Effects of Melatonin in the Non-Surgical Treatment of Periodontitis: A Systematic Review
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Focus Question
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Selection of Articles and Data Extraction
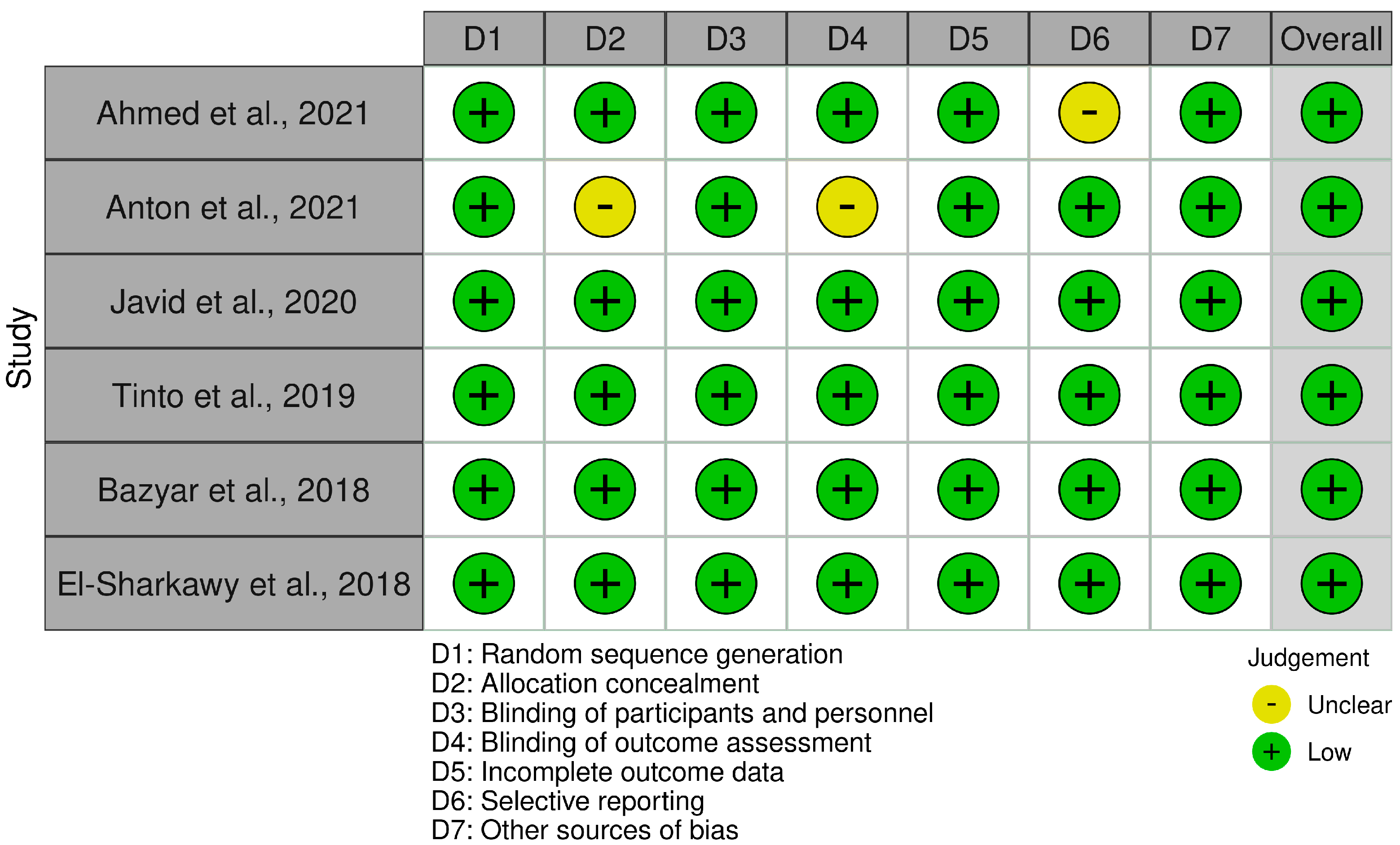
2.5. Risk of Bias
3. Results
3.1. Groups of Treatment: Control vs. Test
3.2. Period of Treatment and Follow-Up
3.3. Clinical Parameters
3.3.1. PD and CAL
3.3.2. Plaque Index (PI), BOP, and GI
3.4. Biomarkers
3.5. Diseases
3.6. Risk Assessment
4. Discussion
4.1. Proteins Analysis
4.2. Clinical Parameters
4.3. Adverse Effects
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Zhang, M.; Weintraub, S.T.; Cabrera, J.; Sainz, R.M.; Mayo, J.C. Identification of highly elevated levels of melatonin in bone marrow: Its origin and significance. Biochim. Biophys. Acta 1999, 1472, 206–214. [Google Scholar] [CrossRef]
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Khurshid, Z.; Zohaib, S.; Zafar, M.S. Therapeutic potential of melatonin in oral medicine and periodontology. Kaohsiung J. Med. Sci. 2016, 32, 391–396. [Google Scholar] [CrossRef]
- Maria, S.; Witt-Enderby, P.A. Melatonin effects on bone: Potential use for the prevention and treatment for osteopenia, osteoporosis, and periodontal disease and for use in bone-grafting procedures. J. Pineal Res. 2014, 56, 115–125. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Cutando, A.; Gómez-Moreno, G.; Arana, C.; Muñoz, F.; Lopez-Peña, M.; Stephenson, J.; Reiter, R.J. Melatonin stimulates osteointegration of dental implants. J. Pineal Res. 2008, 45, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Girgert, R.; Hanf, V.; Emons, G.; Gründker, C. Membrane-bound melatonin receptor MT1 down-regulates estrogen responsive genes in breast cancer cells. J. Pineal Res. 2009, 47, 23–31. [Google Scholar] [CrossRef]
- Lodhi, K.; Saimbi, C.S.; Khan, M.A.; Nath, C.; Shukla, R. Evaluation of melatonin levels in saliva in gingivitis and periodontitis cases: A pilot study. Contemp. Clin. Dent. 2016, 7, 519–523. [Google Scholar]
- Acuña-Castroviejo, D.; Escames, G.; Macías, M.; Muñoz Hoyos, A.; Molina Carballo, A.; Arauzo, M.; Montes, R. Cell protective role of melatonin in the brain. J. Pineal Res. 1995, 19, 57–63. [Google Scholar] [CrossRef]
- Antón-Tay, F.; Ramírez, G.; Martínez, I.; Benítez-King, G. In vitro stimulation of protein kinase C by melatonin. Neurochem. Res. 1998, 23, 601–606. [Google Scholar] [CrossRef]
- Huerto-Delgadillo, L.; Anton-Tay, F.; Benitz-King, G. Effects of melatonin on microtubule assembly depend on hormone concentration: Role of melatonin as a calmodulin antagonist. J. Pineal Res. 1992, 13, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Macías, M.; Escames, G.; Leon, J.; Coto, A.; Sbihi, Y.; Osuna, A.; Acuña-Castroviejo, D. Calreticulin-melatonin. An unexpected relationship. Eur. J. Biochem. 2003, 270, 832–840. [Google Scholar] [CrossRef]
- Mehta, A.; Kaur, G. Potential role of melatonin in prevention and treatment of oral carcinoma. Indian J. Dent. 2014, 5, 56–61. [Google Scholar] [CrossRef]
