Current State of the Gold Mining Waste from the Ores of the Ursk Deposit (Western Siberia, Russia)
Abstract
:1. Introduction
2. Materials and Methods
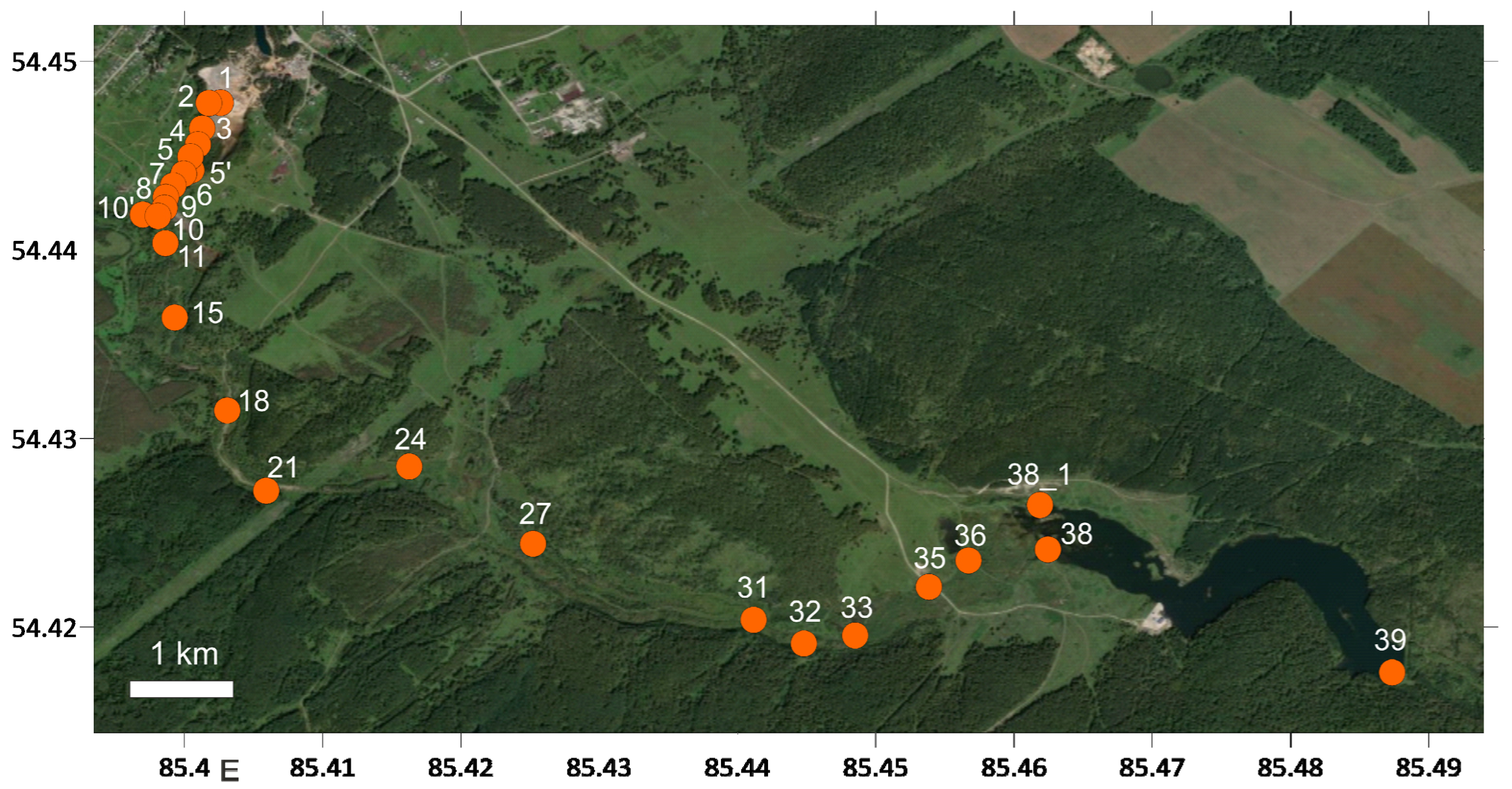
2.1. Field Work
2.1.1. Vertical Electrical Sounding
2.1.2. Magnetic Survey
2.1.3. Aerial Photography and Digital Elevation Model
2.1.4. Geochemical and Hydrochemical Sampling
2.2. Laboratory Studies
2.2.1. Analysis of Chemical Elements Concentrations in Liquid Samples
2.2.2. Analysis of Anion Concentrations in Water Samples
3. Results
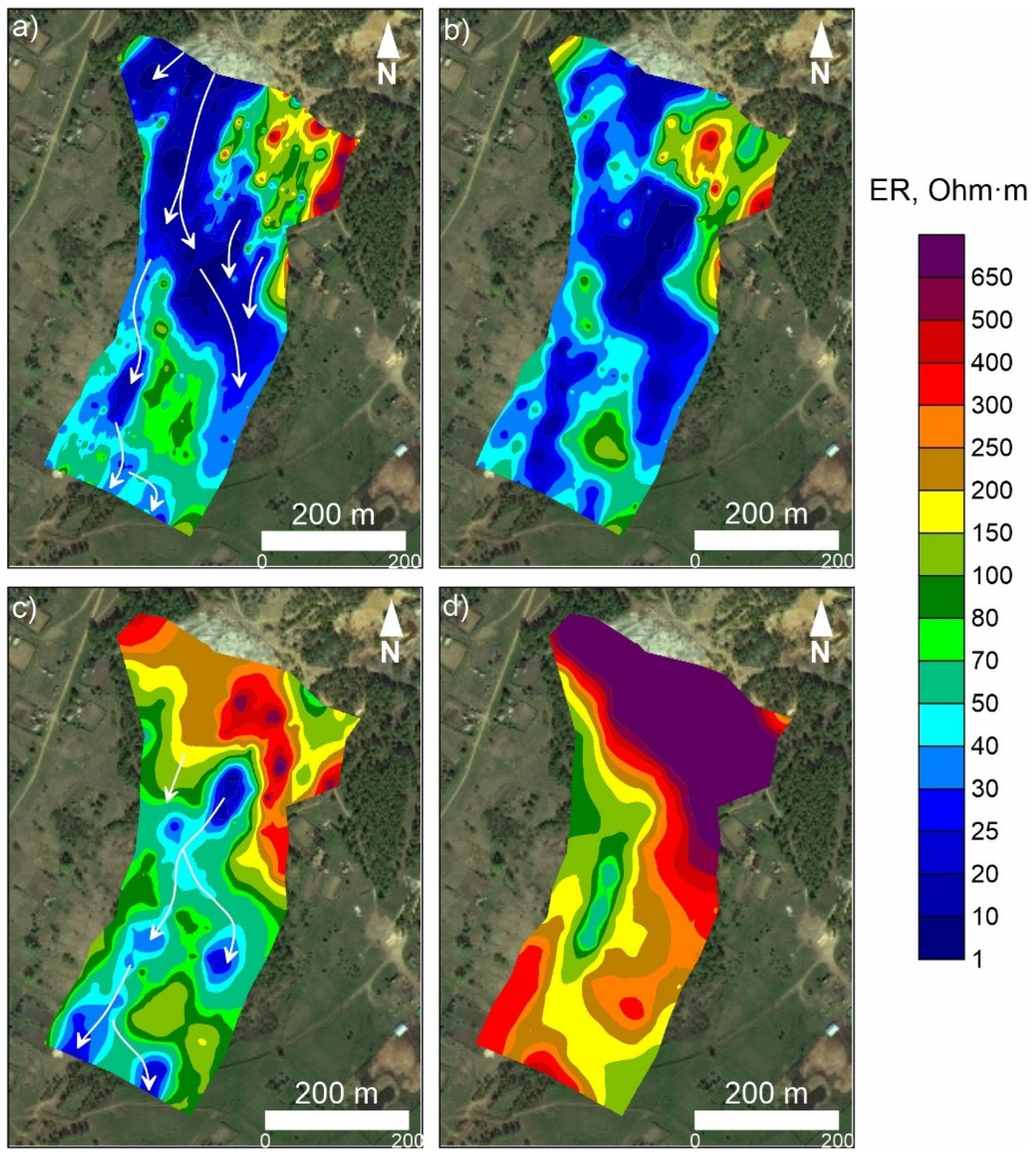
3.1. Results of ERT Study
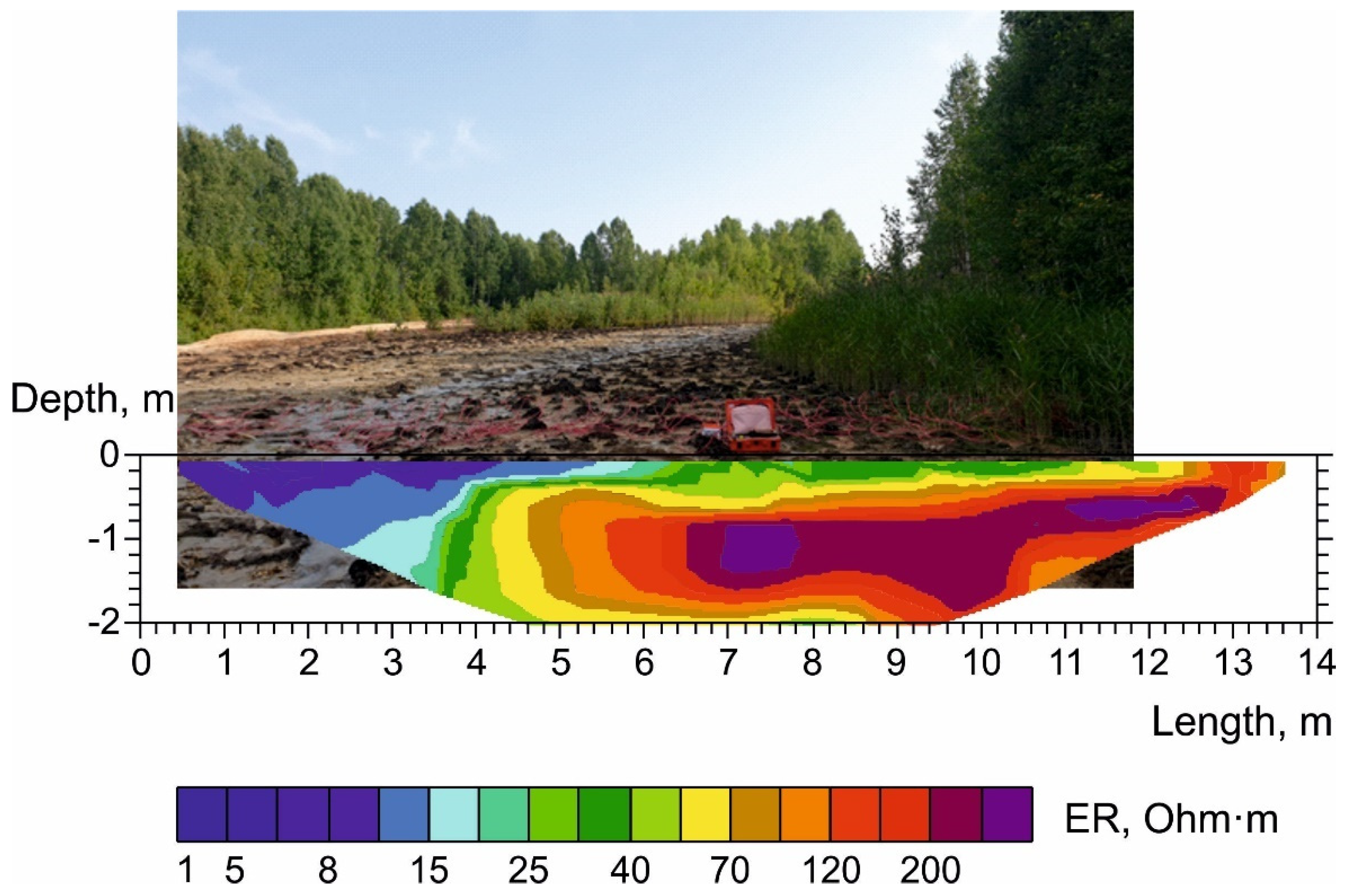
3.2. Results of the Micro-ERT Survey
3.3. Magnetic Survey Results
3.4. Results of Aerial Survey
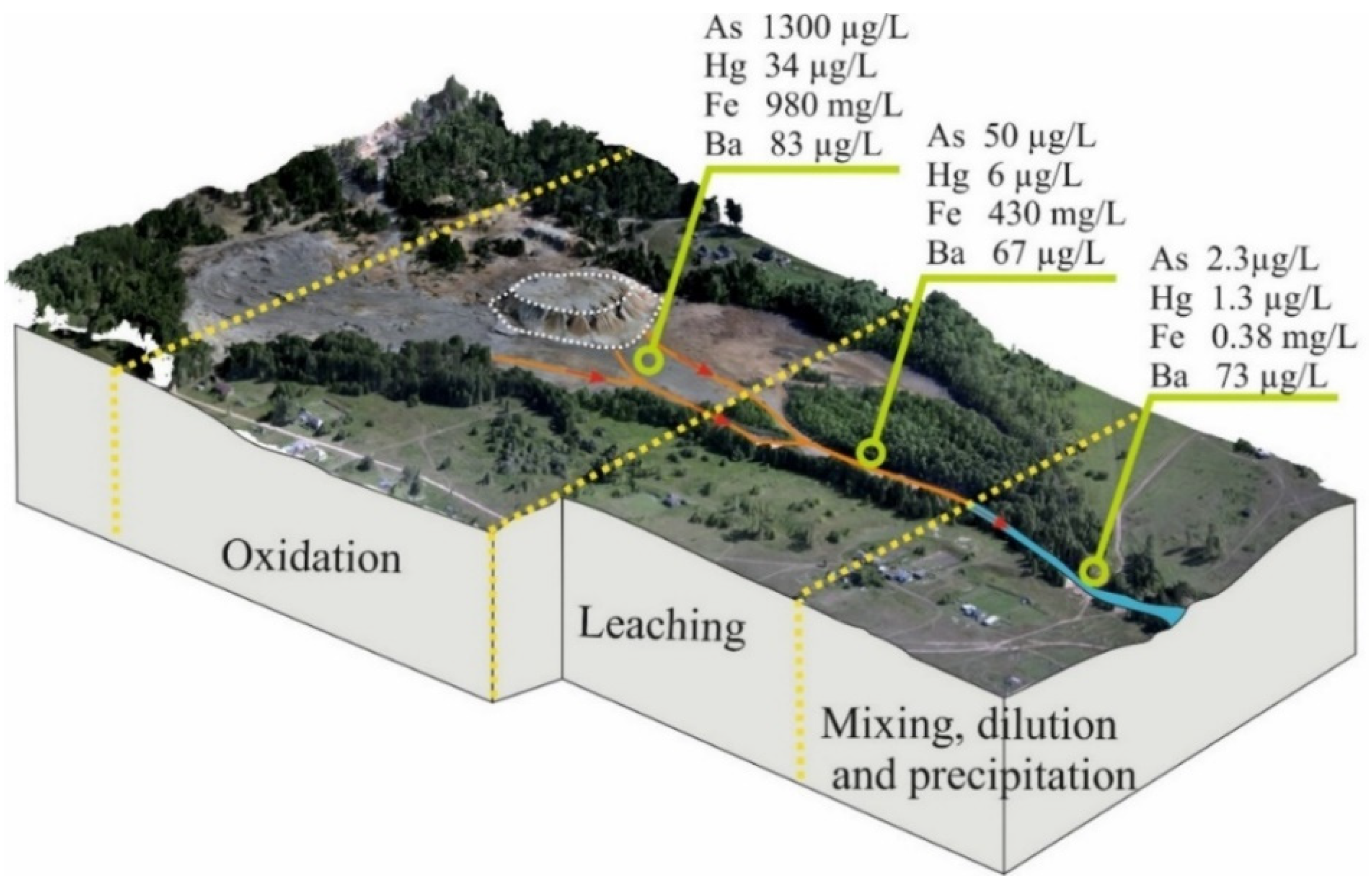
