Crosslinking Mechanisms of Phenol, Catechol, and Gallol for Synthetic Polyphenols: A Comparative Review
Abstract
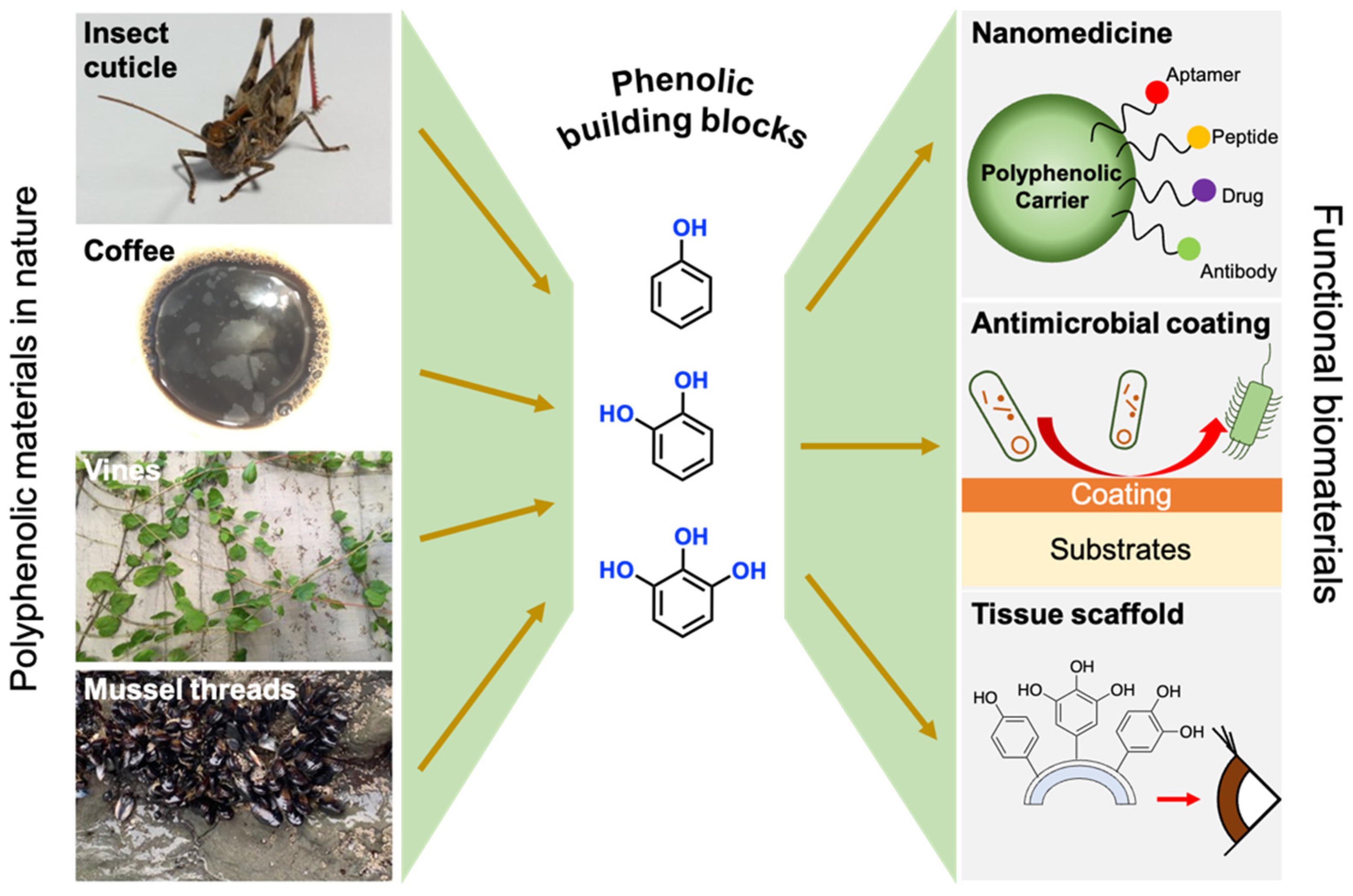
1. Introduction
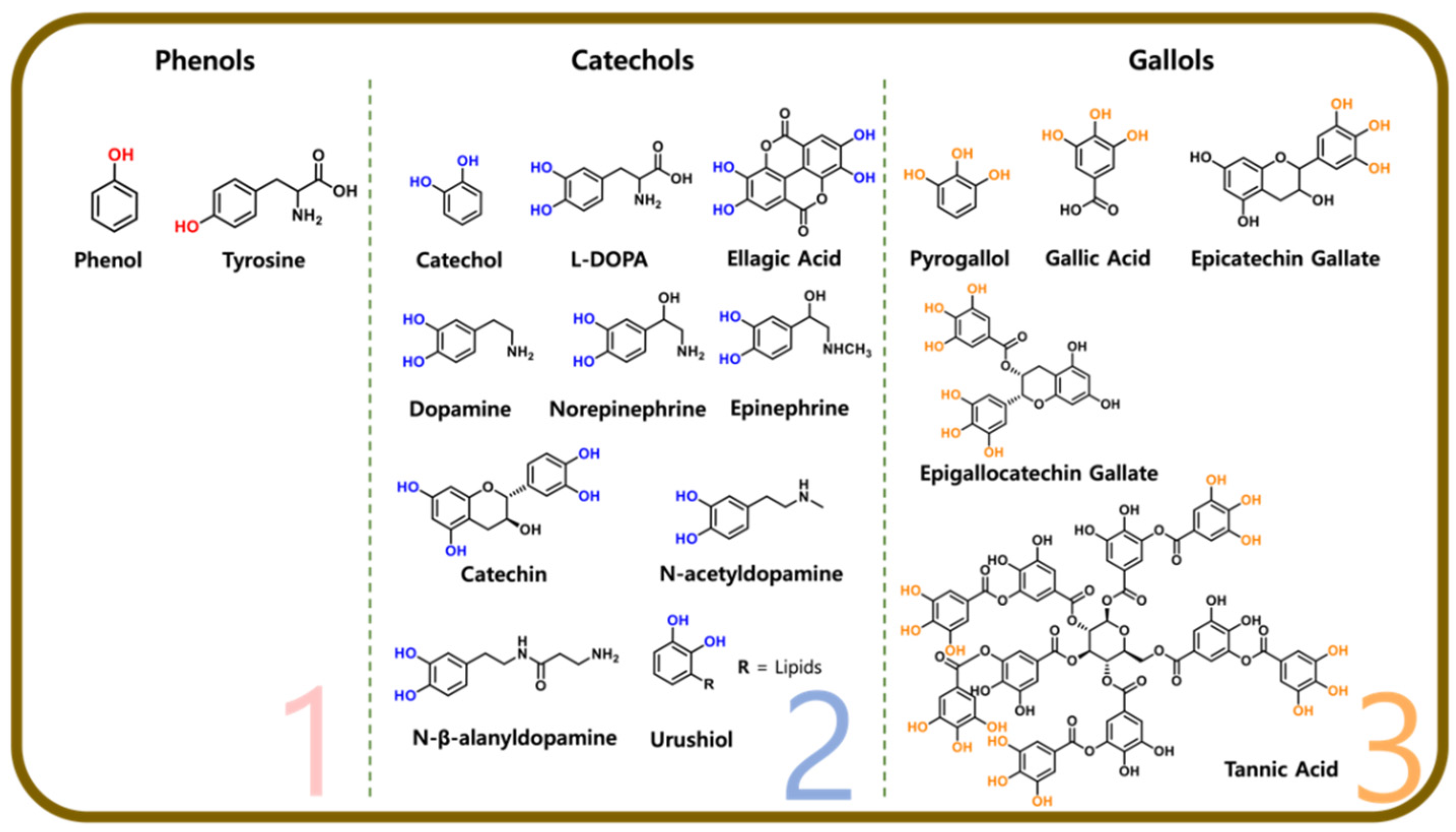
2. Hydroxybenzene-Based Building Blocks (Phenol)
3. Dihydroxybenzene-Based Building Blocks (Catechol)
3.1. Dopamine
3.2. Dopamine Derivatives
3.3. Catechin
3.4. Alkylcatechols
4. 1,2,3-Trihydroxybenzene (Gallol)
4.1. Pyrogallol and Gallic Acid
4.2. Tannic Acid
5. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, M.; Park, E.; Lee, H. Plant-inspired pyrogallol-containing functional materials. Adv. Funct. Mater. 2019, 29, 1903022. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Z.; Ju, Y.; Rahim, M.A.; Richardson, J.J.; Caruso, F. Polyphenol-mediated assembly for particle engineering. Acc. Chem. Res. 2020, 53, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Chanphai, P.; Tajmir-Riahi, H. Tea polyphenols bind serum albumins: A potential application for polyphenol delivery. Food Hydrocoll. 2019, 89, 461–467. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Yang, J.; Stuart, M.A.C.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef]
- Lee, K.; Prajatelistia, E.; Hwang, D.S.; Lee, H. Role of dopamine chemistry in the formation of mechanically strong mandibles of grasshoppers. Chem. Mater. 2015, 27, 6478–6481. [Google Scholar] [CrossRef]
- Hopkins, T.L.; Morgan, T.D.; Aso, Y.; Kramer, K.J. N-β-Alanyldopamine: Major role in insect cuticle tanning. Science 1982, 217, 364–366. [Google Scholar] [CrossRef]
- Kobayashi, T.; Urabe, K.; Winder, A.; Jiménez-Cervantes, C.; Imokawa, G.; Brewington, T.; Solano, F.; García-Borrón, J.; Hearing, V. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994, 13, 5818–5825. [Google Scholar] [CrossRef]
- Kwon, I.S.; Bettinger, C.J. Polydopamine nanostructures as biomaterials for medical applications. J. Mater. Chem. B 2018, 6, 6895–6903. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Batul, R.; Tamanna, T.; Khaliq, A.; Yu, A. Recent progress in the biomedical applications of polydopamine nanostructures. Biomater. Sci. 2017, 5, 1204–1229. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Bisht, H.; Ryu, S.; Hong, D. Development of a versatile, uniform, and stable initiator layer by the functionalization of a polydopamine/polyethyleneimine film. Bull. Korean Chem. Soc. 2022, 43, 788–791. [Google Scholar] [CrossRef]
