Reduction of Water Color in a Spinning Disc Reactor
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Colored Water Treatment with Selected Adsorptive Materials in SD Experimental Setup
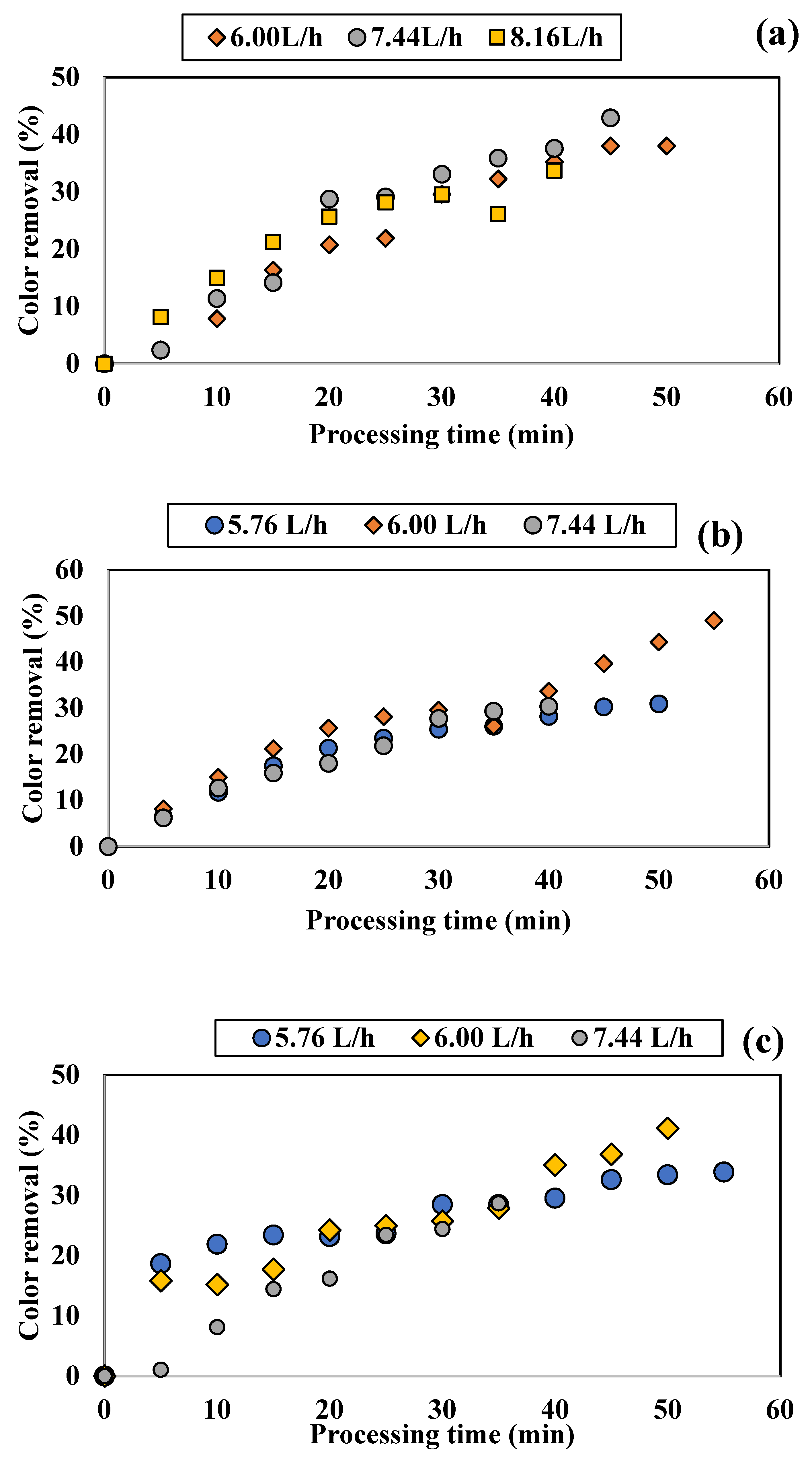
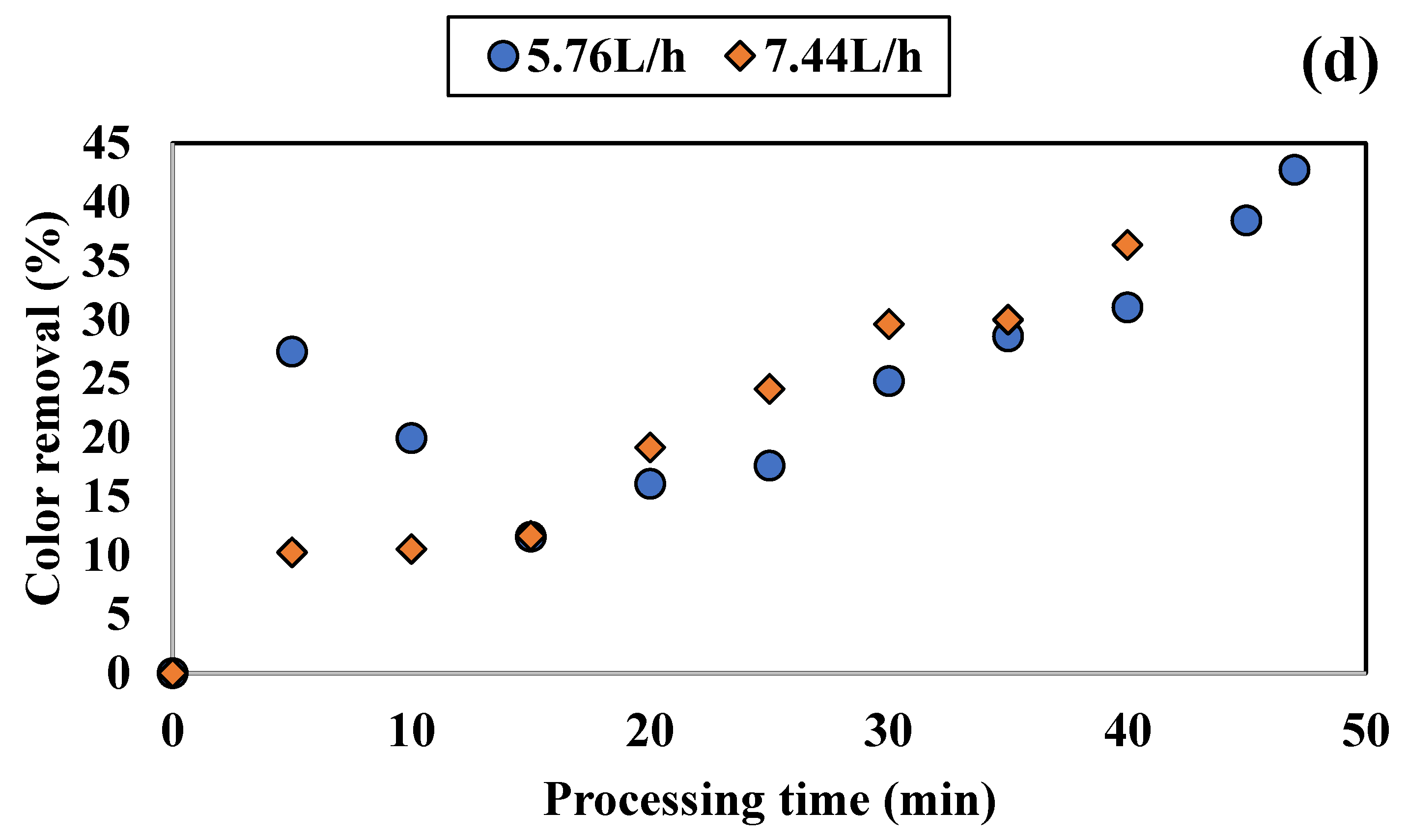
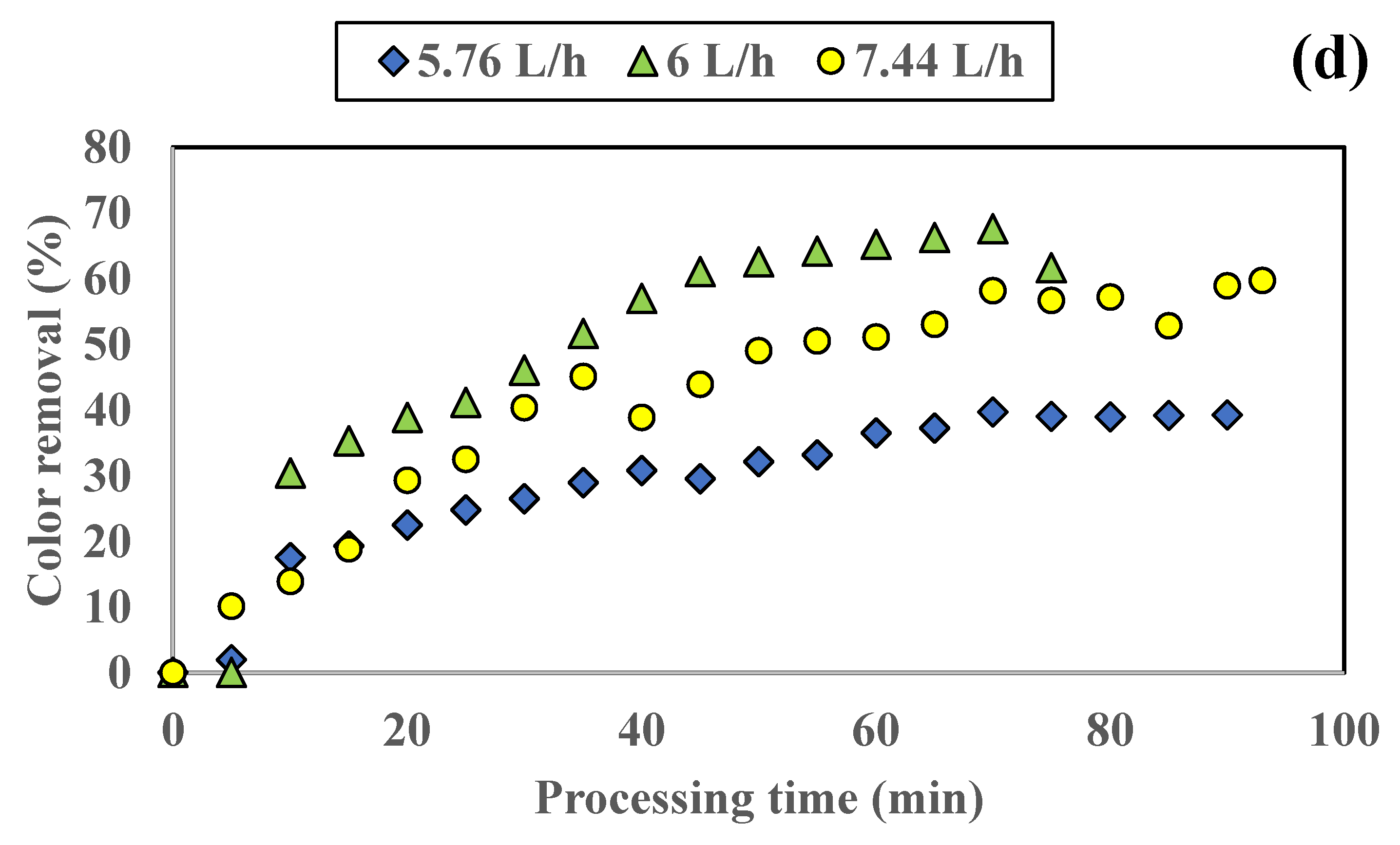
3.1.1. Influence of Colored Water Flow Rate on Discoloration for All Tested Adsorbents