3.5. Hydrochemical Composition of Surface Watercourses in the Ursk Drainage System
4. Discussion
- Drainage stream at the foot of the dump, with a temperature of about +12 to +13 °C (see small bright areas along the stream, Figure 11).
- Sun-warmed western slopes of the embankment (+23 to +24 °C).
- The increased temperature in the upper part of the slope (+20 to +24 °C) relative to the lower (+17 to +22 °C) due to exothermic reactions, because, in the upper part, the scree is “fresh”, whereas, in the lower part, there is material that has crumbled earlier, in which the reactions have already stopped.
- Areas of low temperatures in the northern part of the image are mainly associated to shaded areas.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nordstrom, D.K. Effects of microbiological and geochemical interactions in mine drainage. Environ. Asp. Mine Wastes Short Course Ser. 2003, 31, 227–238. [Google Scholar]
- Lottermoser, B.G. Sulfidic mine wastes. In Mine Wastes; Springer: Berlin/Heidelberg, Germany, 2010; pp. 43–117. [Google Scholar]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Murray, J.; Nordstrom, D.K.; Dold, B.; Kirschbaum, A. Seasonal fluctuations and geochemical modeling of acid mine drainage in the semi-arid Puna region: The Pan de Azúcar Pb–Ag–Zn mine, Argentina. J. S. Am. Earth Sci. 2021, 109, 103197. [Google Scholar] [CrossRef]
- Bortnikova, S.B.; Yurkevich, N.V.; Abrosimova, N.A.; Devyatova, A.Y.; Edelev, A.V.; Makas, A.L.; Troshkov, M.L. Assessment of emissions of trace elements and sulfur gases from sulfide tailings. J. Geochem. Explor. 2018, 186, 256–269. [Google Scholar] [CrossRef]
- Yurkevich, N.; Bortnikova, S.; Abrosimova, N.; Makas, A.; Olenchenko, V.; Yurkevich, N.; Edelev, A.; Saeva, O.; Shevko, A. Sulfur and nitrogen gases in the vapor streams from ore cyanidation wastes at a sharply continental climate, Western Siberia, Russia. Water Air Soil Pollut. 2019, 230, 307. [Google Scholar] [CrossRef]
- Davé, N.; Blanchette, M.; Giziewicz, E.; Review and Assessment of the Roles of Ice, in the Water Cover Option, and Permafrost in Controlling the Acid Generation from Sulphide Tailings. MEND Project 1.61.1. 1996. Available online: http://mend-nedem.org/mend-report/roles-of-ice-in-the-water-cover-option-and-permafrost-in-controlling-acid-generation-from-sulphide-tailings/ (accessed on 12 October 2022).