- Acharya, A.; Kumar, A.; Lee, I.S. Yolk@ Shell Nanoreactors Carrying a Cluster of Metal Nanocrystals Stabilized Inside the Hollow Carbon Shell. Bull. Korean Chem. Soc. 2021, 42, 915–918. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.-T.; Buehler, M.J. Polydopamine and eumelanin: From structure–property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Rho, J.; Choi, I.S.; Messersmith, P.B.; Lee, H. Norepinephrine: Material-independent, multifunctional surface modification reagent. J. Am. Chem. Soc. 2009, 131, 13224–13225. [Google Scholar] [CrossRef]
- Hong, S.; Kim, J.; Na, Y.S.; Park, J.; Kim, S.; Singha, K.; Im, G.I.; Han, D.K.; Kim, W.J.; Lee, H. Poly (norepinephrine): Ultrasmooth material-independent surface chemistry and nanodepot for nitric oxide. Angew. Chem. 2013, 125, 9357–9361. [Google Scholar] [CrossRef]
- Cho, S.; Yun, S.H. Poly(catecholamine) Coated CsPbBr3 Perovskite Microlasers: Lasing in Water and Biofunctionalization. Adv. Funct. Mater. 2021, 31, 2101902. [Google Scholar] [CrossRef]
- Bentley, R. A fresh look at natural tropolonoids. Nat. Prod. Rep. 2008, 25, 118–138. [Google Scholar] [CrossRef]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew. Chem. 2013, 125, 10966–10970. [Google Scholar] [CrossRef]
- Jeong, Y.; Jeong, S.; Nam, Y.K.; Kang, S.M. Development of Freeze-resistant Aluminum Surfaces by Tannic Acid Coating and Subsequent Immobilization of Antifreeze Proteins. Bull. Korean Chem. Soc. 2018, 39, 559–562. [Google Scholar] [CrossRef]
- Acharya, A.; Lee, I.S. Designing plasmonically integrated nanoreactors for efficient catalysis. Bull. Korean Chem. Soc. 2022. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, J.; Li, H.; Li, J.; Jin, Q.; Ren, K.; Ji, J. Mussel-inspired polydopamine: A biocompatible and ultrastable coating for nanoparticles in vivo. ACS Nano 2013, 7, 9384–9395. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Bruggeman, J.P.; Misra, A.; Borenstein, J.T.; Langer, R. Biocompatibility of biodegradable semiconducting melanin films for nerve tissue engineering. Biomaterials 2009, 30, 3050–3057. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.S.; Lee, C.; Park, H.J.; Yang, K.; Jin, Y.; Ryu, J.H.; Hong, K.S.; Moon, S.H.; Chung, H.M. Tissue adhesive catechol-modified hyaluronic acid hydrogel for effective, minimally invasive cell therapy. Adv. Funct. Mater. 2015, 25, 3814–3824. [Google Scholar] [CrossRef]
- Oh, D.X.; Kim, S.; Lee, D.; Hwang, D.S. Tunicate-mimetic nanofibrous hydrogel adhesive with improved wet adhesion. Acta Biomater. 2015, 20, 104–112. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Panzella, L.; Oscurato, S.L.; Salvatore, M.; Avolio, R.; Errico, M.E.; Maddalena, P.; Napolitano, A.; D’Ischia, M. The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay. Biomimetics 2018, 3, 26. [Google Scholar] [CrossRef]
