Biological Monitoring via Urine Samples to Assess Healthcare Workers’ Exposure to Hazardous Drugs: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Databases and Inclusion Criteria
2.2. Search Strategy
2.3. Data Analysis and Reporting
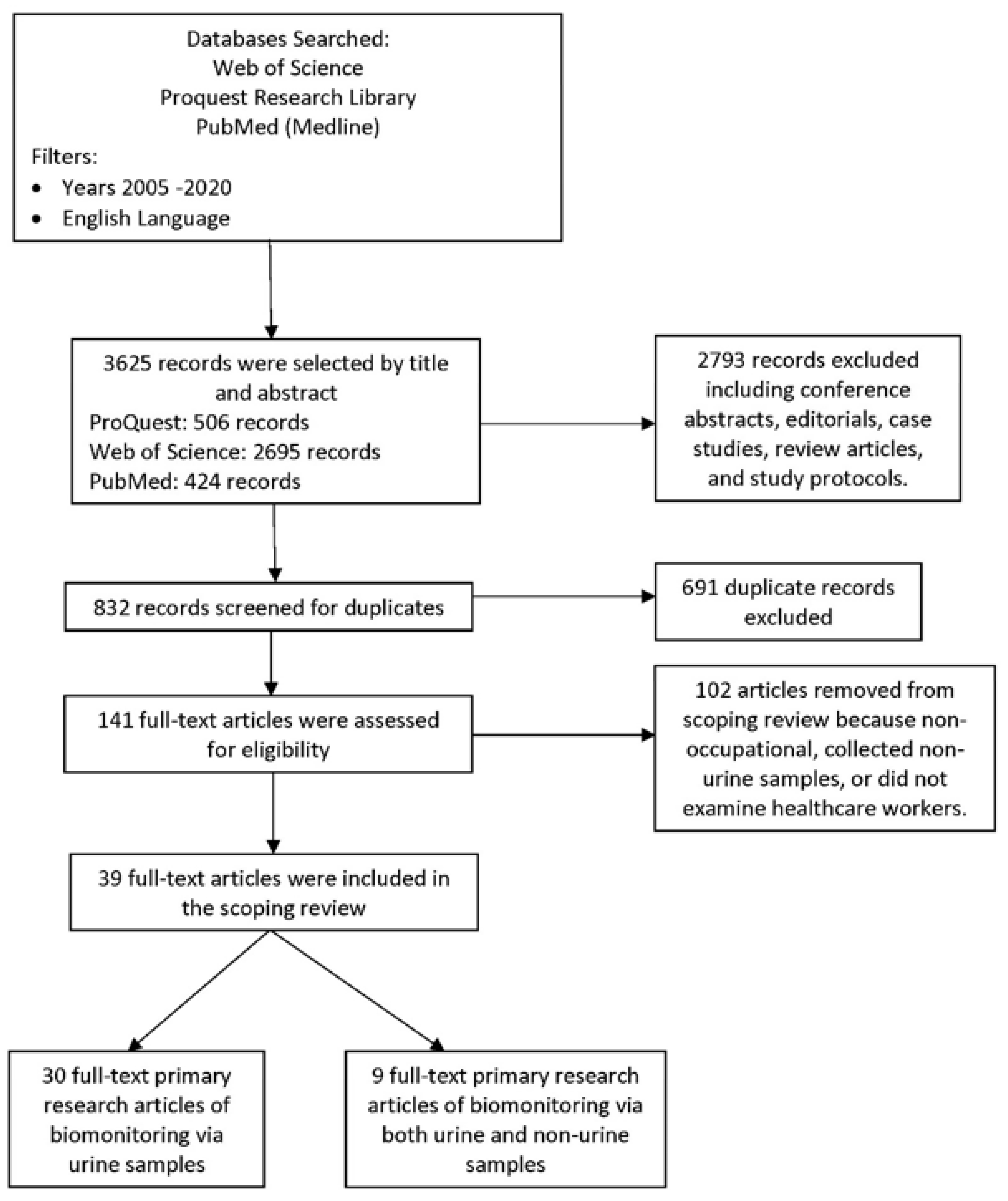
3. Results
3.1. Literature Identified
3.2. How Studies Were Conducted
3.3. Exposure Findings
3.4. Knowledge Gaps
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Connor, T.H.; McDiarmid, M.A. Preventing Occupational Exposures to Antineoplastic Drugs in Health Care Settings. CA Cancer J. Clin. 2006, 56, 354–365. [Google Scholar] [CrossRef]
- Friese, C.R.; McArdle, C.; Zhao, T.; Sun, D.; Spasojevic, I.; Polovich, M.; McCullagh, M.C. Antineoplastic drug exposure in an ambulatory setting: A pilot study. Cancer Nurs. 2015, 38, 111–117. [Google Scholar] [CrossRef]
- Sorsa, M.; Hämeilä, M.; Järviluoma, E. Handling anticancer drugs: From hazard identification to risk management? Ann. N. Y. Acad. Sci. 2006, 1076, 628–634. [Google Scholar] [CrossRef]
- Graeve, C.U.; McGovern, P.M.; Alexander, B.; Church, T.; Ryan, A.; Polovich, M. Occupational Exposure to Antineoplastic Agents. Work Health Saf. 2017, 65, 9–20. [Google Scholar] [CrossRef]
- Hon, C.Y.; Teschke, K.; Chua, P.; Venners, S.; Nakashima, L. Occupational exposure to antineoplastic drugs: Identification of job categories potentially exposed throughout the hospital medication system. Saf. Health Work 2011, 2, 273–281. [Google Scholar] [CrossRef]
- Roussel, C.; Witt, K.L.; Shaw, P.B.; Connor, T.H. Meta-analysis of chromosomal aberrations as a biomarker of exposure in healthcare workers occupationally exposed to antineoplastic drugs. Mutat. Res. 2019, 781, 207–217. [Google Scholar] [CrossRef]
- Santos, A.N.; Oliveira, R.J.; Pessatto, L.R.; Gomes, R.D.; de Freitas, C.A.F. Biomonitoring of pharmacists and nurses at occupational risk from handling antineoplastic agents. Int. J. Pharm. Pract. 2020, 28, 506–511. [Google Scholar] [CrossRef]
- Connor, T.H.; Lawson, C.C.; Polovich, M.; McDiarmid, M.A. Reproductive health risks associated with occupational exposures to antineoplastic drugs in health care settings a review of the evidence. J. Occup. Environ. Med. 2014, 56, 901–910. [Google Scholar] [PubMed]
- Huang, Y.W.; Jian, L.; Zhang, M.B.; Zhou, Q.; Yan, X.F.; Hua, X.D.; Zhou, Y.; He, J.L. An investigation of oxidative dna damage in pharmacy technicians exposed to antineoplastic drugs in two Chinese hospitals using the urinary 8-OHdG assay. Biomed. Environ. Sci. 2012, 25, 109–116. [Google Scholar]
- Connor, T.H.; DeBord, G.; Pretty, J.R.; Oliver, M.S.; Roth, T.S.; Lees, P.S.J.; Krieg, E.F., Jr.; Rogers, B.; Escalante, C.P.; Toennis, C.A.; et al. Evaluation of antineoplastic drug exposure of health care workers at three university-based US cancer centers. J. Occup. Environ. Med. 2010, 52, 1019–1027. [Google Scholar]
- Sottani, C.; Porro, B.; Comelli, M.; Imbriani, M.; Minoia, C. An analysis to study trends in occupational exposure to antineoplastic drugs among health care workers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2593–2605. [Google Scholar] [CrossRef]
- Jakubowski, M.; Trzcinka-Ochocka, M. Biological monitoring of exposure: Trends and key developments. J. Occup. Health 2005, 47, 22–48. [Google Scholar] [CrossRef] [PubMed]
- WHO. Human Biomonitoring: Facts and Figures; WHO: Geneva, Switzerland, 2015; pp. 1–88. Available online: http://www.euro.who.int/__data/assets/pdf_file/0020/276311/Human-biomonitoring-facts-figures-en.pdf (accessed on 9 June 2022).
- Dhersin, A.; Atgé, B.; Martinez, B.; Titier, K.; Rousset, M.; El Moustaph, M.S.C.; Verdun-Esquer, C.; Molimard, M.; Villa, A.; Canal-Raffin, M. Biomonitoring of occupational exposure to 5-FU by assaying α-fluoro-β-alanine in urine with a highly sensitive UHPLC-MS/MS method. Analyst 2018, 143, 4110–4117. [Google Scholar] [CrossRef] [PubMed]
- Mathias, P.I.; Connor, T.H.; B’Hymer, C. A review of high performance liquid chromatographic-mass spectrometric urinary methods for anticancer drug exposure of health care workers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1060, 316–324. [Google Scholar] [CrossRef] [PubMed]
- CDC-NIOSH Publications and Products—Preventing Occupational Exposure to Antineoplastic and Other Hazardous Drugs in Health Care Settings (2004-165). Available online: http://www.cdc.gov/niosh/docs/2004-165/ (accessed on 30 November 2011).
- Hon, C.Y.; Teschke, K.; Demers, P.A.; Venners, S. Antineoplastic drug contamination on the hands of employees working troughout the hospital medication system. Ann. Occup. Hyg. 2014, 58, 761–770. [Google Scholar] [PubMed]