- Elberling, B.; Schippers, A.; Sand, W. Bacterial and chemical oxidation of pyritic mine tailings at low temperatures. J. Contam. Hydrol. 2000, 41, 225–238. [Google Scholar] [CrossRef]
- Amos, R.T.; Blowes, D.W.; Bailey, B.L.; Sego, D.C.; Smith, L.; Ritchie, A.I.M. Waste-rock hydrogeology and geochemistry. Appl. Geochem. 2015, 57, 140–156. [Google Scholar] [CrossRef]
- Elberling, B. Disposal of mine tailings in continuous permafrost areas: Environmental aspects and future control strategies. In Cryosols; Springer: Berlin/Heidelberg, Germany, 2004; pp. 677–698. [Google Scholar]
- Dawson, R.F.; Morin, K.A.; Acid Mine Drainage in Permafrost Regions: Issues, Control Strategies and Research Requirements. MEND Project 1.61.2. 1996. Available online: http://mend-nedem.org/mend-report/acid-mine-drainage-in-permafrost-regions-issues-control-strategies-and-research-requirements/ (accessed on 12 October 2022).
- Cabala, J.; Zogala, B.; Dubiel, R. Geochemical and Geophysical Study of Historical Zn-Pb Ore Processing Waste Dump Areas (Southern Poland). Pol. J. Environ. Stud. 2008, 17, 693–700. [Google Scholar]
- Pierwoła, J.; Szuszkiewicz, M.; Cabala, J.; Jochymczyk, K.; Żogała, B.; Magiera, T. Integrated geophysical and geochemical methods applied for recognition of acid waste drainage (AWD) from Zn-Pb post-flotation tailing pile (Olkusz, southern Poland). Environ. Sci. Pollut. Res. 2020, 27, 16731–16744. [Google Scholar] [CrossRef] [Green Version]
- Casagrande, M.F.S.; Moreira, C.A.; Targa, D.A.; Alberti, H.L.C. Integration of geophysical methods in the study of acid drainage in uranium mining waste. Braz. J. Geophys. 2018, 36, 439–450. [Google Scholar] [CrossRef] [Green Version]
- Moreira, C.A.; Casagrande MF, S.; de Siqueira Büchi, F.M.; Targa, D.A. Hydrogelogical characterization of a waste rock pile and bedrock affected by acid mine drainage from geophysical survey. SN Appl. Sci. 2020, 2, 1236. [Google Scholar] [CrossRef]
- Merritt, P.; Power, C. Assessing the long-term evolution of mine water quality in abandoned underground mine workings using first-flush based models. Sci. Total Environ. 2022, 846, 157390. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, J.J.; Lepper, C.M. Geophysical characterization of mineral waste sites. In Proceedings of the 6th EEGS Symposium on the Application of Geophysics to Engineering and Environmental Problems, San Diego, CA, USA, 18–22 April 1993. [Google Scholar]
- Warchulski, R.; Mendecki, M.; Gawęda, A.; Sołtysiak, M.; Gadowski, M. Rainwater-induced migration of potentially toxic elements from a Zn–Pb slag dump in Ruda Śląska in light of mineralogical, geochemical and geophysical investigations. Appl. Geochem. 2019, 109, 104396. [Google Scholar] [CrossRef]
- Power, C.; Tsourlos, P.; Ramasamy, M.; Nivorlis, A.; Mkandawire, M. Combined DC resistivity and induced polarization (DC-IP) for mapping the internal composition of a mine waste rock pile in Nova Scotia, Canada. J. Appl. Geophys. 2018, 150, 40–51. [Google Scholar] [CrossRef]