- Marmelstein, A.M.; Lobba, M.J.; Mogilevsky, C.S.; Maza, J.C.; Brauer, D.D.; Francis, M.B. Tyrosinase-Mediated Oxidative Coupling of Tyrosine Tags on Peptides and Proteins. J. Am. Chem. Soc. 2020, 142, 5078–5086. [Google Scholar] [CrossRef]
- Wang, Y.; Park, J.P.; Hong, S.H.; Lee, H. Biologically Inspired Materials Exhibiting Repeatable Regeneration with Self-Sealing Capabilities without External Stimuli or Catalysts. Adv. Mater. 2016, 28, 9961–9968. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.S.; Shin, J.; Jeon, E.J.; An, S.; Choi, Y.S.; Cho, S.W. Ascidian-Inspired Fast-Forming Hydrogel System for Versatile Biomedical Applications: Pyrogallol Chemistry for Dual Modes of Crosslinking Mechanism. Adv. Funct. Mater. 2018, 28, 1705244. [Google Scholar] [CrossRef]
- Ruan, C.; Rodgers, M. Cation− π interactions: Structures and energetics of complexation of Na+ and K+ with the aromatic amino acids, phenylalanine, tyrosine, and tryptophan. J. Am. Chem. Soc. 2004, 126, 14600–14610. [Google Scholar] [CrossRef] [PubMed]
- Fry, S. Isodityrosine, a new cross-linking amino acid from plant cell-wall glycoprotein. Biochem. J. 1982, 204, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Elvin, C.M.; Carr, A.G.; Huson, M.G.; Maxwell, J.M.; Pearson, R.D.; Vuocolo, T.; Liyou, N.E.; Wong, D.C.; Merritt, D.J.; Dixon, N.E. Synthesis and properties of crosslinked recombinant pro-resilin. Nature 2005, 437, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Neff, D.; Frazier, S.F.; Quimby, L.; Wang, R.-T.; Zill, S. Identification of resilin in the leg of cockroach, Periplaneta americana: Confirmation by a simple method using pH dependence of UV fluorescence. Arthropod Struct. Dev. 2000, 29, 75–83. [Google Scholar] [CrossRef]
- Appel, E.; Heepe, L.; Lin, C.P.; Gorb, S.N. Ultrastructure of dragonfly wing veins: Composite structure of fibrous material supplemented by resilin. J. Anat. 2015, 227, 561–582. [Google Scholar] [CrossRef]
- Malencik, D.A.; Anderson, S.R. Dityrosine formation in calmodulin: Cross-linking and polymerization catalyzed by Arthromyces peroxidase. Biochemistry 1996, 35, 4375–4386. [Google Scholar] [CrossRef]
- Lee, D.-I.; Choi, J.-Y.; Kim, C.-J.; Ahn, I.-S. Large-scale production of N, N′-diBoc-dityrosine and dityrosine by HRP-catalyzed N-Boc-l-tyrosine oxidation and one-step chromatographic purification. Process Biochem. 2011, 46, 142–147. [Google Scholar] [CrossRef]
