Oxidative Stability Analysis of Selected Oils from Unconventional Raw Materials Using Rancimat Apparatus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Materials
2.2. Chemicals
2.3. Acid, Peroxide, p-Anisidine, and TOTOX Values
2.4. Specific Ultraviolet Extinction
2.5. Determination of Carotenoid and Chlorophyll Pigments
2.6. Colour Measurement Using the CIE L*a*b* Method
2.7. Fatty Acid Composition Analysis
2.8. Oxidizability Value—COX
2.9. Determination of Nutritional Quality Index of Oils
2.10. Oxidative Stability Determination
2.11. Determination of Oxidation Kinetics Parameters
- τRancimat—induction time determined in the Rancimat,
- T—oxidation temperature [K].
- ΔH—enthalpy [kJ/mol],
- ΔS—entropy [J/mol*K],
- h—Planck’s constant [6.63 × 10−34J*s],
- R—gas constant [8.314 J/mol*K],
- kB—Boltzmann constant [1.38 × 10−23 J/K].
2.12. Statistical Analysis
3. Results and Discussion
3.1. Analysed Oils’ Initial Qualities
3.2. Carotenoid and Chlorophyll Pigments Content
3.3. Oil Colour in the CIE L*, a*, b* System
3.4. Fatty Acid Composition of Analysed Oils
3.5. Calculated Oxidizability Value of Oils
3.6. Nutritional Quality Index of Oils
3.7. Oxidative Stability of Analysed Oils
3.8. Kinetics Parameters of Analysed Oils Oxidation under Rancimat Conditions
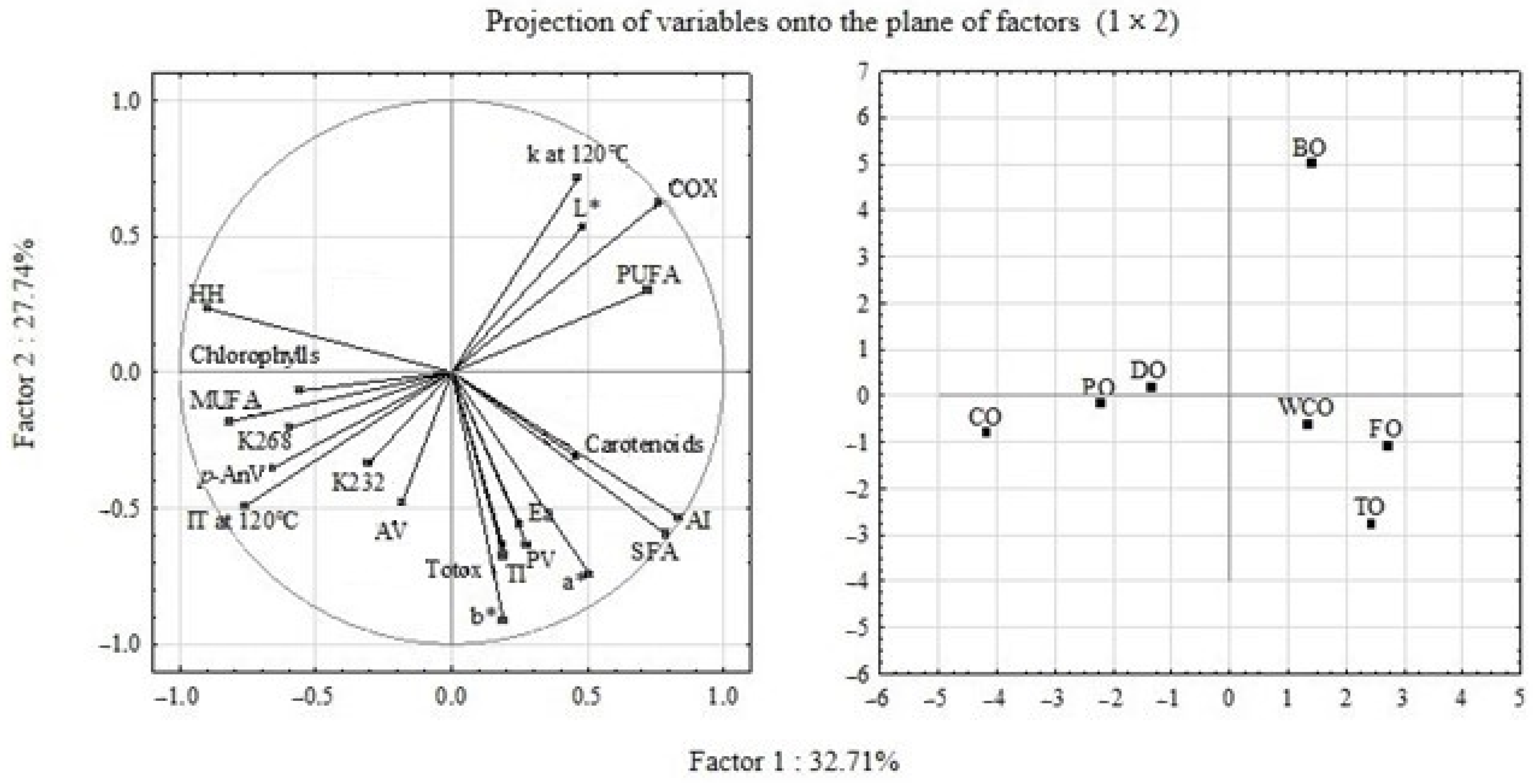
3.9. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 fatty acids and cardiovascular disease. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [Green Version]
- Kozłowska, M.; Gruczyńska, E.; Ścibisz, I.; Rudzińska, M. Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chem. 2016, 213, 450–456. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dłużewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Żbikowska, A. Oxidative stability of selected edible oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Comparison of the oxidative stability of linseed (Linum usitatissimum L.) oil by pressure differential scanning calorimetry and Rancimat measurements. J. Food Sci. Technol. 2016, 53, 3986–3995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EN ISO 660:2010; Animal and Vegetable Fats and Oils–Determination of Acid Value and Acidity. ISO: Geneva, Switzerland, 2010.
- EN ISO 3960:2012; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. ISO: Geneva, Switzerland, 2012.
- EN ISO 6885:2008; Animal and Vegetable Fats and Oils—Determination of Anisidine Value. ISO: Geneva, Switzerland, 2008.
- EN ISO 3656:2011; Animal and Vegetable Fats and Oils—Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction. ISO: Geneva, Switzerland, 2011.
- BS 684-2.20; Methods of Analysis of Fats and Fatty Oils. Other Methods; Determination of Carotene in Vegetable Oils. British Standards Institution: London, UK, 1977.
