Helichrysum arenarium: From Cultivation to Application
Abstract
:1. Introduction
2. Morphology, Taxonomy: Photo, Flowers
3. Area of Spread, Cultivation Techniques, and Applications
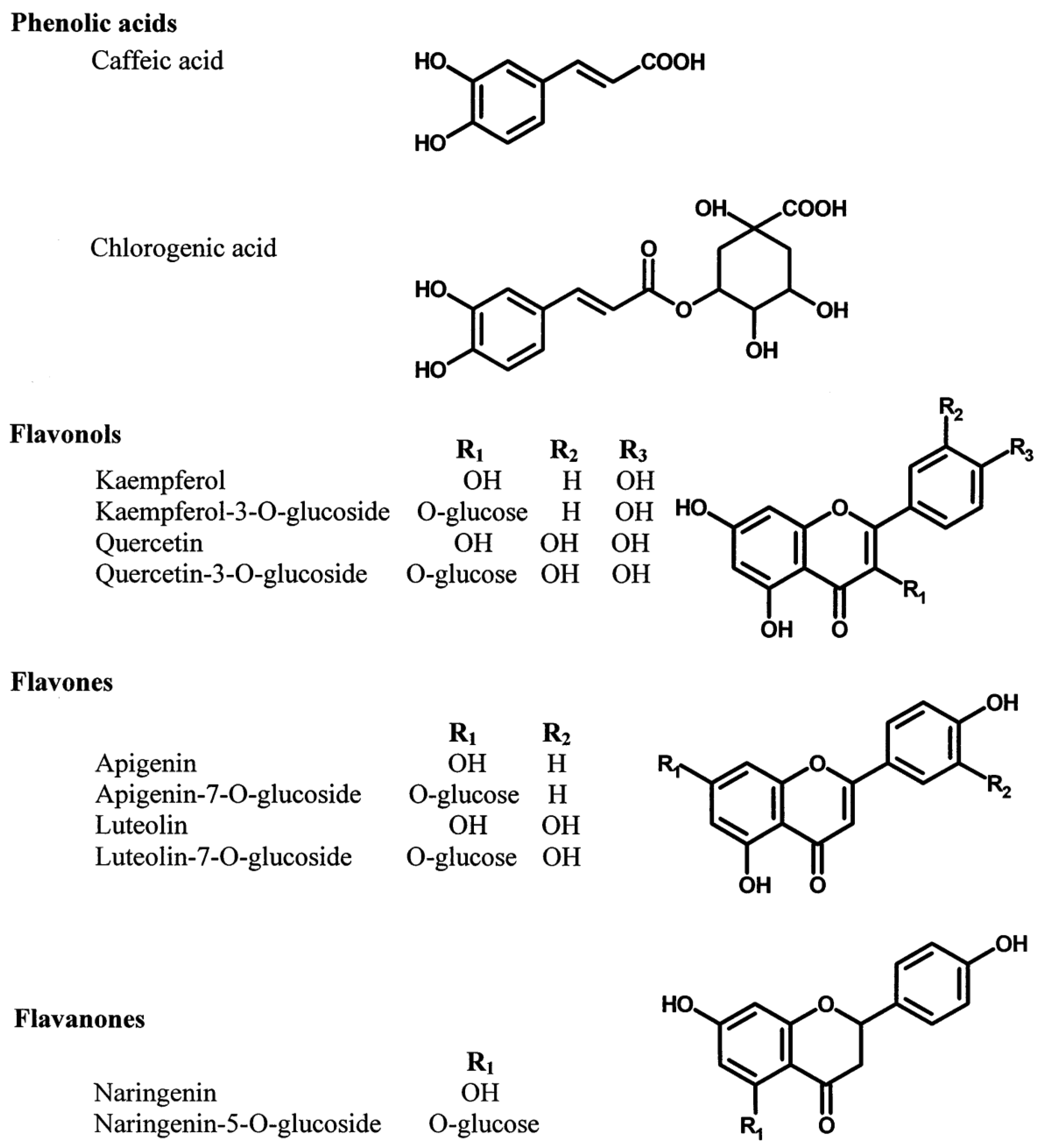
4. Bioactive Compounds
5. Extraction Products
6. Pharmacological Properties
6.1. Choleretic and Cholagogue Activities
6.2. Antioxidant Activities
6.3. Anti-Inflammatory Activities
6.4. Antimicrobial Activities
6.5. Pharmacoeconomic Benefits
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Les, F.; Venditti, A.; Cásedas, G.; Frezzac, C.; Guiso, M.; Sciubba, F.; Serafini, M.; Bianco, A.; Marta Sofía, V.; López, V. Everlasting flower (Helichrysum stoechas Moench) as a potential source of bioactive molecules with antiproliferative, antioxidant, antidiabetic and neuroprotective properties. Ind. Crops. Prod. 2017, 108, 295–302. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal Plants of the Russian Pharmacopoeia; Their history and applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef] [Green Version]
- Ph. Eur. 7.0. European Pharmacopoeia 7.0; EDQM: Strasbourg, France, 2011. [Google Scholar]
- Ph. Helv. VII. Pharmacopoeia Helvetica, 7th ed.; Département fédéral de l’intérieur: Berne, Switzerland, 1987; Volume 1. [Google Scholar]
- Ph USSR. State Pharmacopoeia of the USSR, 11th ed.; Part 2; USSR: Moscow, Russia, 1990. [Google Scholar]
- Polish Ph VI. Farmakopea Polska–Wydanie VI/Polish Pharmacopoeia−6th Edn, Rzeczpospolita Polska Minister Zdrowa, Urzad Rejestracji Produktów Leczniczych; Wyrobów Medycznych i Produktów Biobójczych: Warszawa, Poland, 2002. [Google Scholar]
- Olsson, K.; Pihlik, U.; Radušiene, J.; Wedelsbäck, B.K. Helichrysum arenarium (L.) Moench (Everlasting) in Spice and Medicinal Plants in the Nordic and Baltic Countries Conservation of Genetic Resources; Report from the SPIMED-project group at the Nordic Gene Bank: Alnarp, Sweden, 2005; pp. 55–65. [Google Scholar]
- Sawilska, A.; Figas, A. Micropropagation of Helichrysum arenarium (L.) Moench. In Proceedings of the International Conference Biotechnology, Česke Budejovice, Czech Republic, 15–16 February 2006; Scientific Pedagogical Publishing: Česke Budějovice, Czech Republic, 2006; pp. 721–723. [Google Scholar]