- Fransman, W.; Vermeulen, R.; Kromhout, H. Dermal exposure to cyclophosphamide in hospitals during preparation, nursing and cleaning activities. Int. Arch. Occup. Environ. Health 2005, 78, 403–412. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.; Colquhoun, H.; Levac, D. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef]
- Hama, K.; Fukushima, K.; Hirabatake, M.; Hashida, T.; Kataoka, K. Verification of surface contamination of Japanese cyclophosphamide vials and an example of exposure by handling. J. Oncol. Pharm. Pract. 2012, 18, 201–206. [Google Scholar] [CrossRef]
- Cavallo, D.; Ursini, C.L.; Perniconi, B.; Di Francesco, A.; Giglio, M.; Rubino, F.M.; Marinaccio, A.; Iavicoli, S. Evaluation of genotoxic effects induced by exposure to antineoplastic drugs in lymphocytes and exfoliated buccal cells of oncology nurses and pharmacy employees. Mutat. Res. 2005, 587, 45–51. [Google Scholar] [CrossRef]
- Mason, H.J.; Blair, S.; Sams, C.; Jones, K.; Garfitt, S.J.; Cuschieri, M.J.; Baxter, P.J. Exposure to antineoplastic drugs in two UK hospital pharmacy units. Ann. Occup. Hyg. 2005, 49, 603–610. [Google Scholar]
- Ursini, C.L.; Cavallo, D.; Colombi, A.; Giglio, M.; Marinaccio, A.; Iavicoli, S. Evaluation of early DNA damage in healthcare workers handling antineoplastic drugs. Int. Arch. Occup. Environ. Health 2006, 80, 134–140. [Google Scholar] [CrossRef]
- Fransman, W.; Peelen, S.; Hilhorst, S.; Roeleveld, N.; Heederik, D.; Kromhout, H. A pooled analysis to study trends in exposure to antineoplastic drugs among nurses. Ann. Occup. Hyg. 2007, 51, 231–239. [Google Scholar]
- Rekhadevi, P.V.; Sailaja, N.; Chandrasekhar, M.; Mahboob, M.; Rahman, M.F.; Grover, P. Genotoxicity assessment in oncology nurses handling antineoplastic drugs. Mutagenesis 2007, 22, 395–401. [Google Scholar] [CrossRef]
- Hedmer, M.; Tinnerberg, H.; Axmon, A.; Jönsson, B.A.G. Environmental and biological monitoring of antineoplastic drugs in four workplaces in a Swedish hospital. Int. Arch. Occup. Environ. Health 2008, 81, 899–911. [Google Scholar]
- Tanimura, M.; Yamada, K.; Sugiura, S.I.; Mori, K.; Nagata, H.; Tadokoro, K.; Miyake, T.; Hamaguchi, Y.; Sessink, P.; Nabeshima, T. An environmental and biological study of occupational exposure to cyclophosphamide in the pharmacy of a Japanese community hospital designated for the treatment of cancer. J. Health Sci. 2009, 55, 750–756. [Google Scholar] [CrossRef]
- Yoshida, J.; Tei, G.; Mochizuki, C.; Masu, Y.; Koda, S.; Kumagai, S. Use of a closed system device to reduce occupational contamination and exposure to antineoplastic drugs in the hospital work environment. Ann. Occup. Hyg. 2009, 53, 153–160. [Google Scholar]
- Maeda, S.; Miyawaki, K.; Matsumoto, S.; Oishi, M.; Miwa, Y.; Kurokawa, N. Evaluation of environmental contaminations and occupational exposures involved in preparation of chemotherapeutic drugs. Yakugaku Zasshi 2010, 130, 903–910. [Google Scholar]
- Ndaw, S.; Denis, F.; Marsan, P.; d’Almeida, A.; Robert, A. Biological monitoring of occupational exposure to 5-fluorouracil: Urinary α-fluoro-β-alanine assay by high performance liquid chromatography tandem mass spectrometry in health care personnel. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2630–2634. [Google Scholar] [CrossRef]
- Yoshida, J.; Koda, S.; Nishida, S.; Yoshida, T.; Miyajima, K.; Kumagai, S. Association between occupational exposure levels of antineoplastic drugs and work environment in five hospitals in Japan. J. Oncol. Pharm. Pract. 2011, 17, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Konate, A.; Poupon, J.; Villa, A.; Garnier, R.; Hasni-Pichard, H.; Mezzaroba, D.; Fernandez, G.; Pocard, M. Evaluation of environmental contamination by platinum and exposure risks for healthcare workers during a heated intraperitoneal perioperative chemotherapy (HIPEC) procedure. J. Surg. Oncol. 2011, 103, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.; Nakanishi, H.; Asano, M.; Hashida, T.; Tanimura, M.; Hama, T.; Nabeshima, T. Multicenter study for environmental and biological monitoring of occupational exposure to cyclophosphamide in Japan. J. Oncol. Pharm. Pract. 2011, 17, 20–28. [Google Scholar] [CrossRef]
- Sugiura, S.I.; Asano, M.; Kinoshita, K.; Tanimura, M.; Nabeshima, T. Risks to health professionals from hazardous drugs in Japan: A pilot study of environmental and biological monitoring of occupational exposure to cyclophosphamide. J. Oncol. Pharm. Pract. 2011, 17, 14–19. [Google Scholar]
