Emerging Biomedical Applications of Carbon Dot and Polymer Composite Materials
Abstract
:1. Introduction
2. General Aspects of Carbon Dot/Polymer Composites
2.1. Synthesis of Carbon Dot/Polymer Composites
2.2. Characteristics of Carbon Dots
2.3. Pharmacology of the Carbon Dot—Fate—In the Body
2.4. Carbon Dots and Polymer Hybrid Composite Materials
2.4.1. Features of Carbon Dot/Polymer Composites
2.4.2. Photoluminescent Feature of Carbon Dot and Polymer Composites
2.4.3. Integrative Feature of Carbon Dot and Polymer Composites
3. Biomedical Applications of Carbon Dot/Polymer Composite Materials
4. Emerging Applications of Carbon Dots and Their Composite Materials
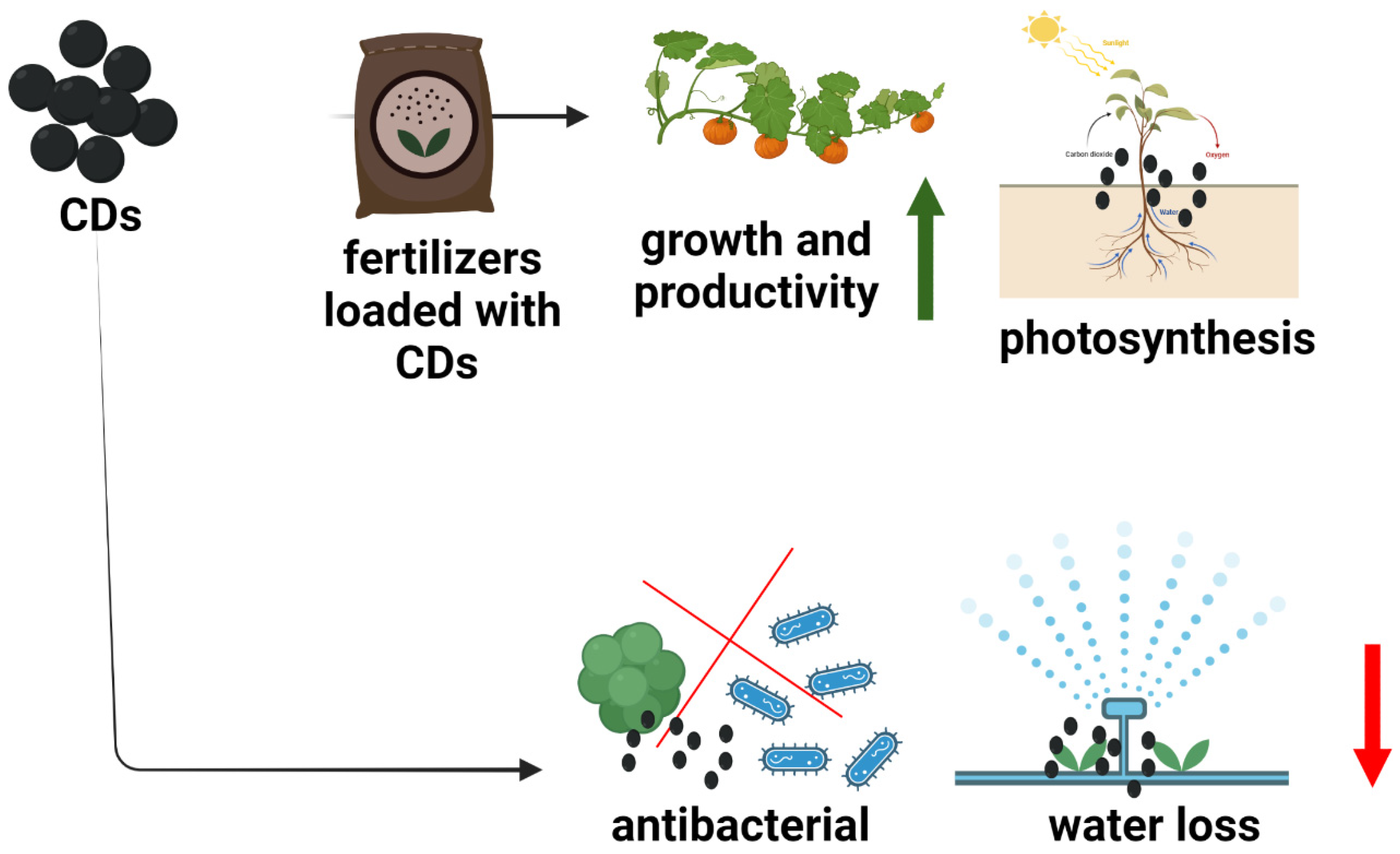
4.1. Agricultural System
4.2. COVID-19 (SARS-2-CoV-2) and Emerging Pandemics
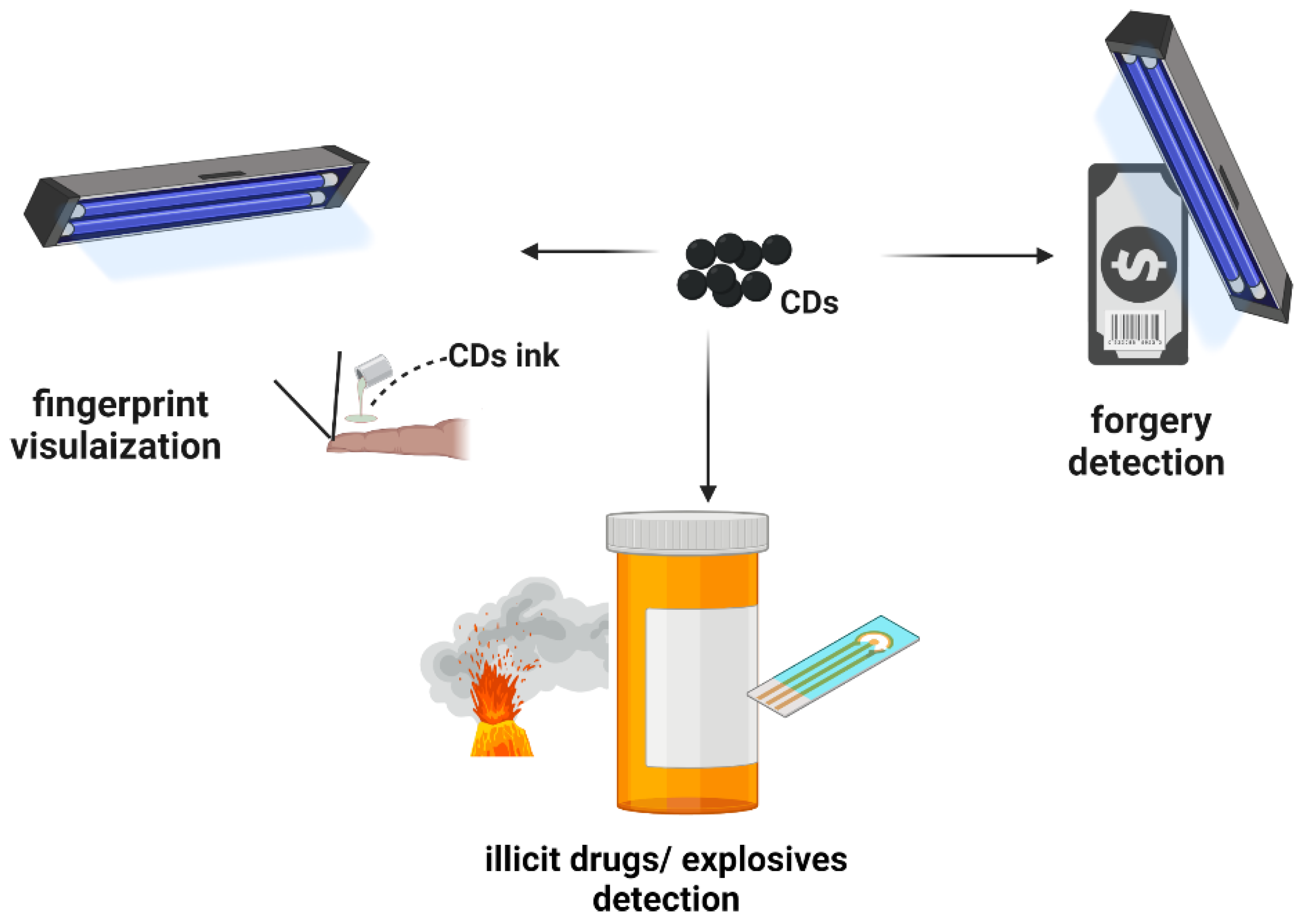
4.3. Anti-Counterfeit and Security
4.4. Treatments of Neurodegenerative Disease
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Boakye-Yiadom, K.O.; Kesse, S.; Opoku-Damoah, Y.; Filli, M.S.; Aquib, M.; Joelle, M.M.B.; Farooq, M.A.; Mavlyanova, R.; Raza, F.; Bavi, R.; et al. Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharm. 2019, 564, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Monte-Filho, S.S.; Andrade, S.I.E.; Lima, M.B.; Araujo, M.C.U. Synthesis of highly fluorescent carbon dots from lemon and onion juices for determination of riboflavin in multivitamin/mineral supplements. J. Pharm. Anal. 2019, 9, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.W.; Lee, S.L.; Chang, C.J.; Liu, L. Recent Progress of Carbon Dot Precursors and Photocatalysis Applications. Polymers 2019, 11, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadesse, A.; Belachew, N.; Hagos, M.; Basavaiah, K. Synthesis of fluorescent nitrogen and phosphorous co-doped carbon quantum dots for sensing of iron, cell imaging and antioxidant activities. J. Fluoresc. 2021, 31, 763–774. [Google Scholar] [CrossRef]
- Doring, A.; Ushakova, E.; Rogach, A.L. Chiral carbon dots: Synthesis, optical properties, and emerging applications. Light Sci. Appl. 2022, 11, 75. [Google Scholar] [CrossRef]
- Qu, J.H.; Wei, Q.; Sun, D.W. Carbon dots: Principles and their applications in food quality and safety detection. Crit. Rev. Food Sci. Nutr. 2018, 58, 2466–2475. [Google Scholar] [CrossRef]
- Cao, L.; Fernando, K.A.S.; Liang, W.X.; Seilkop, A.; Veca, L.M.; Sun, Y.P.; Bunker, C.E. Carbon dots for energy conversion applications. J. Appl. Phys. 2019, 125, 220903. [Google Scholar] [CrossRef] [Green Version]
- Su, W.; Wu, H.; Xu, H.M.; Zhang, Y.; Li, Y.C.; Li, X.H.; Fan, L.N. Carbon dots: A booming material for biomedical applications. Mater. Chem. Front. 2020, 4, 821–836. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Y.; Gong, T.; Cui, K.; Hou, L.; Yuan, C. B, N co-doped carbon dots based fluorescent test paper and hydrogel for visual and efficient dual ion detection. Inorg. Chem. Commun. 2022, 145, 110047. [Google Scholar] [CrossRef]
- Wei, S.; Li, Y.; Liang, H.; Yen, Y.; Lin, Y.; Chang, H. Photoluminescent carbon nanomaterials for sensing of illicit drugs: Focus. Anal. Sci. 2022, 38, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, H.; Ling, L.; Li, G.; Cheng, R.; Lu, X.; Xie, A.-Q.; Li, Q.; Wang, C.-F.; Chen, S. Green synthesis of carbon dots toward anti-counterfeiting. ACS Sust. Chem. Eng. 2020, 8, 1566–1572. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Banerjee, S.; Das, N.C. Advancement in science and technology of carbon dot-polymer hybrid composites: A review. Funct. Compos. Struct. 2019, 1, 022001. [Google Scholar] [CrossRef]