- WHO. Monographs on Medicinal Plants Commonly Used in the Newly Independent States (NIS); World Health Organization: Geneva, Switzerland, 2010.
- Pljevljakušic’, D.; Bigovic’, D.; Jankovic’, T.; Šavikin, K. Sandy Everlasting (Helichrysum arenarium (L.) Moench): Botanical, Chemical and Biological Properties. Front. Plant Sci. 2018, 9, 1123. [Google Scholar] [CrossRef] [Green Version]
- Clasquin, S.; Henry, M. Micropropagation of Helichrysum arenarium L. Moench. Acta Bot. Gallica 2002, 149, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Figas, A.; Tomaszewska-Sowa, M.; Sawilska, A.; Keutgen, A.J. Improvement of in vitro propagation and acclimation of Helichrysum arenarium L. Moench. Acta Sci. Pol. Hortorum Cultus 2016, 15, 17–26. [Google Scholar]
- Bryksa-Godzisz, M.; Pawełczak, A. In vitro propagation of the yellow everlasting (Helichrysum arenarium (L.) Moench) from root explants. Propag. Ornam. Plants 2010, 10, 14–17. [Google Scholar]
- Roşu, A. Elemente de Biotehnologii Vegetale-Aplicaţii în Ameliorare; Ametist-92: Bucureşti, Romania, 1999. [Google Scholar]
- Cachiţă, C.D.; Ardelean, A. Tratat de Biotehnaologie Vegetală; Dacia: Cluj-Napoca, Romania, 2009; Volume II. [Google Scholar]
- Cachiţă, C.D.; Deliu, C.; Rakosy, T.L.; Ardelean, A. Tratat de Biotehnologie Vegetală; Dacia: Cluj-Napoca, Romania, 2004; Volume I. [Google Scholar]
- Smirnova, L.P.; Pervykh, L.N. Quantitative determination of the total content of flavonoids in the flowers of immortelle Helichrysum arenarium. Pharm. Chem. J. 1998, 32, 321–324. [Google Scholar] [CrossRef]
- Czinner, E.; Hagymasi, K.; Blazovics, A.; Kery, A.; Szoke, E. In Vitro antioxidant properties of Helichrysum arenarium (L.) Moench. J. Ethnopharmacol. 2000, 73, 437–443. [Google Scholar] [CrossRef]
