Therapeutic Potential of Myrrh, a Natural Resin, in Health Management through Modulation of Oxidative Stress, Inflammation, and Advanced Glycation End Products Formation Using In Vitro and In Silico Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental
2.2.1. Myrrh Methanolic Extract Preparation
2.2.2. Phytochemical Screening
2.2.3. Polyphenol Content Evaluation
2.2.4. Total Flavonoid Content
2.2.5. Reference Drug Preparation
2.2.6. Assessment of Reduction Capacity
2.2.7. Hydrogen Peroxide Scavenging (H2O2) Activity
2.2.8. DPPH Assay
2.2.9. Inhibition Potential of Myrrh Extract against Heat Induced Albumin Denaturation
2.2.10. Test for Proteinase Action Inhibition
2.2.11. Egg Albumin Denaturation Inhibition for Anti-Arthritic Activity
2.2.12. Screening for Antiglycation Property of Myrrh Extracts
- Incubation of Myrrh extracts with in vitro glycation system
- b.
- Effect of Myrrh extracts on browning in glycated samples
- c.
- Effect of Myrrh extract on protein aggregation index
- d.
- Determination of fibrillar state by Congo red assay
2.2.13. Biophysical Investigations
- a.
- UV Absorption
- b.
- AGEs-specific fluorescence study
- c.
- Thioflavin T-specific fluorescence study
2.2.14. Statistical Analysis
2.2.15. Docking Studies
- a.
- The Receptors
- b.
- The Ligands
- c.
- Molecular Docking
- d.
- Validation of Docking Study
3. Results
3.1. Preliminary Observations, Flavonoid, and Phenolic Content
3.2. H2O2 Reducing Ability
3.3. Reducing Power Estimation by FRAP Assay
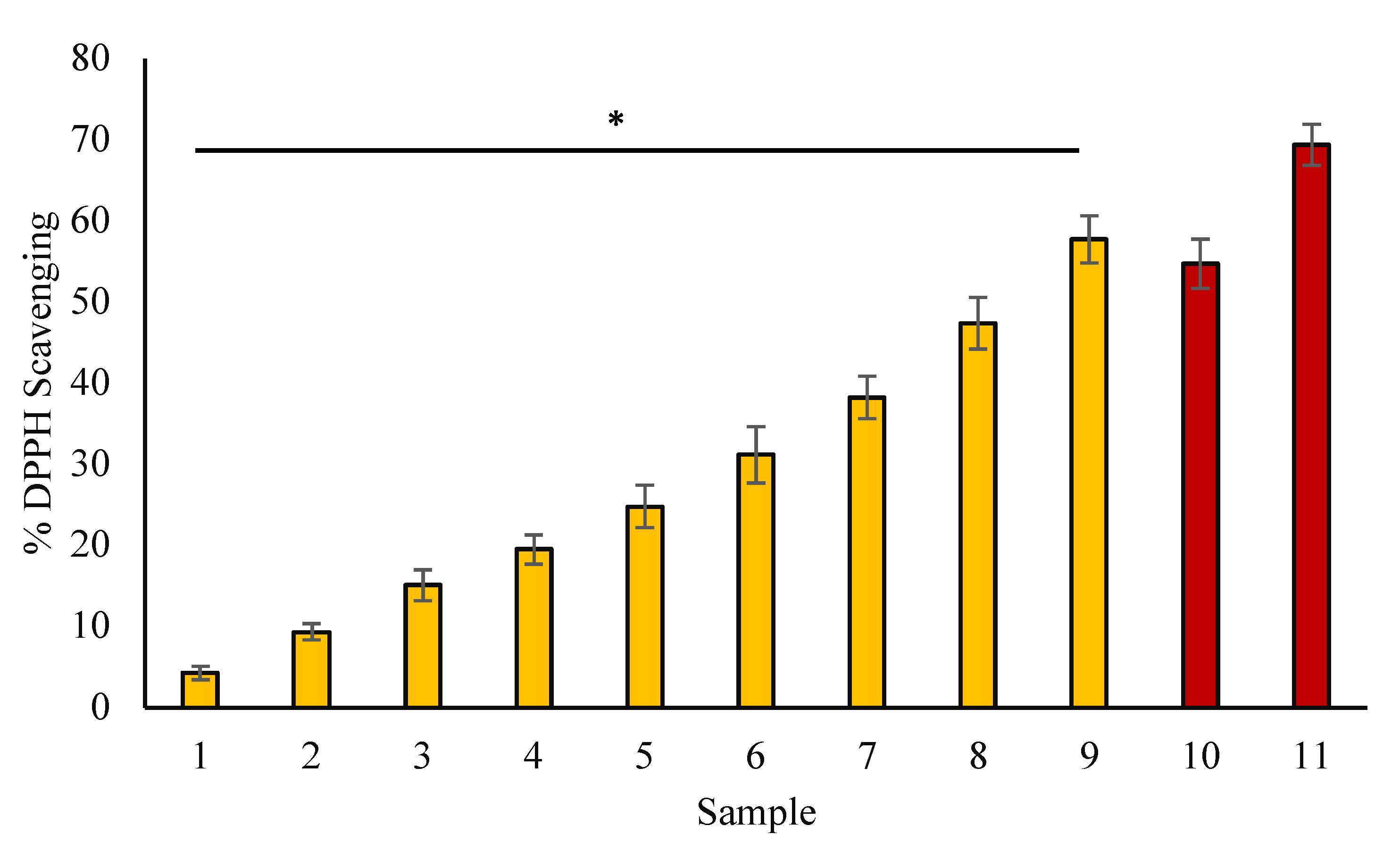
3.4. DPPH Assay
3.5. Albumin Denaturation Inhibition
3.6. Inhibition of Proteinase Action
3.7. Inhibition of Egg Albumin Denaturation
3.8. Effect of Myrrh Extract on Browning Intensity in Glycated Samples
3.9. Effect of Myrrh Extract on Protein Aggregation Index
3.10. Congo Red Test
3.11. UV-Absorption Investigations
3.12. Effects of Extract on Fluorescent AGE Formation-Antiglycating Property
3.13. Effect of Myrrh Extract against Glycation Induced Fibrils Formation
3.14. Investigation of Ligand-Receptor Interaction Using Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yalcin Kehribar, D.; Cihangiroglu, M.; Sehmen, E.; Avci, B.; Capraz, A.; Yildirim Bilgin, A.; Gunaydin, C.; Ozgen, M. The receptor for advanced glycation end product (RAGE) pathway in COVID-19. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2021, 26, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Younus, H.; Anwar, S. Prevention of non-enzymatic glycosylation (glycation): Implication in the treatment of diabetic complication. Int. J. Health Sci. 2016, 10, 261–277. [Google Scholar] [CrossRef]
- Sartore, G.; Ragazzi, E.; Faccin, L.; Lapolla, A. A role of glycation and methylation for SARS-CoV-2 infection in diabetes? Med. Hypotheses 2020, 144, 110247. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Rodriguez, J.R.; Garza-Veloz, I.; Flores-Morales, V.; Badillo-Almaraz, J.I.; Rocha-Pizaña, M.R.; Valdés-Aguayo, J.J.; Martinez-Fierro, M.L. Hyperglycemia and Angiotensin-Converting Enzyme 2 in Pulmonary Function in the Context of SARS-CoV-2 Infection. Front. Med. 2022, 8, 758414. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Raut, R.; Alsahli, M.A.; Almatroudi, A.; Alfheeaid, H.; Alzahrani, F.M.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Role of Ajwa Date Fruit Pulp and Seed in the Management of Diseases through In Vitro and In Silico Analysis. Biology 2022, 11, 78. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroudi, A.; Allemailem, K.S.; Jacob Joseph, R.; Khan, A.A.; Rahmani, A.H. Protective Effects of Ginger Extract against Glycation and Oxidative Stress-Induced Health Complications: An In Vitro Study. Processes 2020, 8, 468. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, S.; Almatroudi, A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. A review on mechanism of inhibition of advanced glycation end products formation by plant derived polyphenolic compounds. Mol. Biol. Rep. 2021, 48, 787–805. [Google Scholar] [CrossRef]
- Ansari, N.A.; Rasheed, Z. Non-enzymatic glycation of proteins: From diabetes to cancer. Biomeditsinskaia Khimiia 2010, 56, 168–178. [Google Scholar] [CrossRef]
- Alsahli, M.A.; Anwar, S.; Alzahrani, F.M.; Almatroudi, A.; Alfheeaid, H.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Health Promoting Effect of Phyllanthus emblica and Azadiractha indica against Advanced Glycation End Products Formation. Appl. Sci. 2021, 11, 8819. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Meenatchi, P.; Purushothaman, A.; Maneemegalai, S. Antioxidant, antiglycation and insulinotrophic properties of Coccinia grandis (L.) in vitro: Possible role in prevention of diabetic complications. J. Tradit. Complement. Med. 2016, 7, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, C.; Ge, Z.; Liu, Y.; Liu, Y.; Feng, W.; Li, S.; Chen, G.; Wei, T. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol. Lett. 2013, 6, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.A.A. Efficacy of Commiphora myrrh mouthwash on early wound healing after tooth extraction: A randomized controlled trial. Saudi Dent. J. 2021, 33, 44–54. [Google Scholar] [CrossRef]
- Sotoudeh, R.; Hadjzadeh, M.-A.-R.; Gholamnezhad, Z.; Aghaei, A. The anti-diabetic and antioxidant effects of a combination of Commiphora mukul, Commiphora myrrha and Terminalia chebula in diabetic rats. Avicenna J. Phytomed. 2019, 9, 454–464. [Google Scholar] [PubMed]
- Shalaby, M.A.; Hammouda, A.A.-E. Analgesic, anti-inflammatory and anti-hyperlipidemic activities of Commiphora molmol extract (Myrrh). J. Intercult. Ethnopharmacol. 2014, 3, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Nohr, L.A.; Rasmussen, L.B.; Straand, J. Resin from the mukul myrrh tree, guggul, can it be used for treating hypercholesterolemia? A randomized, controlled study. Complement. Ther. Med. 2009, 17, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Almatroudi, A.; Anwar, S.; Yousif Babiker, A.; Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Antioxidant, anti-inflammatory and hepatoprotective effects of olive fruit pulp extract: In vivo and in vitro study. J. Taibah Univ. Sci. 2020, 14, 1660–1670. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroodi, S.A.; Almatroudi, A.; Allemailem, K.S.; Joseph, R.J.; Khan, A.A.; Alrumaihi, F.; Alsahli, M.A.; Husain Rahmani, A. Biosynthesis of silver nanoparticles using Tamarix articulata leaf extract: An effective approach for attenuation of oxidative stress mediated diseases. Int. J. Food Prop. 2021, 24, 677–701. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chandra, S.; Dey, P.; Bhattacharya, S. Evaluation of anti-inflammatory effects of green tea and black tea: A comparative in vitro study. J. Adv. Pharm. Technol. Res. 2012, 3, 136–138. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Anwar, S.; Almatroudi, A.; Khan, A.A.; Alrumaihi, F.; Alsahli, M.A.; Rahmani, A.H. Hepatoprotective Effects of Garlic Extract against Carbon Tetrachloride (CCl4)-Induced Liver Injury via Modulation of Antioxidant, Anti-Inflammatory Activities and Hepatocyte Architecture. Appl. Sci. 2020, 10, 6200. [Google Scholar] [CrossRef]
- Sakat, S.; Juvekar, A.; Gambhire, M. In vitro antioxidant and anti inflammatory activity of methanol extract of Oxalis corniculata Linn. Chemistry 2010, 2, 146–155. [Google Scholar] [CrossRef]
- Brownlee, M.; Vlassara, H.; Kooney, A.; Ulrich, P.; Cerami, A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science 1986, 232, 1629–1632. [Google Scholar] [CrossRef]
- Schrodel, A.; de Marco, A. Characterization of the aggregates formed during recombinant protein expression in bacteria. BMC Biochem. 2005, 6, 10. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, M.A.; Sadaf, A.; Younus, H. A structural study on the protection of glycation of superoxide dismutase by thymoquinone. Int. J. Biol. Macromol. 2014, 69, 476–481. [Google Scholar] [CrossRef]
- Anwar, S.; Younus, H. Anti-glycating potential of ellagic acid against glucose and methylglyoxal-induced glycation of superoxide dismutase. J. Proteins Proteom. 2017, 8, 1–12. [Google Scholar]
- Anwar, S.; Younus, H. Inhibitory effect of alliin from Allium sativum on the glycation of superoxide dismutase. Int. J. Biol. Macromol. 2017, 103, 182–193. [Google Scholar] [CrossRef]
- Dar, A.M.; Mir, S. Molecular Docking: Approaches, Types, Applications and Basic Challenges. J. Anal. Bioanal. Tech. 2017, 8, 356. [Google Scholar] [CrossRef]
- Schröter, D.; Höhn, A. Role of Advanced Glycation End Products in Carcinogenesis and their Therapeutic Implications. Curr. Pharm. Des. 2018, 24, 5245–5251. [Google Scholar] [CrossRef]
- Putnam, C.D.; Arvai, A.S.; Bourne, Y.; Tainer, J.A. Active and inhibited human catalase structures: Ligand and NADPH binding and catalytic mechanism11Edited by R. Huber. J. Mol. Biol. 2000, 296, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Manjula, R.; Wright, G.S.A.; Strange, R.W.; Padmanabhan, B. Assessment of ligand binding at a site relevant to SOD1 oxidation and aggregation. FEBS Lett. 2018, 592, 1725–1737. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, S.R.; Al-Ghadeer, A.R.; Ali, R.; Qamar, W.; Aljarboa, S. Analysis of inorganic and organic constituents of myrrh resin by GC–MS and ICP-MS: An emphasis on medicinal assets. Saudi Pharm. J. 2017, 25, 788–794. [Google Scholar] [CrossRef]
- Sunghwan, K.; Chen, j.; Cheng, T.; Gindulyte, A.; Jia, H. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.N.; Ramakrishnan, C.; Sasisekharan, V. Stereochemistry of polypeptide chain configurations. J. Mol. Biol. 1963, 7, 95–99. [Google Scholar] [CrossRef]
