeterior. Biodegrad. 1998, 41, 101–109. [Google Scholar] [CrossRef]
- Parker, C.D. The corrosion of concrete. Aust. J. Exp. Biol. Med. Sci. 1945, 23, 81. [Google Scholar] [CrossRef]
- Fonseca, A.J.; Pina, F.; Macedo, M.F.; Leal, N.; Romanowska-Deskins, A.; Laiz, L.; Gómez-Bolea, A.; Saiz-Jimenez, C. Anatase as an alternative application for preventing biodeterioration of mortars: Evaluation and comparison with other biocides. Int. Biodeterior. Biodegrad. 2010, 64, 388–396. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Guan, K. Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of TiO2/SiO2 films. Surf. Coat. Technol. 2005, 191, 155–160. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Kazuhito, H.; Hiroshi, I.; Akira, F. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Br. Commun. Rev. Pap. JJAP 2005, 44, 8269. [Google Scholar]
- Carré, G.; Hamon, E.; Ennahar, S.; Estner, M.; Lett, M.-C.; Horvatovich, P.; Gies, J.-P.; Keller, V.; Keller, N.; Andre, P. TiO2 photocatalysis damages lipids and proteins in Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Huang, Z.; Maness, P.-C.; Blake, D.M.; Wolfrum, E.J.; Smolinski, S.L.; Jacoby, W.A. Bactericidal mode of titanium dioxide photocatalysis. J. Photochem. Photobiol. A: Chem. 2000, 130, 163–170. [Google Scholar] [CrossRef]
- Kiwi, J.; Nadtochenko, V. Evidence for the mechanism of photocatalytic degradation of the bacterial wall membrane at the TiO2 interface by ATR-FTIR and laser kinetic spectroscopy. Langmuir 2005, 21, 4631–4641. [Google Scholar] [CrossRef]
- Lower, B.H.; Yongsunthon, R.; Shi, L.; Wildling, L.; Gruber, H.J.; Wigginton, N.S.; Reardon, C.L.; Pinchuk, G.E.; Droubay, T.C.; Boily, J.-F.; et al. Antibody recognition force microscopy shows that outer membrane cytochromes OmcA and MtrC are expressed on the exterior surface of Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 2009, 75, 2931–2935. [Google Scholar] [CrossRef]
- Reardon, C.L.; Dohnalkova, A.C.; Nachimuthu, P.; Kennedy, D.; Saffarini, D.A.; Arey, B.W.; Shi, L.; Wang, Z.; Moore, D.; McLean, J.; et al. Role of outer-membrane cytochromes MtrC and OmcA in the biomineralization of ferrihydrite by Shewanella oneidensis MR-1. Geobiology 2010, 8, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.; Wang, X.; Marshall, M.J.; Zachara, J.M.; Rosso, K.M.; Dupuis, M.; Fredrickson, J.K.; Heald, S.; Shi, L. Kinetics of reduction of Fe(III) complexes by outer membrane cytochromes MtrC and OmcA of Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 2008, 74, 6746–6755. [Google Scholar] [CrossRef] [PubMed]
- Jani, R.; Colberg, P.; Eggleston, C.; Shi, L.; Reardon, C. Microbial fuel cell study of the role of OmcA and MtrC in electron transfer from Shewanella oneidensis to oxide electrodes. Geochim. Cosmochim. Acta 2010, 74, A457. [Google Scholar]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Wagner, V.E.; Iglewski, B.H. P. aeruginosa biofilms in CF infection. Clin. Rev. Allergy Immunol. 2008, 35, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Haagensen, J.A.J.; Jelsbak, L.; Johansen, H.K.; Sternberg, C.; Høiby, N.; Molin, S. In situ growth rates and biofilm development of Pseudomonas aeruginosa populations in chronic lung infections. J. Bacteriol. 2008, 190, 2767–2776. [Google Scholar] [CrossRef]