- Galindo Moreno, P.; Avila Ortiz, G.; Wang, H.L.; Padial Molina, M.; Ortega Oller, I.; O’Valle, F. The role of melatonin in periodontal and periimplant bone homeostasis and regeneration. J. Oral Sci. Rehabil. 2016, 2, 8–15. [Google Scholar]
- Czesnikiewicz-Guzik, M.; Konturek, S.J.; Loster, B.; Wisniewska, G.; Majewski, S. Melatonin and its role in oxidative stress related diseases of oral cavity. J. Physiol. Pharmacol. 2007, 58, 5–19. [Google Scholar] [PubMed]
- Reiter, R.J. Melatonin The chemical expression of darkness. Mol. Cell Endocrinol. 1991, 79, 153–158. [Google Scholar] [CrossRef]
- Reiter, R.J. Pineal melatonin. Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef]
- Okatani, Y.; Okamoto, K.; Hayashi, K.; Wakatsuki, A.; Sagara, Y. Maternal-fetal transfer of melatonin in human pregnancy near term. J. Pineal Res. 1998, 25, 129–134. [Google Scholar]
- Redman, J.; Armstrong, S.; Ng, K.T. Free-running activity rhythms in the rat: Entrainment by melatonin. Science 1983, 219, 1089–1091. [Google Scholar] [CrossRef]
- McArthur, A.J.; Hunt, A.E.; Gillette, M.U. Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: Activation of protein kinase C at dusk and dawn. Endocrinology 1997, 138, 627–634. [Google Scholar] [CrossRef]
- Dollins, A.B.; Zhdanova, I.V.; Wurtman, R.J.; Lynch, H.J.; Deng, M.H. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc. Natl. Acad. Sci. USA 1994, 91, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- García-Mauriño, S.; Pozo, D.; Calvo, J.R.; Guerrero, J.M. Correlation between nuclear melatonin receptor expression and enhanced cytokine production in human lymphocytic and monocytic cell lines. J. Pineal Res. 2000, 29, 129–137. [Google Scholar] [CrossRef]
- Guerrero, J.M.; Reiter, R.J. Melatonin-immune system relationships. Curr. Top. Med. Chem. 2002, 2, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Tresguerres, I.F.; Clemente, C.; Donado, M.; Gómez-Pellico, L.; Blanco, L.; Alobera, M.A.; Tresguerres, J.A.F. Local administration of growth hormone enhances periimplant bone reaction in an osteoporotic rabbit model. Clin. Oral Implants Res. 2002, 13, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A review of the multiple actions of melatonin on the immune system. Endocrine 2005, 27, 189–200. [Google Scholar] [CrossRef]
- Arabaci, T.; Kermen, E.; Özkanlar, S.; Köse, O.; Kara, A.; Kızıldağ, A.; Duman, S.B.; Ibişoğlu, E. Therapeutic effects of melatonin on alveolar bone resorption after experimental periodontitis in rats: A biochemical and immunohistochemical study. J. Periodontol. 2015, 86, 874–881. [Google Scholar] [CrossRef]
- Cengiz, M.I.; Cengiz, S.; Wang, H.L. Melatonin and oral cavity. Int. J. Dent. 2012, 2012, 491872. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Ahmed, E.; Shaker, O.G.; Yussif, N.; Ghalwash, D.M. Effect of locally delivered melatonin as an adjunct to nonsurgical therapy on GCF antioxidant capacity and MMP-9 in stage II periodontitis patients: A randomized controlled clinical trial. Int. J. Dent. 2021, 2021, 8840167. [Google Scholar] [CrossRef]
- Anton, D.-M.; Martu, M.-A.; Maris, M.; Maftei, G.-A.; Sufaru, I.-G.; Tatarciuc, D.; Luchian, I.; Ioanid, N.; Martu, S. Study on the effects of melatonin on glycemic control and periodontal parameters in patients with type II diabetes mellitus and periodontal disease. Medicina 2021, 57, 140. [Google Scholar] [PubMed]