- Foubert, A.; Beloglazova, N.V.; Rajkovic, A.; Sas, B.; Madder, A.; Goryacheva, I.Y.; De Saeger, S. Bioconjugation of quantum dots: Review & impact on future application. Trac-Trends Analyt. Chem. 2016, 83, 31–48. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Sharma, S.K.; Peng, Z.; Leblanc, R.M. Polymers in carbon dots: A review. Polymers 2017, 9, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharker, S.M.; Do, M. Nanoscale carbon-polymer dots for theranostics and biomedical exploration. J. Nanotheranostics 2021, 2, 118–130. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Jayanthi, M.; Megarajan, S.; Subramaniyan, S.B.; Kamlekar, R.K.; Anbazhagan, V. A convenient green method to synthesize luminescent carbon dots from edible carrot and its application in bioimaging and preparation of nanocatalyst. J. Mol. Liq. 2019, 278, 175–182. [Google Scholar] [CrossRef]
- Ghosh, D.; Sarkar, K.; Devi, P.; Kim, K.H.; Kumar, P. Current and future perspectives of carbon and graphene quantum dots: From synthesis to strategy for building optoelectronic and energy devices. Renew. Sust. Energy Rev. 2021, 135, 110391. [Google Scholar] [CrossRef]
- Xie, J.D.; Lai, G.W.; Huq, M.M. Hydrothermal route to graphene quantum dots: Effects of precursor and temperature. Diam. Relat. Mater. 2017, 79, 112–118. [Google Scholar] [CrossRef]
- Ross, S.; Wu, R.S.; Wei, S.C.; Ross, G.M.; Chang, H.T. The analytical and biomedical applications of carbon dots and their future theranostic potential: A review. J. Food Drug Anal. 2020, 28, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulfajri, M.; Sudewi, S.; Ismulyati, S.; Rasool, A.; Adlim, M.; Huang, G.G. Carbon dot/polymer composites with various precursors and their sensing applications: A Review. Coatings 2021, 11, 1100. [Google Scholar] [CrossRef]
- Konwar, A.; Gogoi, N.; Majumdar, G.; Chowdhury, D. Green chitosan–carbon dots nanocomposite hydrogel film with superior properties. Carbohydr. Polym. 2015, 115, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Z.-Z.; Weng, Y.; Tan, H. Pyrophosphate ion-responsive alginate hydrogel as an effective fluorescent sensing platform for alkaline phosphatase detection. Chem. Commun. 2019, 55, 11450–11453. [Google Scholar] [CrossRef]
- Bi, R.; Zhang, R.; Shen, J.; Liu, Y.-N.; He, M.; You, X.; Su, Y.; Jiang, Z. Graphene quantum dots engineered nanofiltration membrane for ultrafast molecular separation. J. Membr. Sci. 2019, 572, 504–511. [Google Scholar] [CrossRef]
- Yang, W.J.; Shao, D.D.; Zhou, Z.; Xia, Q.C.; Chen, J.; Cao, X.L.; Zheng, T.; Sun, S.P. Carbon quantum dots (CQDs) nanofiltration membranes towards efficient biogas slurry valorization. Chem. Eng. J. 2020, 385, 123993. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Feng, B.; Zhong, X.; Ostrikov, K.K. Photoluminescence mechanism of carbon dots: Triggering high-color-purity red fluorescence emission through edge amino protonation. Nat. Commun. 2021, 12, 6856. [Google Scholar] [CrossRef]
- Linehan, K.; Doyle, H. Size controlled synthesis of carbon quantum dots using hydride reducing agents. J. Mater. Chem. C 2014, 2, 6025–6031. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Siddique, A.B.; Pramanick, A.K.; Chatterjee, S.; Ray, M. Amorphous carbon dots and their remarkable ability to detect 2,4,6-trinitrophenol. Sci. Rep. 2018, 8, 9770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.B.; Liu, K.K.; Song, S.Y.; Zhou, R.; Shan, C.X. Fluorescent nano-biomass dots: Ultrasonic-assisted extraction and their application as nanoprobe for Fe3+ detection. Nanoscale Res. Lett. 2019, 14, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulfajri, M.; Dayalan, S.; Li, W.Y.; Chang, C.J.; Chang, Y.P.; Huang, G.G. Nitrogen-doped carbon dots from averrhoa carambola fruit extract as a fluorescent probe for methyl orange. Sensors 2019, 19, 5008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Claudel, M.; Ronzani, C.; Arezki, Y.; Lebeau, L.; Pons, F. Physicochemical characteristics that affect carbon dot safety: Lessons from a comprehensive study on a nanoparticle library. Int. J. Pharm. 2019, 569, 118521. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, T. Photoluminescence tuning in carbon dots: Surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C 2018, 6, 7944–7970. [Google Scholar] [CrossRef]
- Mihalache, I.; Radoi, A.; Pascu, R.; Romanitan, C.; Vasile, E.; Kusko, M. Engineering graphene quantum dots for enhanced ultraviolet and visible light p-si nanowire-based photodetector. ACS Appl. Mater. Interfaces 2017, 9, 29234–29247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Tan, Z. Fluorescent carbon dots: Fantastic electroluminescent materials for light-emitting diodes. Adv. Sci. 2021, 8, 2001977. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yan, F.; Xu, J.; Zhang, H.; Chen, L. Solvent-controlled synthesis strategy of multicolor emission carbon dots and its applications in sensing and light-emitting devices. Nano Res. 2021, 15, 414–422. [Google Scholar] [CrossRef]
- Ding, Y.; Yu, J.; Chen, X.; Wang, S.; Tu, Z.; Shen, G.; Wang, H.; Jia, R.; Ge, S.; Ruan, J.; et al. Dose-dependent carbon-dot-induced ros promote uveal melanoma cell tumorigenicity via activation of mtor signaling and glutamine metabolism. Adv. Sci. 2021, 8, 2002404. [Google Scholar] [CrossRef]
- Luo, W.K.; Zhang, L.L.; Yang, Z.Y.; Guo, X.H.; Wu, Y.; Zhang, W.; Luo, J.K.; Tang, T.; Wang, Y. Herbal medicine derived carbon dots: Synthesis and applications in therapeutics, bioimaging and sensing. J. Nanobiotechnol. 2021, 19, 320. [Google Scholar] [CrossRef]
- Martin, C.; Jun, G.; Schurhammer, R.; Reina, G.; Chen, P.; Bianco, A.; Menard-Moyon, C. Enzymatic degradation of graphene quantum dots by human peroxidases. Small 2019, 15, e1905405. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truskewycz, A.; Yin, H.; Halberg, N.; Lai, D.T.H.; Ball, A.S.; Truong, V.K.; Rybicka, A.M.; Cole, I. Carbon dot therapeutic platforms: Administration, distribution, metabolism, excretion, toxicity, and therapeutic potential. Small 2022, 18, e2106342. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gao, Z.; Gao, G.; Wo, Y.; Wang, Y.; Shen, G.; Cui, D. Systematic safety evaluation on photoluminescent carbon dots. Nanoscale Res. Lett. 2013, 8, 122. [Google Scholar] [CrossRef] [Green Version]
- Tabish, T.A.; Lin, L.; Ali, M.; Jabeen, F.; Ali, M.; Iqbal, R.; Horsell, D.W.; Winyard, P.G.; Zhang, S. Investigating the bioavailability of graphene quantum dots in lung tissues via Fourier transform infrared spectroscopy. Interface Focus 2018, 8, 20170054. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.B.; Zhu, S.J.; Shao, J.R.; Yang, B. Polymer carbon dots-a highlight reviewing their unique structure, bright emission and probable photoluminescence mechanism. J. Polym. Sci. Part A-Polym. Chem. 2017, 55, 610–615. [Google Scholar] [CrossRef]
- Bellani, S.; Bartolotta, A.; Agresti, A.; Calogero, G.; Grancini, G.; Di Carlo, A.; Kymakis, E.; Bonaccorso, F. Solution-processed two-dimensional materials for next-generation photovoltaics. Chem. Soc. Rev. 2021, 50, 11870–11965. [Google Scholar] [CrossRef]
