Abstract
One of the solutions implemented in order to improve the quality of soils exposed to pesticides is the application of sewage sludge, which is a by-product of wastewater treatment. As an organic substrate, it provides soil with important nutrients, such as nitrogen and phosphorus, and enriches it with organic matter, thanks to which it can be a valuable fertilizer. The aim of the presented research was to evaluate the influence of granulated sewage sludge (GSS) on the biological properties of soil treated with herbicides (MCPA and dicamba) and fungicides (thiophanate-methyl and azoxystrobin). The following aspects were investigated: the activity of selected soil enzymes, the genetic biodiversity of bacteria and fungi, and the abundance of the bacterial gene responsible for ammonia oxidation. A field experiment was conducted, in which granulated sewage sludge (GSS) was applied to soil at a single dose of 3 t/ha. Wheat (Triticum aestivum L.) was sown on the prepared plots. The herbicides (H) and fungicides (F) as well as their mixture (F + H) were applied to the plants in the appropriate growth phases in the doses recommended by the producer. The control was soil without sewage sludge (C). The samples taken were tested for: dehydrogenases, catalases and urease activities, genetic biodiversity structure of bacteria and fungi by TRFLP assay, and the abundance of the bacterial amoA gene by qPCR. On the basis of the obtained results, it was found that the application of pesticides to soil fertilized with sewage sludge influenced the enzymatic activity of soil, and their activity differed depending on the tested enzyme. The activity of URE and DHA on the plots with GSS was higher by approx. 20% and 30%, respectively, as compared to the plots without GSS application. Moreover, both the genetic biodiversity of microorganisms and the abundance of amoA gene differed depending on the variant of the experiment. The GSS treatment of soil significantly influenced the growth of the studied gene as compared to C, and its abundance was 9.15 log10 gene copies/g DW of soil. Due to the content of nutrients in sewage sludge, it can be a valuable fertilizer in agricultural crops treated with pesticides.
1. Introduction
In agriculture, various types of fertilizers are applied to enrich soil for crops. The most commonly used kinds are natural fertilizers such as slurry and liquid manure, mineral and organic fertilizers made of a single organic substance, or a mixture such as composts, vermicomposts and municipal sewage sludge [1]. The limiting factor in the use of both organic and mineral fertilizers is the content of N and P; therefore, their doses should always be calculated on the basis of the content of these elements in soil and fertilizers [2]. It cannot be ignored that the crucial moment is their application method, which should be carried out very efficiently, and fertilizers should be quickly covered with soil in order to minimize nitrogen losses in the form of ammonia, which contributes to environmental contamination.
The sludge as a by-product from wastewater treatment plants is characterized by typical fertilizing and cariogenic properties, such as the presence of many nutrients necessary for plants (macro- and micronutrients, including nitrogen and phosphorus) and a high content of organic matter [3]. Currently, the most popular method of sludge disposal is incineration [4]. However, in the case of sewage sludge from areas with low industrialization and low content of heavy metals, it is encouraged to use them as a substrate to improve the quality of soils by enriching them with organic matter and minerals necessary for plants.
Unfortunately, the use of sludge is still associated with the disapproval of society due to the place of its formation and the possibility of occurrence of various pollutants. Moreover, the risk associated with the application of sludge to soil as a fertilizer is the possibility of leaching contaminants (e.g., heavy metals) and other components from soil into the groundwater, which can lead to water contamination. The leaching process depends mainly on the mineral composition of the waste product and soil chemical composition, pH and texture [5]. Therefore, in order to use sludge for agricultural purposes in a safe way, its dose and method of application should be adjusted to the type and properties of soil [6].
Obviously, the content of fertilizers in the sewage sludge may be varied and it mainly depends on the quality of the incoming sewage and the treatment technology used [6,7]. The mean content of macroelements in the sewage sludge for nitrogen is approx. 2.5%, with fluctuations from 0.9 to 7.6% DS, phosphorus (P2O5) approx. 3.2% in weight, while potassium (K2O) is approx. 0.4% on a dry-weight basis [8]. Moreover, it is estimated that approx. 35% of municipal waste is an organic fraction, which, when applied to soil, improves properties such as: bulk density, porosity, and stability of soil aggregates or water retention capacity [6,9]. Panasiewicz et al. [10] indicate that all these factors contribute to the growth and development of various groups of soil microorganisms, which, also by transforming organic and mineral compounds, enrich soil with nitrogen and biologically active, antibiotic and growth substances [11]. Many studies confirm the positive effect of sewage sludge on the growth of oligo- and macrotrophic bacteria and soil fungi, such as Geotrichum, Fusarium, Mucor, Penicillium, Mortierelta, Verlicillium and Trichoderma after the direct application of sludge. However, there are also reports that the microorganisms present in sludge are not able to fully inhabit soil environment and in a short time most of them die, becoming an additional source of organic matter for native bacteria [11,12]. According to Wydro et al. [3], the growth of microorganisms in soil treated with sludge contributes to the stimulation of biochemical activity, which guarantees plant vegetation and the final yield at a satisfactory level [12].
However, during the growing season, various agrophages appear in crops, which significantly affects their size and quality. To counteract their occurrence, farmers use various biological, chemical, agronomic and mechanical methods. Among them, the chemical ones tend to be chosen due to their easy application, speed and efficiency [13]. Their use ensures obtaining the right amount of good-quality food and allows for its storage and transport for a long time. The usage of pesticides does not only contribute to the elimination of agrophages, but can also significantly change the soil microflora by disrupting basic biochemical processes [14]. Moreover, the presence of active substances (a.s.) in soil may cause complications in succeeding crops, while washing out pesticides deep into the soil profile carries the risk of contamination of groundwater [13].
Herbicides (MCPA and dicamba) and fungicides (thiophanate-methyl and azoxystrobin) are often used in agricultural crops. MCPA and dicamba are herbicides with a broad spectrum of activity against various species of weeds. From another point of view, these herbicides may affect resistance to biodegradation processes and the number of specific species of microorganisms, and thus the growth and quality of crops [15,16]. In turn, thiophanate methyl and azoxystrobin are used in the fight against diseases during plant growth that inhibit the growth of the fungus by stopping the electron transfer between Cytb gene (cytochrome b) and Cytc (cytochrome c), which reduces the oxidation of NADH (nicotinamide adenine dinucleotide) and ATP synthesis (adenosine triphosphate) [17,18]. The application of pesticides as substances that improve the quality of the crop also has negative environmental effects. There are reports in the literature which indicate the effect of pesticide use, including the biodiversity of soil microflora, abundance of ammonia-oxidizing bacteria, and enzymatic activity of microorganisms [13,19].
The root zone is one of the areas with the most intense biological activity, characterized by a rich bacterial and fungal flora. It has a large impact on the uptake of nutrients by the roots [10,13]. Soil biodiversity plays an important role in regulating and maintaining a high functional potential, contributing to soil stability and productivity. According to Verma et al. [19], different groups of soil organisms play different roles in maintaining multi-functionality. For example: soil invertebrates are responsible for processing large amounts of plant and animal residues, while protists, bacteria and fungi are important in ensuring proper energy flow. It cannot be ignored that the activity of soil microorganisms depends, to a large extent, on the oxygen conditions of soil, determined by its physical and chemical properties and the influx of xenobiotic factors. The activities of dehydrogenases, catalases and ureases are considered to be important indicators determining the number and activity of microorganisms, which provide important information on the biochemical processes taking place in the medium. The activity of dehydrogenases increases proportionally to the amount of sewage sludge introduced into soil, as it is strongly correlated with the presence of organic matter and allows for assessing the influence of factors adversely affecting soil microorganisms [13]. Moreover, ureases provide information on the decomposition of nitrogenous organic compounds. According to Gleń and Gondek [20], there are significant positive correlations between soil catalase activity and organic matter content, microbial biomass, O2 uptake, CO2 release, ATP content, soil fertility, and dehydrogenase activity [21].
In the literature, there are many reports describing the advantages and disadvantages of using sewage sludge in crops, but there are still insufficient data available explaining the effects of sludge and pesticides on the changes that occur in soil microbial population. Therefore, the aim of the presented research was to evaluate the influence of granulated sewage sludge on the biological properties of soils treated with herbicides (MCPA and dicamba) and fungicides (thiophanate-methyl and azoxystrobin). The study analyses three parameters: activity of selected soil enzymes, genetic biodiversity of bacteria and fungi, and abundance of the bacterial gene responsible for ammonia oxidation.