- EMA. Assessment Report on Helichrysum arenarium (L.) Moench Flos (Rapporteur: Wojciech Dymowski); European Medicines Agency: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Moench, C. Methodus Plantas Horti Botanici et Agri Marburgensis; Cattorum, M., Ed.; Nova Libraria Academiae: Marburg, Germany, 1794; p. 780. Available online: https://species.wikimedia.org/wiki/Methodus_Plantas_Horti_Botanici_et_Agri_Marburgensis (accessed on 8 January 2022).
- Erbar, C.; Leins, P. Diversity of styles and mechanisms of secondary pollen presentation in basal Asteraceae—New insights in phylogeny and function. Flora Morphol. Distrib. Funct. Ecol. Plants 2015, 217, 109–130. [Google Scholar] [CrossRef]
- Babotă, M.; Mocan, A.; Vlase, L.; Crișan, O.; Ielciu, I.; Gheldiu, A.M.; Vodnar, D.C.; Crișan, G.; Păltinean, R. Phytochemical Analysis, Antioxidant and Antimicrobial Activities of Helichrysum arenarium (L.) Moench and Antennaria dioica (L.) Gaertn. Flowers. Molecules 2018, 23, 409. [Google Scholar] [CrossRef] [Green Version]
- Stanescu, U.; Hancianu, M.; Cioanca, O.; Aprostosoaie, A.C.; Miron, A. Plante Medicinale de la A la Z, 3rd ed.; Polirom Publishing House: Iasi, Romania, 2018; pp. 313–315. [Google Scholar]
- Camen-Comanescu Petronela, Ph.D. taken at the University of Bucharest’s “Dimitrie Brândză” Botanical Garden in July and August, 2013, Photos personal collection. Unpublished work.
- Yousheng, C.; Shixin, Z.; Bayer, R.J. Tribe Gnaphalieae, Genus Helichrysum, Asteraceae (Compositae), Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2011; pp. 20–21. [Google Scholar]
- Flora von Deutschland Österreich und der Schweiz (1885)—BioLib.de. Available online: http://www.biolib.de/thome/icon_page_00294.html] (accessed on 16 July 2022).
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europea, Plantaginaceae to Compositae (and Rubiaceae); Cambridge University Press: Cambridge, UK, 2006; Volume IV. [Google Scholar]
- Maznev, N.I. Entsiklopediia Lekarstvennykh Rastenii. In Encyclopedia of Medicinal Plants; Martin Press: Moscow, Russia, 2004. [Google Scholar]
- Lilleleht, V. Red Data Book of Estonia; Eesti Teaduste Akadeemia Looduskaitse Komisjon: Tartu, Estonia, 1998; Available online: http://www.zbi.ee/punane/liigid/soontaimed_e.html (accessed on 3 August 2022)(In Estonian with English Summary).