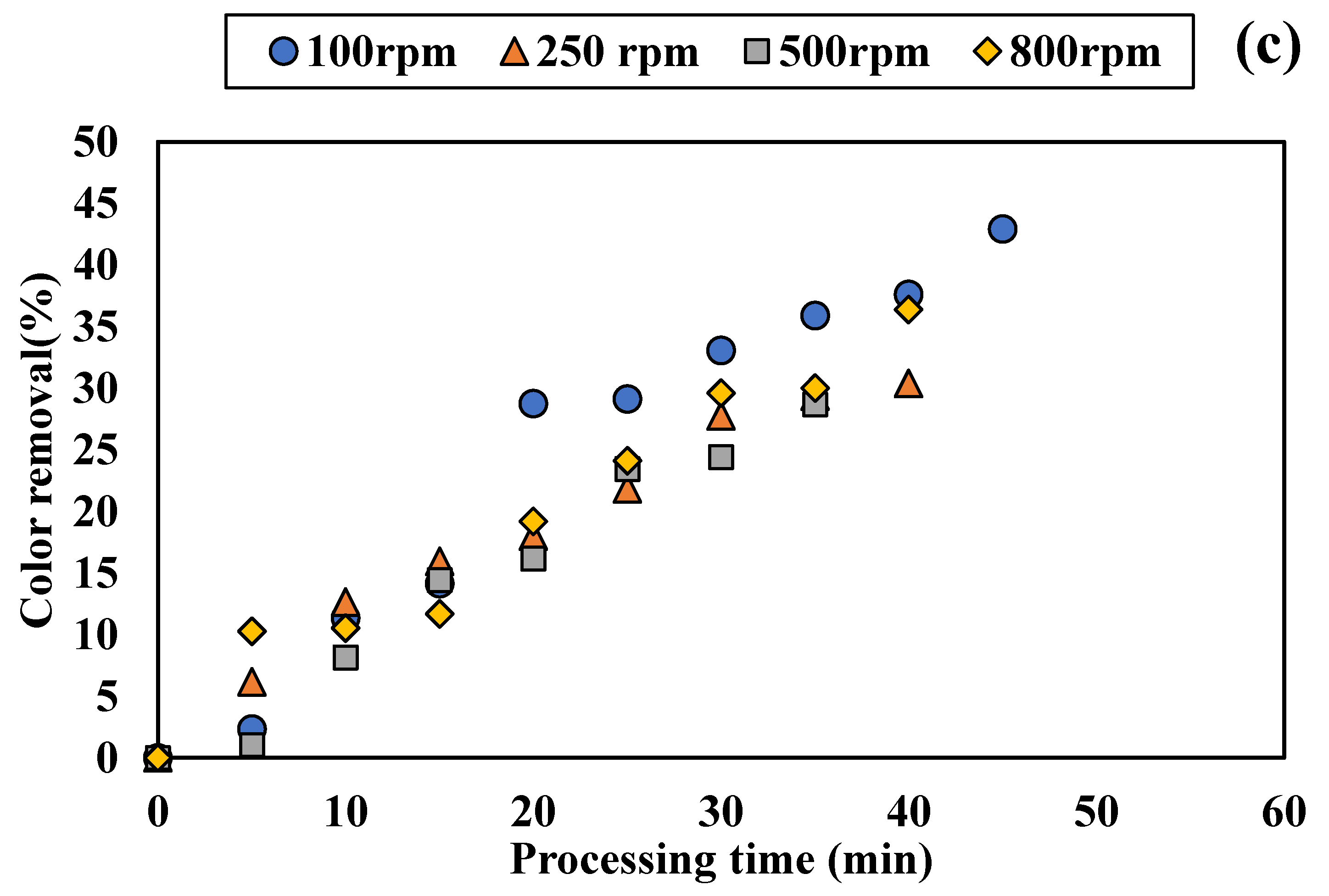
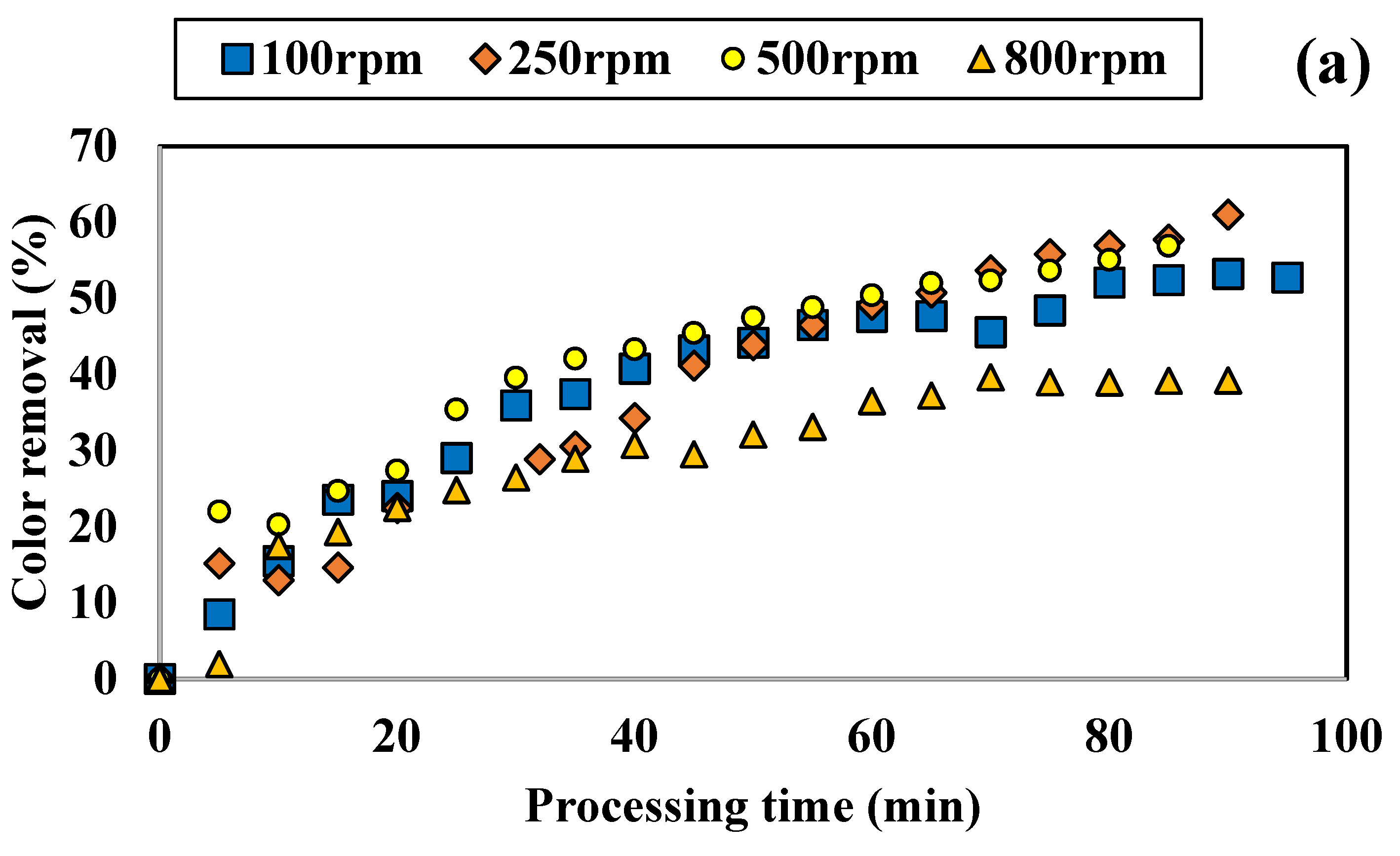
3.1.2. Influence of Disc Rotational Speed on Discoloration for All Tested Adsorbents
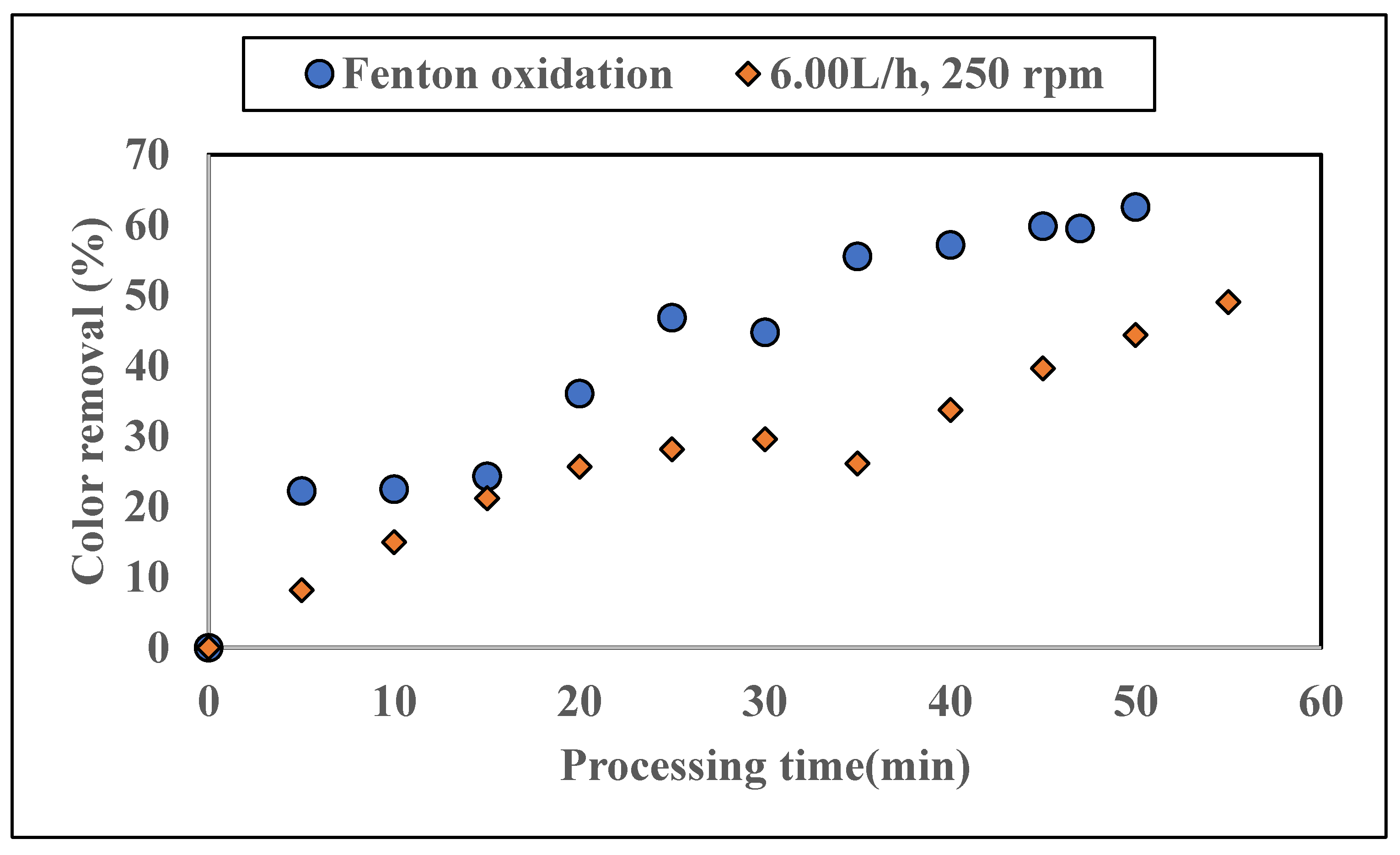
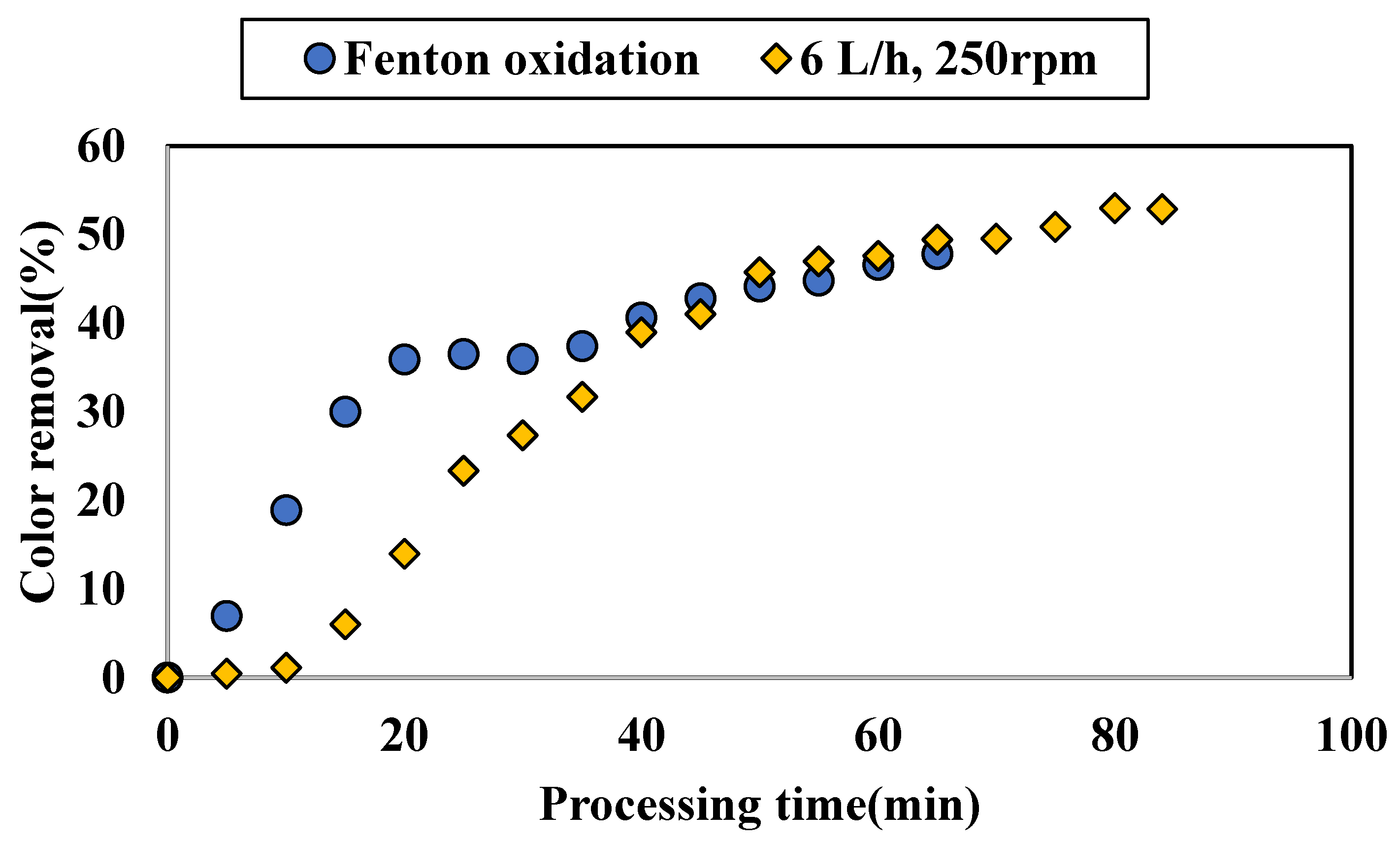
3.1.3. Application of Additional Fenton Oxidation on Colored Water Tested with ZnAlLDH in the SD Setup