- AOCS Official Method Cc 13i-96. Methods and Recommended Practice of the American Oil Chemists Society, 5th ed.; AOCS Press: Champaign, IL, USA, 1998. [Google Scholar]
- AOAC Official Method 996.06. Fat (Total, Saturated, and Unsaturated) in Foods; Hydrolytic Extraction Gas Chromatographic Method. In Methods and Recommended Practices of the AOCS; AOCS International: Arlington, MA, USA, 2001. [Google Scholar]
- Fatemi, S.H.; Hammond, E.G. Analysis of oleate, linoleate and linolenate hydroperoxides in oxidised ester mixtures. Lipids 1980, 15, 379–385. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype: Feeding system and slaughter weight on the quality of light lambs. II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- AOCS Official Method Cd 12b-92. Oil Stability Index. Sampling and Analysis of Commercial Fats and Oils. In Methods and Recommended Practices of the AOCS, 6th ed.; Firestone, D., Ed.; AOCS Press: Champaign, IL, USA, 1997; pp. 1–2. [Google Scholar]
- Kowalski, B.; Ratusz, K.; Kowalska, D.; Bekas, W. Determination of the oxidative stability of vegetable oils by differential scanning calorimetry and Rancimat measurements. Eur. J. Lipid Sci. Technol. 2004, 106, 165–169. [Google Scholar] [CrossRef]
- Ahmed, F.B.M.; Alla, A.E.-M.J. Phytochemical screening of Coriandrum sativum extract and influence in chemical properties of sunflower oil. Am. J. Appl. Chem. 2019, 3, 15–21. [Google Scholar]
- Milczarek, A.; Osek, M. Zmiany liczby kwasowej i nadtlenkowej tłuszczu produktów rzepakowych przechowywanych w różnych warunkach bez i z dodatkiem przeciwutleniacza. Rośliny Oleiste 2012, 33, 295–306. [Google Scholar] [CrossRef]
- Wroniak, M.; Ptaszek, A.; Ratusz, K. Ocena wpływu warunków tłoczenia w prasie ślimakowej na jakość i skład chemiczny olejów rzepakowych. Żywność. Nauka. Technologia. Jakość 2013, 1, 92–104. [Google Scholar]
- Lazos, E.S.; Tsaknis, J.; Lalas, S. Characteristics and composition of tomato seed oil. Grasas Y Aceites 1998, 49, 440–445. [Google Scholar] [CrossRef]
- Yilmaz, E.; Aydeniz, B.; Güneşer, O.; Arsunar, E.S. Sensory and physico-chemical properties of cold press-produced tomato (Lycopersicon esculentum L.) seed oils. J. Am. Oil Chem. Soc. 2015, 92, 833–842. [Google Scholar] [CrossRef]
- Makareviciene, V.; Janulis, P. Analiza jakości olejów jadalnych oraz obowiązkowe wymagania. Tłuszcze Jadalne 1999, 34, 15–31. [Google Scholar]
- Kalyna, V.S.; Lutsenko, M.V.; Kharytonov, M.M. Feasibility study of the technology of fatty coriander oil complex processing. Ann. Agrar. Sci. 2018, 16, 95–100. [Google Scholar] [CrossRef]
- Dedebas, T.; Ekici, L.; Sagdic, O. Chemical characteristics and storage stabilities of different cold-pressed seed oils. J. Food Process. Preserv. 2020, 45, e15107. [Google Scholar] [CrossRef]