2. Materials and Methods
2.1. Experimental Design
The experiment was conducted in Dobrzyniewo in Podlaskie (53°11′45.2″ N 23°00′40.4″ E) using randomized blocks with 5 replications. Fertilization had been performed before the sowing of wheat, using organic fertilizer P–133 kg/ha, N–87.5 kg/ha, and organic substance 1400 kg/ha. In addition, on the plots, we used single-dose granulated sewage sludge (GSS) from a local sewage treatment plant. The dose of GSS (3 t/ha) was adjusted according to Regulation of the Minister of the Environment of 6 February 2015 on the use of municipal sewage sludge [22].
In this experiment, weather conditions were characterized by low rainfall, the sum of which amounted to 30 mm in April, 89 mm in May, 78 mm in June, and 68 mm in July. The observed amount of rainfall was lower on average by 33% with respect to rainfall the in same period in previous years. Average daily temperatures in April, May, June, and July were, respectively, 6, 12, 18 and 21 °C.
Subsequently, herbicides (MCPA and dicamba-H) and fungicides (thiophanate-methyl and azoxystrobin-F) were added to plots in the form of a spray using a knapsack compressed-air sprayer AP-1/p. Considering fertilization with GSS and the application of F and H, eight studied variants were obtained (Table 1).

Table 1.
The chemical protection treatment variants.
2.2. Parameters of Sewage Sludge and Soil with Sewage Sludge
The granulated sludge was dried at a temperature of 130 °C. The sludge did not contain Salmonella and invasive nematode ova (Ascaris sp., Trichuris sp., Toxocara sp.) and was applied on the experimental plots at dose 3 t/ha DW (dry matter). The levels of heavy metals of granulated sludge were: Pb—21.5 mg/kg DW, Cd—1.15 mg/kg DW, Cu—202 mg/kg DW, Cr—64.8 mg/kg DW, Ni—29.8 mg/kg DW, and Zn—1100 mg/kg DW. The properties of the sludge used in the experiment was pH—7.5, dry matter—89.1%, organic matter—56.4 g/kg DW, ammonium N—0.33% DW, total N—4.7% DW, total P—3.1% DW, Mg—0.71% DW, and Ca—4.18% DW.
After mixing GSS with soil, the pH was 7.2, while the metal content was: Pb—17.4 mg/kg DW, Cd—0.78 mg/kg DW, Cu—36 mg/kg DW, Cr—43.3 mg/kg DW, Ni—6.74 mg/kg DW, and Zn—77.21 mg/kg DW.
2.3. Soil Sampling and Preparing
Samples for physicochemical, enzymatic and microbiological tests were collected in one time after all procedures were performed between 77 and 83 phases of the growing plant according to Zadoks [23]. Soil samples from individual research variants for biological tests were collected from 40 plots at a depth of up to 10 cm. Then, samples from the same test variants were mixed and 3 representative samples were taken. Samples were collected after wheat harvest. Soil samples for physicochemical and enzymatic analyses were dried to dry matter, then sieved (2 mm sieve) and stored in plastic containers for analysis. Samples for microbiological testing were transferred to sterile plastic bags and stored at −20 °C to further analysis.
2.3.1. Chemical Analyses of Soil
The organic carbon in samples was measured in Multi N/C 3100 Analytik Jena and the content of total phosphorus was determined by using Agilent 8800 ICP–MS Triple Quad (ICP–QQQ) after sample digestion in 65% HNO3 (7 mL) with 30% H2O2 (2 mL) using the Advanced Microwave Digestion System Ethos Easy (Milestone, Sorisole, Italy). The values of pH were measured by 1 M KCl using a pH meter. The particular fraction in soil was measured by using the laser particle size analyzer Analysette 22 NanoTec plus (Fritsch GmbH, Weimar, Germany) according to the manufacturer’s instructions. The samples were analyzed in triplicate.
2.3.2. Soil Enzyme Activities
Dehydrogenase activities (DHA) were determined in soil samples according to Casida et al. [24], modified by Tabatabai [25] using as a substrate 3% 2,3,5-triphenyltetrazolium chloride (TTC). All results were expressed on the basis of dry weight of soil in μmol TPF g/D.M soil 20 h.
Catalase activities (CAT) were estimated according to Johnson and Temple [26] using as a substrate disodium p-nitrophenyl phosphate hexahydrate (Acros Organics, Vernon Hills, IL, USA). The content of p-nitrophenol (p-NP) (Merck KGaA, Darmstadt, Germany) in samples was analysed photometrically at 400 nm by using a microplate reader GloMax®-Multi (Promega Corporation, Madison, WI, USA).
Urease activity (URE) was determined in soil samples according to Hoffman and Teicher [27]. Absorbance measurements were performed using by a Lambda Bio+ spectrophotometer (PerkinElmer, Boston, MA, USA) at a wavelength of 630 nm after 30 min. URE was expressed in μg N formed in 1 g of soil for 1h (μg N/g DW h).
2.4. DNA Extraction, Polymerase Chain Reaction (PCR), and TRFLP Analysis
Genomic soil DNA was extracted with the use of a GeneMATRIX Soil DNA Purification Kit (EURx, Gdańsk, Poland) according to the manufacturer’s protocol. The amount of DNA obtained was determined using the QuantiFluor® dsDNA Dye (Promega, WI, USA) on a GloMax®-Multi Microplate Multimode Reader (Promega Corporation, Madison, WI, USA). The quality of the soil DNA was assessed using a Lambda Bio+ spectrophotometer (PerkinElmer, Boston, MA, USA) with an ultra-micro sample volume spectrometer cell TrayCell (Hellma GmbH, Müllheim/Baden, Germany).
Bacterial (16S rDNA) and fungal (ITS) gene amplification was performed using the primers and conditions reported by Mattan et al. [28]. The 25 µL reaction mixture consisted of: 15 µL GoTaq® G2 Colorless Master Mix (Promega Corporation, Madison, WI, USA), 0.5 µM of each primer, and 2 µL (5 ng/µL) of soil DNA. The purity of the PCR product was determined by agarose gel electrophoresis (1.2%, w/v).
The amplification products were purified using Exo-BAP Mix (EURx, Gdańsk, Poland) according to the manufacturer’s assay, and then 7 µL of the purified product was digested with 6U of the restriction enzyme MspI (10 U/µL) (Promega Corporation, Madison, WI, USA) in restriction buffer in a final volume of 10 µL for 2 h at 37 °C. The resulting product was desalted using the PCR/DNA Clean-Up Purification Kit (Promega Corporation, Madison, WI, USA). Further procedures, including the TRFLP protocol, are described in Wydro et al. [3].
The relative number of restriction fragments (TRFs) was determined by the quotient of the area of each peak and the sum of the area of all peaks in one sample. The analysis included peaks in the 50–600 bp range with a relative abundance greater than 1%.
2.5. Bacteria amoA Gene Abundance
Abundance of the bacterial gene amoA was determined using quantitative polymerase chain reaction (qPCR). The analysis was performed on a CFX96 Touch Real-Time PCR Detection System thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using the primers provided by Mattan et al. [28]. The amplification reaction was carried out in a final volume of the reaction mixture of 20 µL, consisting of: 10 µL of Go-Taq® qPCR Master Mix (Promega), 0.5 µL of reverse and forward primer (10 µM), 0.5 µL of dimethyl sulfoxide (DMSO), 6 µL of PCR-grade water, and 2 µL of purified DNA (4 ng/µL). The operating conditions of the thermal cycler were as follows: polymerase activation at 95 °C for 2 min (1 cycle); then, 40 cycles: denaturation at 95 °C for 15 s, annealing, and extension 60 °C for 1 min. Melt curve analysis of the PCR product was performed (65–95 °C, 0.5 °C increments at 2–5 s/step) to confirm the specificity of the PCR product. The standard curve was generated according to the protocol provided by Shahsavari et al. [29]. The standard curve PCR product was purified using Wizard® SV Gel and PCR Clean-Up System (Promega). Sample and standard analyses were performed in triplicate; NTC (no-template control) also performed. The amplification efficiency was calculated according to the formula: E = [10(−1/slope) − 1] × 100, which in our study was 95%. The abundance of the bacterial amoA gene is expressed as log10 gene copy number per soil dry weight (log10 gene copies/g DW).