- Muthurasu, A.; Ganesh, V. Tuning optical properties of nitrogen-doped carbon dots through fluorescence resonance energy transfer using Rhodamine B for the ratiometric sensing of mercury ions. Analyt. Methods 2021, 13, 1857–1865. [Google Scholar] [CrossRef]
- He, M.; Zhang, J.; Wang, H.; Kong, Y.; Xiao, Y.; Xu, W. Material and optical properties of fluorescent carbon quantum dots fabricated from lemon juice via hydrothermal reaction. Nanoscale Res. Lett. 2018, 13, 175. [Google Scholar] [CrossRef]
- Tao, S.; Zhu, S.; Feng, T.; Xia, C.; Song, Y.; Yang, B. The polymeric characteristics and photoluminescence mechanism in polymer carbon dots: A review. Mater. Today Chem. 2017, 6, 13–25. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cui, K.; Gong, T.; Zhang, J.; Zhai, Z.; Hou, L.; Zaman, F.U.; Yuan, C. Ultrasonic-assisted synthesis of n-doped, multicolor carbon dots toward fluorescent inks, fluorescence sensors, and logic gate operations. Nanomaterials 2022, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.J.; Song, Y.B.; Zhao, X.H.; Shao, J.R.; Zhang, J.H.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Phatake, R.S.; Nabha Barnea, S.; Zerby, N.; Zhu, J.J.; Shikler, R.; Lemcoff, N.G.; Jelinek, R. Fluorescent self-healing carbon dot/polymer gels. ACS Nano 2019, 13, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Xiong, H.M. Exploring the blue luminescence origin of nitrogen-doped carbon dots by controlling the water amount in synthesis. RSC Adv. 2015, 5, 66528–66533. [Google Scholar] [CrossRef]
- Liu, C.J.; Zhang, P.; Tian, F.; Li, W.C.; Li, F.; Liu, W.G. One-step synthesis of surface passivated carbon nanodots by microwave assisted pyrolysis for enhanced multicolor photoluminescence and bioimaging. J. Mater. Chem. 2011, 21, 13163–13167. [Google Scholar] [CrossRef]
- Sharker, S.M. Hexagonal Boron Nitrides (White Graphene): A promising method for cancer drug delivery. Int. J. Nanomed. 2019, 14, 9983–9993. [Google Scholar] [CrossRef] [Green Version]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef]
- Duan, Q.Q.; Ma, Y.; Che, M.X.; Zhang, B.Y.; Zhang, Y.X.; Li, Y.; Zhang, W.D.; Sang, S.B. Fluorescent carbon dots as carriers for intracellular doxorubicin delivery and track. J. Drug Deliv. Sci. Technol. 2019, 49, 527–533. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, W.; Zhang, B.; Zhou, D.; Fan, X.; Wang, X.; Liu, X. Carbon dots embedded hybrid microgel with phenylboronic acid as monomer for fluorescent glucose sensing and glucose-triggered insulin release at physiological pH. Nanomaterials 2022, 12, 3065. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Wu, H.C.; Kuan, C.H.; Lin, C.J.; Wang, L.W.; Chang, C.W.; Wang, T.W. Multi-functionalized carbon dots as theranostic nanoagent for gene delivery in lung cancer therapy. Sci. Rep. 2016, 6, 21170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, J.; Yi, G.; Yoo, J.; Park, C.; Koo, H.; Choi, H.S. Light-responsive nanomedicine for biophotonic imaging and targeted therapy. Adv. Drug Deliv. Rev. 2019, 138, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Liu, H.R.; Lou, Q.; Wang, F.; Liu, K.K.; Dong, L.; Shan, C.X. Recent progress of carbon dots in targeted bioimaging and cancer therapy. Theranostics 2022, 12, 2860–2893. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, Y.; Wang, L. Synthesis and applications of red-emissive carbon dots. Chem. Rec. 2019, 19, 2083–2094. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal treatment of grass: A low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu(II) ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef]