- Dubinina, E.; Gavrovskaya, S.; Kuzmich, E.; Leonova, N.; Morozova, M.; Kovrugina, S.; Smirnova, T. Oxidative modification of proteins: Oxidation of tryptophan and production of dityrosine in purified proteins using Fenton’s system. Biochemistry 2002, 67, 343–350. [Google Scholar]
- Fancy, D.A.; Kodadek, T. Chemistry for the analysis of protein–protein interactions: Rapid and efficient cross-linking triggered by long wavelength light. Proc. Natl. Acad. Sci. USA 1999, 96, 6020–6024. [Google Scholar] [CrossRef]
- Mrówczyński, R.; Markiewicz, R.; Liebscher, J. Chemistry of polydopamine analogues. Polym. Int. 2016, 65, 1288–1299. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Micillo, R.; Panzella, L.; Crescenzi, O.; Oscurato, S.L.; Maddalena, P.; Napolitano, A.; Ball, V.; d’Ischia, M. Structural basis of polydopamine film formation: Probing 5, 6-dihydroxyindole-based eumelanin type units and the porphyrin issue. ACS Appl. Mater. Interfaces 2017, 10, 7670–7680. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, W.; Wu, M.; Gong, L.; Tang, A.; Xiang, L.; Zhu, B.; Zhu, L.; Zeng, H. Cost-effective strategy for surface modification via complexation of disassembled polydopamine with Fe (III) ions. Langmuir 2019, 35, 4101–4109. [Google Scholar] [CrossRef] [PubMed]
- Sadi, M.; Pan, J.; Xu, A.; Cheng, D.; Cai, G.; Wang, X. Direct dip-coating of carbon nanotubes onto polydopamine-templated cotton fabrics for wearable applications. Cellulose 2019, 26, 7569–7579. [Google Scholar] [CrossRef]
- Wang, H.; Lin, C.; Zhang, X.; Lin, K.; Wang, X.; Shen, S.G. Mussel-inspired polydopamine coating: A general strategy to enhance osteogenic differentiation and osseointegration for diverse implants. ACS Appl. Mater. Interfaces 2019, 11, 7615–7625. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, J.-H.; Cho, H.-J.; Kim, H.K.; Yoon, T.R.; Shin, H. Electrospun fibers immobilized with bone forming peptide-1 derived from BMP7 for guided bone regeneration. Biomaterials 2013, 34, 5059–5069. [Google Scholar] [CrossRef]
- Lee, J.-h.; Lee, Y.J.; Cho, H.-j.; Shin, H. Guidance of in vitro migration of human mesenchymal stem cells and in vivo guided bone regeneration using aligned electrospun fibers. Tissue Eng. Part A 2014, 20, 2031–2042. [Google Scholar] [CrossRef]
- Cho, H.-j.; Madhurakkat Perikamana, S.K.; Lee, J.-h.; Lee, J.; Lee, K.-M.; Shin, C.S.; Shin, H. Effective immobilization of BMP-2 mediated by polydopamine coating on biodegradable nanofibers for enhanced in vivo bone formation. ACS Appl. Mater. Interfaces 2014, 6, 11225–11235. [Google Scholar] [CrossRef]