2.6. Data Analysis
The differences in the variance of the experiment for the tested soil properties were determined using ANOVA. If there were significant differences between the experimental factors, the means were compared using the HSD post hoc Tukey test for p < 0.05. The PCA (principal component analysis) was used in order to determine the relationships between the studied variables and the experimental factors.
The genetic diversity of bacteria/fungi was assessed by calculating the Shannon (H′) and Margalef indices. The similarity between the defined TRFs for bacteria and fungi was determined by calculating the Jaccard index, taking into account the presence or absence of a given TRF. Results are presented as a dendrogram which was constructed using the unweighted pair group mean (UGMA). The analyses and calculations were performed using the Statistica 13.3 and PAST 4.03 packages (https://www.nhm.uio.no/english/research/resources/past/, accessed on 3 October 2022).
3. Results
3.1. Soil Enzyme Activities
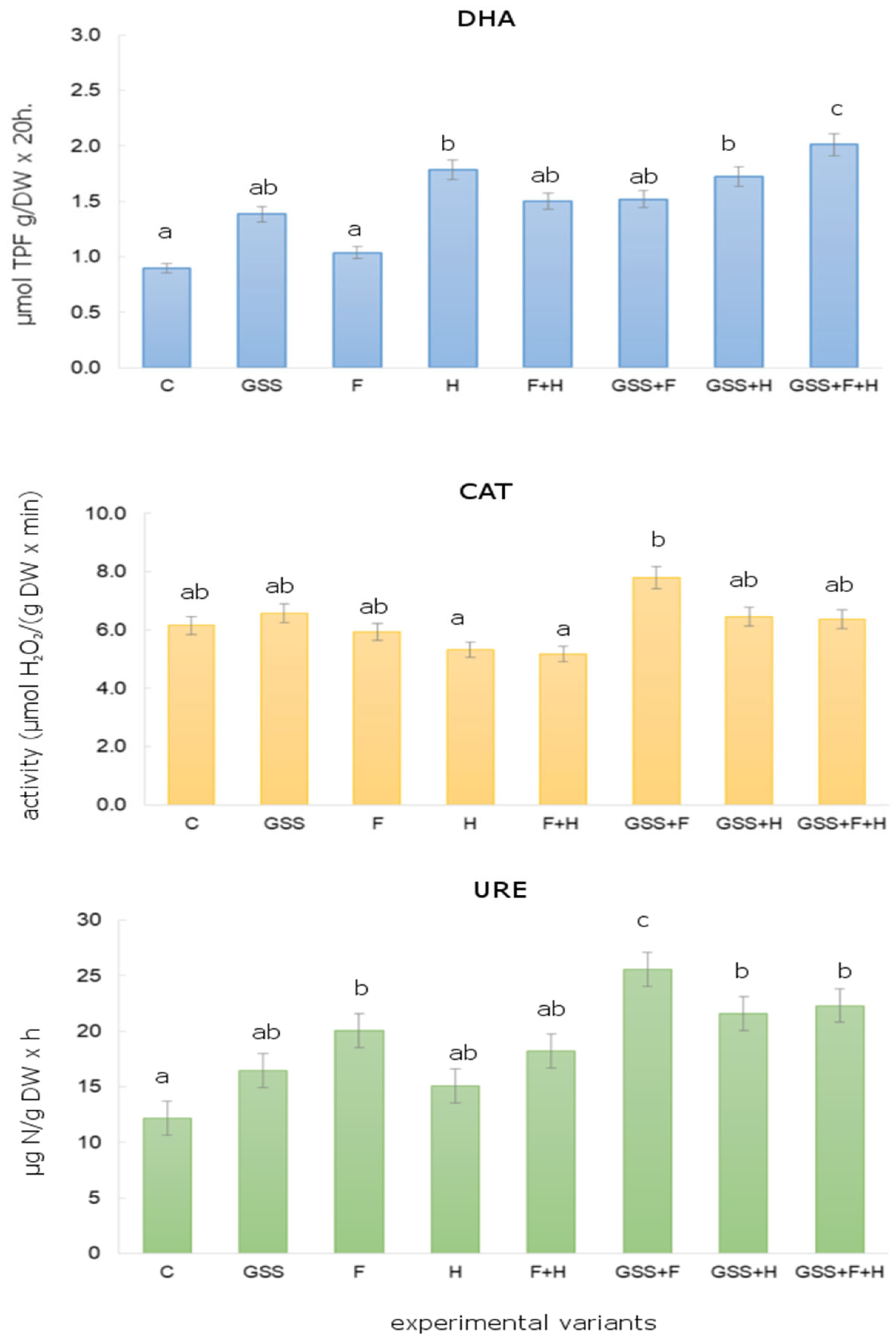
Figure 1 shows the soil enzyme activity without GSS application and after GSS application, depending on the tested variant of herbicides/fungicides. In the present research study, it was shown that in the soil treated with GSS, GSS + F, GSS + H, and GSS + F + H, the activity of DHA was significantly higher than in the control plot. The highest activity of DHA recorded in the soil treated with GSS + F + H amounted to 2.01 μmol TPF g/d.m. 20 h. The results indicate that the application of herbicides/fungicides affected DHA activity. There were statistically significant differences for DHA depending on the dose of a.s. pesticides. DHA activity in the soil without GSS fluctuated depending on the applied a.s. of pesticides from 1.03 μmol TPF g/DW 20 h (after F application) to 1.78 μmol TPF g/DW 20 h (after H application). On average, the DHA activity on plots without GSS was lower by approx. 20% compared to GSS plots.
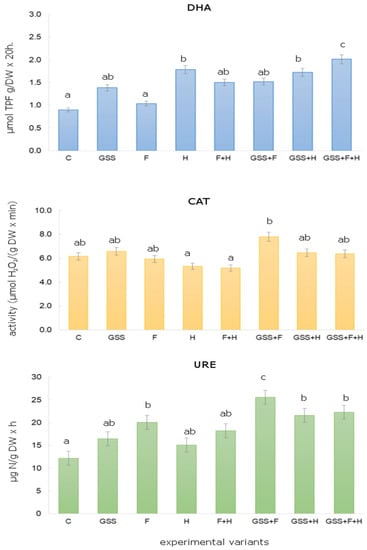
Figure 1.
Changes in DHA, CAT and URE activity after GSS and herbicides (MCPA/dicamba) and fungicides (thiophanate-methyl/azoxystrobin) application. (The same letters above bars mean non-significant differences between treatments estimated by Tukey post hoc test at p < 0.05).
It was observed that after the application of H and F + H, there was a decrease in CAT activity by approx. 10% as compared to the control untreated plots. On the other hand, the addition of GSS to soil increased the activity of CAT in comparison to the control plots. The highest statistically significant activity was recorded on the plots where GSS + F was applied. It was 7.80 µmol H2O2/g DW × min, thus approx. 26% higher compared to the control plots. In turn, the activity of URE was the lowest in the control plots (12.20 μg N/g DW h), while being the highest where GSS + F was applied, as it was 26.60 μg N/g DW h (Figure 1). On average, the activity of URE on the plots with GSS was higher by approx. 30% as compared to the plots without GSS application.
3.2. Biodiversity of Bacteria and Fungi in Soil
The results showed that the patterns characterizing the community of bacteria and fungi in the soil determined by the tRFLP method were different depending on the research variant. This dissimilarity was demonstrated in terms of relative abundance and by calculating the biodiversity and similarity indices (Figure 2 and Figure 3).