- Sawilska, A.K.; Jendrzejczak, E. Efficiency of sandy everlasting Helichrysum arenarium (L.) Moench cultivation from in vitro seedlings and achenes. Ind. Crops Prod. 2013, 43, 50–55. [Google Scholar] [CrossRef]
- Greuter, W. Compositae (pro parte majore), Compositae. Euro+Med PlantBase—The Information Resource for Euro-Mediterranean Plant Diversity; Greuter, W., von Raab-Straube, E., Eds. 2006. Available online: http://www.emplantbase.org/ (accessed on 8 August 2022).
- Dihoru, G.; Negrean, G. The Red Book of Vascular Plants from Romania; Romanian Academy: Bucharest, Romania, 2009. [Google Scholar]
- Dihoru, G.; Boruz, V. The List of Main Spontaneous Medicinal Plants from Romania; Annals of the University of Craiova—Agriculture, Montanology, Cadastre Series; University of Craiova: Craiova, Romania, 2014; Volume XLIV. [Google Scholar]
- Fijalkowski, D.; Seroczynska, M. Sandy everlasting [Helichrysum arenarium (L.) Moench] in Lubelskie voivodship and its cultivation. Herba Pol. 1974, 20, 363–372. [Google Scholar]
- Sawilska, A.K.; Jendrzejczak, E.; Welc, M.; Kieliszewska-Rokicka, B. Influence of mycorrhizal fungi on the growth and development of sandy everlasting [Helichrysum arenarium (L.) Moench]. Acta Agrobot. 2009, 62, 67–76. [Google Scholar] [CrossRef]
- Buchwald, W. Sandy everlasting as a vanishing species. Wiad. Ziel. 1992, 34, 5–6. [Google Scholar]
- Sawilska, A.; Jendrzejczak, E. Adaptation of Helichrysum arenarium (Asteraceae) microseedlings to mineral and organic soils. Rare Relic Endanger. Plants Fungi Pol. 2009, 2009, 473–477. [Google Scholar]
- Tyszynska-Kownacka, D. Let us replenish the natural stands of medicine plants. Wiad. Ziel. 1972, 11, 8–9. [Google Scholar]
- Sawilska, A.K. The impact of environmental factors on the course of flowering Helichrysum arenarium (L.) Moench. Acta Agrobot. 2006, 59, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Sawilska, A.K. The influence of origin of Helichrysum arenarium (L.) Moench individuals on their inflorescence yield and germination ability. Acta Agrobot. 2007, 60, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Sawilska, A.K. Dynamics of Helichrysum arenarium (L.) Moench populations growing in fallow field on barren soil. Ecol. Quest. 2008, 9, 93–101. [Google Scholar] [CrossRef]
- Crisan, I.; Vidican, R.; Stoie, A.; Simea, S.A. Spring-autumn arbuscular mycorrhiza colonization dynamic in Iris germanica L. from urban microclimate. AgroLife Sci. J. 2020, 9, 82–90. [Google Scholar]
- Bisset, N.G.; Wichtl, M. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis; Medpharm Scientific Publishers: Stuttgart, Germany, 1994. [Google Scholar]
- Sõukand, R.; Hrynevich, Y.; Prakofjewa, J.; Valodzina, T.; Vasilyeva, I.; Paciupa, J.; Shrubok, A.; Hlushko, A.; Knureva, Y.; Litvinava, Y.; et al. Use of cultivated plants and non-plant remedies for human and animal home-medication in Liubań district, Belarus. J. Ethnobiol. Ethnomed. 2017, 13, 54. [Google Scholar] [CrossRef] [Green Version]
- Rančić, A.; Soković, M.; Vukojević, J.; Simić, A.; Marin, P.; Duletić-Laušević, S.; Djoković, D. Chemical Composition and Antimicrobial Activities of Essential Oils of Myrrhis odorata (L.) Scop, Hypericum perforatum L and Helichrysum arenarium (L.) Moench. J. Essent. Oil Res. 2005, 17, 341–345. [Google Scholar] [CrossRef]