- Turci, R.; Minoia, C.; Sottani, C.; Coghi, R.; Severi, P.; Castriotta, C.; Del Bianco, M.; Imbriani, M. Occupational exposure to antineoplastic drugs in seven Italian hospitals: The effect of quality assurance and adherence to guidelines. J. Oncol. Pharm. Pract. 2011, 17, 320–332. [Google Scholar] [CrossRef]
- Villarini, M.; Dominici, L.; Piccinini, R.; Fatigoni, C.; Ambrogi, M.; Curti, G.; Morucci, P.; Muzi, G.; Monarca, S.; Moretti, M. Assessment of primary, oxidative and excision repaired DNA damage in hospital personnel handling antineoplastic drugs. Mutagenesis 2011, 26, 359–369. [Google Scholar] [CrossRef]
- Sottani, C.; Porro, B.; Imbriani, M.; Minoia, C. Occupational exposure to antineoplastic drugs in four Italian health care settings. Toxicol. Lett. 2012, 213, 107–115. [Google Scholar]
- Kopp, B.; Crauste-Manciet, S.; Guibert, A.; Mourier, W.; Guerrault-Moro, M.N.; Ferrari, S.; Jomier, J.-Y.; Brossard, D.; Schierl, R. Environmental and biological monitoring of platinum-containing drugs in two hospital pharmacies using positive air pressure isolators. Ann. Occup. Hyg. 2013, 57, 374–383. [Google Scholar]
- Miyake, T.; Iwamoto, T.; Tanimura, M.; Okuda, M. Impact of closed-system drug transfer device on exposure of environment and healthcare provider to cyclophosphamide in Japanese hospital. Springerplus 2013, 2, 273. [Google Scholar] [CrossRef]
- Yoshida, J.; Koda, S.; Nishida, S.; Nakano, H.; Tei, G.; Kumagai, S. Association between occupational exposure and control measures for antineoplastic drugs in a pharmacy of a hospital. Ann. Occup. Hyg. 2013, 57, 251–260. [Google Scholar]
- Ramphal, R.; Bains, T.; Vaillancourt, R.; Osmond, M.H.; Barrowman, N. Occupational exposure to cyclophosphamide in nurses at a single center. J. Occup. Environ. Med. 2014, 56, 304–312. [Google Scholar] [CrossRef]
- Sessink, P.J.; Leclercq, G.M.; Wouters, D.M.; Halbardier, L.; Hammad, C.; Kassoul, N. Environmental contamination, product contamination and workers exposure using a robotic system for antineoplastic drug preparation. J. Oncol. Pharm. Pract. 2014, 21, 118–127. [Google Scholar] [CrossRef]
- Hon, C.Y.; Teschke, K.; Shen, H.; Demers, P.A.; Venners, S. Antineoplastic drug contamination in the urine of Canadian healthcare workers. Int. Arch. Occup. Environ. Health 2015, 88, 933–941. [Google Scholar] [CrossRef]
- Moretti, M.; Grollino, M.G.; Pavanello, S.; Bonfiglioli, R.; Villarini, M.; Appolloni, M.; Carrieri, M.; Sabatini, L.; Dominici, L.; Stronati, L.; et al. Micronuclei and chromosome aberrations in subjects occupationally exposed to antineoplastic drugs: A multicentric approach. Int. Arch. Occup. Environ. Health 2015, 88, 683–695. [Google Scholar] [CrossRef]
- Villa, A.F.; El Balkhi, S.; Aboura, R.; Sageot, H.; Hasni-Pichard, H.; Pocard, M.; Elias, D.; Joly, N.; Payen, D.; Blot, F.; et al. Evaluation of oxaliplatin exposure of healthcare workers during heated intraperitoneal perioperative chemotherapy (HIPEC). Ind. Health 2015, 53, 28–37. [Google Scholar] [CrossRef]
- Zhang, J.; Bao, J.; Wang, R.; Geng, Z.; Chen, Y.; Liu, X.; Xie, Y.; Jiang, L.; Deng, Y.; Liu, G.; et al. A multicenter study of biological effects assessment of pharmacy workers occupationally exposed to antineoplastic drugs in Pharmacy Intravenous Admixture Services. J. Hazard Mater. 2016, 315, 86–92. [Google Scholar] [CrossRef]
- Poupeau, C.; Tanguay, C.; Plante, C.; Gagné, S.; Caron, N.; Bussières, J.F. Pilot study of biological monitoring of four antineoplastic drugs among Canadian healthcare workers. J. Oncol. Pharm. Pract. 2017, 23, 323–332. [Google Scholar] [CrossRef]
- Baniasadi, S.; Alehashem, M.; Yunesian, M.; Rastkari, N. Biological monitoring of healthcare workers exposed to antineoplastic drugs: Urinary assessment of cyclophosphamide and ifosfamide. Iran. J. Pharm. Res. 2018, 17, 1458–1464. [Google Scholar]
- Koller, M.; Böhlandt, A.; Haberl, C.; Nowak, D.; Schierl, R. Environmental and biological monitoring on an oncology ward during a complete working week. Toxicol. Lett. 2018, 298, 158–163. [Google Scholar] [CrossRef]
- Ndaw, S.; Hanser, O.; Kenepekian, V.; Vidal, M.; Melczer, M.; Remy, A.; Robert, A.; Bakrin, N. Occupational exposure to platinum drugs during intraperitoneal chemotherapy. Biomonitoring and surface contamination. Toxicol. Lett. 2018, 298, 171–176. [Google Scholar] [CrossRef]
- Azari, M.R.; Akbari, M.E.; Abdollahi, M.B.; Mirzaei, H.R.; Sahlabadi, A.S.; Tabibi, R.; Rahmati, A.; Panahi, D. Biological monitoring of the oncology healthcare staff exposed to cyclophosphamide in two hospitals in Tehran. Int. J. Cancer Manag. 2019, 12, e86537. [Google Scholar]