- Brochot, A.; Guilbot, A.; Haddioui, L.; Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. MicrobiologyOpen 2017, 6, e00459. [Google Scholar] [CrossRef] [PubMed]
- Madia, V.N.; De Angelis, M.; De Vita, D.; Messore, A.; De Leo, A.; Ialongo, D.; Tudino, V.; Saccoliti, F.; De Chiara, G.; Garzoli, S.; et al. Investigation of Commiphora myrrha (Nees) Engl. Oil and Its Main Components for Antiviral Activity. Pharmaceuticals 2021, 14, 243. [Google Scholar] [CrossRef]
- Messina, F.; Gigliarelli, G.; Palmier, A.; Marcotullio, M.C. Furanodienone: An Emerging Bioactive Furanosesquiterpenoid. Curr. Org. Chem. 2017, 21, 305–310. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroudi, A.; Alsahli, M.A.; Khan, M.A.; Khan, A.A.; Rahmani, A.H. Natural Products: Implication in Cancer Prevention and Treatment through Modulating Various Biological Activities. Anticancer Agents Med. Chem. 2020, 20, 2025–2040. [Google Scholar] [CrossRef]
- Hanuka Katz, I.; Eran Nagar, E.; Okun, Z.; Shpigelman, A. The Link between Polyphenol Structure, Antioxidant Capacity and Shelf-Life Stability in the Presence of Fructose and Ascorbic Acid. Molecules 2020, 25, 225. [Google Scholar] [CrossRef]
- Sirangelo, I.; Iannuzzi, C. Understanding the Role of Protein Glycation in the Amyloid Aggregation Process. Int. J. Mol. Sci. 2021, 22, 6609. [Google Scholar] [CrossRef]
- Chu, Y.-F.; Sun, J.; Wu, X.; Liu, R.H. Antioxidant and Antiproliferative Activities of Common Vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef]
- Derouiche, S. Oxidative Stress Associated with SARS-Cov-2 (COVID-19) Increases the Severity of the Lung Disease—A Systematic Review. J. Infect. Dis. Epidemiol. 2020, 6. [Google Scholar] [CrossRef]
- Schreck, R.; Albermann, K.; Baeuerle, P.A. Nuclear Factor Kb: An Oxidative Stress-Responsive Transcription Factor of Eukaryotic Cells (A Review). Free. Radic. Res. Commun. 1992, 17, 221–237. [Google Scholar] [CrossRef]
- Pourahmad, J.; Salimi, A.; Seydi, E. Role of Oxygen Free Radicals in Cancer Development and Treatment. In Free Radicals and Diseases; Ahmad, R., Ed.; IntechOpen: Vienna, Austria, 2016. [Google Scholar] [CrossRef]
- Williams, L.A.D.; O’Connar, A.; Latore, L.; Dennis, O.; Ringer, S.; Whittaker, J.A.; Conrad, J.; Vogler, B.; Rosner, H.; Kraus, W. The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process. West Indian Med. J. 2008, 57, 327–331. [Google Scholar]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Brown, J.H.; Mackey, H.K. Inhibition of Heat-Induced Denaturation of Serum Proteins by Mixtures of Nonsteroidal Anti-Inflammatory Agents and Amino Acids. Proc. Soc. Exp. Biol. Med. 1968, 128, 225–228. [Google Scholar] [CrossRef]
- Turner, D.P. Advanced glycation end-products: A biological consequence of lifestyle contributing to cancer disparity. Cancer Res. 2015, 75, 1925–1929. [Google Scholar] [CrossRef]
- Liu, R.H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]















| Phytochemical Constituents | Resin |
|---|---|
| Alkaloids | + |
| Saponins | - |
| Tannins | + |
| Flavonoids | + |
| Glycosides | + |