- Lu, S.; Sui, L.; Liu, J.; Zhu, S.; Chen, A.; Jin, M.; Yang, B. Near-Infrared photoluminescent polymer-carbon nanodots with two-photon fluorescence. Adv. Mater. 2017, 29, 1603443. [Google Scholar] [CrossRef]
- Li, Q.; Ohulchanskyy, T.Y.; Liu, R.L.; Koynov, K.; Wu, D.Q.; Best, A.; Kumar, R.; Bonoiu, A.; Prasad, P.N. Photoluminescent carbon dots as biocompatible nanoprobes for targeting cancer cells in vitro. J. Phys. Chem. C 2010, 114, 12062–12068. [Google Scholar] [CrossRef]
- Tao, S.; Feng, T.; Zheng, C.; Zhu, S.; Yang, B. Carbonized polymer dots: A brand new perspective to recognize luminescent carbon-based nanomaterials. J. Phys. Chem. Lett. 2019, 10, 5182–5188. [Google Scholar] [CrossRef]
- Zhou, L.; He, B.; Huang, J. Amphibious fluorescent carbon dots: One-step green synthesis and application for light-emitting polymer nanocomposites. Chem. Commun. 2013, 49, 8078–8080. [Google Scholar] [CrossRef]
- Zhang, P.; Li, W.; Zhai, X.; Liu, C.; Dai, L.; Liu, W. A facile and versatile approach to biocompatible “fluorescent polymers” from polymerizable carbon nanodots. Chem. Commun. 2012, 48, 10431–10433. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Y.; Liu, H.J.; Jiang, S.; Chen, Y.; Yao, Y. Hyperbranched polymer functionalized carbon dots with multistimuli-responsive property. ACS Macro Lett. 2013, 2, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, P.; Zhai, X.; Tian, F.; Li, W.; Yang, J.; Liu, Y.; Wang, H.; Wang, W.; Liu, W. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 2012, 33, 3604–3613. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, J.; Kim, H.; Singha, K.; Kim, W.J. Transfection and intracellular trafficking properties of carbon dot-gold nanoparticle molecular assembly conjugated with PEI-pDNA. Biomaterials 2013, 34, 7168–7180. [Google Scholar] [CrossRef]
- Sachdev, A.; Matai, I.; Gopinath, P. Carbon dots incorporated polymeric hydrogels as multifunctional platform for imaging and induction of apoptosis in lung cancer cells. Colloids Surf. B 2016, 141, 242–252. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, A.; Kumari, R.; Sinha, K.K.; Singh, M.K.; Das, P. Carbon dots assisted formation of DNA hydrogel for sustained release of drug. Carbon 2017, 114, 169–176. [Google Scholar] [CrossRef]
- Hu, M.; Gu, X.Y.; Hu, Y.; Wang, T.; Huang, J.; Wang, C.Y. Low chemically cross-linked PAM/C-dot hydrogel with robustness and superstretchability in both as-prepared and swelling equilibrium states. Macromolecules 2016, 49, 3174–3183. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Xue, Y.N.; Li, S.R.; Zhang, X.H.; Fei, H.X.; Wu, X.G.; Sang, S.B.; Li, X.N.; Wei, M.; Chen, W.Y. Nanocomposite carbon dots/PAM fluorescent hydrogels and their mechanical properties. J. Polym. Res. 2017, 24, 224. [Google Scholar] [CrossRef]
- Wang, H.; Yi, J.; Velado, D.; Yu, Y.; Zhou, S. Immobilization of carbon dots in molecularly imprinted microgels for optical sensing of glucose at physiological pH. ACS Appl. Mater. Interfaces 2015, 7, 15735–15745. [Google Scholar] [CrossRef]
- Wang, H.; Di, J.; Sun, Y.; Fu, J.; Wei, Z.; Matsui, H.; del C. Alonso, A.; Zhou, S. Biocompatible PEG-chitosan@ carbon dots hybrid nanogels for two-photon fluorescence imaging, near-infrared light/pH dual-responsive drug carrier, and synergistic therapy. Adv. Funct. Mater. 2015, 25, 5537–5547. [Google Scholar] [CrossRef]