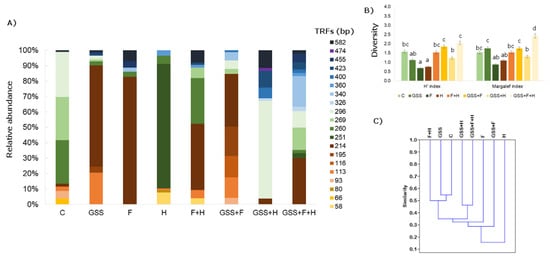
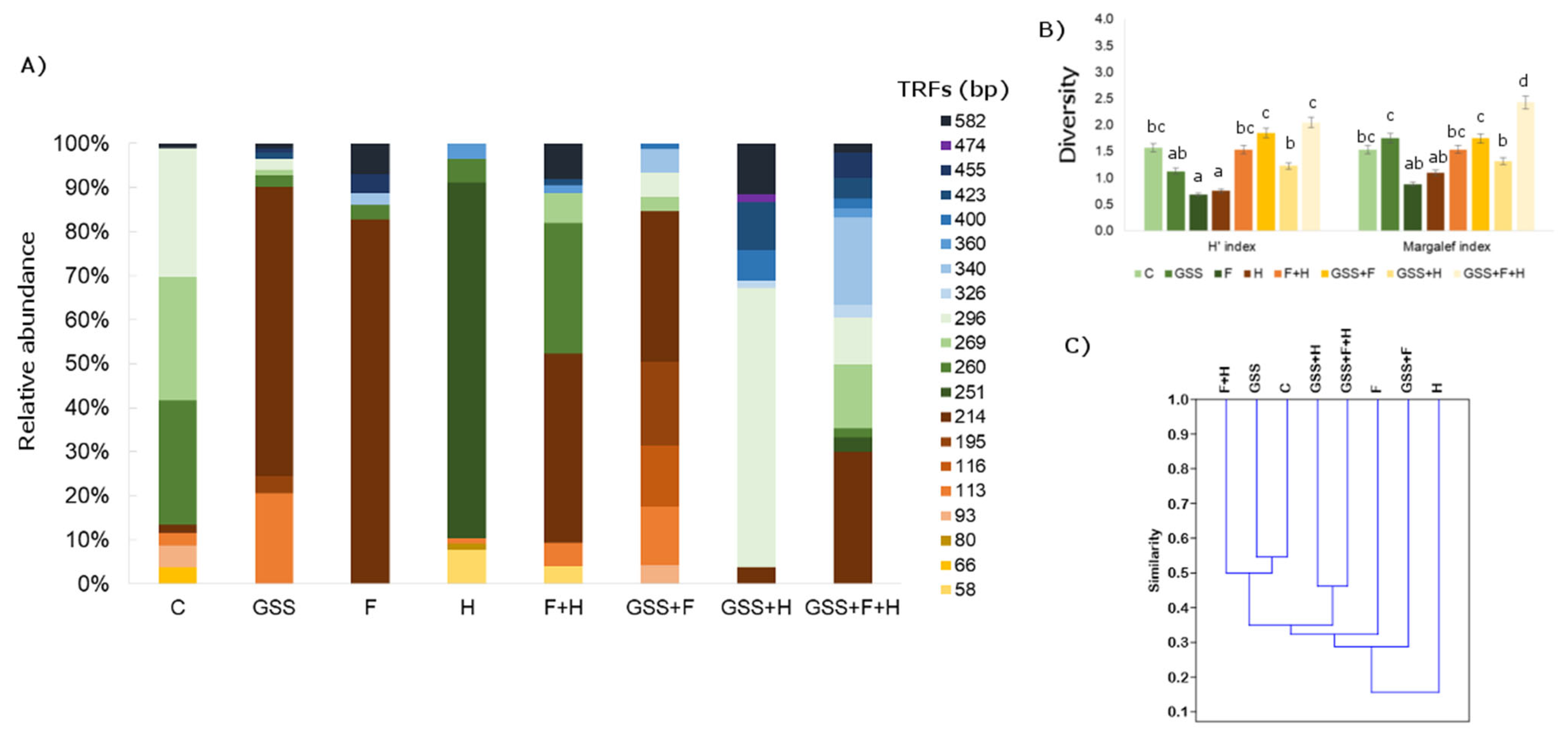
Figure 2.
(A) Relative abundance (%) of bacteria TRFs fragments after MspI digestion; (B) Shannon (H′) and Margalef diversity indexes and (C) dendrogram presenting Jaccard similarity index (C). The data presented in (B) are means ± SD; the same letters mean non-significant differences between treatments assessed by Tukey post hoc HSD test at p < 0.05.
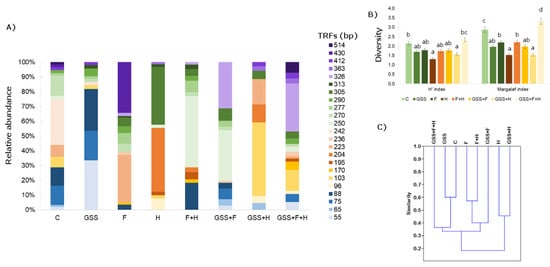
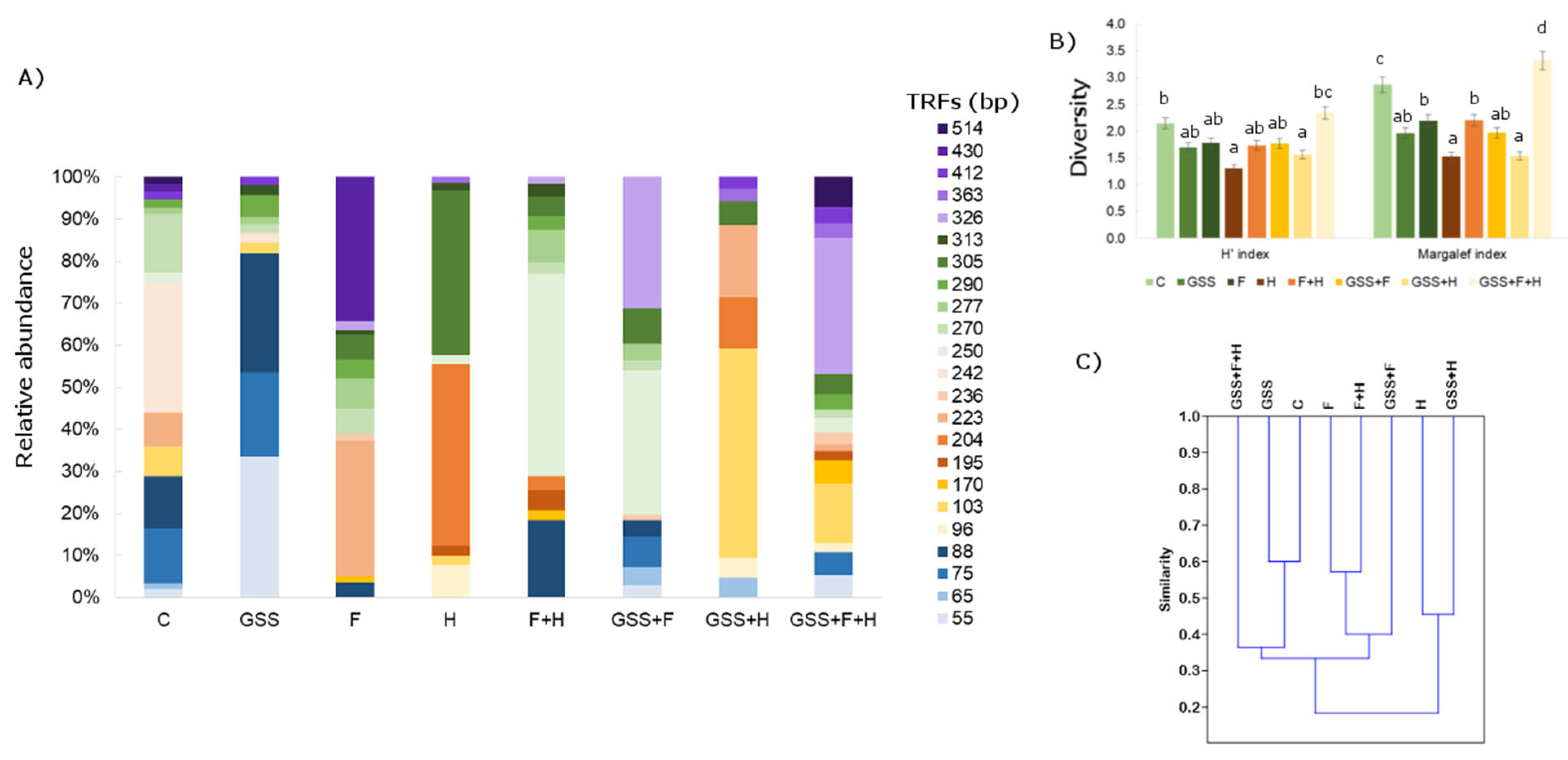
Figure 3.