- Ursini, C.L.; Omodeo Salè, E.; Fresegna, A.M.; Ciervo, A.; Jemos, C.; Maiello, R.; Buresti, G.; Colosio, C.; Rubino, F.M.; Mandić-Rajčević, S.; et al. Antineoplastic drug occupational exposure: A new integrated approach to evaluate exposure and early genotoxic and cytotoxic effects by no-invasive Buccal Micronucleus Cytome Assay biomarker. Toxicol. Lett. 2019, 316, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Palamini, M.; Dufour, A.; Therrien, R.; Delisle, J.F.; Mercier, G.; Gagné, S.; Caron, N.; Bussières, J.F. Quantification of healthcare workers’ exposure to cyclophosphamide, ifosfamide, methotrexate, and 5-fluorouracil by 24-h urine assay: A descriptive pilot study. J. Oncol. Pharm. Pract. 2020, 26, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Villarini, M.; Gianfredi, V.; Levorato, S.; Vannini, S.; Salvatori, T.; Moretti, M. Occupational exposure to cytostatic/antineoplastic drugs and cytogenetic damage measured using the lymphocyte cytokinesis-block micronucleus assay: A systematic review of the literature and meta-analysis. Mutat. Res. 2016, 770, 35–45. [Google Scholar] [CrossRef]
- Kibby, T. A review of surface wipe sampling compared to biologic monitoring for occupational exposure to antineoplastic drugs. J. Occup. Environ. Hyg. 2017, 14, 159–174. [Google Scholar] [CrossRef]
- Ye, X.; Wong, L.Y.; Bishop, A.M.; Calafat, A.M. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ. Health Perspect. 2011, 119, 983–988. [Google Scholar] [CrossRef]
- de Jonge, M.E.; Huitema, A.D.R.; Rodenhuis, S.; Beijnen, J.H. Clinical pharmacokinetics of cyclophosphamide. Clin. Pharmacokinet. 2005, 44, 1135–1164. [Google Scholar] [CrossRef]
- Dugheri, S.; Bonari, A.; Pompilio, I.; Boccalon, P.; Tognoni, D.; Cecchi, M.; Ughi, M.; Mucci, N.; Arcangeli, G. Analytical strategies for assessing occupational exposure to antineoplastic drugs in healthcare workplaces. Medyca Pracy 2018, 69, 589–603. [Google Scholar] [CrossRef]
- Poupeau, C.; Tanguay, C.; Caron, N.J.; Bussières, J.F. Multicenter study of environmental contamination with cyclophosphamide, ifosfamide, and methotrexate in 48 Canadian hospitals. J. Oncol. Pharm. Pract. 2018, 24, 9–17. [Google Scholar] [CrossRef]
- Hurst, J.; McIntyre, J.; Tamauchi, Y.; Kinuhata, H.; Kodama, T. A summary of the ’ALARP’ principle and associated thinking. J. Nucl. Sci. Technol. 2019, 56, 241–253. [Google Scholar] [CrossRef]
- Hon, C.-Y.; Teschke, K.; Chu, W.; Demers, P.; Venners, S. Antineoplastic Drug Contamination of Surfaces Throughout the Hospital Medication System in Canadian Hospitals. J. Occup. Environ. Hyg. 2013, 10, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Cancer Care Ontario. A Quality Initiative of the Safe Handling of Cytotoxics; Cancer Care Ontario: Toronto, ON, Canada, 2018. [Google Scholar]
| Author | Population | Sample Size | Study Type | Urine Sampling Strategy | Drugs Tested and Corresponding Detection Limit (LOD Unless Otherwise Indicated) | Laboratory Analytical Method | Summary of Results | Detectable Levels? |
|---|---|---|---|---|---|---|---|---|
| Cavallo D. et al. (2005) * [23] | Healthcare workers (nurses, pharmacy technicians) | 60 | Case-Control 30 exposed and 30 controls | Collected at the beginning of the work shift after 3 working days. | AFBA: 18 ng/mL | GC-MS | Both exposed and control subjects showed detectable levels of AFBA only in the urine of nurses administering drugs. AFBA values of 30 and 1140 µg/L were found in 2 day-care hospital nurses and a value of 20 µg/L in a ward nurse. | Yes |
| Mason H. et al. (2005) [24] | Pharmacy technicians | 46 | Case-Control 6 exposed and 40 controls | Collected daily pre-shift and post-shift samples. | CP: 1 ng/mL IF: 1 ng/mL MTX: 10 ng/mL (LLOQ) Pt: 0.022 ng/mL | GC-MS (CP and IF), ICP-MS (Pt), ELISA (MTX) | All urinary CP, IF, and MTX results were less than the detection limits of the assays. However, the urinary creatinine-corrected, post-shift Pt results from cases were significantly higher (p < 0.01) than control group. | No |
| Ursini C. et al. (2006) * [25] | Nurses and pharmacy technicians | 60 | Case-Control 30 exposed and 30 controls | Collected in the morning at the beginning of the work shift on the third working day. | AFBA: 18 ng/mL | GC-MS | Both exposed and control subjects showed detectable levels of AFBA only in the urine of nurses administering drugs. Authors found 30 and 1140 µg/L AFBA in 2 day-hospital nurses and 20 µg/L AFBA in a ward nurse. | Yes |