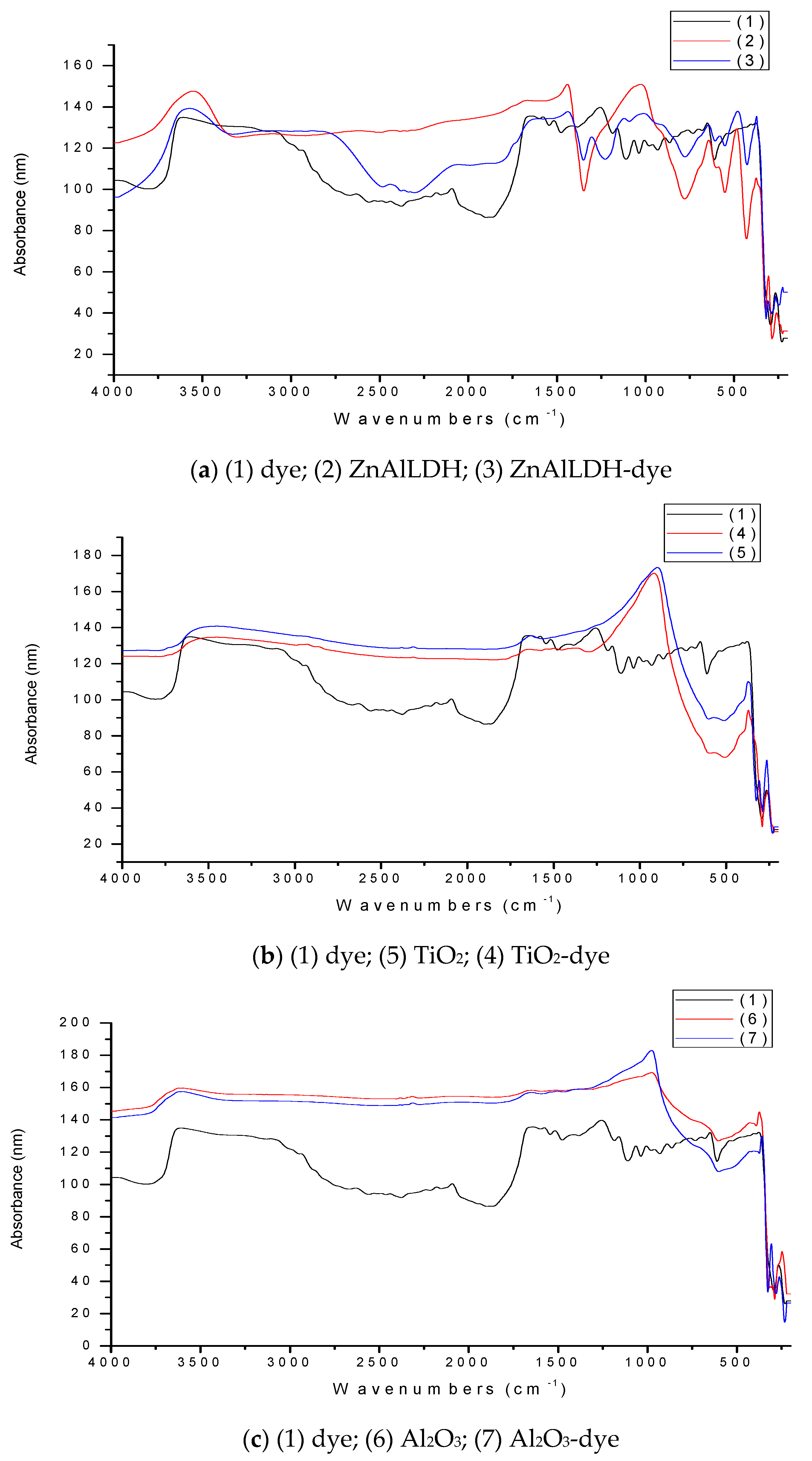
3.2. Validation of Dye Retention onto Selected Materials by FTIR Spectroscopy
4. Discussion
4.1. FTIR Spectra of Adsorbents before and after Dye Adsorption in the SD Setup
- -
- The peak at 3608 cm−1 can be seen in the spectrum of the pure dye (1), it being assigned to N-H in the amine groups due to strong stretching vibrations, since no OH was present in the dye structure. In addition, the peak at 3555 cm−1 from ZnAlLDH structure was assigned to strong OH stretching vibrations, and the ones at 3571 and 3104 cm−1, from the ZnAlLDH loaded with dye (ZnAlLDH-dye), were assigned to OH stretching vibrations in the LDHs structure or water molecules and to the aromatic benzene nucleus of adsorbed dye, respectively. The peaks at 3453 cm−1 from the TiO2 structure, and also at 3454 cm−1 from the structure of TiO2 loaded with dye, were assigned to OH stretching vibrations in the TiO2-based adsorbent and water molecules, and the same considerations were made for the peaks at 3621 cm−1 from the Al2O3 structure and 3598 cm−1 from the Al2O3-dye structure, but no significant dye adsorption inside of the used adsorbent was shown.
- -
- The peak at 2441 cm−1 for the ZnAlLDH structure after treatment in the SD setup was assigned to the presence of a carboxylic acid (COOH) originating from the studied dye structure adsorbed onto adsorbent surface.
- -
- The 1630 cm−1 peak for the TiO2 structure was assigned to the strong stretching vibrations of C=N double bonds in the aromatic species adsorbed on it.