- Bazyar, H.; Gholinezhad, H.; Moradi, L.; Salehi, P.; Abadi, F.; Ravanbakhsh, M.; Javid, A.Z. The effects of melatonin supplementation in adjunct with non-surgical periodontal therapy on periodontal status, serum melatonin and inflammatory markers in type 2 diabetes mellitus patients with chronic periodontitis: A double-blind, placebo-controlled trial. Inflammopharmacology 2019, 27, 67–76. [Google Scholar] [PubMed]
- Javid, A.Z.; Hosseini, S.A.; Gholinezhad, H.; Moradi, L.; Haghighi-Zadeh, M.H.; Hadi Bazyar, H. Antioxidant and anti-inflammatory properties of melatonin in patients with type 2 diabetes mellitus with periodontal disease under non-surgical periodontal therapy: A double-blind, placebo-controlled trial. Diabetes Metab. Syndr. Obes. 2020, 13, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Tinto, M.; Sartori, M.; Pizzi, I.; Verga, A.; Longoni, S. Melatonin as host modulating agent supporting nonsurgical periodontal therapy in patients affected by untreated severe periodontitis: A preliminary randomized, triple-blind, placebo-controlled study. J. Periodontal Res. 2020, 55, 61–67. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Elmeadawy, S.; Elshinnawi, U.; Anees, M. Is dietary melatonin supplementation a viable adjunctive therapy for chronic periodontitis? A randomized controlled clinical trial. J. Periodontal Res. 2019, 54, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.T.R.; Saavedra-Silva, M.; López-Marcos, J.F.; Veiga, N.J.; Castilho, R.d.M.; Fernandes, G.V.O. Blood and Salivary Inflammatory Biomarkers Profile in Patients with Chronic Kidney Disease and Periodontal Disease: A Systematic Review. Diseases 2022, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Mayo, J.C.; Sainz, R.M.; Leon, J.; Czarnocki, Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003, 50, 1129–1146. [Google Scholar] [CrossRef]
- Balaji, T.M.; Varadarajan, S.; Jagannathan, R.; Gupta, A.A.; Raj, A.T.; Patil, S.; Fageeh, H.I.; Fageeh, H.N. Melatonin levels in periodontitis vs. the healthy state: A systematic review and meta-analysis. Oral Dis. 2022, 28, 284–306. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Buranasin, P.; Mikami, R.; Takeda, K.; Kido, D.; Watanabe, K.; Takemura, S.; Nakagawa, K.; Kominato, H.; Saito, N.; et al. Effects of antioxidant in adjunct with periodontal therapy in patients with type 2 diabetes: A systematic review and meta-analysis. Antioxidants 2021, 10, 1304. [Google Scholar] [CrossRef]
- Mohan, N.; Sadeghi, K.; Reiter, R.J.; Meltz, M.L. The neurohormone melatonin inhibits cytokine, mitogen and ionizing radiation induced NF-kappa B. Biochem. Mol. Biol. Int. 1995, 37, 1063–1070. [Google Scholar]
- Bertuglia, S.; Marchiafava, P.L.; Colantuoni, A. Melatonin prevents ischemia reperfusion injury in hamster cheek pouch microcirculation. Cardiovasc. Res. 1996, 31, 947–952. [Google Scholar] [CrossRef]