- Baldwin, F.; Craig, T.J.; Shiel, A.I.; Cox, T.; Lee, K.; Mansell, J.P. Polydopamine-Lysophosphatidate-Functionalised Titanium: A Novel Hybrid Surface Finish for Bone Regenerative Applications. Molecules 2020, 25, 1583. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Li, K.; Shen, S.; Liu, Z.; Wu, D. Ultralong circulating lollipop-like nanoparticles assembled with gossypol, doxorubicin, and polydopamine via π–π stacking for synergistic tumor therapy. Adv. Funct. Mater. 2019, 29, 1805582. [Google Scholar] [CrossRef]
- Tan, X.; Gao, P.; Li, Y.; Qi, P.; Liu, J.; Shen, R.; Wang, L.; Huang, N.; Xiong, K.; Tian, W. Poly-dopamine, poly-levodopa, and poly-norepinephrine coatings: Comparison of physico-chemical and biological properties with focus on the application for blood-contacting devices. Bioact. Mater. 2021, 6, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Fan, Q.; Fan, B.; Zhang, Y.; Fan, D.; Wu, D.; Wei, Q. Formation of homogeneous epinephrine-melanin solutions to fabricate electrodes for enhanced photoelectrochemical biosensing. Langmuir 2018, 34, 7744–7750. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Zhang, J.; Wang, W.; Gong, L.; Zhang, L.; Yan, B.; Zeng, H. Nanomechanics of π-cation-π interaction with implications for bio-inspired wet adhesion. Acta Biomater. 2020, 117, 294–301. [Google Scholar] [CrossRef]
- Behboodi-Sadabad, F.; Zhang, H.; Trouillet, V.; Welle, A.; Plumeré, N.; Levkin, P.A. Bioinspired strategy for controlled polymerization and photopatterning of plant polyphenols. Chem. Mater. 2018, 30, 1937–1946. [Google Scholar]
- Lee, J.S.; Lee, J.S.; Lee, M.S.; An, S.; Yang, K.; Lee, K.; Yang, H.S.; Lee, H.; Cho, S.-W. Plant flavonoid-mediated multifunctional surface modification chemistry: Catechin coating for enhanced osteogenesis of human stem cells. Chem. Mater. 2017, 29, 4375–4384. [Google Scholar] [CrossRef]
- Herrero-Martínez, J.M.; Sanmartin, M.; Rosés, M.; Bosch, E.; Ràfols, C. Determination of dissociation constants of flavonoids by capillary electrophoresis. Electrophoresis 2005, 26, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Wang, Y.; Park, S.Y.; Lee, H. Progressive fuzzy cation-π assembly of biological catecholamines. Sci. Adv. 2018, 4, eaat7457. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, W.; Li, B.; Li, T.; Chang, H.; Cheng, Y. Natural polyphenols augment cytosolic protein delivery by a functional polymer. Chem. Mater. 2019, 31, 1956–1965. [Google Scholar] [CrossRef]
- Wang, C.; Sang, H.; Wang, Y.; Zhu, F.; Hu, X.; Wang, X.; Wang, X.; Li, Y.; Cheng, Y. Foe to friend: Supramolecular nanomedicines consisting of natural polyphenols and bortezomib. Nano Lett. 2018, 18, 7045–7051. [Google Scholar] [CrossRef]