| Fransman W. et al. (2007) [26] | Nurses | 26 in 1997 13 in 2000 | Longitudinal 1997–2000 | 1997-Starting at the beginning of a work shift, urine samples were collected for 24 h. 2000-Collected urine samples during three workdays in separate fractions for 24 h. | CP: 0.1 ng/mL | GC-MS/MS | The percentage of positive urine fractions had decreased by a factor of 4 between 1997 and 2000 (factor difference = 0.24; 95% CI = 0.10–0.57). The CP levels in the nurses’ positive urine samples decreased 3-fold between 1997 and 2000. The percentage of detectable urine samples did not differ statistically significantly between nurses who reported having performed one of the CP-related tasks and nurses who reported not having performed one of the CP-related tasks. | Yes |
| Rekhadevi P. et al. (2007) * [27] | Nurses | 120 | Case-Control 60 exposed and 60 controls | Samples were collected from all the exposed personnel on the last day of their 6-day work shift in the morning hours before the beginning of their work shift. Six controls and six exposed samples were taken every 7 days. | CP: 0.04 ng/mL; 0.13 ng/mL (LOQ) | GC-MS | Varying concentrations of CP in the range of 0.08–0.9 µg/mL of urine were found in 42 subjects only. The mean CP concentration in 42 subjects was 0.44 +/− 0.26 µg/mL. The rest of the samples had CP below the limit of detection. | Yes |
| Hedmer M. et al. (2008) [28] | Pharmacy workers, nurses, cleaners | 22 | Cross-Sectional | Spot samples of urine were collected before and after work. | CP: 0.01 ng/mL IF 0.03 ng/L | LC-MS/MS | No CP or IF was detected in any of the pre- and post-shift urine samples from workers. | No |
| Tanimura M et al. (2009) [29] | Pharmacists | 4 | Cross-Sectional | 24-h urine samples. | CP: N/A | GC-MS/MS | The urinary concentration of CP decreased for 3 pharmacists and increased for 1 pharmacist after the revision of the compounding standard operating procedure. The mean urinary CP before the revision was 165.3 ng/24 h and decreased to 47.4 ng/24 h after the revision, although this difference was not statistically significant (p = 0.15). | Yes |
| Yoshida J. et al. (2009) [30] | Pharmacists | 6 | Cross-Sectional | 24-h urine samples. | CP: 0.01 ng/mL | HPLC-MS | The mean and median values among the six pharmacists during the conventional method were 39 and 12 ng/day, respectively. The mean and median values during the closed-system method were 4.9 and 2.0 ng/day, respectively. The mean CP of urine samples when using closed-system method was reduced to 13% of the conventional method. | Yes |
| Connor T et al. (2010) * [10] | Nurses, pharmacists, pharmacy technicians, nurse assistants | 121 | Case-Control 68 exposed and 53 controls | Collected every void for the last 4 h of the work shift and the first 4 h after the end of the shift. | CP: 0.015 ng/mL PTX: 0.015 ng/mL | HPLC-MS/MS | Two of the urine samples from exposed pharmacists demonstrated concentrations of CP above LOD of 0.015 ng/mL and one pharmacy technician had a concentration of PTX at the LOD of 0.015 ng/mL. None of the urine samples from non-exposed demonstrated concentrations of either drug greater than LOD. | Yes |
| Maeda S. et al. (2010) [31] | Pharmacists and nurses | 8 | Cross-Sectional | (1) 24-h sampling (1 pharmacist). (2) Spot sampling (6 pharmacists): 6–10 h or 20–24 h after preparing CP. (3) Spot sampling (2 nurses): collected from each voiding when on duty after treating patients with CP. | CP: 0.4 ng/mL IF: 0.4 ng/mL | LC-MS/MS | CP and IF were not detected in any urine samples. | No |
| Ndaw S. et sl. (2010) [32] | Pharmacy technicians, nurses, and auxiliary nurses | 19 | Cross-Sectional | Urine samples were collected before and after work shifts. Five consecutive days were considered in this study. | AFBA: 1 ng/mL | HPLC-MS/MS | AFBA was found at least once in 74% of subjects, mainly in the post-shift samples. In the hospital pharmacy, AFBA was detected in 83% of the pharmacy technicians, the concentrations ranging from 1.17 to 6.06 µg/L. In the oncology ward, AFBA was found in the urine of 69% of the nurses and auxiliary nurses with concentrations ranging from 1.00 to 22.7 µg/L. The pharmacy technicians had 15 positive results, the nurses 4, and the auxiliary nurses 16 positive urine samples. | Yes |
| Sottani C. et al. (2010) [11] | Pharmacy technicians | 385 (1998–2007) 263 (2002–2007) | Longitudinal 1998–2007 2002–2007 | Pre- and post-work shift samples were collected from workers after 6 h of their work shift. | CP: 0.02 ng/mL (LLOQ) DOXO: 0.10 ng/mL EPI: 0.10 ng/mL | HPLC–ESI–MS/MS | The percentage of positive urine samples was found to be around 30% in the 1990s and 2% in the 2000s. Moreover, no positive samples were detected in 2006 or 2007. | Yes |
| Yoshida J. et al. (2010) [33] | Pharmacists | 17 | Cross-Sectional | 24-h urine samples. | CP: 0.1 ng/mL Pt: 2.0 ng/mL | GC-MS (CP), ICP-MS (Pt) | CP was detected from one pharmacist each in Hospital B, Hospital D, and Hospital E. Pt was not detected in any samples. | Yes (CP) No (Pt) |