- Reiter, R.J.; Calvo, J.R.; Karbownik, M.; Qi, W.; Tan, D.X. Melatonin and its relation to the immune system and inflammation. Ann. N. Y. Acad. Sci. 2000, 917, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.M.; Pozo, D.; García-Mauriño, S.; Osuna, C.; Molinero, P.; Calvo, J.R. Involvement of nuclear receptors in the enhanced IL-2 production by melatonin in Jurkat cells. Ann. N. Y. Acad. Sci. 2000, 917, 397–403. [Google Scholar] [CrossRef]
- Garcia-Mauriño, S.; Gonzalez-Haba, M.G.; Calvo, J.R.; Goberna, R.; Guerrero, J.M. Involvement of nuclear binding sites for melatonin in the regulation of IL-2 and IL-6 production by human blood mononuclear cells. J. Neuroimmunol. 1998, 92, 76–84. [Google Scholar] [CrossRef]
- Cutando, A.; Montero, J.; Diego, R.G.; Ferrera, M.-J.; Lopez-Valverde, A. Effect of topical application of melatonin on serum levels of C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) in patients with type 1 or type 2 diabetes and periodontal disease. J. Clin. Exp. Dent. 2015, 7, e628–e633. [Google Scholar] [CrossRef]
- Kara, A.; Akman, S.; Ozkanlar, S.; Tozoglu, U.; Kalkan, Y.; Canakci, C.F.; Tozoglu, S. Immune modulatory and antioxidant effects of melatonin in experimental periodontitis in rats. Free Radic. Biol. Med. 2013, 55, 21–26. [Google Scholar] [CrossRef]
- Köse, O.; Arabaci, T.; Kizildag, A.; Erdemci, B.; Eminoğlu, D.O.; Gedikli, S.; Özkanlar, S.; Zihni, M.; Albayrak, M.; Kara, A.; et al. Melatonin prevents radiation-induced oxidative stress and periodontal tissue breakdown in irradiated rats with experimental periodontitis. J. Periodontal Res. 2017, 52, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Rudra, D.S.; Pal, U.; Maiti, N.C.; Reiter, R.J.; Swarnakar, S. Melatonin inhibits matrix metalloproteinase-9 activity by binding to its active site. J. Pineal Res. 2013, 54, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Ostadmohammadi, V.; Soleimani, A.; Bahmani, F.; Aghadavod, E.; Ramezani, R.; Reiter, R.J.; Mansournia, M.A.; Banikazemi, Z.; Soleimani, M.; Zaroudi, M.; et al. The effects of melatonin supplementation on parameters of mental health, glycemic control, markers of cardiometabolic risk, and oxidative stress in diabetic hemodialysis patients: A randomized, double-blind, placebo-controlled trial. J. Ren. Nutr. 2020, 30, 242–250. [Google Scholar] [CrossRef]
- Prado, N.J.; Ferder, L.; Manucha, W.; Diez, E.R. Anti-inflammatory effects of melatonin in obesity and hypertension. Curr. Hypertens. Rep. 2018, 20, 45. [Google Scholar] [CrossRef]
- Konečná, B.; Chobodová, P.; Janko, J.; Baňasová, L.; Bábíčková, J.; Celec, P.; Tóthová, L. The Effect of Melatonin on Periodontitis. Int. J. Mol. Sci. 2021, 22, 2390. [Google Scholar] [CrossRef] [PubMed]
- Nardi, G.M.; Petruzzi, M.; Potenza, P.; Santantonio, M.; Coscia, F.M.; De Vito, D.; Grassia, F.R. Efficacia di un collutorio e un dentifricio nella gestione della malattia parodontale. Studio clinico-microbiologico monocentricoMouthwash and toothpaste efficacy in the management of periodontal disease. Prev. Assist. Dent. 2009, 35, 34–44. [Google Scholar]