- Czinner, E.; Kery, A.; Hagymási, K.; Blázovics, A.; Lugasi, A.; Szoke, E.; Lemberkovics, E. Biologically active compounds of Helichrysum arenarium (L.) Moench. Eur. J. Drug Metab. Pharmacokinet. 1999, 24, 309–313. [Google Scholar] [CrossRef]
- Czinner, E.; Kusinszki, L.; Baumann, D.; Hamburger, M. Phytochemical study of phenolic compounds from Helichrysi flos by LC-DAD –MS. In Natural Products in the New Millenium: Prospects and Industrial Application; Rauter, A.P., Palma, F.B., Justino, J., Araújo, M.E., Santos, S.P.d., Eds.; Kluwer Acadenic Publishers: Amsterdam, The Netherlands, 2002; pp. 99–109. [Google Scholar]
- Kurkina, A.; Ryzhov, V.; Avdeeva, E. Assay of isosalipurposide in raw material and drugs from the dwarf everlast (Helichrysum arenarium). Pharm. Chem. J. 2012, 46, 171–176. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Katan, M.B. Bioavilibilty and health effects of dietary flavonols in man. Arch. Toxicol. 1998, 20, 237–248. [Google Scholar]
- Czinner, E.; Lemberkovics, É.; Bihátsi-Karsai, É.; Vitányi, G.; Lelik, L. Composition of the essential oil from the inflorescence of Helichrysum arenarium (L.) Moench. J. Essent. Oil Res. 2000, 12, 728–730. [Google Scholar] [CrossRef]
- Gadjalova, A.V.; Mihaylova, D. Ultrasound-assisted extraction of medicinal plants and evaluation of their biological activity. Food Res. 2019, 3, 530–536. [Google Scholar] [CrossRef]
- Liu, X.; Jing, X.; Li, G. A process to acquire essential oil by distillation concatenated liquid-liquid extraction and flavonoids by solid-liquid extraction simultaneously from Helichrysum arenarium (L.) Moench inflorescences under ionic liquid microwave mediated. Sep. Purif. Technol. 2019, 209, 164–174. [Google Scholar] [CrossRef]
- Oman, M.; Škerget, M.; Knez, Ž. Application of supercritical fluid extraction for the separation of nutraceuticals and other phytochemicals from plant material. Maced. J. Chem. Chem. Eng. 2013, 32, 183–226. [Google Scholar] [CrossRef] [Green Version]
- Scalia, S.; Giuffreda, L.; Pallado, P. Analytical and preparative supercritical fluid extraction of chamomile flowers and its comparison with conventional methods. J. Pharm. Biomed. Anal. 1999, 21, 549–558. [Google Scholar] [CrossRef]
- Chiu, K.L.; Cheng, Y.C.; Chen, J.H.; Chang, C.M.J.; Yang, P.W. Supercritical fluids extraction of ginkgo ginkgolides and flavonoids. J. Supercrit. Fluids 2002, 24, 77–87. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, L.; Ye, W. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Dorene Petersen, R.H. Immortelle Essential Oil and Extract: Are Two Preparations Better than One? J. Am. Herb. Guild 2015, 13, 21–27. [Google Scholar]
- Azar, P.A.; Torabbeigi, M.; Tehrani, M.S.; Husain, S.W. Hydrodistillation, Solvent Free Microwave Assisted Extraction and Headspace-Solid Phase Microextraction for Analysis of Essential Oil of Flowers of Helichrysum aucheri. Asian J. Chem. 2011, 23, 1209–1211. [Google Scholar]
- Goldansaz, S.M.; Mahboubi, A.; Yazdi-nejad, A.; Jahanbakhshi, M.; Mojab, F. Investigation on total phenolic content, antibacterial, and antioxidant activity of ethanolic extract of Helichrysum leucocephalum Boiss. Am. J. Essent. Oil. Nat. Prod. 2018, 6, 20–24. [Google Scholar]