- Maury-Ramirez, A.; De Muynck, W.; Stevens, R.; Demeestere, K.; De Belie, N. Titanium dioxide based strategies to prevent algal fouling on cementitious materials. Cem. Concr. Compos. 2013, 36, 93–100. [Google Scholar] [CrossRef]
- Sikora, P.; Cendrowski, K.; Markowska-Szczupak, A.; Horszczaruk, E.; Mijowska, E. The effects of silica/titania nanocomposite on the mechanical and bactericidal properties of cement mortars. Constr. Build. Mater. 2017, 150, 738–746. [Google Scholar] [CrossRef]
- Folli, A.; Pochard, I.; Nonat, A.; Jakobsen, U.H.; Shepherd, A.M.; Macphee, D.E. Engineering photocatalytic cements: Understanding TiO2 surface chemistry to control and modulate photocatalytic performances. J. Am. Ceram. Soc. 2010, 93, 3360–3369. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdorster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. Interdiscip. Forum Nanoscale Sci. Technol. 2009, 11, 77. [Google Scholar] [CrossRef]
- Yousefi, A.; Allahverdi, A.; Hejazi, P. Effective dispersion of nano-TiO2 powder for enhancement of photocatalytic properties in cement mixes. Constr. Build. Mater. 2013, 41, 224–230. [Google Scholar] [CrossRef]
- Lackhoff, M.; Prieto, X.; Nestle, N.; Dehn, F.; Niessner, R. Photocatalytic activity of semiconductor-modified cement—Influence of semiconductor type and cement ageing. Appl. Catal. B Environ. 2003, 43, 205–216. [Google Scholar] [CrossRef]
- Lucas, S.; Ferreira, V.; de Aguiar, J.B. Incorporation of titanium dioxide nanoparticles in mortars—Influence of microstructure in the hardened state properties and photocatalytic activity. Cem. Concr. Res. 2013, 43, 112–120. [Google Scholar] [CrossRef]
- Gallus, M.; Akylas, V.; Barmpas, F.; Beeldens, A.; Boonen, E.; Boréave, A.; Cazaunau, M.; Chen, H.; Daële, V.; Doussin, J.; et al. Photocatalytic de-pollution in the Leopold II tunnel in Brussels: NOx abatement results. Build. Environ. 2015, 84, 125–133. [Google Scholar] [CrossRef]
- Arandigoyen, M.; Bicer-Simsir, B.; Alvarez, J.; Lange, D. Variation of microstructure with carbonation in lime and blended pastes. Appl. Surf. Sci. 2006, 252, 7562–7571. [Google Scholar] [CrossRef] [Green Version]
- Johannesson, B.; Utgenannt, P. Microstructural changes caused by carbonation of cement mortar. Cem. Concr. Res. 2001, 31, 925–931. [Google Scholar] [CrossRef]
- Dias, W.P.S. Reduction of concrete sorptivity with age through carbonation. Cem. Concr. Res. 2000, 30, 1255–1261. [Google Scholar] [CrossRef]
- Jun, C.; Chi-Sun, P. Photocatalytic cementitious materials: Influence of the microstructure of cement paste on photocatalytic pollution degradation. Environ. Sci. Technol. 2009, 43, 8948–8952. [Google Scholar]
- Torgal, F.P. Nanotechnology in Eco-Efficient Construction. Woodhead Publishing in Materials; Woodhead Publishing: Cambridge, UK; Philadelphia, PA, USA, 2013. [Google Scholar]
- Bo Yeon, L.; Kurtis, K.E. Influence of TiO2 Nanoparticles on Early C3S Hydration. J. Am. Ceram. Soc. 2010, 93, 3399–3405. [Google Scholar]
- Gutteridge, W.A.; Dalziel, J.A. Filler cement: The effect of the secondary component on the hydration of Portland cement. Cem. Concr. Res. 1990, 20, 778–782. [Google Scholar] [CrossRef]
- Sauer, M.L.; Ollis, D.F. Catalyst deactivation in gas-solid photocatalysis. J. Catal. 1996, 163, 215–217. [Google Scholar] [CrossRef]
- Mendez-Roman, R.; Cardona-Martínez, N. Relationship between the formation of surface species and catalyst deactivation during the gas-phase photocatalytic oxidation of toluene. Catal. Today 1998, 40, 353–365. [Google Scholar] [CrossRef]