- Symes, W.F.; Dawson, C.R. Poison ivy “urushiol”. J. Am. Chem. Soc. 1954, 76, 2959–2963. [Google Scholar] [CrossRef]
- Lu, R.; Yoshida, T.; Miyakoshi, T. Oriental lacquer: A natural polymer. Polym. Rev. 2013, 53, 153–191. [Google Scholar] [CrossRef]
- Lu, R.; Ishimura, T.; Tsutida, K.; Honda, T.; Miyakoshi, T. Development of a fast drying hybrid lacquer in a low-relative-humidity environment based on kurome lacquer sap. J. Appl. Polym. Sci. 2005, 98, 1055–1061. [Google Scholar] [CrossRef]
- Watanabe, H.; Fujimoto, A.; Takahara, A. Characterization of catechol-containing natural thermosetting polymer “urushiol” thin film. J. Polym. Sci. Part A: Polym. Chem. 2013, 51, 3688–3692. [Google Scholar] [CrossRef]
- Xia, J.; Lin, J.; Xu, Y.; Chen, Q. On the UV-induced polymeric behavior of Chinese lacquer. ACS Appl. Mater. Interfaces 2011, 3, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Bussotti, F.; Gravano, E.; Grossoni, P.; Tani, C. Occurrence of tannins in leaves of beech trees (Fagus sylvatica) along an ecological gradient, detected by histochemical and ultrastructural analyses. New Phytol. 1998, 138, 469–479. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Taylor, S.W.; Kammerer, B.; Bayer, E. New perspectives in the chemistry and biochemistry of the tunichromes and related compounds. Chem. Rev. 1997, 97, 333–346. [Google Scholar] [CrossRef]
- Zhan, K.; Kim, C.; Sung, K.; Ejima, H.; Yoshie, N. Tunicate-inspired gallol polymers for underwater adhesive: A comparative study of catechol and gallol. Biomacromolecules 2017, 18, 2959–2966. [Google Scholar] [CrossRef]
- Kambourakis, S.; Draths, K.; Frost, J. Synthesis of gallic acid and pyrogallol from glucose: Replacing natural product isolation with microbial catalysis. J. Am. Chem. Soc. 2000, 122, 9042–9043. [Google Scholar] [CrossRef]
- Behboodi-Sadabad, F.; Zhang, H.; Trouillet, V.; Welle, A.; Plumeré, N.; Levkin, P.A. UV-Triggered polymerization, deposition, and patterning of plant phenolic compounds. Adv. Funct. Mater. 2017, 27, 1700127. [Google Scholar] [CrossRef]
- Wang, Y.; Jeon, E.J.; Lee, J.; Hwang, H.; Cho, S.W.; Lee, H. A phenol-amine superglue inspired by insect sclerotization process. Adv. Mater. 2020, 32, 2002118. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mittal, D.; Verma, A.K.; Roy, I. Copper-gallic acid nanoscale metal–organic framework for combined drug delivery and photodynamic therapy. ACS Appl. Bio Mater. 2019, 2, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Schaber, C.F.; Dening, K.; Appel, E.; Gorb, S.N.; Lee, H. Air/Water Interfacial Formation of Freestanding, Stimuli-Responsive, Self-Healing Catecholamine Janus-Faced Microfilms. Adv. Mater. 