| Hama K. et al. (2011) [22] | Pharmacist | 1 | Single-Subject Study | 29-h urine samples. | CP: 0.05 ng/mL | GC-MS/MS | Over the 29-h period, seven separate urine samples were collected from the pharmacist. The total amount of CP excreted was 13.5 ng/24 h, but CP was detected in only the 7th urine sample, 13.5 ng (0.05 ng/mL). | Yes |
| Konate A. et al. (2011) [34] | Surgeons, anesthesiologist, surgical nurse, anesthesiologist’s nurse, and cleaner | 17 | Case-Control 11 exposed and 6 controls | A sample was obtained from each of the control subjects in the morning. Two samples were obtained from each member of the exposed group: the first was taken in the morning before surgery and the second in the evening after the HIPEC procedure. | Pt: 0.0015 ng/mL | ICP-MS | For the 11 exposed cases, Pt concentrations were above LOD (1.5 ng/L) but below LOQ (5 ng/L). In the control group, the Pt concentration was below the LOD In five instances and was 5.8 ng/L in one subject. | Yes |
| Sugiura S. et al. (2011) [35] | Physicians, pharmacists, nurses | 41 | Cross-Sectional | 24-h urine samples. | CP: N/A | GC-MS/MS | Overall, 276 samples were collected. CP was detected in 90 samples obtained from 23 subjects. The excretion of CP per staff member was between 2.7 and 462.8 ng/24 h. | Yes |
| Sugiura S. et al. (2011) [36] | Doctors, pharmacists, nurses | 10 | Cross-Sectional | 24-h urine samples. | CP: 0.01 ng/mL | GC-MS/MS | A total of 62 urine samples were collected from the ten hospital workers. CP was detected in 11 urine samples from two nurses from Department A and a medical doctor from Department B. The mean amount of CP excreted on a group basis was 29.3 ng/24 h. | Yes |
| Turci R. et al. (2011) [37] | Nurses and pharmacists | 102 | Cross-Sectional | Samples were collected at the beginning and at the end of the work shifts. | CP: 20 ng/mL (LOQ) 5-FU: 0.1 ng/mL (LOQ) AFBA: 2 ng/mL (LOQ) DOXO: 0.1 ng/mL (LOQ) EPI: 0.1 ng/mL (LOQ) Pt-compounds: 0.4 ng/mL (LOQ) | HPLC-MS/MS (CP, TAX, DOXO, and EPI), Q-DRC (Pt), HPLC-UV (5-FU), GC-MS (AFBA) | No analytes (drug or metabolite) were detected above the LOQ in any of the collected urine samples. | No |
| Villarini M. et al. (2011) * [38] | Pharmacy technicians, day hospital nurses, ward nurses, attendants | 104 | Case-Control 52 exposed and 52 controls | Samples were collected at the end of the work shifts. | CP: 0.1 ng/mL | GC-MS | Exposed subjects showed detectable levels of CP in the post-shift urine samples in 17.5% of participants, with CP concentrations in the range of 0.1–0.2 µg/L. One subject had a urinary CP concentration of 1.2 ug/L. | Yes |
| Huang Y. et al. (2012) [9] | Pharmacy technicians | 80 | Case-Control 40 exposed and 40 controls | A spot urine sample was obtained from each subject before lunch on the last working day. | Urinary 8-OHdG: N/A | ELISA | The urinary 8-OHdG concentration in exposed group I was significantly higher than that in control group I and exposed group II (p < 0.01). Moreover, there was a significant correlation between urinary 8-OHdG concentrations and spill frequencies per person (p < 0.01). | Yes |
| Sottani C. et al. (2012) [39] | Pharmacy technicians and nursing personnel | 36 | Cross-Sectional | Pre- and post-work shift samples were collected from workers after 6 h of their work shift. | GEM: 0.2 ng/mL (LLOQ) CP: 0.2 ng/mL (LLOQ) IF: 0.2 ng/mL (LLOQ) | HPLC-MS/MS | Pre- and post-shift urine samples did not show significant concentrations of CP, IF, and GEM for any of the healthcare workers involved in manipulating hazardous drugs. | No |
| Kopp B. et al. (2013) [40] | Pharmacy personnel | 17 | Case-Control 12 exposed and 5 controls | Collected on Mondays and Fridays before starting work, as well as an additional sample on Fridays after work. | Pt: 0.002 ng/mL | Voltammetry | None of the urine samples contained an increased amount of platinum. There was no statistical difference between the platinum concentrations in the urine taken from exposed or non-exposed pharmacy personnel. | Yes |
| Miyake T. et al. (2013) [41] | Pharmacists | 4 | Cross-Sectional | 24-h urine samples. | CP: 0.01 ng/mL | GC-MS/MS | Before PhaSeal, CP was detected in 76% of samples. The mean value of CP excreted was 47.4 ng/24 h. After PhaSeal, CP was detected in 6% of samples. The mean value for these samples was 3.6 ng/24 h. | Yes |