- Gao, Q.; Han, J.; Ma, Z. Polyamidoamine dendrimers-capped carbon dots/Au nanocrystal nanocomposites and its application for electrochemical immunosensor. Biosens. Bioelectron. 2013, 49, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Hu, S.; Zhang, H.; Chen, J.; He, Y.; Li, F.; Weng, W.; Ni, J.; Bao, X.; Lin, Y. Carbon dots and chitosan composite film based biosensor for the sensitive and selective determination of dopamine. Analyst 2013, 138, 5417–5423. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Das, K.; Das, P.K. Label-free fluorimetric detection of histone using quaternized carbon dot–DNA nanobiohybrid. Chem. Commun. 2013, 49, 8851–8853. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, S.; Kumar, M.; Mandal, B.B.; Karak, N. High performance luminescent thermosetting waterborne hyperbranched polyurethane/carbon quantum dot nanocomposite with in vitro cytocompatibility. Compos. Sci. Technol. 2015, 118, 39–46. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Adolfsson, K.H.; Wu, D.; Hakkarainen, M. Supramolecular assembly of biobased graphene oxide quantum dots controls the morphology of and induces mineralization on poly (ε-caprolactone) films. Biomacromolecules 2016, 17, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.; Erdal, N.B.; Hakkarainen, M.; Nandan, B.; Srivastava, R.K. Cellulose-derived nanographene oxide reinforced macroporous scaffolds of high internal phase emulsion-templated cross-Linked poly (ε-caprolactone). Biomacromolecules 2019, 21, 589–596. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A mini review on carbon quantum dots: Preparation, properties, and electrocatalytic application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Wu, D.; Samanta, A.; Srivastava, R.K.; Hakkarainen, M. Nano-graphene oxide functionalized bioactive poly (lactic acid) and poly (ε-caprolactone) nanofibrous scaffolds. Materials 2018, 11, 566. [Google Scholar] [CrossRef] [Green Version]
- Mazrad, Z.A.I.; Phuong, P.T.M.; Choi, C.A.; In, I.; Lee, K.D.; Park, S.Y. pH/redox-triggered photothermal treatment for cancer therapy based on a dual-responsive cationic polymer dot. Chem. Med. Chem. 2018, 13, 2437–2447. [Google Scholar] [CrossRef]
- Ardekani, S.M.; Dehghani, A.; Hassan, M.; Kianinia, M.; Aharonovich, I.; Gomes, V.G. Two-photon excitation triggers combined chemo-photothermal therapy via doped carbon nanohybrid dots for effective breast cancer treatment. Chem. Eng. J. 2017, 330, 651–662. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Q.; Dai, X.; Sun, J.; Gao, F. Dual-emission carbonized polymer dots for ratiometric ph sensing, ph-dependent generation of singlet oxygen, and imaging-guided dynamics monitoring of photodynamic therapy. ACS Appl. Bio. Mater. 2021, 4, 7663–7672. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, F.; Jin, H.; Han, Y.; Wang, L.; Bao, L.; Chen, T.; Yan, Y.; Qiu, Y.; Chen, Z.-L. Incorporation of green emission polymer dots into pyropheophorbide-α enhance the PDT effect and biocompatibility. Photodiagnosis Photodyn. Ther. 2022, 37, 102562. [Google Scholar] [CrossRef] [PubMed]
- Agnol, L.D.; Neves, R.M.; Maraschin, M.; Moura, S.; Luiz, H.L.; Dias, F.T.G.; Bianchi, O. Green synthesis of Spirulina-based carbon dots for stimulating agricultural plant growth. Sustain. Mater. Technol. 2021, 30, e00347. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Z.; Wang, X.; Peng, X.; Zheng, J. Fluorescent carbon-dots enhance light harvesting and photosynthesis by overexpressing PsbP and PsiK genes. J. Nanobiotechnol. 2021, 19, 260. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Zhang, M.L.; Song, Y.X.; Li, H.; Huang, H.; Shao, M.W.; Liu, Y.; Kang, Z.H. Carbon dots promote the growth and photosynthesis of mung bean sprouts. Carbon 2018, 136, 94–102. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Fan, D.; Jiang, F. Effect of carbon dots with chitosan coating on microorganisms and storage quality of modified-atmosphere-packaged fresh-cut cucumber. J. Sci. Food Agric. 2019, 99, 6032–6041. [Google Scholar] [CrossRef]