- Barago, N.; Covelli, S.; Mauri, M.; Oberti di Valnera, S.; Forte, E. Prediction of Trace Metal Distribution in a Tailings Impoundment Using an Integrated Geophysical and Geochemical Approach (Raibl Mine, Pb-Zn Alpine District, Northern Italy). Int. J. Environ. Res. Public Health 2021, 18, 1157. [Google Scholar] [CrossRef]
- Arcila, E.J.A.; Moreira, C.A.; Camarero, P.L.; Casagrande, M.F.S. Identification of flow zones inside and at the base of a uranium mine tailings Dam using geophysics. Mine Water Environ. 2021, 40, 308–319. [Google Scholar] [CrossRef]
- Targa, D.A.; Moreira, C.A.; Casagrande, M.F.S. Hydrogeological analysis of sulfide tailings at a uranium mine using geophysical and hydrochemical methods. Mine Water Environ. 2021, 40, 671–689. [Google Scholar] [CrossRef]
- Martínez, J.; Mendoza, R.; Rey, J.; Sandoval, S.; Hidalgo, M.C. Characterization of Tailings Dams by Electrical Geophysical Methods (ERT, IP): Federico Mine (La Carolina, Southeastern Spain). Minerals 2021, 11, 145. [Google Scholar] [CrossRef]
- Tavakoli, S.; Rasmussen, T.M. Geophysical tools to study the near-surface distribution of the tailings in the Smaltjärnen repository, south-central Sweden; a feasibility study. Acta Geophysica 2022, 70, 141–159. [Google Scholar] [CrossRef]
- Olenchenko, V.V.; Kucher, D.O.; Bortnikova, S.B.; Gas’kova, O.L.; Edelev, A.V.; Gora, M.P. Vertical and lateral spreading of highly mineralized acid drainage solutions (Ur dump, Salair): Electrical resistivity tomography and hydrogeochemical data. Russ. Geol. Geophys. 2016, 57, 617–628. [Google Scholar] [CrossRef]
- Myagkaya, I.N.; Lazareva, E.V.; Gustaytis, M.A.; Zhmodik, S.M. Gold and silver in a system of sulfide tailings. Part 1: Migration in water flow. J. Geochem. Explor. 2016, 160, 16–30. [Google Scholar] [CrossRef]
- Loke, M.H. Res2Dinv and Res3dinv Software Version 3.59; Geoelectrical Imaging 2D&3D: Penang, Malaysia, 2009. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; OSTI: Oak Ridge, TN, USA, 1985. [Google Scholar]
- WHO. Guidelines for Drinking Water Quality; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- A General List. In General List of Fisheries Norms: Maximum Permissible Concentrations and Approximately Safe Levels of Harmful Substances in Water of Fishery Water Bodies; VNIRO: Moscow, Russia, 1999; p. 304.
- Kelly, B.C.; Tuovinen, O.H. Chemistry and Biology of Solid Waste; Springer: Berlin/Heidelberg, Germany, 1988; p. 33. [Google Scholar]
- Gogoi, H.; Leiviskä, T.; Heiderscheidt, E.; Postila, H.; Tanskanen, J. The effectiveness of metal and metalloid sorption from mining influenced waters by natural and modified peat. Mine Water Environ. 2018, 37, 734–743. [Google Scholar] [CrossRef]
- Yurak, V.; Apakashev, R.; Dushin, A.; Usmanov, A.; Lebzin, M.; Malyshev, A. Testing of natural sorbents for the assessment of heavy metal ions’ adsorption. Appl. Sci. 2021, 11, 3723. [Google Scholar] [CrossRef]