- Giuffrè, A.M. Tomato seed oil: A comparison of extraction systems and solvents on its biodiesel and edible properties. Rivista Italiana Delle Sostanze Grasse 2017, 94, 149–160. [Google Scholar]
- Wroniak, M.; Kwiatkowska, M.; Krygier, K. Charakterystyka wybranych olejów tłoczonych na zimno. Żywność. Nauka. Technologia. Jakość 2016, 2, 46–58. [Google Scholar]
- Krajewska, M.; Zdybel, B.; Andrejko, D.; Ślaska-Grzywna, B.; Tańska, M. Właściwości chemiczne wybranych olejów tłoczonych na zimno. Acta Agrophys. 2017, 24, 579–590. [Google Scholar]
- Wroniak, M.; Krygier, K. Cold-pressed oils. Food Ind. 2006, 60, 30–32. [Google Scholar]
- Rój, A.; Przybyłowski, P. Assessment of the colour of energy drinks. Bromatol. Toxicol. Chem. 2012, 45, 817–821. [Google Scholar]
- Uitterhaegen, E.; Sampaio, K.; Delbeke, E.; De Greyt, W.; Cerny, M.; Evon, P.; Merah, O.; Talou, T.; Stevens, C.V. Characterization of French coriander oil as source of petroselinic acid. Molecules 2016, 21, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mińkowski, K.; Grześkiewicz, S.; Jerzewska, M.; Ropelewska, M. Charakterystyka składu chemicznego olejów roślinnych o wysokiej zawartości kwasów linolenowych. Żywność. Nauka. Technologia. Jakość 2010, 17, 146–157. [Google Scholar]
- Sikorska, E.; Caponio, F.; Bilancia, M.T.; Summo, C.; Pasqualone, A.; Khmelinskii, I.V.; Sikorski, M. Changes in colour of extra-virgin olive oil during storage. Polish J. Food Nutr. Sci. 2007, 57, 495–498. [Google Scholar]
- Wahidu, Z.; Silvia, D.; Wan, N.; Yang, T.A. Physicochemical and Quality Characteristics of Cold and Hot Press of Nigella Sativa L Seed Oil Using Screw Press. J. Appl. Sci. Res. 2014, 10, 36–45. [Google Scholar]
- Idris, A.A.; Nour, A.H.; Ali, M.M.; Erwa, I.Y.; Omer Ishag, O.A.; Nour, A.H. Physicochemical Properties and Fatty Acid Composition of Ocimum basilicum L. Seed Oil. Asian J. Phys. Chem. Sci. 2020, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Nour, A.H.; Elhussein, S.A.; Osman, N.A.; Nour, A.H. Characterization and Chemical Composition of the Fixed Oil of Fourteen Basil (Ocimum basilicum L.) Accessions Grown in Sudan. Int. J. Chem. Technol. 2009, 1, 52–58. [Google Scholar] [CrossRef]
- Sulieman, A.M.E.; Ali, A.O.; Hemavathy, J. Lipid content and fatty acid composition of fenugreek (Trigonellafoenum-graecum L.) seeds grown in Sudan. Int. J. Food Sci. 2008, 43, 380–382. [Google Scholar] [CrossRef]
- Al-Jasass, F.M.; Al-Jasser, M.S. Chemical Composition and Fatty Acid Content of Some Spices and Herbs under Saudi Arabia Conditions. Sci. World J. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Munshi, M.; Arya, P.; Kumar, P. Physico-chemical analysis and fatty acid profiling of fenugreek (Trigonella Foenum-Graecum) seed oil using different solvents. J. Oleo Sci. 2020, 69, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, A.M.; Capocasale, M.; Zappia, C. Physicochemical composition of tomato seed oil for an edible use: The effect of cultivar. Int. Food Res. J. 2016, 23, 583–591. [Google Scholar]