- Montero, J.; López-Valverde, N.; Ferrera, M.-J.; López-Valverde, A. Changes in crevicular cytokines after application of melatonin in patients with periodontal disease. J. Clin. Exp. Dent. 2017, 9, e1081–e1087. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Ramírez-Fernández, M.P.; Gómez-Moreno, G.; Maté-Sánchez, J.E.; Delgado-Ruiz, R.; Guardia, J.; López-Marí, L.; Barone, A.; Ortiz-Ruiz, A.J.; Martínez-González, J.M.; et al. Retracted: Melatonin stimulates the growth of new bone around implants in the tibia of rabbits. J. Pineal Res. 2010, 49, 356–363. [Google Scholar] [CrossRef]
- Gómez-Moreno, G.; Aguilar-Salvatierra, A.; Boquete-Castro, A.; Guardia, J.; Piattelli, A.; Perrotti, V.; Delgado-Ruiz, R.A.; Calvo-Guirado, J.L. Outcomes of topical applications of melatonin in implant dentistry: A systematic review. Implant Dent. 2015, 24, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Allegra, M.; Reiter, R.J.; Tan, D.-X.; Gentile, C.; Tesoriere, L.; Livrea, M.A. The chemistry of melatonin’s interaction with reactive species. J. Pineal Res. 2003, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, F.; He, H.W. Melatonin effects on hard tissues: Bone and tooth. Int. J. Mol. Sci. 2013, 14, 10063–10074. [Google Scholar] [CrossRef]

| Population | Patients over 18 years old, both genders, with periodontal disease |
| Intervention | Periodontal treatment with the application of melatonin |
| Comparison | Periodontal treatment without the application of melatonin or healthy control |
| Outcomes | Clinical parameters and biomarkers after melatonin application in non-surgical periodontal treatment |
| Authors (Year) | n Control | n Test Group | Patients | Diagnosis | Systemic Condition | Type of Study | Dropout | NSPT Description | Exclusion Criteria | Period of Treatment | Follow-Up | Side Effects |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmed et al. (2021) [30] | 24 | 24 | 30 patients. In the end, 24 patients (17F and 7M with age range 32–55) | Stage II periodontitis (interdental CAL is detectable at ≥2 nonadjacent teeth, or buccal or oral CAL ≥ 3 mm with pocketing >3 mm is detectable at ≥2 teeth and CAL 3–4 mm and maximum probing depth ≤ 5 mm) | Systemically free according to the modified Cornell Medical Index | Split-mouth randomized controlled clinical trial | 6 | Test: NSPT + Intrapocket application of 5% melatonin gel using a plastic disposable syringe. Control was treated by nonsurgical therapy, followed by intrapocket application of placebo gel using a plastic disposable syringe. | Smokers, pregnant and lactating, patients who received any periodontal treatment in the past 6 months prior to the examination, patients who used antibiotic or anti-inflammatory drugs or antioxidants within the 6 months preceding the beginning of the study, and patients working in night shifts or received any medication that known to alter melatonin levels (e.g., for sleeping disorders). | Weekly once received the application, for 4 weeks | 3 months | NR |
| Anton et al., 2021 [31] | 27 | 27 | 54 patients. In the end, 50 patients (21F and 29M) Test: 53.24 ± 3.4 Control: 52.21 ± 3.1 years old | With periodontal disease | Type 2 diabetes (Glycated hemoglobin A1c (HbA1c) | A double-blind, placebo-controlled, single-center study | 4 | Non-surgical periodontal debridement that involved ultrasonic scaling (Woodpecker UDS-A-LED, Guilin Woodpecker Medical Instrument Co., Ltd., Guilin, China) and manual root planing (Gracey Standard and Mini curettes—Hu-Friedy, Chicago, IL, USA) (SRP) in one session. | Subjects who had undergone periodontal or anti-inflammatory treatment in the last 6 months, insulin treatment, significant change in drug use, and treatment of their diabetes or diet. | Daily for 8 weeks, 1 h before bedtime | 8 weeks | No adverse effects of melatonin were observed during the study. |