| Yoshida J. et al. (2013) [42] | Pharmacists | 11 | Cross-Sectional | 24-h urine samples. | CP: 0.003 ng/mL; 0.02 ng/mL (LOQ) AFBA: 0.016 ng/mL; 0.04 ng/mL (LOQ) | GC-MS | During the non-attainment period (<80%), urinary CP and AFBA were detected in 45% and 55% of the pharmacists, respectively. During the attainment period, urinary CP and AFBA were detected in 0% and 17% of the pharmacists, respectively. The median urinary CP and AFBA concentrations of the pharmacists tended to be lower in the attainment versus non-attainment period; however, they were not statistically significant (p = 0.061 and 0.061, respectively). | Yes |
| Ramphal R. et al. (2014) [43] | Nurses | 90 | Case-Control 41 exposed and 49 controls | 24-h urine samples. | CP: 0.01 ng/mL | GC-MS | CP was detected in at least one urine sample in 34% of oncology nurses and 33% of control nurses (p = 1.0). No CP was detected in any of the urine samples from the 10 community controls. There was a statistically significant difference in the proportion of participants who tested positive between the nurses (combined oncology and control nurses) and the community controls (p = 0.03), between the oncology nurses and the community controls (p = 0.05), control nurses and the community controls (p = 0.04), and between the oncology nurses and the community controls (p = 0.05). | Yes |
| Sessink P. et al. (2014) [44] | Pharmacy technician | 2 | Cross-Sectional | 24-h urine samples. | CP: 0.10 ng/mL | GC-MS | CP was not detected in any of the 14 urine samples. | No |
| Friese C. et al. (2015) [2] | Nurses, medical assistants, pharmacists, and pharmacy technicians | 40 | Cross-Sectional | Participants saved all urine voids for a total of 8 h. They began their urine collection 4 h after drug exposure occurred. | ETOP: 0.02 ng/mL (LLOQ) PTR: 0.10 ng/mL (LLOQ) DOCE: 0.025 ng/mL (LLOQ) | LC-MS/MS | Of the 6 urine samples from workers who reported ETOP exposure, 1 sample exceeded the LOD but not the LLOQ. The samples from workers without a reported drug spill did not yield detectable levels of ETOP. Of the 3 samples analyzed from workers with exposure to DOCE, PTR, and CIS, all were above the LOD for DOCE and no samples were above the LOD for PTR. All 3 of these samples exceeded the LLOQ and were expressed as drug levels: 0.58, 0.10, and 0.03 ng/mL, respectively. Four samples from workers who did not report a drug spill were above the LOD for DOCE, but not above the LLOQ. Urine samples from cancer center employees who did not report a drug spill had detectable but no quantifiable levels of docetaxel. | Yes |
| Hon C. et al. (2015) [45] | Pharmacists, pharmacy receiver, pharmacy technician, nurse, transport staff, unit clerks, and others working in drug administration units (volunteers, ward aides, oncologists, and dieticians) | 103 | Cross-Sectional | 24-h urine samples. A total of 103 provided the 1st sample and 98 participants provided a 2nd sample. | CP: 0.05 ng/mL | HPLC-MS/MS | A total of 55% of samples had CP levels greater than the LOD with a maximum reported concentration of 2.37 ng/mL. The mean urinary CP concentration was 0.156 ng/mL, the GM was 0.067 ng/mL, the GSD was 3.18, and the 75th percentile was 0.129 ng/mL. All eight job categories examined had a maximum urinary concentration level above the LOD of 0.05 ng/mL. | Yes |
| Moretti M, et al. (2015) * [46] | Nurses | 148 | Case-Control 71 exposed and 77 controls | Samples collected at the end of the work shift. | CP: 0.05 ng/mL | LC–ESI–MS/MS | Samples from two exposed nurses had CP levels which exceeded LOD of 0.05 µg/L (0.08 and 0.12 µg/L). | Yes |
| Villa A, et al. (2015) [47] | Surgeons, anesthesiologist, operating room nurse, and nurse anesthetist, room cleaner) | 51 | Case-Control 44 exposed and 7 controls | Sample collected from the first void in the morning after the procedure. | Pt: 0.005 ng/mL; 0.016 ng/mL (LOQ) | ICP-MS | Pt was undetectable (<5 ng/L) in all workers. The Pt concentration was situated between the LOD and the LOQ (16 ng/L). In 1 of the 42 samples (2.38%) obtained before hyperthermic intraperitoneal chemotherapy (HIPEC), the worker concerned had participated in another HIPEC procedure one month previously. | No |
| Zhang J. et al. (2016) * [48] | Pharmacists and nurses | 301 | Case-Control 158 exposed and 143 controls | A 5 mL urine sample was collected from each subject before lunch. | 8-OHdG: N/A | ELISA | The urinary 8-OHdG mean concentration in 158 workers occupationally exposed to antineoplastic drugs was 22.05 ± 17.89 ng/mg creatinine, which was significantly higher than the levels observed in a control population (17.36 ± 13.50 ng/mg creatinine (p = 0.014)). | Yes |