- Knothe, G.; Steidley, K.R. composition of some apiaceae seed oils includes phytochemicals, and mass spectrometry of fatty acid 2-methoxyethyl esters. Eur. J. Lipid Sci. Technol. 2019, 121, 1800386. [Google Scholar] [CrossRef]
- Badar, N.; Arshad, M.; Farooq, U. characteristics of anethum graveolens (Umbelliferae) seed oil: Extraction, composition and antimicrobial activity. Int. J. Agric. Biol. 2008, 10, 329–332. [Google Scholar]
- Drăghici, O.; Păcală, M.-L.; Oancea, S. Kinetic studies on the oxidative stabilisation effect of red onion skins anthocyanins extract on parsley (Petroselinum crispum) seed oil. Food Chem. 2018, 265, 337–343. [Google Scholar] [CrossRef]
- Parry, J.; Hao, Z.; Luther, M.; Su, L.; Zhou, K.; Yu, L. Characterization of cold-pressed onion, parsley, cardamom, mullein, roasted pumpkin, and milk thistle seed oils. J. Americ. Oil Chem. Soc. 2007, 84, 613. [Google Scholar] [CrossRef] [Green Version]
- Ying, Q.; Wojciechowska, P.; Siger, A.; Kaczmarek, A.; Rudzińska, M. Phytochemical content, oxidative stability, and nutritional properties of unconventional cold-pressed edible oils. J. Food Nutr. Res. 2018, 6, 476–485. [Google Scholar] [CrossRef] [Green Version]
- Diwakar, B.T.; Dutta, P.K.; Lokesh, B.R.; Naidu, K.A. physicochemical properties of garden cress (Lepidium sativum L.) seed oil. J. Americ. Oil Chem. Soc. 2010, 87, 539–548. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Amer, M.M.A.; Awad, A.E.-S. Coriander (Coriandrum sativum L.) seed oil improves plasma lipid profile in rats fed a diet containing cholesterol. Eur. Food Res. Technol. 2008, 227, 1173–1182. [Google Scholar] [CrossRef]
- Chatoui, K.; Harhar, H.; El Kamli, T.; Tabyaoui, M. Chemical composition and antioxidant capacity of Lepidium Sativum seeds from four regions of Morocco. Evid. Based Complement. Altern. Med. 2020, 2020, 7302727. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; Medici, F.; Piga, L. Use of cell wall degrading enzymes for the production of high-quality functional products from tomato processing waste. Asian J. Chem. Sci. 2014, 38, 355–360. [Google Scholar] [CrossRef]
- Xu, T.; Li, J.; Fan, Y.-W.; Zheng, T.; Deng, Z.-Y. Comparison of oxidative stability among edible oils under continuous frying conditions. Int. J. Food Prop. 2015, 18, 1478–1490. [Google Scholar] [CrossRef]