- Brookins, D.G. Eh-pH Diagrams for Geochemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Linnik, P.M.; Zubenko, I.B. Role of bottom sediments in the secondary pollution of aquatic environments by heavy-metal compounds. Lakes Reserv. Res. Manag. 2000, 5, 11–21. [Google Scholar] [CrossRef]








| Power supply, W | 1550 |
| Detector response, s per ion | 0.12 |
| The temperature of the spray chamber | 2.0 ± 0.1° |
| The speed of peristaltic pump, rpm | 30 |
| The sample feed rate to the spray chamber, mL∙min−1 | 0.2 |
| Argon flow rate, L∙min−1 | 15 |
| Sample | Distance, m | EC, µS/cm | pH | SO42−, mg/L | µg/L | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na | Mg | Al | K | Ca | Fe | |||||
| 1/20 | 0 | 16,000 | 3.61 | 4500 | 24 | 140 | 350 | 1.4 | 230 | 970 |
| 2/20 | 50 | 1200 | 3.82 | 1450 | 19 | 35 | 20 | 0.94 | 100 | 12 |
| 3/20 | 200 | 2500 | 3.55 | 1100 | 23 | 49 | 43 | 0.43 | 81 | 420 |
| 4/20 | 310 | 3900 | 3.62 | 2300 | 22 | 90 | 220 | 0.67 | 120 | 530 |
| 5′/20 | 410 | 3980 | 3.48 | 3200 | 22 | 90 | 220 | 0.67 | 110 | 560 |
| 6/20 | 500 | 3130 | 3.55 | 2100 | 31 | 84 | 150 | 1.4 | 140 | 370 |
| 7/20 | 600 | 3100 | 3.71 | 2000 | 32 | 84 | 150 | 1.6 | 140 | 360 |
| 8/20 | 650 | 3130 | 3.68 | 1800 | 23 | 84 | 150 | 0.54 | 120 | 380 |
| 9/20 | 720 | 3260 | 3.49 | 2500 | 20 | 70 | 160 | 0.77 | 120 | 380 |
| 10/20 | 780 | 430 | 7.62 | 22 | 6.8 | 13 | 0.06 | 0.95 | 46 | 0.03 |
| 11/20 | 940 | 450 | 7.81 | 21 | 7.7 | 14 | 0.09 | 1.06 | 49 | 0.05 |
| 15/20 | 1400 | 340 | 7.81 | 49 | 7.7 | 13 | 0.22 | 1.66 | 79 | 0.64 |
| 18/20 | 1900 | 460 | 7.85 | 40 | 6.7 | 13 | 0.10 | 0.93 | 49 | 0.47 |
| 21/20 | 2500 | 450 | 7.58 | 32 | 6.9 | 13 | 0.08 | 1.0 | 50 | 0.30 |
| 35/20 | 5900 | 460 | 7.45 | 35 | 7.6 | 13 | 0.01 | 1.4 | 53 | 0.78 |
| 36/20 | 6200 | 370 | 8.27 | 29 | 6.4 | 11 | 0.03 | 1.5 | 84 | 0.15 |
| 38/20 | 6500 | 350 | 7.59 | 27 | 5.6 | 12 | 0.01 | 0.65 | 35 | 0.10 |
| 38-1/20 | 6800 | 250 | 7.95 | 20 | 5.0 | 12 | 0.02 | 0.43 | 27 | 0.33 |
| 39/20 | 7700 | 280 | 7.87 | 17 | 6.2 | 9.4 | 0.01 | 1.1 | 50 | 0.02 |
| min | 235 | 252 | 3.4 | 17 | 5.0 | 9.4 | 0.01 | 0.43 | 27 | 0.02 |
| max | 320 | 16000 | 8.3 | 4500 | 9.7 | 14 | 0.22 | 1.7 | 86 | 0.78 |
| average | 281 | 1869 | 6.0 | 900 | 6.9 | 12 | 0.06 | 1.2 | 62 | 0.21 |
| Clark * | 6.3 | 4.1 | 0.05 | 2.3 | 15 | 40 | ||||
| Sample | Height, m | Distance, m | mg/L | µg/L | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn | Zn | Cu | Cd | Pb | Ba | Ni | Co | Be | As | Sb | Hg | |||
| 0/20 | 316 | 0 | 19 | 14 | 3000 | 9.1 | 1200 | 83 | 290 | 260 | 3.5 | 1300 | 9.2 | 34 |
| 2/20 | 320 | 50 | 2.5 | 0.83 | 190 | 1.5 | 150 | 59 | 33 | 24 | 0.50 | 110 | 2.0 | 6.0 |
| 3/20 | 324 | 200 | 5.7 | 1.8 | 580 | 3.0 | 240 | 14 | 62 | 47 | 2.1 | 48 | 0.10 | 6.0 |
| 4/20 | 311 | 310 | 17 | 13 | 1700 | 18 | 250 | 100 | 100 | 150 | 4.5 | 83 | 2.0 | 6.0 |
| 5′/20 | 304 | 410 | 17 | 13 | 1700 | 18 | 210 | 78 | 100 | 150 | 3.8 | 82 | 2.0 | 6.0 |
| 6/20 | 304 | 500 | 14 | 9.8 | 1300 | 15 | 170 | 13 | 90 | 120 | 3.2 | 28 | 0.10 | 6.0 |
| 7/20 | 298 | 600 | 14 | 11 | 1300 | 14 | 140 | 110 | 88 | 120 | 4.0 | 19 | 0.10 | 6.0 |