(A) Relative abundance (%) of fungal TRFs fragments in soil after MspI digestion; (B) Shannon (H′) and Margalef diversity indexes and (C) dendrogram presenting Jaccard similarity index (C). The data presented in (B) are means ± SD; the same letters mean non-significant differences between treatments assessed by Tukey post hoc HSD test at p < 0.05.
Considering the genetic biodiversity of soil bacteria, the length of the defined TRFs ranged in size from 55 to 582 bp (Figure 2). After digestion with MspI, 20 TRFs were found. In the tested soil, TRF (214) bp was the most characteristic, except for the soil treated with H. This restriction fragment also had a high relative abundance (from 2 to 83%). Analysing the H′ and Margalef biodiversity indexes, it was found that the highest indexes occurred in the GSS + F + H variant, while the lowest was in the F and H variants. Taking into account Jaccard’s similarity indexes, it was observed that they were not high and the highest variants were C and GSS, and between GSS and F + H (0.54).
When analysing the genetic profile of soil fungi, 23 TRFs were noted and the length of the defined fragments ranged from 55 to 514 bp (Figure 3). The highest biodiversity, according to the calculated indicators, was found in the GSS + F + H variant (H′ index: 2.34; Margalef index: 3.32), while the lowest was in the H′ variant (H′ index: 1.31; Margalef index: 1.53). The most specific TRFs were 270 bp and 305 b; however, their relative abundance was not high, as it ranged from 2 to 14% and 5 to 39%, respectively. Considering the similarity index calculated according to Jaccard, the highest was recorded between the GSS and C variants (0.60), and between F + H and F (0.57).
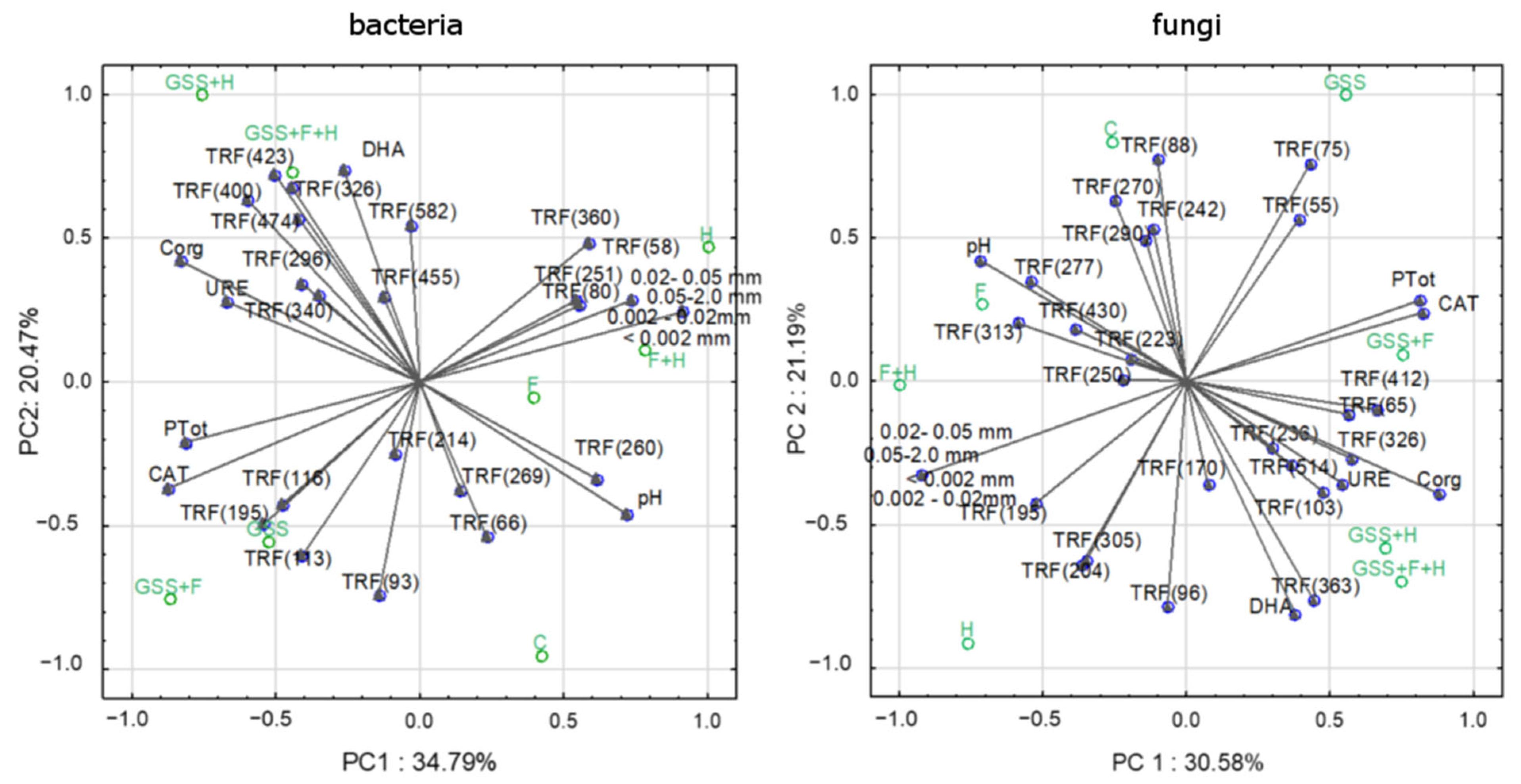
BiPlot performed for bacterial TRFs and the main properties of the soil explained a total of 58.26% of the variability of the data (Figure 4). In turn, principal components designed for fungal TRFs and soil properties explained a total of 51.77% of the variable’s variance. The presented results indicate that the occurrence of selected bacterial and fungal genetic patterns is related to some soil properties and may be specific for the research variant.
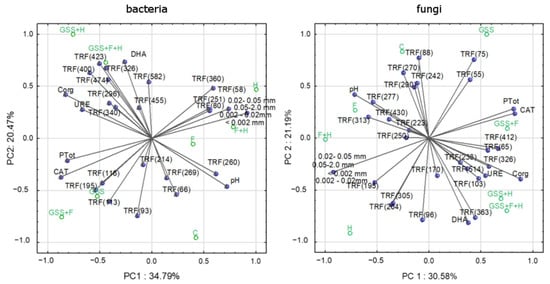
Figure 4.
PCA analysis presented as BiPlot. Factor coordinates of cases represent the analyzed properties of soil and TRFs defined for bacterial and fungal community; factor coordinates of variables represent the research variants.