- Abril, D.; Mirabal-Gallardo, Y.; González, A.; Marican, A.; Durán-Lara, E.F.; Silva Santos, L.; Valdés, O. Comparison of the oxidative stability and antioxidant activity of extra-virgin olive oil and oils extracted from seeds of Colliguaya integerrima and Cynara cardunculus under normal conditions and after thermal treatment. Antioxidants 2019, 8, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, G.; Sharma, M.P. Potential and limitation of straight vegetable oils as engine fuel- An Indian perspective. Renew. Sust. Energ. Rev. 2014, 33, 316–322. [Google Scholar] [CrossRef]
- Ganzaroli, J.F.; Sanchez, J.L.; Da Silva, M.V.; Tanamati, A.A.C.; Fuchs, R.H.B.; Tanamati, A. Absolute quantification of fatty acids in chia seeds produced in brazil. Bol. Cent. Pesqui. Process. Aliment. 2017, 35, 1–9. [Google Scholar] [CrossRef]
- Ratusz, K.; Symoniuk, E.; Wroniak, M.; Rudzińska, M. Bioactive compounds, nutritional quality and oxidative stability of cold-pressed camelina (Camelina sativa L.) oils. Appl. Sci. 2018, 8, 2606. [Google Scholar] [CrossRef] [Green Version]
- Moser, B.R.; Vaughn, S.F. Coriander seed oil methyl esters as biodiesel fuel: Unique fatty acid composition and excellent oxidative stability. Biomass Bioenergy 2010, 34, 550–558. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation, 2nd ed.; Oily Press lipid library; Oily Press: Bridgwater, UK, 2005; pp. 103–106. [Google Scholar]
- Iqbal, M.J.; Butt, M.S.; Suleria, H.A.R. Coriander (Coriandrum sativum L.): Bioactive molecules and health effects. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2019; pp. 2199–2235. [Google Scholar] [CrossRef]
- Antoniak, K.; Bylka, W. Aktywność biologiczna wybranych składników olejków eterycznych. Cz. 2. Postępy Fitoter. 2020, 1, 42–48. [Google Scholar] [CrossRef]
- Ghaleshahi, A.Z.; Ezzatpanah, H.; Rajabzadeh, G.; Ghavami, M. Comparison and analysis characteristics of flax, perilla and basil seed oils cultivated in Iran. J. Food Sci. Technol. 2020, 57, 1258–1268. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Alara, O.R.; Abayomi, O.O. extraction, characterisation and antioxidant activity of fenugreek (Trigonella-Foenum graecum) seed oil. Mater. Sci. Technol. 2019, 2, 349–355. [Google Scholar] [CrossRef]
- Gu, L.-B.; Liu, X.-N.; Liu, H.-M.; Pang, H.-L.; Qin, G.-Y. Extraction of fenugreek (Trigonella Foenum-Graceum L.) seed oil using subcritical butane: Characterisation and process optimisation. Molecules 2017, 22, 228. [Google Scholar] [CrossRef] [Green Version]
- Hassanien, M.M.; Abdel-Razek, A.G.; Rudzińska, M.; Siger, A.; Ratusz, K.; Przybylski, R. Phytochemical contents and oxidative stability of oils from non-traditional sources. Eur. J. Lipid Sci. Technol. 2014, 116, 1563–1571. [Google Scholar] [CrossRef]
- Nogańska, N. Analiza Składu Kwasów Tłuszczowych i Stabilności Oksydacyjnej Wybranych Olejów z Nasion ziół, Przypraw i Pestek Owoców. Master’s Thesis, Warsaw University of Life Sciences, Warszawa, Poland, 2020. [Google Scholar]