| Bazyar et al., 2018 [32], and Javid et al. (2020) [33] | 25 | 25 | 50 patients (30F, 20M) with T2DM M and F (aged 30–60 years) Control: 51.45 ± 5.03 Test: 53.72 ± 6.68 | Severity periodontitis of mild to moderate (PD ≥ 4 mm and CAL = 1–4 mm) | Type-2 diabetes mellitus (T2DM) and body mass index of 18.5–30 kg/m2 | Double-blinded, placebo-controlled, and single-center trial | 6 | Both groups underwent the NSPT, and instructions for dental hygiene (how to brush and use dental floss correctly) were explained to patients. The patients were asked to avoid using mouthwash. | Patients with kidney failure, pregnancy, breastfeeding, thyroid disease, traveling more than two weeks, smoking, using any immuno-suppressive medications, antioxidants, anti-inflammatory agents, insulin, any mouthwash and antibiotic, and noticeable change in consumption of drugs, patients with severe periodontitis, and following a specific diet over the past six months. | 8 weeks (melatonin and placebo tablets were recommended to use one hour before sleeping at night) | 8 weeks | NR |
| Tinto et al., 2020 [34] | 10 | 10 | 12M and 8F, with a mean age of 45.6 years (between 30 years and 70 years old) | Untreated severe stage III (interdental CAL ≥ 5 mm, ≤4 teeth lost, maximum PD ≥ 6 mm) periodontitis | Healthy adult patients | Randomized, placebo-controlled, triple-blind, monocentric clinical trial | 0 | NSPT was performed following a one-stage full-mouth protocol under local anesthesia with ultrasonic instruments and periodontal curettes. The time spent per quadrant was nearly 45 min. Patients were motivated toward oral hygiene and plaque control and instructed to rinse twice per day for 14 days with a 0.20% solution of chlorhexidine. | • Smoking >20 cigarettes per day • Uncontrolled diabetes; • Immunosuppression (pathological or drug-induced); • Current therapy with antiresorptive drugs (bisphosphonates); • Pregnancy and breastfeeding; • In need of antibiotic therapy or treatment with antibiotics in the previous month; • Therapy with mood modulators or sedatives. | 30 days | 6 months | Sleepiness (20%); headache (10%); symptoms resolved spontaneously after a few days. |
| El-Sharkawy et al., 2019 [35] | 40 | 40 | 41M and 33F, with mean age: Control: 46.7 ± 8.3 Test: 45.6 ± 7.1 | At least 20 teeth were diagnosed to have moderate to severe generalized chronic periodontitis (i.e., radiographic evidence of bone loss and presence of PD ≥ 5 mm and at least three sites in each quadrant with attachment loss ≥ 4 mm) | AIS score ≥ 6 (provided that individuals have primary insomnia, which is not related to any other systemic causes or drug intake according to the International Classification of Sleep Disorders, ICSD-3) | Randomized Controlled Trial | 6 | All patients