3.3. Abundance of amoA Bacteria Gene
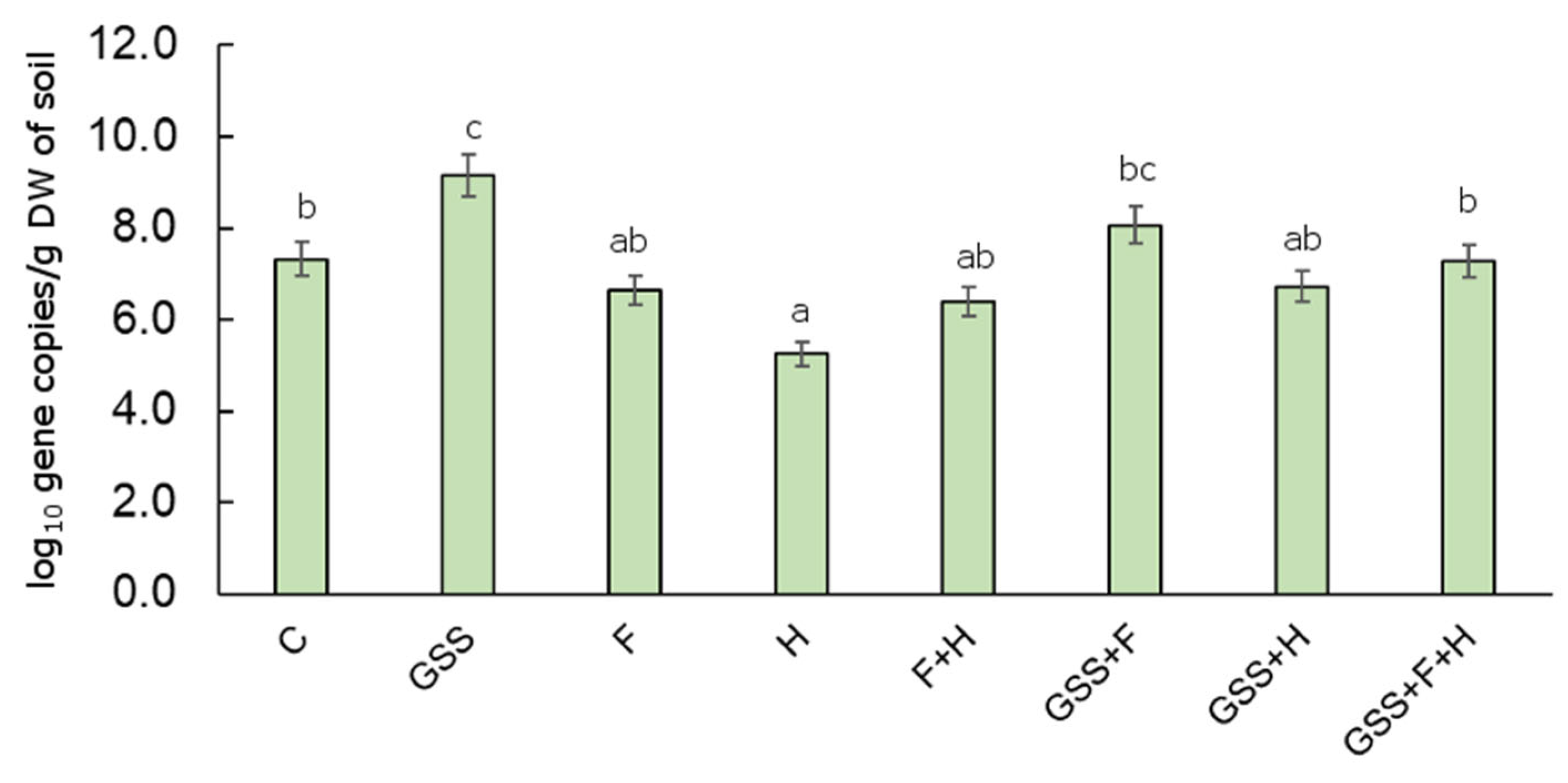
The amoA bacteria gene abundance was calculated as the log10 copy number of the gene encoding bacterial ammonia monoxygenase. Significantly, the lowest gene abundance was recorded in variant H (5.25 log10 gene copies/g DW of soil). It was also found that the GSS treatment of soil significantly influenced the growth of the studied gene as compared to C, and its abundance was 9.15 log10 gene copies/g DW of soil (Figure 5).
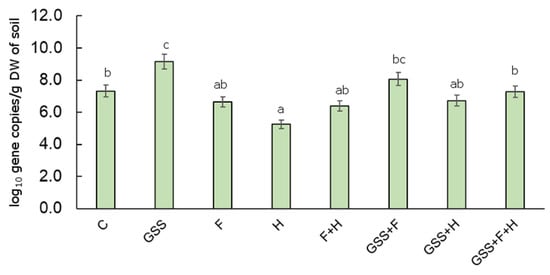
Figure 5.
The abundance of amoA gene copy number (log10 gene copy numbers/g DW of soil) in the soil samples collected for experimental plots. The data are presented as means ± SD; the same letters mean non-significant differences between treatments assessed by Tukey post hoc HSD test at p < 0.05.
4. Discussion
On the one hand, the application of sludge to agricultural soil is a rational solution that increases the sorption capacity and thus improves soil and water properties, which has a positive effect on the microbiological parameters [3,6]. On the other hand, according to Farsang et al. [30], sludge can also introduce toxic elements into the soil environment, which has an adverse effect on biological activities occurring in soil. Therefore, each portion of sludge used to fertilise soil should be analysed for heavy-metal content and sanitary pollutants.
There are various reports in the literature describing the influence of sludge on the enzymatic activity of soil. As reported by Wallenstein and Vilgalys, [31], enzyme activity, abundance, diversity and structure of soil microorganisms, and the abundance of functional genes in soil are important parameters reflecting changes in soil. Hence, they are often used to study soil response to various treatments and environmental pollution. Gleń and Gondek [20] report that various biochemical changes occurring in soil are a key indicator of the quality and productivity which provides important information on the specific metabolic activity and functions of soil microorganism population. On the basis of changes in the activity of microorganisms, it is possible to assess the course of the decomposition of the organic substance introduced into soil as well as changes in the metabolic activity of microorganisms in soil under the influence of anthropogenic stress factors which also include the application of pesticides [32].
Our previous research indicates [2] that the application of GSS and herbicides influences soil enzyme activity in a manner analogous to the present study. Therefore, it can be concluded that enzyme activity is a suitable biomarker of the state of the soil environment after pesticide application [13]. The addition of GSS to the agricultural soil improves its quality and prevents the negative effects of pesticides, which may subsequently change the activity of enzymes and influence bacterial diversity [6].
The activity of dehydrogenase and catalase is a good indicator for the assessment of metabolic activity in the presence of soil contaminants. These indicators express the intensity of the respiratory metabolism of microorganisms as well as provide information on, among others, aerobic and moisture conditions in soil. Moreover, the activity of urease informs on the rate of mineralization of N introduced into soil [3]. Based on our own research, it can be concluded that the addition of GSS will increase DHA, CAT and URE. It may indicate an increase in microbial biomass and the rate of denitrification as a result of using GSS, which was also observed in the studies by Yao et al. [33]. In addition, an increase in the enzyme activity under the influence of GSS may be due to the fact that organic matter was additionally introduced into soil along with the sludge with a pool of macro- and microelements, which could additionally stimulate the growth of soil microorganisms. However, such a relationship was not observed in the studies conducted by Farsang et al. [30], because after the sludge application, they did not notice any significant increases in the activity of DHA and CAT in particular.
According to the literature, the a.s. of pesticides introduced into the environment may undergo multidirectional changes. Their migration, persistence and synergistic effect mean that they can significantly affect microbial activity by disrupting the correct sequence of biochemical pathways in the biogeochemical cycles of soil [13,20,34]. Moreover, according to Baćmaga et al. [35] the application of herbicides to soil stimulated the activity of microorganisms; however, it will depend on the properties of the herbicide as well as agrotechnical treatments, the physicochemical properties of the soil, the date of its application, and also on climatic conditions. On the other hand, as reported by Zhang et al. [34] the application of fungicides onto plants may disrupt their metabolic pathways by altering the photosynthetic pathway as well as disrupting soil biological homeostasis and altering the diversity and structure of their community. In our own research, on the plots where the F, H and F + H treatments were applied, an increase in the activity of DHA and URE was observed, which could be due to the fact that the microorganisms could actively use the introduced herbicides/fungicides as an additional source of carbon necessary for their growth and development. However, the effect of pesticides on soil enzyme activity is inconclusive according to the literature data. Du et al. [36] found no effect of mesotrione on the activity of URE but Sviridov et al. [37] observed positive influence of glyphosate on URE activities. Baćmaga et al. [38] showed that azoxystrobin significantly inhibits changes in enzymatic activity for URE and CAT, but no decreasing effect on DHA was obtained. In turn, Hu et al. [39] observed in their research that after the application of fomesafen, the activity of URE was inhibited, while it had no significant influence on the activity of CAT. In our research, in the case of CAT, the F, H and F + H treatments reduced the activity of this enzyme, which suggests that microorganisms that had the ability to decompose thiophanate-methyl and azoxystrobin could have displaced those species that were responsible for CAT activity in soil. Moreover, as was indicated by literature data and own research, the activity of soil enzymes in soil largely dependent on the active substance of the pesticide.