- Ibusuki, T.; Takeuchi, K. Toluene oxidation on u.v.-irradiated titanium dioxide with and without O2, NO2 OR H2O at ambient temperature. Atmospheric Environ. 1986, 20, 1711–1715. [Google Scholar] [CrossRef]
- Obee, T.N.; Hay, S.O. Effects of moisture and temperature on the photooxidation of ethylene on titania. Environ. Sci. Technol. 1997, 31, 2034–2038. [Google Scholar] [CrossRef]
- Akhter, P.; Hussain, M.; Saracco, G.; Russo, N. Novel nanostructured-TiO2 materials for the photocatalytic reduction of CO2 greenhouse gas to hydrocarbons and syngas. Fuel 2015, 149, 55–65. [Google Scholar] [CrossRef]
- Kamali, S.; Gérard, B.; Moranville, M. Modelling the leaching kinetics of cement-based materials—Influence of materials and environment. Cem. Concr. Compos. 2003, 25, 451–458. [Google Scholar] [CrossRef]
- Kamali, S.; Moranville, M.; Leclercq, S. Material and environmental parameter effects on the leaching of cement pastes: Experiments and modelling. Cem. Concr. Res. 2008, 38, 575–585. [Google Scholar] [CrossRef]
- Kamali-Bernard, S.; Bernard, F.; Prince, W. Computer modelling of tritiated water diffusion test for cement based materials. Comput. Mater. Sci. 2009, 45, 528–535. [Google Scholar] [CrossRef]
- Bernard, F.; Fu, J.; Kamali-Bernard, S. Multiscale modelling approach to determine the specific heat of cementitious materials. Eur. J. Environ. Civ. Eng. 2019, 23, 535–551. [Google Scholar] [CrossRef]
- Shahzamanian, M.M.; Tadepalli, T.; Rajendran, A.M.; Hodo, W.D.; Mohan, R.; Valisetty, R.; Chung, P.W.; Ramsey, J.J. Representative volume element based modeling of cementitious materials. J. Eng. Mater. Technol. 2013, 136, 011007. [Google Scholar] [CrossRef]
- Zhai, Q.; Ye, W.; Rahardjo, H.; Satyanaga, A.; Dai, G.; Zhao, X. Theoretical method for the estimation of vapor conductivity for unsaturated soil. Eng. Geol. 2021, 295, 106447. [Google Scholar] [CrossRef]
- Zhai, Q.; Rahardjo, H.; Satyanaga, A.; Dai, G. Estimation of tensile strength of sandy soil from soil-water characteristic curve. Acta Geotech. 2020, 15, 3371–3381. [Google Scholar] [CrossRef]
- Zhai, Q.; Rahardjo, H.; Satyanaga, A. Estimation of the air permeability function from the Soil-Water Characteristic Curve. Can. Geotech. J. 2019, 56, 505–513. [Google Scholar] [CrossRef]
- Zhai, Q.; Rahardjo, H.; Satyanaga, A.; Priono; Dai, G. Role of the pore-size distribution function on water flow in unsaturated soil. J. Zhejiang Univ. Sci. A 2019, 20, 10–20. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdany, A.H.; Satyanaga, A.; Zhang, D.; Kim, Y.; Kim, J.R. Photocatalytic Cementitious Material for Eco-Efficient Construction—A Systematic Literature Review. Appl. Sci. 2022, 12, 8741. https://doi.org/10.3390/app12178741
Hamdany AH, Satyanaga A, Zhang D, Kim Y, Kim JR. Photocatalytic Cementitious Material for Eco-Efficient Construction—A Systematic Literature Review. Applied Sciences. 2022; 12(17):8741. https://doi.org/10.3390/app12178741
Chicago/Turabian StyleHamdany, Abdul Halim, Alfrendo Satyanaga, Dichuan Zhang, Yongmin Kim, and Jong R. Kim. 2022. "Photocatalytic Cementitious Material for Eco-Efficient Construction—A Systematic Literature Review" Applied Sciences 12, no. 17: 8741. https://doi.org/10.3390/app12178741