- Dan, X.; HongMei, P.; Hao, T.; DeZhi, Y.; YaLing, Y.; Hong, L. Application of carbon dots-chitosan coating in preservation of mango. Food Ferment. Ind. 2019, 45, 130–135. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Hendrix, B.; Hoffer, P.; Sanders, R.A.; Zheng, W. carbon dots for efficient small interfering RNA delivery and gene silencing in plants. Plant Physiol. 2020, 184, 647–657. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Wong, L.R.; Li, K.; Verma, A.K.; Ortiz, M.E.; Wohlford-Lenane, C.; Leidinger, M.R.; Knudson, C.M.; Meyerholz, D.K.; McCray, P.B.; et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 2021, 589, 603–607. [Google Scholar] [CrossRef]
- Ye, C.; Qi, L.; Wang, J.; Zheng, S. COVID-19 Pandemic: Advances in diagnosis, treatment, organoid applications and impacts on cancer patient management. Front. Med. 2021, 8, 606755. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Alhakamy, N.A.; Md, S.; Nair, A.B.; Deb, P.K. Exploring the potential of carbon dots to combat COVID-19. Front. Mol. Biosci. 2020, 7, 616575. [Google Scholar] [CrossRef] [PubMed]
- Kalkal, A.; Allawadhi, P.; Pradhan, R.; Khurana, A.; Bharani, K.K.; Packirisamy, G. Allium sativum derived carbon dots as a potential theranostic agent to combat the COVID-19 crisis. Sensors Int. 2021, 2, 100102. [Google Scholar] [CrossRef]
- Belza, J.; Opletalova, A.; Polakova, K. Carbon dots for virus detection and therapy. Microchim. Acta 2021, 188, 430. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Shauloff, N.; Sharma, C.P.; Shimoni, R.; Arnusch, C.J.; Jelinek, R. Carbon dot-polymer nanoporous membrane for recyclable sunlight-sterilized facemasks. J. Colloid. Interface Sci. 2021, 592, 342–348. [Google Scholar] [CrossRef]
- Meilia, P.D.I.; Freeman, M.D.; Herkutanto; Zeegers, M.P. A review of the diversity in taxonomy, definitions, scope, and roles in forensic medicine: Implications for evidence-based practice. Forensic Sci. Med. Pathol. 2018, 14, 460–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.; Ren, G.; Zhu, B.; Yu, L.; Liu, X.; Chai, F.; Wu, H.; Wang, C. Facile synthesis of orange emissive carbon dots and their application for mercury ion detection and fast fingerprint development. Analyt. Methods 2019, 11, 2072–2081. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, L.; Lu, J.; Xu, C.; Cai, C.; Lin, H. Triple-mode emission of carbon dots: Applications for advanced anti-counterfeiting. Angew. Chem. Int. Ed. 2016, 55, 7231–7235. [Google Scholar] [CrossRef]
- Verhagen, A.; Kelarakis, A. Carbon dots for forensic applications: A critical review. Nanomaterials 2020, 10, 1535. [Google Scholar] [CrossRef]
- Khan, F.; Oloketuyi, S.F. A future perspective on neurodegenerative diseases: Nasopharyngeal and gut microbiota. J. Appl. Microbiol. 2017, 122, 306–320. [Google Scholar] [CrossRef]
- Maneu, V.; Lax, P.; Cuenca, N. Current and future therapeutic strategies for the treatment of retinal neurodegenerative diseases. Neural. Regen. Res. 2022, 17, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xu, N.; Fan, J.; Sun, W.; Peng, X. Carbon dots for in vivo bioimaging and theranostics. Small 2019, 15, e1805087. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Ruan, S.; Liu, S.; Sun, T.; Qu, D.; Zhao, H.; Xie, Z.; Gao, H.; Jing, X.; Sun, Z. Self-targeting fluorescent carbon dots for diagnosis of brain cancer cells. ACS Nano 2015, 9, 11455–11461. [Google Scholar] [CrossRef]