The fingerprint technique—TRFLP analysis was used in the studies on the biodiversity of soil bacteria and fungi. It is an indirect method that allows for assessing the dynamics of the community of soil microorganisms depending on changes in environmental conditions, including the presence of pollutants [40]. Our research showed that various agrotechnical treatments (organic fertilization; the application of pesticides) can affect the richness and diversity of soil microflora, which was also confirmed in the research conducted by Gryta et al. [41]. In addition, studies by Wydro et al. [3], in which the popular herbicide (glyphosate) was used, showed that the application of this agent may reduce the genetic biodiversity of bacteria and at the same time affect the growth of fungi biodiversity over time. In the present research study, the determined indicators of biodiversity for bacteria (H′ and Margalef indices) in variants with F and H are significantly lower than in the control, while in the case of fungi, this relationship applies to almost all research variants (excluding GSS + F + H). There was also no observed increase in the biodiversity of fungi as compared to bacteria, which does not support the previous studies on glyphosate. Moreover, the authors’ own research showed a beneficial effect of the sludge applied simultaneously with the herbicide and fungicide on the level of diversity of the bacterial population. A lower effect was noticed in case of fungi. It can be assumed that nutrients such as C, N and P contained in sewage sludge as well as micronutrients may improve the activity of soil microorganisms and thus eliminate the negative impact of pesticide application. Moreover, according to Wang et al. [42], both sludge and pesticides are a source of carbon and some consortia of microorganisms have the ability to acclimatize in difficult conditions and, at the same time, are able to decompose pollutants.
Du et al. [32] and Sun et al. [36] demonstrated that exposure to herbicide, even at very low concentrations, can reduce the abundance of bacteria and fungi in the soil. They also noticed that under the influence of mesotrione, the diversity and evenness of soil microbiota were slightly reduced. Similar observations were obtained in the authors’ own research, which showed a decrease in the biodiversity of bacteria and fungi in soils after the application of H. On the other hand, the research study presented by Prudnikova et al. [43] showed a reduction in bacteria and fungi abundance with azoxystrobin as compared to control. Moreover, the fungicide application increased the share of Bacillus sp. oraz Penicillum and Aspergillus.
The analysis of the similarity expressed by the Jaccard coefficient indicates that both the application of sewage sludge and pesticides can significantly change the relative abundance of soil bacteria and fungi with respect to control, as reflected by the low Jaccard similarity coefficients. This can be explained by the fact that the application of sewage sludge, on the one hand, may be a source of foreign microorganisms, some of which may survive in soil, and the supplied organic matter with the sludge may stimulate the activity of native microflora. On the other hand, both sewage sludge and pesticides can cause stress, which can result in the reduction in less resistant species of microorganisms.
One of the most important cycles of organic matter in nature is the nitrogen cycle. Ammonia oxidation is an inseparable stage of nitrification and determines its proper course. Hence, it is important to control the abundance of amoA genes reflecting the presence of ammonia-oxidizing bacteria [44]. Crouzet et al. [45] investigated the effect of mesotrione (a herbicide) on microorganisms involved in the nitrogen cycle. They found that the action of this substance was dose-dependent and at higher levels, it could inhibit the nitrification process. In turn, Du et al. [32] report that herbicides can inhibit the nitrification process, which is manifested by a reduction in the abundance of the amoA gene. Similar results were obtained in our own research, while a significant reduction in the number of copies of the amoA bacterial gene was noted both in the case of the application of the herbicide and the fungicide.
5. Conclusions
The present study allowed for learning about the changes that take place in the activity and diversity of soil microbiome after the application of sewage sludge and herbicides/fungicides. The obtained results show that GSS and GSS with herbicide (MCPA/dicamba) and fungicide (thiophanate-methyl/azoxystrobin) application has a positive effect on the microbiological parameters of soil by significantly increasing the activity of the tested enzymes and increasing the genetic diversity of bacteria and soil fungi as compared to the controls and variants with only pesticides (F, H, F + H). Moreover, it has been shown that the application of F, H and F + H reduces the abundance of microorganisms capable of nitrifying processes, which means that the nitrogen cycle in such soil can be significantly disturbed.
However, there is still a need for long-term studies conducted after the application of GSS and herbicides/fungicides that would further explain the dynamics of changes in the microbial community. In addition, future research should focus more on whether the long-term application of GSS to agricultural soils will have a positive effect on soil microbiome structure, plant quality and soil chemistry. In addition, it is important whether the GSS application will also counteract the negative impact of pesticides, whose development and introduction into use takes place in a dynamic manner, on the discussed biological parameters.
Author Contributions
Conceptualization, E.W. and U.W.; visualization, methodology, writing—original draft, and writing—review and editing, E.W., U.W.; A.J.-T. and B.Ł.; methodology, P.K. (Paweł Kondzior) and M.J.; visualization, writing—review and editing, A.P., A.C. and P.K. (Piotr Kaczyński); writing—review and editing, J.R. and W.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Ministry of Education and Science in Poland, under the research project number WZ/WB-IIŚ/6/2022, SWB-5 and on the subsidy within statutory activities Institute of Plant Protection (SIB-03).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adegoke, A.A.; Awolusi, O.O.; Stenström, T.A. Organic Fertilizers: Public Health Intricacies. In Organic Fertilizers-From Basic Concepts to Applied Outcomes; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Chivenge, P.; Vanlauwe, B.; Six, J. Does the Combined Application of Organic and Mineral Nutrient Sources Influence Maize Productivity? A Meta-Analysis. Plant Soil 2011, 342, 1–30. [Google Scholar] [CrossRef]
- Wydro, U.; Wołejko, E.; Łozowicka, B.; Jabłońska-Trypuć, A. Microbial Diversity and P Content Changes after the Application of Sewage Sludge and Glyphosate to Soil. Minerals 2021, 11, 1423. [Google Scholar] [CrossRef]
- Catarino, M.; Soares Dias, A.P.; Ramos, M. Double Benefit Biodiesel Produced from Waste Frying Oils and Animal Fats. In WASTES–Solutions, Treatments and Opportunities II; CRC Press: Boca Raton, FL, USA, 2017; pp. 153–159. [Google Scholar]
- Bauman-Kaszubska, H.; Sikorski, M. The Impact of Selected Sewage Treatment Methods on the Change in Parameters of Sewage Sludge Originating from Municipal Sewage Treatment Plants. Inż. Ekol. 2018, 19, 199–207. [Google Scholar] [CrossRef]
- Wieczorek, J.; Gambuś, F.; Baran, A. Heavy Metal Content and Yielding of Italian Ryegrass Cultivated in the Soil Intensively Fertilized with Municipal Sewage Sludges. E3S Web Conf. 2013, 1, 15005. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Potential Benefits and Risks of Land Application of Sewage Sludge. Waste Manag. 2008, 28, 347–358. [Google Scholar] [CrossRef]
- Czech, T.; Gambuś, F.; Wieczorek, J. Assessment of Chemical Composition of Waste Materials from Hard Coal Burning in View of Their Agricultural and Environmental Applications. Inż. Ekol. 2013, 34, 89–95. [Google Scholar] [CrossRef]
- Li, X.; Li, F.Y.; Rong, X.M. Risk and Leaching Characteristics of Nitrogen and Phosphorus in Sandy Soil Amended with Sewage Sludge. J. Soil Water Conserv. 2013, 27, 93–97. [Google Scholar] [CrossRef]