- Tan, C.P.; Che Man, Y.B.; Selamat, J.; Yusoff, M.S.A. application of arrhenius kinetics to evaluate oxidative stability in vegetable oils by isothermal differential scanning calorimetry. J. Am. Oil Chem. Soc. 2001, 78, 1133–1138. [Google Scholar] [CrossRef]
- Aktar, T.; Adal, E. Determining the Arrhenius kinetics of avocado oil: Oxidative stability under Rancimat test conditions. Foods 2019, 8, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratusz, K.; Popis, E.; Ciemniewska-Żytkiewicz, H.; Wroniak, M. Oxidative stability of camelina (Camelina sativa L.) oil using Pressure Differential Scanning Calorimetry and Rancimat method. J. Therm. Anal. Calorim. 2016, 126, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Farhoosh, R.; Niazmand, R.; Rezaei, M.; Sarabi, M. Kinetic parameter determination of vegetable oil oxidation under Rancimat test conditions. Eur. J. Lipid Sci. Technol. 2008, 110, 587–592. [Google Scholar] [CrossRef]
| Oil Sample | AV [mg KOH/g] | PV [mEq O2/kg] | p-AnV | TOTOX | Chlorophylls [mg/kg] | Carotenoids [mg Pheophytin a/kg] | K232 | K268 |
|---|---|---|---|---|---|---|---|---|
| CO | 4.61 g | 9.43 b | 12.01 e | 30.69 c | 23.43 e | 23.03 c | 4.49 a | 1.21 a |
| FO | 2.25 d | 9.98 b | 3.22 c | 23.17 b | 12.59 d | 153.68 a | 3.40 b | 0.69 b |
| BO | 1.34 b | 3.41 a | 2.83 b | 9.65 a | 2.60 a | 5.40 g | 0.56 c | 0.43 c |
| PO | 1.54 c | 11.73 c | 6.55 d | 30.02 c | 93.27 f | 20.66 d | 17.11 d | 2.37 d |
| WCO | 2.64 e | 17.38 d | 2.05 a | 36.82 d | 7.52 b | 58.16 b | 0.80 e | 0.47 c |
| DO | 0.34 a | 26.10 e | 2.83 b | 55.09 e | 102.20 g | 20.26 e | 1.17 f | 0.67 b |
| TO | 3.25 f | 44.91 f | 6.82 d | 95.92 f | 10.74 c | 18.18 f | 8.06 g | 0.77 e |
| Oil Sample | Colour Components | ||
|---|---|---|---|
| L* | a* | b* | |
| CO | 73.03 d ± 0.07 | 15.04 c ± 0.02 | 104.06 c ± 0.06 |
| FO | 71.67 c ± 0.01 | 40.73 f ± 0.01 | 122.40 e ± 0.04 |
| BO | 96.69 g ± 0.01 | –4.57 a ± 0.01 | 50.79 a ± 0.07 |
| PO | 65.45 b ± 0.01 | 12.71 bc ± 0.00 | 108.18 d ± 0.07 |
| WCO | 83.29 f ± 0.02 | 23.43 d ± 0.00 | 137.85 f ± 0.02 |
| DO | 58.70 a ± 0.10 | 6.99 b ± 0.03 | 97.59 b ± 0.14 |
| TO | 79.09 e ± 0.01 | 29.87 e ± 0.01 | 131.86 g ± 0.11 |
| Fatty Acid | Oil Sample | ||||||
|---|---|---|---|---|---|---|---|
| CO | FO | BO | PO | WCO | DO | TO | |
| C4:0 | 0.85 ± 0.02 | - | - | - | - | - | - |
| C6:0 | 0.26 ± 0.02 | - | - | - | - | - | - |
| C8:0 | 4.42 ± 0.20 | - | - | - | - | - | |
| C10:0 | 0.34 ± 0.02 | - | - | - | - | - | - |
| C12:0 | 0.21 ± 0.02 | 0.16 ± 0.04 | - | - | - | - | - |
| C14:0 | - | 0.17 ± 0.00 | - | <0.1 ± 0.00 | <0.1 ± 0.01 | - | <0.1 ± 0.01 |
| C16:0 | 3.11 ± 0.03 | 9.19 ± 0.15 | 5.80 ± 0.11 | 5.24 ± 0.10 | 7.82 ± 0.04 | 5.55 ± 0.04 | 11.60 ± 0.05 |
| C16:1 | 0.41 ± 0.01 | - | <0.1 ± 0.01 | 0.69 ± 0.03 | 0.20 ± 0.00 | 0.38 ± 0.36 | 0.18 ± 0.01 |