- Seven, E.S.; Seven, Y.B.; Zhou, Y.; Poudel-Sharma, S.; Diaz-Rucco, J.J.; Kirbas Cilingir, E.; Mitchell, G.S.; Van Dyken, J.D.; Leblanc, R.M. Crossing the blood-brain barrier with carbon dots: Uptake mechanism and in vivo cargo delivery. Nanoscale Adv. 2021, 3, 3942–3953. [Google Scholar] [CrossRef] [PubMed]
- Damian Guerrero, E.; Lopez-Velazquez, A.M.; Ahlawat, J.; Narayan, M. Carbon quantum dots for treatment of amyloid disorders. ACS Appl. Nano Mater. 2021, 4, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; Liu, X.Y.; Li, S.L.; Jiang, P.; Jiang, F.L.; Liu, Y. High-oxygen-content carbon dots as a high-efficiency inhibitor of human insulin aggregation. ACS Appl. Bio Mater. 2019, 2, 4067–4076. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.A.; Praveena, P.; Dhanavel, S.; Manikandan, R.; Senthilkumar, S.; Stephen, A. Luminescent chitosan/carbon dots as an effective nano-drug carrier for neurodegenerative diseases. RSC Adv. 2020, 10, 24386–24396. [Google Scholar] [CrossRef]




| CD/Polymer Composites | Biomedical Application | References |
|---|---|---|
| CD/poly(N-isopropylacrylamide) (PNIPAM) | Thermo-responsive drug delivery and bioimaging | [70,71] |
| CD/polyethylenimine (PEI) | Drug delivery with bioimaging | [72] |
| CD/PEI | Gene delivery and bioimaging | [73] |
| CD/gold nanoparticle and PEI | pDNA delivery and bioimaging | [74] |
| CD/Chitosan | Hydrogel-based 5-fluorouracil drug delivery | [75] |
| CD/deoxyribonucleic acid (DNA) | Hydrogel-based doxorubicin drug delivery | [76] |
| CD/polyacrylamide (PAM) | Fluorescence hydrogel with excellent mechanical stability | [77,78] |
| CD/poly(N-isopropylacrylamide-acrylamide-vinylphenylboronic acid) | Hydrogel based glucose sensing and measurement | [79] |
| CD/PEG and chitosan | pH sensitive fluorescence biosensing system | [80] |
| CD/polyamidoamine and gold nanocrystal | Immune-sensing of alpha-fetoprotein biosensor | [81] |
| CD/chitosan | Carbon electrode (biosensor) for selective determination of dopamine | [82] |
| CD/DNA | Nanobiohybrid (biosensor) for fluorimetric detection of histone | [83] |
| CD/polyurethane | Bone tissue engineering | [84] |
| CD/poly(ε-caprolactone) | Biomineralization for bone cells growth | [85,86] |
| CD/starch | Tissue scaffold for tissue engineering | [87] |
| CD/PLA and PCL electrospun fibers | Osteo-bioactivity for bone cells growth | [88] |
| CD/PEI and folic acid | Photothermal therapy | [89] |
| CD/PEG | Photothermal therapy and doxorubicin drug delivery | [90] |
| CD/PEI and 4, 4′, 4″, 4‴-(porphine-5, 10, 15, 20-tetrayl) tetrakis (benzoic acid) (TCPP) | Photodynamic therapy | [91] |
| CD/pyropheophorbide-a (PPa) | Photodynamic therapy | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam, G.O.; Sharker, S.M.; Ryu, J.H. Emerging Biomedical Applications of Carbon Dot and Polymer Composite Materials. Appl. Sci. 2022, 12, 10565. https://doi.org/10.3390/app122010565
Adam GO, Sharker SM, Ryu JH. Emerging Biomedical Applications of Carbon Dot and Polymer Composite Materials. Applied Sciences. 2022; 12(20):10565. https://doi.org/10.3390/app122010565
Chicago/Turabian StyleAdam, Gareeballah Osman, Shazid Md. Sharker, and Ji Hyun Ryu. 2022. "Emerging Biomedical Applications of Carbon Dot and Polymer Composite Materials" Applied Sciences 12, no. 20: 10565. https://doi.org/10.3390/app122010565