- -
- The strong deformation vibrations at 1441 and 1428 cm−1 from the structure of the ZnAlLDH before and after treatment in the SD experimental setup were assigned either to the C-H deformation vibrations from the dye structure, indicating dye adsorption onto the solid adsorbent surface, or to the aliphatic hydroxyl group deformation due to the adsorbent and water molecules. The peaks at 1304 cm−1 from the ZnAlLDH structure after treatment and at 1370 cm−1 from the TiO2 structure after treatment in the SD experimental setup could be assigned either to the O=S=O in the sulfonic groups of the studied dye as result of antisymmetric stretching vibrations, or to NO2 deformation vibrations due to the active sites of the dye adsorbed onto the TiO2 surface with the help of water molecules. The 1027 cm−1 band for the ZnAlLDH structure before treatment and the 1011 or 1121 cm−1 bands from the ZnAlLDH structure after treatment in the SD experimental setup corresponded to the symmetric stretching vibrations of the adsorbent overlapping with other functional groups present in the structure of the adsorbed dye as a sulfonic SO3- group. These are valuable indicators that suggest the retention of the dye onto the adsorbent.
- -
- For the region of 1000–250 cm−1 (fingerprint), all the peaks corresponded to each characteristic spectrum of tested solid material (with no modifications after treatment), except in the case of the 919 cm−1 band for the TiO2 structure, with possible weak dye traces, or of the 644–650 cm−1/266–267 cm−1 bands for the ZnAlLDH material.
4.2. Performance of SD Reactor in Water Color Reduction in the Selected Working Regime
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crini, G.; Lichtfouse, E. Wastewater treatment: An overview. Green Adsorbents for Pollutant Removal. In Environmental Chemistry for a Sustainable World; Springer Nature: London, UK; Berlin, Germany; New York, NY, USA, 2018; pp. 1–22. [Google Scholar]
- Zaharia, C.; Suteu, D. Textile Organic Dyes-Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents-A Critical Overview. In Organic Pollutants-Ten Years after the Stockholm Convention. Environmental and Analytical Update; Puzyn, T., Mostrag-Szlichtyng, A., Eds.; InTech Open Science/Open Minds: Rijeka, Croatia, 2012; pp. 55–86. Available online: www.intechopen.com (accessed on 9 October 2022).
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Musteret, C.; Fighir, D.; Gavrilescu, D.; Zaharia, C.; Teodosiu, C. Water and Wastewater Treatment: Practice Applications; Politehnium: Iasi, Romania, 2014; pp. 131–225. (In Romanian) [Google Scholar]
- Iacob Tudose, E.T.; Zaharia, C. Textile Wastewater Treatment on a Spinning Disc Reactor: Characteristics, Performance and Empirical Modeling. Appl. Sci. 2020, 10, 8687. [Google Scholar] [CrossRef]
- Zaharia, C.; Leon, L.; Curteanu, S.; Iacob Tudose, E.T. Textile Wastewater Treatment in a Spinning Disc Reactor: Improved Performances-Experimental, Modeling and SVM Optimization. Processes 2021, 9, 2003. [Google Scholar] [CrossRef]
- Boiarkina, I.; Norris, S.E.; Patterson, D.A. Investigation into the effect of flow structure on the photocatalytic degradation of Methylene Blue and Dehydroabietic Acid in a spinning disc reactor. Chem. Eng. J. 2013, 222, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Boodhoo, K.V.K.; Al-Hengari, S. Micromixing characteristics in a small scale spinning disk reactor. Chem. Eng. Technol. 2012, 35, 1229–1237. [Google Scholar] [CrossRef]
- Iacob Tudose, E.T. Observations on the Spinning Disc Micromixing Characteristics. Bull. Polytech. Inst. Iasi Ser. Chem. Chem. Eng. 2021, 67, 23–32. [Google Scholar]
- Li, R.; Li, T.; Zhou, Q. Impact of Titanium Dioxide (TiO2) Modification on Its Application to Pollution Treatment-A Review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Abbas, M. Experimental investigation of titanium dioxide as an adsorbent for removal of Congo red from aqueous solution, equilibrium and kinetics modelling. J. Water Reuse Desalin. 2020, 10, 251–266. [Google Scholar] [CrossRef]
- Mogal, S.I.; Shah, D.O.; Mukherjee, T.; Sgripathi, T.; Mishra, M.K. Enhanced Photocatalytic Efficiency of a Least Active AgTiO2 by Amine Adsorption. ACS Omega 2018, 3, 12802–12812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheed, M.A.; Hussein, F.H. Adsorption of Reactive Black 5 on Synthesized Titanium Dioxide Nanoparticles: Equilibrium Isotherm and Kinetic Studies. J. Nanomater. 2014, 2014, 198561. [Google Scholar] [CrossRef]