| C17:0 | - | 0.24 ± 0.05 | <0.1 ± 0.02 | 0.40 ± 0.01 | - | - | <0.1 ± 0.01 |
| C17:1 | - | 0.13 ± 0.02 | - | 0.13 ± 0.02 | - | - | 0.32 ± 0.00 |
| C18:0 | 0.81 ± 0.00 | 4.14 ± 0.04 | 3.28 ± 0.03 | 3.00 ± 0.02 | 2.81 ± 0.02 | 3.30 ± 0.02 | 4.49 ± 0.01 |
| C18:1 | 73.01 ± 0.18 | 14.50 ± 0.01 | 17.77 ± 0.01 | 32.47 ± 0.08 | 24.17 ± 1.06 | 30.93 ± 0.52 | 17.64 ± 0.02 |
| C18:2 | 14.68 ± 0.12 | 39.70 ± 0.07 | 20.16 ± 0.14 | 53.71 ± 0.32 | 11.48 ± 0.13 | 58.03 ± 0.93 | 61.07 ± 0.02 |
| C18:3 | - | 0.16 ± 0.06 | 0.24 ± 0,01 | 2.35 ± 0.05 | 0.11 ± 0.01 | 0.52 ± 0.05 | 1.65 ± 0.00 |
| C18:3 | 0.45 ± 0.01 | 28.50 ± 0.22 | 52.10 ± 0.20 | 0.29 ± 0.12 | 29.52 ± 0.42 | - | 1.96 ± 0.02 |
| C20:0 | - | 1.31 ± 0.01 | 0.24 ± 0.00 | 0.25 ± 0.00 | 2.94 ± 0.02 | 0.23 ± 0.01 | 0.42 ± 0.00 |
| C20:1 | 0.25 ± 0.01 | 0.31 ± 0.01 | 0.17 ± 0.00 | 0.27 ± 0.11 | 11.99 ± 0.01 | 0.14 ± 0.01 | 0.13 ± 0.00 |
| C20:3 | - | - | - | <0.1 ± 0.00 | 0.51 ± 0.01 | - | - |
| C22:0 | <0.1 ± 0.01 | 0.62 ± 0.00 | 0.06 ± 0.00 | 0.67 ± 0.02 | 0.84 ± 0.02 | 0.66 ± 0.04 | 0.11 ± 0.00 |
| C22:1 | - | 0.19 ± 0.01 | - | <0.1 ± 0.01 | 4.28 ± 0.06 | - | - |
| C24:0 | <0.1 ± 0.00 | 0.25 ± 0.00 | - | 0.25 ± 0.02 | 0.69 ± 0.02 | 0.24 ± 0.01 | 0.16 ± 0.00 |
| C24:1 | - | <0.1 ± 0.02 | - | <0.1 ± 0.00 | 1.17 ± 0.11 | - | - |
| ∑SFA | 8.89 ± 0.32 | 16.08 ± 0.29 | 9.32 ± 0.13 | 9.81 ± 0.17 | 15.10 ± 0.13 | 9.98 ± 0.12 | 16.78 ± 0.08 |
| ∑MUFA | 73.67 ± 0.2 | 15.13 ± 0.07 | 17.94 ± 0.02 | 33.56 ± 0.25 | 41.81 ± 1.24 | 31.45 ± 0.89 | 18.27 ± 0.03 |
| ∑PUFA | 14.93 ± 0.13 | 68.36 ± 0.35 | 72.50 ± 0.35 | 56.35 ± 0.49 | 41.62 ± 0.57 | 58.55 ± 0.98 | 64.68 ± 0.04 |
| n-3 | 0.45 ± 0.01 | 28.50 ± 0.22 | 52.10 ± 0.20 | 0.29 ± 0.12 | 29.52 ± 0.42 | 0.00 | 1.96 ± 0.02 |
| n-6 | 14.68 ± 0.12 | 39.86 ± 0.13 | 20.40 ± 0.15 | 56.06 ± 0.37 | 11.59 ± 0.14 | 58.55 ± 0.98 | 62.72 ± 0.02 |
| n6/n3 | 33:1 | 1.5:1 | 1:2.5 | 193:1 | 1:2.5 | 1:0 | 32:1 |
| n-3/n-6 | 0.03 | 0.72 | 2.55 | 0.01 | 2.55 | 0.00 | 0.03 |
| COX | 2.35 | 10.43 | 13.56 | 6.44 | 8.11 | 6.40 | 7.25 |
| AI | 0.04 | 0.12 | 0.06 | 0.06 | 0.10 | 0.06 | 0.14 |
| TI | 0.09 | 0.12 | 0.05 | 0.18 | 0.09 | 0.20 | 0.35 |
| HH | 28.34 | 8.84 | 15.52 | 16.19 | 8.23 | 16.03 | 6.89 |
| Oil Sample | Induction Time [h] | ||||
| 90 °C | 100 °C | 105 °C | 110 °C | 120 °C | |
| FO | 16.26 b ± 0.11 | 8.02 b ± 0.11 | 5.83 b ± 0.57 | 4.23 b ± 0.01 | 2.24 a ± 0.01 |
| BO | 8.55 a ± 0.21 | 4.10 a ± 0.14 | 2.94 a ± 0.13 | 2.11 a ± 0.01 | 1.07 c ± 0.07 |
| PO | 28.59 d ± 0.13 | 12.77 d ± 0.13 | 8.63 c ± 0.16 | 5.87 c ± 0.16 | 2.91 d ± 0.04 |
| WCO | 15.59 b ± 0.00 | 7.70 b ± 0.38 | 5.42 b ± 0.28 | 4.14 b ± 0.04 | 2.26 b ± 0.01 |
| DO | 33.33 e ± 0.32 | 14.42 e ± 0.30 | 10.06 d ± 0.15 | 7.12 d ± 0.34 | 3.60 e ± 0.01 |
| TO | 20.71 c ± 0.24 | 9.27 c ± 0.15 | 6.24 b ± 0.25 | 4.12 b ± 0.06 | 2.10 a ± 0.14 |