- Panasiewicz, K.; Niewiadomska, A.; Sulewska, H.; Wolna-Maruwka, A.; Borowiak, K.; Budka, A.; Ratajczak, K. The Effect of Sewage Sludge and BAF Inoculant on Plant Condition and Yield as Well as Biochemical and Microbial Activity of Soil in Willow (Salix Viminalis L.) Culture as an Energy Crop. PeerJ 2019, 7, e6434. [Google Scholar] [CrossRef]
- Janvier, C.; Villeneuve, F.; Alabouvette, C.; Edel-Hermann, V.; Mateille, T.; Steinberg, C. Soil Health through Soil Disease Suppression: Which Strategy from Descriptors to Indicators? Soil Biol. Biochem. 2007, 39, 1–23. [Google Scholar] [CrossRef]
- Grobelak, A.; Placek, A.; Grosser, A.; Singh, B.R.; Almås, Å.R.; Napora, A.; Kacprzak, M. Effects of Single Sewage Sludge Application on Soil Phytoremediation. J. Clean. Prod. 2017, 155, 189–197. [Google Scholar] [CrossRef]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil Biological Activity as an Indicator of Soil Pollution with Pesticides—A Review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Wolejko, E.; Wydro, U.; Butarewicz, A.; Lozowicka, B. MCPA (2-Methyl-4-Chlorophenoxyacetic Acid) and Sulfosulfuron—Pesticides with Potential Endocrine Disrupting Compounds Properties. Dwt 2018, 117, 194–201. [Google Scholar] [CrossRef]
- Pernak, J.; Syguda, A.; Janiszewska, D.; Materna, K.; Praczyk, T. Ionic Liquids with Herbicidal Anions. Tetrahedron 2011, 67, 4838–4844. [Google Scholar] [CrossRef]
- Ławniczak, Ł.; Syguda, A.; Borkowski, A.; Cyplik, P.; Marcinkowska, K.; Wolko, Ł.; Praczyk, T.; Chrzanowski, Ł.; Pernak, J. Influence of Oligomeric Herbicidal Ionic Liquids with MCPA and Dicamba Anions on the Community Structure of Autochthonic Bacteria Present in Agricultural Soil. Sci. Total Environ. 2016, 563–564, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Conrado, N. Hydrolysis Study and Extraction of Spiroxamine from Soils of Different Physico-Chemical Properties. Chemosphere 2009, 77, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tsuji, S.S.; Li, Y.; Hu, M.; Bandeira, M.A.; Câmara, M.P.S.; Michereff, S.J.; Schnabel, G. Reduced Sensitivity of Azoxystrobin and Thiophanate-Methyl Resistance in Lasiodiplodia Theobromae from Papaya. Pestic. Biochem. Physiol. 2020, 162, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Yadav, A.N.; Kazy, S.K.; Saxena, A.K.; Suman, A. Evaluating the Diversity and Phylogeny of Plant growth Promoting Bacteria Associated with Wheat (Triticum Aestivum) Growing in Central Zone of India. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 432–447. [Google Scholar]
- Gleń, K.; Gondek, K. Sewage Sludge Influence on Microbiological and Biochemical Soil Activity. Eng. Environ. Prot. 2014, 17, 619–630. [Google Scholar] [CrossRef]
- Pepper, I.L.; Brooks, J.P.; Gerba, C.P. Pathogens in Biosolids. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–41. [Google Scholar]
- The Regulation of the Minister of Environment on the on the Municipal Sewage Sludge (own translation)—Rozporządzenie Ministra Środowiska z dnia 6 lutego 2015 r. w sprawie komunalnych osadów ściekowych; O.J. 2015 poz. 257. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150000257 (accessed on 26 July 2022). (In Polish)
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Casida, L.E., Jr.; Klein, D.A.; Santoro, T. Soil Dehydrogenase Activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis: Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Botttomley, P.S., Eds.; Soil Science Society of America: Madison, WI, USA, 1994. [Google Scholar]
- Johnson, J.I.; Temple, K.L. Some variables affecting the measurements of catalase activity in soil. Soil Sci. Soc. Am. J. 1964, 28, 207–216. [Google Scholar] [CrossRef]
- Hoffman, G.; Teicher, K. Ein kolorimetrisches Verfahren zur Bestimmung der Ureaseaktivität in Böden. Zeit. Pflanzenernahr. Dung. Bodenkund. 1961, 95, 55–63. [Google Scholar] [CrossRef]
- Mattana, S.; Chelinho, S.; Sousa, J.P.; Alcañiz, J.M.; Domene, X. Nonylphenol Causes Shifts in Microbial Communities and Nitrogen Mineralization in Soil Microcosms. Ecotoxicol. Environ. Saf. 2019, 181, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Shahsavari, E.; Aburto-Medina, A.; Taha, M.; Ball, A.S. A Quantitative PCR Approach for Quantification of Functional Genes Involved in the Degradation of Polycyclic Aromatic Hydrocarbons in Contaminated Soils. MethodsX 2016, 3, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Farsang, A.; Babcsányi, I.; Ladányi, Z.; Perei, K.; Bodor, A.; Csányi, K.T.; Barta, K. Evaluating the Effects of Sewage Sludge Compost Applications on the Microbial Activity, the Nutrient and Heavy Metal Content of a Chernozem Soil in a Field Survey. Arab. J. Geosci. 2020, 13, 982. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Vilgalys, R.J. Quantitative Analyses of Nitrogen Cycling Genes in Soils. Pedobiologia 2005, 49, 665–672. [Google Scholar] [CrossRef]
- Du, Z.; Zhu, Y.; Zhu, L.; Zhang, J.; Li, B.; Wang, J.; Wang, J.; Zhang, C.; Cheng, C. Effects of the Herbicide Mesotrione on Soil Enzyme Activity and Microbial Communities. Ecotoxicol. Environ. Saf. 2018, 164, 571–578. [Google Scholar] [CrossRef]
- Yao, R.; Yuan, Q.; Wang, K. Enrichment of Denitrifying Bacterial Community Using Nitrite as an Electron Acceptor for Nitrogen Removal from Wastewater. Water 2019, 12, 48. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, W.; Jian, Q.; Song, W.; Zheng, Z.; Wang, D.; Liu, X. Residues and Dissipation Kinetics of Triazole Fungicides Difenoconazole and Propiconazole in Wheat and Soil in Chinese Fields. Food Chem. 2015, 168, 396–403. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. Bacterial Diversity and Enzymatic Activity in a Soil Recently Treated with Tebuconazole. Ecol. Indic. 2021, 123, 107373. [Google Scholar] [CrossRef]
- Sun, Y.B.; Wang, L.; Xu, Y.M.; Liang, X.F.; Zheng, S.N. Ecotoxicological Effect of Mesotrione on Enzyme Activity and Microbial Community in Agricultural Soils. Appl. Ecol. Environ. Res. 2020, 18, 3525–3541. [Google Scholar] [CrossRef]
- Sviridov, A.V.; Shushkova, T.V.; Ermakova, I.T.; Ivanova, E.V.; Epiktetov, D.O.; Leontievsky, A.A. Microbial degradation of glyphosate herbicides. Prikl. Biochem. Mikrobiol. 2015, 51, 183–190. [Google Scholar] [PubMed]
- Baćmaga, M.; Borowik, A.; Kucharski, J.; Tomkiel, M.; Wyszkowska, J. Microbial and enzymatic activity of soil contaminated with a mixture of diflufenican + mesosulfuron-methyl + iodosulfuron-methyl-sodium. Environ. Sci. Pollut. Res. 2015, 22, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhou, H.; Zhou, S.; Li, Z.; Wei, C.; Yu, Y.; Hay, A.G. Fomesafen Impacts Bacterial Communities and Enzyme Activities in the Rhizosphere. Environ. Pollut. 2019, 253, 302–311. [Google Scholar] [CrossRef]
- De Vrieze, J.; Ijaz, U.Z.; Saunders, A.M.; Theuerl, S. Terminal Restriction Fragment Length Polymorphism Is an “Old School” Reliable Technique for Swift Microbial Community Screening in Anaerobic Digestion. Sci. Rep. 2018, 8, 16818. [Google Scholar] [CrossRef]
- Gryta, A.; Frąc, M.; Oszust, K. Community Shift in Structure and Functions across Soil Profile in Response to Organic Waste and Mineral Fertilization Strategies. Appl. Soil Ecol. 2019, 143, 55–60. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Z.; Liu, H.; Li, J.; Wang, Y.; Xu, H. Application of Acclimated Sewage Sludge as a Bio-Augmentation/Bio-Stimulation Strategy for Remediating Chlorpyrifos Contamination in Soil With/without Cadmium. Sci. Total Environ. 2017, 579, 657–666. [Google Scholar] [CrossRef]
- Prudnikova, S.; Streltsova, N.; Volova, T. The Effect of the Pesticide Delivery Method on the Microbial Community of Field Soil. Environ. Sci. Pollut. Res. Int. 2021, 28, 8681–8697. [Google Scholar] [CrossRef]
- Könneke, M.; Bernhard, A.E.; de La Torre, J.R.; Walker, C.B.; Waterbury, J.B.; Stahl, D.A. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 2005, 437, 543–546. [Google Scholar] [CrossRef]
- Crouzet, O.; Poly, F.; Bonnemoy, F.; Bru, D.; Batisson, I.; Bohatier, J.; Philippot, L.; Mallet, C. Functional and Structural Responses of Soil N-Cycling Microbial Communities to the Herbicide Mesotrione: A Dose-Effect Microcosm Approach. Environ. Sci. Pollut. Res. Int. 2016, 23, 4207–4217. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).