| 8/20 | 294 | 650 | 14 | 11 | 1400 | 12 | 170 | 24 | 95 | 130 | 3.6 | 22 | 0.10 | 6.0 |
| 9/20 | 294 | 750 | 14 | 9.2 | 1200 | 13 | 140 | 140 | 91 | 130 | 3.4 | 24 | 2.0 | 6.0 |
| 15/20 | 256 | 1400 | 0.60 | 0.04 | 8.4 | 0.30 | 2.1 | 55 | 3.9 | 2.4 | 0.10 | 2.5 | 0.20 | 2.0 |
| 18/20 | 283 | 1900 | 0.03 | 0.01 | 3.3 | 0.30 | 0.19 | 72 | 1.5 | 0.40 | 0.10 | 1.3 | 0.10 | 0.60 |
| 21/20 | 282 | 2500 | 0.01 | 0.01 | 3.5 | 0.30 | 0.35 | 73 | 1.3 | 0.40 | 0.10 | 1.6 | 0.10 | 0.60 |
| 23/20 | 260 | 2900 | 0.05 | 0.01 | 2.8 | 0.30 | 0.74 | 91 | 2.3 | 0.40 | 0.10 | 3.9 | 0.20 | 2.0 |
| 27/20 | 251 | 3800 | 0.21 | 0.01 | 3.6 | 0.30 | 0.85 | 94 | 2.0 | 0.40 | 0.10 | 2.9 | 0.20 | 1.6 |
| 31/20 | 244 | 4900 | 0.06 | 0.01 | 3.3 | 0.30 | 0.68 | 91 | 1.7 | 0.40 | 0.10 | 3.3 | 0.20 | 2.0 |
| 32/20 | 242 | 5200 | 0.06 | 0.01 | 3.1 | 0.30 | 0.75 | 92 | 1.8 | 0.40 | 0.10 | 3.2 | 0.20 | 1.3 |
| 33/20 | 241 | 5400 | 0.05 | 0.01 | 3.2 | 0.30 | 0.42 | 92 | 2.0 | 0.40 | 0.10 | 2.9 | 0.20 | 1.7 |
| 35/20 | 275 | 5900 | 0.004 | 0.01 | 2.0 | 0.30 | 0.20 | 90 | 0.8 | 0.40 | 0.10 | 1.3 | 0.10 | 0.60 |
| 36/20 | 235 | 6200 | 0.06 | 0.01 | 2.9 | 0.30 | 0.35 | 88 | 1.5 | 0.40 | 0.10 | 2.5 | 0.20 | 2.0 |
| 38/20 | 267 | 6500 | 0.002 | 0.01 | 2.0 | 0.30 | 0.20 | 63 | 1.0 | 0.40 | 0.10 | 2.2 | 0.10 | 0.60 |
| 38-1/20 | 273 | 6800 | 0.001 | 0.01 | 2.0 | 0.30 | 0.31 | 48 | 1.0 | 0.40 | 0.10 | 1.6 | 0.10 | 0.60 |
| 39/20 | 236 | 7700 | 0.02 | 0.01 | 2.7 | 0.30 | 0.70 | 46 | 1.1 | 0.40 | 0.10 | 3.3 | 0.20 | 1.8 |
| 10/20 | 294 | 780 | 0.02 | 0.01 | 2.0 | 0.30 | 0.20 | 60 | 1.0 | 0.40 | 0.10 | 1.9 | 0.10 | 0.60 |
| 10′/20 | 294 | 820 | 0.003 | 0.01 | 2.0 | 0.30 | 0.20 | 60 | 1.4 | 0.40 | 0.10 | 2.4 | 0.10 | 0.60 |
| 10″/20 | 266 | 870 | 0.15 | 0.01 | 3.3 | 0.30 | 2.5 | 54 | 2.4 | 0.46 | 0.10 | 4.3 | 0.20 | 2.2 |
| 11/20 | 280 | 940 | 0.01 | 0.01 | 2.0 | 0.30 | 0.20 | 61 | 1.1 | 0.40 | 0.10 | 1.7 | 0.10 | 0.60 |
| MPC WHO * | - | - | 0.40 | 3.0 | 2000 | 3.0 | 10 | 700 | 70 | n.d. | 12 | 10 | 20 | 6.0 |
| MPC RF ** | - | - | 0.10 | 1.0 | 1000 | 1.0 | 10.00 | 700 | 20 | 100 | 0.20 | 10 | 5.0 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurkevich, N.; Osipova, P.; Tsibizov, L.; Tsibizova, E.; Fadeeva, I.; Volynkin, S.; Tulisova, K.; Kuleshova, T. Current State of the Gold Mining Waste from the Ores of the Ursk Deposit (Western Siberia, Russia). Appl. Sci. 2022, 12, 10610. https://doi.org/10.3390/app122010610
Yurkevich N, Osipova P, Tsibizov L, Tsibizova E, Fadeeva I, Volynkin S, Tulisova K, Kuleshova T. Current State of the Gold Mining Waste from the Ores of the Ursk Deposit (Western Siberia, Russia). Applied Sciences. 2022; 12(20):10610. https://doi.org/10.3390/app122010610
Chicago/Turabian StyleYurkevich, Nataliya, Polina Osipova, Leonid Tsibizov, Ekaterina Tsibizova, Irina Fadeeva, Sergey Volynkin, Kristina Tulisova, and Tatyana Kuleshova. 2022. "Current State of the Gold Mining Waste from the Ores of the Ursk Deposit (Western Siberia, Russia)" Applied Sciences 12, no. 20: 10610. https://doi.org/10.3390/app122010610