- Cheng, M.; Ding, L.; Kan, H.; Zhang, H.; Jiang, B.; Sun, Y.; Cao, S.; Li, W.; Koike, K.; Qiu, F. Isolation, structural elucidation and in vitro hepatoprotective activity of flavonoids from Glycyrrhiza uralensis. J. Nat. Med. 2019, 73, 847–854. [Google Scholar] [CrossRef]
- Jarzycka, A.; Lewin’ska, A.; Gancarz, R.; Wilk, A.K. Assessment of extracts of Helichrysum arenarium, Crataegus monogyna, Sambucus nigra in photoprotective UVA and UVB; photostability in cosmetic emulsions. J. Photochem. Photobiol. B Biol. 2013, 128, 50–57. [Google Scholar] [CrossRef]
- Liu, Z.; Kong, L.; Lu, S.; Zou, Z. Application of a Combined Homogenate and Ultrasonic Cavitation System for the Efficient Extraction of Flavonoids from Cinnamomum camphora Leaves and Evaluation of Their Antioxidant Activity In Vitro. J. Anal. Methods Chem. 2019, 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Kroeber, L. Cat’s Foot, Gnaphalium dioecum L., Antennaria dioeca, Gnaphalium arenarium, Helichrysum arenarium DC as cholagogues and choleretics. Pharmazie 1951, 6, 615–617. [Google Scholar]
- Szadowska, A. Pharmacology of galenic preparations and flavonoids from Helichrysum arenarium. Acta Pol. Pharm. 1962, 19, 465–479. [Google Scholar]
- Wagner, H. Pharmazeutische Biologie, Drogen und ihre Inhaltsstoffe; Gustav Fischer Verlag: Stuttgart, Germany, 1993. [Google Scholar]
- Wichtl, M. Herbal Medicines and Phytopharmaceuticals, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Shass, E.Y. Phytotherapy; Academy of Medicinal Science of USSR: Moscow, Russia, 1952. [Google Scholar]
- Vereschagin, V.I.; Sobolevskaya, K.A.; Yakubova, A.I. Useful Plants of West Siberia; Academy of Science of USSR: Moscow, Russia, 1959. [Google Scholar]
- Sokolov, S.Y. Phytotherapy and Phytopharmacology: The Manual for Doctors; Medical News Agency: Moscow, Russia, 2000. [Google Scholar]
- Krivenko, V.V.; Potebnia, G.P.; Loiko, V.V. Experience in treating digestive organ diseases with medicinal plants. Vracebnoe Delo 1989, 3, 76–78. [Google Scholar]
- Litvinenko, V.I.; Popova, T.P.; Popova, N.V.; Bubenchikova, V.N. Medicinal plants and preparations derived from them. Farmaseotychnyi Zhurnal 1992, 3, 83–84. [Google Scholar]
- Skakun, N.P.; Stepanov, N.Y. Comparative evaluation of the hepatoprotective, antioxidant and choleretic activity of flavonoid drugs. Vracebnoe Delo 1988, 12, 52–54. [Google Scholar]
- Ionescu, D.; Spînu, S.; Orţan, A.; Moraru, I.; Fîntîneru, G.; Fierăscu, R.C.; Fierăscu, I.; Drugulescu, M. Evaluation of biological active compounds found in Silybi mariani fructus. AgroLife Sci. J. 2017, 6, 141–145. [Google Scholar]
- Catana, R.; Helepciuc, F.E.; Zamfir, M.; Florescu, L.; Mitoi, M. In Vivo and in vitro antioxidant activity of Cnicus benedictus. AgroLife Sci. J. 2020, 9, 73–78. [Google Scholar]
- Mao, Z.; Gan, C.; Zhu, J.; Ma, N.; Wu, L.; Wang, L.; Wang, X. Anti-atherosclerotic activities of flavonoids from the flowers of Helichrysum arenarium L. Moench through the pathway of anti-inflammation. Bioorg. Med. Chem. Lett. 2017, 27, 2812–2817. [Google Scholar] [CrossRef]
- Drewes, S.E.; Van Vuuren, S.F. Antimicrobial acylphloroglucinols and dibenzyloxyflavonoids from flowers of Helichrysum gymnocomum. Phytochem. Lett. 2008, 69, 1745–1794. [Google Scholar] [CrossRef]