2014, 26, 7581–7587. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.; Lee, J.; Yoon, S.; Kim, W.J. Tannic acid-based nanogel as an efficient anti-inflammatory agent. Biomater. Sci. 2020, 8, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, S.; Moon, H.C.; Seo, H.; Kim, J.Y.; Hong, S.-P.; Lee, B.S.; Kang, E.; Lee, J.; Ryu, D.H. Antimicrobial spray nanocoating of supramolecular Fe (III)-tannic acid metal-organic coordination complex: Applications to shoe insoles and fruits. Sci. Rep. 2017, 7, 6980. [Google Scholar] [CrossRef]
- Schlaich, C.; Li, M.; Cheng, C.; Donskyi, I.S.; Yu, L.; Song, G.; Osorio, E.; Wei, Q.; Haag, R. Mussel-inspired polymer-based universal spray coating for surface modification: Fast fabrication of antibacterial and superhydrophobic surface coatings. Adv. Mater. Interfaces 2018, 5, 1701254. [Google Scholar] [CrossRef]
- Sileika, T.S.; Kim, H.-D.; Maniak, P.; Messersmith, P.B. Antibacterial performance of polydopamine-modified polymer surfaces containing passive and active components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. [Google Scholar] [CrossRef]
- Xu, G.; Pranantyo, D.; Zhang, B.; Xu, L.; Neoh, K.-G.; Kang, E.-T. Tannic acid anchored layer-by-layer covalent deposition of parasin I peptide for antifouling and antimicrobial coatings. RSC Adv. 2016, 6, 14809–14818. [Google Scholar] [CrossRef]
- Shin, M.; Lee, H.; Lee, M.; Shin, Y.; Song, J.-J.; Kang, S.-W.; Nam, D.-H.; Jeon, E.J.; Cho, M.; Do, M. Targeting protein and peptide therapeutics to the heart via tannic acid modification. Nat. Biomed. Eng. 2018, 2, 304–317. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Lee, K. Crosslinking Mechanisms of Phenol, Catechol, and Gallol for Synthetic Polyphenols: A Comparative Review. Appl. Sci. 2022, 12, 11626. https://doi.org/10.3390/app122211626
Choi H, Lee K. Crosslinking Mechanisms of Phenol, Catechol, and Gallol for Synthetic Polyphenols: A Comparative Review. Applied Sciences. 2022; 12(22):11626. https://doi.org/10.3390/app122211626
Chicago/Turabian StyleChoi, Hyunbin, and Kyueui Lee. 2022. "Crosslinking Mechanisms of Phenol, Catechol, and Gallol for Synthetic Polyphenols: A Comparative Review" Applied Sciences 12, no. 22: 11626. https://doi.org/10.3390/app122211626
APA StyleChoi, H., & Lee, K. (2022). Crosslinking Mechanisms of Phenol, Catechol, and Gallol for Synthetic Polyphenols: A Comparative Review. Applied Sciences, 12(22), 11626. https://doi.org/10.3390/app122211626