| Oil Sample | Induction Time [h] | ||||
| 120 °C | 130 °C | 140 °C | 145 °C | 150 °C | |
| CO | 28.92 f ± 0.16 | 13.49 a ± 0.58 | 6.51 a ± 0.06 | 4.52 a ± 0.04 | 3.48 a ± 0.20 |
| Oil Sample | Z | k90 °C | k100 °C | k105 °C | k110 °C | k120 °C | Ea [KJ/mol] | ΔH [kJ/mol] | ΔS [J/mol*K] |
| FO | 9.7 × 109 | 0.2144 | 0.4138 | 0.5674 | 0.7716 | 1.3941 | 94.18 | 77.2 | −118.2 |
| BO | 6.5 × 1010 | 0.4303 | 0.8578 | 1.1946 | 1.6494 | 3.0679 | 74.08 | 80.9 | −102.3 |
| PO | 4.6 × 1011 | 0.1486 | 0.3212 | 0.4650 | 0.6668 | 1.3340 | 77.72 | 90.0 | −86.4 |
| WCO | 6.1 × 109 | 0.2193 | 0.4178 | 0.5694 | 0.7697 | 1.3743 | 86.84 | 75.8 | −121.8 |
| DO | 1.1 × 1011 | 0.1277 | 0.2667 | 0.3798 | 0.5360 | 1.0397 | 72.61 | 86.1 | −98.3 |
| TO | 7.6 × 1011 | 0.2077 | 0.4510 | 0.6545 | 0.9404 | 1.8889 | 82.97 | 90.5 | −82.4 |
| CO | Z | k120 °C | k130 °C | k140 °C | k145 °C | k150 °C | Ea [KJ/mol] | ΔH [kJ/mol] | ΔS [J/mol*K] |
| 4.9 × 1011 | 0.1504 | 0.3073 | 0.6066 | 0.8420 | 1.1596 | 87.34 | 97.6 | −85.2 |
| Quality Parameter | IT at 120 °C | Ea | k at 120 °C |
|---|---|---|---|
| AV | 0.10 | 0.66 | −0.16 |
| PV | 0.14 | −0.08 | −0.19 |
| p-AnV | 0.46 | 0.20 | −0.26 |
| TOTOX | 0.20 | −0.06 | −0.22 |
| K232 | 0.22 | −0.07 | −0.21 |
| K268 | 0.43 | −0.11 | −0.35 |
| Chlorophylls | 0.69 * | −0.56 | −0.52 |
| Carotenoids | −0.08 | 0.78 * | −0.28 |
| L* | −0.88 * | 0.02 | 0.85 * |
| a* | −0.04 | 0.86 * | −0.35 |
| b* | 0.30 | 0.64 | −0.65 |
| SFA | −0.33 | 0.61 | −0.07 |
| MUFA | 0.63 | 0.17 | −0.49 |
| PUFA | −0.61 | −0.30 | 0.53 |
| COX | −0.87 * | −0.14 | 0.77 * |
| AI | −0.41 | 0.49 | 0.06 |
| TI | 0.17 | −0.12 | −0.19 |
| HH | 0.53 | −0.18 | −0.18 |
| Ea | −0.01 | - | - |
| k at 120 °C | −0.89 * | - | - |
| IT at 120 °C | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Symoniuk, E.; Ksibi, N.; Wroniak, M.; Lefek, M.; Ratusz, K. Oxidative Stability Analysis of Selected Oils from Unconventional Raw Materials Using Rancimat Apparatus. Appl. Sci. 2022, 12, 10355. https://doi.org/10.3390/app122010355
Symoniuk E, Ksibi N, Wroniak M, Lefek M, Ratusz K. Oxidative Stability Analysis of Selected Oils from Unconventional Raw Materials Using Rancimat Apparatus. Applied Sciences. 2022; 12(20):10355. https://doi.org/10.3390/app122010355
Chicago/Turabian StyleSymoniuk, Edyta, Nour Ksibi, Małgorzata Wroniak, Marta Lefek, and Katarzyna Ratusz. 2022. "Oxidative Stability Analysis of Selected Oils from Unconventional Raw Materials Using Rancimat Apparatus" Applied Sciences 12, no. 20: 10355. https://doi.org/10.3390/app122010355
APA StyleSymoniuk, E., Ksibi, N., Wroniak, M., Lefek, M., & Ratusz, K. (2022). Oxidative Stability Analysis of Selected Oils from Unconventional Raw Materials Using Rancimat Apparatus. Applied Sciences, 12(20), 10355. https://doi.org/10.3390/app122010355