- Ding, L.; Liao, J.; Zhang, Y. Adsorption performance and mechanism of Al2O3 aerogels towards aqueous U(VI) using template synthesis technology. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125980. [Google Scholar] [CrossRef]
- Meshcheryakov, E.P.; Reshetnikov, S.I.; Sandu, M.P.; Knyazev, A.S.; Kurzina, I.A. Efficient Adsorbent-Desiccant Based on Aluminium Oxide. Appl. Sci. 2021, 11, 2457. [Google Scholar] [CrossRef]
- Saria, I.; Taher, T.; Hariani, P.L.; Lesbani, A. Synthesis of Zn/Al layered double hydroxides as adsorbent for congo red and direct violet removal from aqueous solution. AIP Conf. Proc. 2018, 2026, 020043. [Google Scholar] [CrossRef]
- Li, A.; Deng, H.; Ye, C.; Jiang, Y. Fabrication and Characterization of Novel ZnAl-Layered Double Hydroxide for the Superadsorption of Organic Contaminants from Wastewater. ACS Omega 2020, 5, 15152–15161. [Google Scholar] [CrossRef]
- Chiban, M.; Zerbet, M.; Carja, G.; Sinan, F. Application of low-cost adsorbents for arsenic removal: A review. J. Environ. Chem. Ecotoxicol. 2012, 4, 91–102. [Google Scholar] [CrossRef]
- Seftel, E.M.; Puscasu, M.; Mertens, M.; Cool, P.; Carja, G. Photo-responsive behavior of γ-Fe2O3 NPs embedded into ZnAlFe-LDH matrices and their catalytic efficiency in wastewater remediation. Catal. Today 2015, 252, 7–13. [Google Scholar] [CrossRef]
- Puscasu, C.M.; Carja, G.; Zaharia, C. MgZnFeAlLDHs nanoarchitectonics for photocatalytic removal of some organic pollutants by using solar irradiation. Int. J. Mater. Prod. Technol. 2015, 51, 228–240. [Google Scholar] [CrossRef]
- Dartu, L.; Zaharia, C.; Carja, G. Application of new synthesized materials based on anionic clays for industrial effluent decoloration. Adv. Mater. Res. 2014, 837, 271–276. [Google Scholar] [CrossRef]
- Zaghloul, A.; Ichou, A.A.; Abali, M.; Benhiti, R.; Soudani, A.; Carja, G.; Chiban, M.; Zerbet, M.; Sinan, F. Removal and Comparative Adsorption of Anionic Dye on Various MgAl synthetic Clay. Biointerface Res. Appl. Chem. 2021, 11, 14986–14997. [Google Scholar] [CrossRef]



| Material Type | ZnAlLDH | TiO2 | Al2O3 |
|---|---|---|---|
| Concentration (w) % | 20 | 20 | 20 |
| Investigated Samples | t = 0 min | t = 30 min | t = 60 min | t = 90 min | t = 120 min |
|---|---|---|---|---|---|
| Sample 1 (pH = 7) | 0.344 | 0.182 | 0.233 | 1.292 | 2.003 |
| Sample 2 (pH = 2.5) | 0.330 | 1.429 | 1.758 | 2.051 | 2.071 |
| Sample 3 (pH = 4.5) | 0.374 | 0.305 | 0.659 | 1.052 | 1.340 |
| Rotation Speed [rpm] | Flow Rate [L/h] | Color Removal [%] | Time Period [min] |
|---|---|---|---|
| 100 | 6.00 | 38.01 | 60 |
| 7.44 | 42.92 | 45 | |
| 8.16 | 40.00 | 30 | |
| 250 | 5.76 | 30.94 | 50 |
| 6.00 | 49.03 | 55 | |
| 7.44 | 30.39 | 40 | |
| 500 | 5.76 | 33.87 | 55 |
| 6.00 | 41.14 | 50 | |
| 7.44 | 28.68 | 35 | |
| 800 | 5.76 | 42.74 | 47 |
| 7.44 | 36.37 | 40 |
| Rotation Speed [rpm] | Flow Rate [L/h] | Color Removal [%] | Time Period [min] |
|---|---|---|---|
| 100 | 5.76 | 52.74 | 95 |
| 6.00 | 52.84 | 90 | |
| 7.44 | 45.98 | 59 | |
| 8.16 | 56.52 | 55 | |
| 250 | 5.76 | 61.00 | 90 |
| 6.00 | 52.85 | 84 | |
| 7.44 | 46.62 | 60 | |
| 500 | 5.76 | 56.90 | 85 |
| 6.00 | 46.93 | 75 | |
| 7.44 | 56.08 | 95 | |
| 800 | 5.76 | 39.23 | 90 |
| 6.00 | 67.55 | 70 | |
| 7.44 | 59.68 | 93 |
| Solid Material Tested | SD Treatment Status | Relevant Peaks (cm−1)/Its Magnitude (s—Strong; m—Medium; w—Weak) |
|---|---|---|
| RR dye (1) | no | 3608 sh, 2093 s, 1641 sh, 1517 s, 1256 s, 1161 m, 1065 s, 891 w, 650 s, 378 s, 267 s |
| ZnAlLDH | Before (2) | 3555 sh, 1441 s, 1027 s, 642 s, 485 s, 375 s, 306 s, 260 m |
| After (3) | 3571 sh, 3104 w, 2441 m, 1428 s, 1304 s, 1121 m, 1011 s, 644 s, 581 m, 488 s, 373 s, 309 m, 266 m, 226 m | |
| TiO2 | Before (5) | 3453 sh, 1630 w, 898 s, 373 s, 308 s, 265 s |
| After (4) | 3454 sh, 1370 w, 919 s, 371 s, 261 s | |
| Al2O3 | Before (6) | 621 sh, 977 s, 428 m, 374 s, 301 w, 248 s |
| After (7) | 3598 sh, 977 s, 360 s, 308 s, 264 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacob-Tudose, E.T.; Zaharia, C.; Melniciuc-Puica, N. Reduction of Water Color in a Spinning Disc Reactor. Appl. Sci. 2022, 12, 10253. https://doi.org/10.3390/app122010253
Iacob-Tudose ET, Zaharia C, Melniciuc-Puica N. Reduction of Water Color in a Spinning Disc Reactor. Applied Sciences. 2022; 12(20):10253. https://doi.org/10.3390/app122010253
Chicago/Turabian StyleIacob-Tudose, Eugenia Teodora, Carmen Zaharia, and Nicoleta Melniciuc-Puica. 2022. "Reduction of Water Color in a Spinning Disc Reactor" Applied Sciences 12, no. 20: 10253. https://doi.org/10.3390/app122010253