| Terpenoids | + |
| Phenolic compounds | + |
| 1. Catalase (1DGH) | |||
|---|---|---|---|
| Ligands | PubChem CID | Binding Energy (kcal/mol) | Interacting Amino Acids |
| 2,3-Furandione | 13586830 | −5.1 | Phe334, Arg365, Arg72, Arg112 |
| Curzerene | 5316217 | −7.7 | Phe334, His75, Gly147, Ash148, Ala133, Arg354, Val146, Tyr358 |
| delta-Elemene | 12309449 | −6.9 | Val146, His75, Tyr358 |
| Furanoeudesma-1,3-Diene | 643237 | −9.0 | His75, Ala133, Val146, Val74, Tyr358, Arg72, Arg112, Phe334, Val73, Thr361, Ser114, Gly131, Phe132, Gly147, Asn148, Arg354 |
| Positive Control: NADPH | 5884 | −10.2 | Gly216, Arg354, Asp65, Tyr358, His75, Gly147, Ser114, Ala133, Arg72, Ile332, Arg112, Arg365, Phe161, Val146, Arg148, His218, Phe153, Leu299 |
| 2. Superoxide dismutase (5YTU) | |||
| 2,3-Furandione | 13586830 | −3.4 | Asn65, His63 |
| Curzerene | 5316217 | −2.5 | Leu42, Asn86, Thr88 |
| delta-Elemene | 12309449 | −4.2 | Leu144, Glu121, His43, Thr39, Val14, Pro13, Gly12 |
| Furanoeudesma-1,3-Diene | 643237 | −5.0 | Asp11, Cys57, Leu144, Pro13, Val14, Gly12, Arg143, Gly10 |
| Positive Control: Levisoprenaline | 443372 | −5.7 | His63, Lys136, Lys70, Arg69, Asn65, Pro62 |
| 3. Spike Protein S (6VXX) | |||
|---|---|---|---|
| Ligands | PubChem CID | Binding Energy (kcal/mol) | Interacting Amino Acids |
| 2,3-Furandione | 13586830 | −4.9 | Ile870, Gly1059, His1058, Ala1056, Phe782, Val729, Thr778, Ser730, Pro863 |
| Curzerene | 5316217 | −6.0 | Leu1024, Arg1039, Phe1042, Thr1027, Asn1023 |
| delta-Elemene | 12309449 | −5.8 | Gln1010, Arg1014, Ile1013, Glu1017, Gln954, Ile1012, Arg1019, Ala1015, Glu773, Ile770, Gly769, Ala766, Arg765 |
| Furanoeudesma-1,3-Diene | 643237 | −6.7 | Ala1026, Glu780, Gln784, Ser1030, Glu1031, Thr1027, Asp1041, Val1040, Arg1039, Phe1042, Leu1024, Lys1028 |
| Positive control: Acetylglucosamine | 24139 | −6.5 | Arg1014, Arg765, Gln762 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmani, A.H.; Anwar, S.; Raut, R.; Almatroudi, A.; Babiker, A.Y.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A. Therapeutic Potential of Myrrh, a Natural Resin, in Health Management through Modulation of Oxidative Stress, Inflammation, and Advanced Glycation End Products Formation Using In Vitro and In Silico Analysis. Appl. Sci. 2022, 12, 9175. https://doi.org/10.3390/app12189175
Rahmani AH, Anwar S, Raut R, Almatroudi A, Babiker AY, Khan AA, Alsahli MA, Almatroodi SA. Therapeutic Potential of Myrrh, a Natural Resin, in Health Management through Modulation of Oxidative Stress, Inflammation, and Advanced Glycation End Products Formation Using In Vitro and In Silico Analysis. Applied Sciences. 2022; 12(18):9175. https://doi.org/10.3390/app12189175
Chicago/Turabian StyleRahmani, Arshad Husain, Shehwaz Anwar, Ravindra Raut, Ahmad Almatroudi, Ali Yousif Babiker, Amjad Ali Khan, Mohammed A. Alsahli, and Saleh A. Almatroodi. 2022. "Therapeutic Potential of Myrrh, a Natural Resin, in Health Management through Modulation of Oxidative Stress, Inflammation, and Advanced Glycation End Products Formation Using In Vitro and In Silico Analysis" Applied Sciences 12, no. 18: 9175. https://doi.org/10.3390/app12189175