- Recio, M.C.; Giner, R.; Terencio, M.C.; Sanz, M.J.; Rios, J.L. Anti-inflammatory activity of Helichrysum stoechas. Planta Medica 1991, 57, A56. [Google Scholar] [CrossRef]
- Sala, A.; Recio, M.D.C.; Giner, R.M.; Manez, S.; Rios, J.-L. Newacetophenone glucosides isolated from extracts of Helichrysum italicum with anti-inflammatory activity. J. Nat. Prod. 2001, 64, 1360–1362. [Google Scholar] [CrossRef]
- Sala, A.; Recio, M.D.C.; Giner, R.M.; Manez, S.; Tournier, H.; Schinella, G.; Rios, J.-L. Anti-inflammatory and anti-oxidant properties of Helichrysum italicum. J. Pharm. Pharmacol. 2002, 54, 365–371. [Google Scholar] [CrossRef]
- Sala, A.; Recio, M.C.; Schinella, G.R.; Manez, S.; Giner, R.M.; Cerda-Nicolas, M.; Rios, J.-L. Assessment of the anti-inflammatory activity and free radical scavenger activity of tiliroside. Eur. J. Pharmacol. 2003, 461, 53–61. [Google Scholar] [CrossRef]
- Sala, A.; Recio, M.C.; Schinella, G.R.; Manez, S.; Giner, R.M.M.; Rios, J.-L. A new dual inhibitor of arachidonate metabolism isolated from Helichrysum italicum. Eur. J. Pharmacol. 2003, 460, 219–226. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; El-Sayed, H.A.; El-Shahat, M.; El-Sayed, A.A.; Darwesh, O.M. Novel Triazolothiadiazole and Triazolothiadiazine Derivatives Containing Pyridine Moiety: Design, Synthesis, Bactericidal and Fungicidal Activities. Curr. Bioact. Compd. 2018, 14, 169–179. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Mahmoud, R.H.; Abdo, S.M.; Marrez, D.A. Isolation of Haematococcus lacustris as source of novel anti-multi-antibiotic resistant microbes agents; fractionation and identification of bioactive compounds. Biotechnol. Rep. 2022, 35, e00753. [Google Scholar] [CrossRef]
- Khristenko, L.A.; Pertsev, I.M.; Salo, D.P.; Negrash, A.K. Possible use of arenarin in medicated ophthalmological films. Pharm. Chem. J. 1997, 11, 995–998. [Google Scholar] [CrossRef]
- Gradinaru, A.C.; Silion, M.; Trifan, A.; Miron, A.; Aprotosoaie, A.C. Helichrysum arenarium subsp. arenarium: Phenolic composition and antibacterial activity against lower respiratory tract pathogens. Nat. Prod. Res. 2014, 28, 2076–2080. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.M. Inhibitory Effect of Helichrysum arenarium Essential Oil on the Growth of Food Contaminated Microorganisms. Int. J. Agric. Biol. Eng. 2014, 8, 795–799. [Google Scholar]
- Albayrak, S.; Aksoy, A.; Sağdic, O.; Budak, U. Phenolic compounds and antioxidant and antimicrobial properties of Helichrysum species collected from eastern Anatolia, Turkey. Turk. J. Biol. 2010, 34, 463–473. [Google Scholar] [CrossRef]
- Albayrak, S.; Aksoy, A.; Sagdic, O.; Hamzaoglu, E. Compositions, antioxidant and antimicrobial activities of Helichrysum (Asteraceae) species collected from Turkey. Food Chem. 2010, 119, 114–122. [Google Scholar] [CrossRef]
- Kutluk, I.; Aslan, M.; Orhan, I.E.; Özçelik, B. Antibacterial, antifungal and antiviral bioactivities of selected Helichrysum species. S. Afr. J. Bot. 2018, 119, 252–257. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; İspirli, H.; Taylan, O.; Balubaid, M.; Dertli, E. Facile biomimetic synthesis of AgNPs using aqueous extract of Helichrysum arenarium: Characterization and antimicrobial activity. Inorg. Nano-Met. Chem. 2022, 52, 1–12. [Google Scholar] [CrossRef]