| Poupeau C et al. (2017) [49] | Doctors, nurses, pharmacists, or pharmacy technicians | 102 | Case-Control 92 exposed and 9 controls | One sample collected per participant at the end of the work shift. | CP: 0.1 ng/mL Pt: 2.0 ng/mL | UPLC-MS/MS | None of the samples analyzed (0/101) had detectable concentrations of any of the four drugs evaluated. | No |
| Baniasadi S. et al. (2018) [50] | Nurses, nurse assistants, cleaners, and secretary | 30 | Case-Control 15 exposed and 15 controls | Urine samples were collected before the start and at the end of the work shift. | CP: 0.04 ng/mL (LLOQ) IF: 0.05 ng/mL (LLOQ) | GC-MS | The results indicated 46.66% and 16.66% of the subjects′ urine samples were positive for CP and IF, respectively. CP and IF were found in 33.32% and 6.66% of the pre-shift samples, respectively. Large amounts of CP (0.57 ng/mL (0.22–1.04)) and IF (0.26 ng/mL (0.12–0.35)) were found in post-shift urine samples. | Yes |
| Koller M. et al. (2018) [51] | Nurses and physicians | 15 | Cross-Sectional | Collected before and after their daily shift. | CP: 0.05 ng/mL 5-FU: 0.2 ng/mL Pt: 0.001 ng/mL | GC-MS/MS (CP, 5-FU) Inverse voltammetry (Pt) | No AFBA or CP residues were detected in any urine sample. Regarding Pt analysis, most urinary Pt concentrations (96 out of 98 samples) were below the reference value of 10 ng/L. Two nurses had pre-shift urine Pt concentrations of 10.3 and 16.2 ng/L. | No (AFBA or CP) Yes (Pt) |
| Ndaw S. et al. (2018) [52] | Anesthetist, surgeon, an operating room cleaner, an anesthetist nurse, and auxiliary nurse | 15 | Case-Control 10 exposed and 5 controls | 24-h urine samples from those exposed. Pre-shift and post-shift urine samples were obtained from controls. | Pt: 0.01 ng/mL (LOQ) | ICP-MS | Control group: Pt concentrations were above the LOQ (10 ng/L) in 72% of samples. The concentrations ranged from below the LOQ to 91 ng/L. HIPEC treatment: Pt concentrations were above the LOQ in 44% of samples. The concentrations varied from below the LOQ to 87 ng/L. PIPAC treatment: Pt concentrations were above the LOQ in 48% of the samples. The concentrations varied from below the LOQ up to 136 ng/L. | Yes |
| Azari M. et al. (2019) [53] | Pharmacy technicians, nurses, and auxiliary workers | 32 | Cross-Sectional | Specimens were obtained at the end of the work shift. | CP: 0.2 ng/mL (LLOD); 0.5 ng/mL (LLOQ) | GC-ECD which was subsequently confirmed by GC-MS | A total of 10 out of 32 urine samples had CP concentrations higher than LLOD. Most positive samples were from oncology nurses. The highest recorded CP concentration (21.4 µg/L) was from a nurse working in Hospital B. | Yes |
| Santos A. et al. (2019) * [7] | Non-exposed professionals, pharmacist, nurses | 59 | Case-Control 49 exposed and 10 controls | Samples were collected on Friday afternoon at the end of the week’s work shift. | N-trifluoroacetylated CP: 0.03 ng/mL; 0.11 ng/mL (LOQ) | GC | The presence of CP and/or its metabolites were evaluated in the urine samples of exposed and non-exposed individuals (p < 0.05), and 6- and 6.5-fold increases were observed for pharmacists and nurses, respectively, compared with the controls. | Yes |
| Ursini C. et al. (2019) * [54] | Pharmacy technicians and nurses | 95 | Case-Control 42 exposed and 53 controls | 24-h urine samples. | AFBA: 20 ng/mL | LC–MS/MS | No sample with AFBA above its detection limit of 0.02 μg/mL was found. | No |
| Palamini M. et al. (2020) [55] | Nurses and pharmacy technicians | 18 | Cross-Sectional | 24-h urine samples. | CP: 0.09 ng/mL IF: 0.0097 ng/mL MTX: 0.0075 ng/mL AFBA: 0.12 ng/mL | UPLC-MS/MS | A total of 128 urine samples were analyzed for the 18 workers. All urine samples were negative for the 4 antineoplastics drugs tested. | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hon, C.-Y.; Motiwala, N. Biological Monitoring via Urine Samples to Assess Healthcare Workers’ Exposure to Hazardous Drugs: A Scoping Review. Appl. Sci. 2022, 12, 11170. https://doi.org/10.3390/app122111170
Hon C-Y, Motiwala N. Biological Monitoring via Urine Samples to Assess Healthcare Workers’ Exposure to Hazardous Drugs: A Scoping Review. Applied Sciences. 2022; 12(21):11170. https://doi.org/10.3390/app122111170
Chicago/Turabian StyleHon, Chun-Yip, and Naqiyah Motiwala. 2022. "Biological Monitoring via Urine Samples to Assess Healthcare Workers’ Exposure to Hazardous Drugs: A Scoping Review" Applied Sciences 12, no. 21: 11170. https://doi.org/10.3390/app122111170
APA StyleHon, C.-Y., & Motiwala, N. (2022). Biological Monitoring via Urine Samples to Assess Healthcare Workers’ Exposure to Hazardous Drugs: A Scoping Review. Applied Sciences, 12(21), 11170. https://doi.org/10.3390/app122111170