- Cosar, G.; Cubukcu, B. Antibacterial activity of Helichrysum species growing in Turkey. Fitoterapia 1990, 61, 161–164. [Google Scholar]
- Czinner, E.; Hagymási, K.; Blázovics, A.; Kéry, A.; Szoke, E.; Lemberkovics, E. The in vitro effect of Helichrysi flos on microsomal lipid peroxidation. J. Ethnopharmacol. 2001, 77, 31–35. [Google Scholar] [CrossRef]
- Bigovic, D.; Brankovic, S.; Kitic, D.; Radenkovic, M.; Jankovic, T.; Savikin, K.; Zivanovic, S. Relaxant effect of the ethanol extract of Helichrysum plicatum (Asteraceae) on isolated rat ileum contractions. Molecules 2010, 15, 3391–3401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigovic, D.; Savikin, K.; Jankovic, T.; Menkovic, N.; Zdunic, G.; Stanojkovic, T. Antiradical and cytotoxic activity of different Helichrysum plicatum flower extracts. Nat. Prod. Commun. 2011, 6, 819–822. [Google Scholar] [CrossRef]
- Aslanyan, M.; Bobrytska, L.; Hrytsenko, V.; Shpychak, O.; Popova, N.; Germanyuk, T.; Kryvoviaz, O.; Ivko, T. Technological aspects of development of a new drug in tablets called «Lavaflam» and its pharmacoeconomic evaluation. Res. J. Pharm. Biol. Chem. Sci. (RJPBCS) 2017, 8, 808. [Google Scholar]



| Extraction Technique | Solvent | Active Constituents | References |
|---|---|---|---|
| Distillation | Water | Monoterpenoids, sesquiterpenoids, phenolic compounds | [18,52,56] |
| Maceration | Alcohol | Alkaloids, carotenes, flavonoids, tannins | [1,56] |
| Solvent extraction or enfleurage | Solvent organic | Monoterpenes, sesquiterpenes, monoterpenoids, phenolic compounds, carotenes | [52,56,57,59,61] |
| Ultrasonic-assisted extraction (UAE) or sonication | Ethanol aqueous solution | Phenolic acids and flavonoids | [51,54,56,60,62] |
| Supercritical fluid extraction (SFE) | Supercritical carbon dioxide | Nonpolar natural products such as lipid and volatile oil. | [53,54,55,56] |
| Microwave-assisted extraction (MAE): two types of methods: 1. solvent-free extraction; 2. solvent extraction | 1. usually for volatile compounds; 2. usually for nonvolatile compounds | Essential oils: - Monoterpene hydrocarbons - Sesquiterpene hydrocarbons - Oxygenated monoterpenes - Oxygenated sesquiterpenes | [52,56,58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dănăilă-Guidea, S.M.; Eremia, M.C.; Dinu, L.D.; Miu, D.-M. Helichrysum arenarium: From Cultivation to Application. Appl. Sci. 2022, 12, 10241. https://doi.org/10.3390/app122010241
Dănăilă-Guidea SM, Eremia MC, Dinu LD, Miu D-M. Helichrysum arenarium: From Cultivation to Application. Applied Sciences. 2022; 12(20):10241. https://doi.org/10.3390/app122010241
Chicago/Turabian StyleDănăilă-Guidea, Silvana Mihaela, Mihaela Carmen Eremia, Laura Dorina Dinu, and Dana-Maria Miu. 2022. "Helichrysum arenarium: From Cultivation to Application" Applied Sciences 12, no. 20: 10241. https://doi.org/10.3390/app122010241
APA StyleDănăilă-Guidea, S. M., Eremia, M. C., Dinu, L. D., & Miu, D.-M. (2022). Helichrysum arenarium: From Cultivation to Application. Applied Sciences, 12(20), 10241. https://doi.org/10.3390/app122010241