received meticulous thorough SRP with a standard ultrasonic scaler and hand curettes in two visits by an experienced periodontist (MA) prior to giving the capsules. For all participants, strict oral hygiene instructions were given, and 0.12% chlorhexidine mouthwash was prescribed for 2 weeks following SRP. | Diabetes mellitus, smokers, individuals having night work shifts, patients with AIS score <6, cancer patients, patients with autoimmune diseases or osteoporosis, users of antibiotics or non-steroidal anti-inflammatory drugs within the last 3 months, and patients who were subjected to any periodontal therapy during the previous year. | 2 months | 3 and 6 months | Mild adverse reactions observed ranged from zero to a maximum of two cases of melatonin and placebo. - Headache; - Dizziness; - Nausea; - Constipation; - Diarrhea; - Abdominal cramp. Daily oral melatonin intake before bedtime has improved sleep quality and daily life activities for insomniac patients by using the AIS. |
| Authors (Year) | Clinical Features | Treatment | General Results |
|---|---|---|---|
| Ahmed et al., 2021 [30] | -Patients with periodontitis stage II were diagnosed as having interdental CAL, which is detectable at ≥2 nonadjacent teeth or buccal, CAL ≥ 3 mm with pocketing >3 mm, which is detectable at ≥2 teeth, CAL 3–4 mm, and maximum probing depth ≤ 5 mm | -TG: NSPT + OHI + with 5% melatonin gel -CG: NSPT + OHI + placebo gel administration 4 weeks for once a week | Baseline vs. After treatment -TAC: TG (284.5 ± 33.2 vs. 584.4 ± 64.1), CG (283.2 ± 30.9 vs. 437.3 ± 60.4) -MMP-9: TG (77.71 ± 2.86 vs. 31.20 ± 2.3), CG (77.53 ± 3.4 vs. 47.1 ± 1.8) |
| Anton et al., 2021 [31] | -Patients with type 2 diabetes and chronic periodontitis -Subjects with fastening blood glucose levels higher than 126 mg/dL and glycated hemoglobin higher than 6.5% were defined as diabetic | -One a day for 8 weeks -TG: NSPT + OHI + 2 melatonin tablets (250 mg) with 3 mg melatonin -CG: NSPT + OHI + 2 placebo tablets (250 mg) | Baseline vs. After treatment -PD: TG (4.65 ± 1.04 vs. 2.27 ± 0.7), CG (4.53 ± 1.01 vs. 4.40 ± 1.02) -CAL: TG (3.05 ± 0.56 vs. 1.24 ± 0.45), CG (3.02 ± 0.93 vs. 2.98 ± 0.96) -NSPT therapy: CG decrease without reaching statistical significance -PI%: TG (100 vs. 24), CG (100 vs. 48) -BOP%: TG (100 vs. 20), CG (100 vs. 40) -Periodontitis severity: TG significant changes were observed for all severity categories (superficial, moderate, and severe) CG a slight decrease in the number of teeth with moderate and severe periodontitis (p > 0.05) and a significant increase in the number of teeth with superficial periodontitis (p < 0.05). |
| Javid et al., 2020 [33] | -Patients with diabetes 2 with mild to moderate periodontal disease (PD 4 mm and CAL = 1–4 mm) with body mass index of 18.5–30 kg/m2 | -TG: NSPT + OHI+ 250 mg per day (2 tablets) of sodium starch glycolate, magnesium stearate, and 3 mg melatonin -CG: NSPT + OHI+ 250 mg per day placebo tablets with cellulose, silicon dioxide, magnesium stearate, starch, and a few tastes of peppermint oil 1 once/day for 8 weeks | Baseline vs. After treatment -SOD: TG (13.91 ± 2.75 vs. 15.53 ± 4.37 with p = 0.008), CG (14.27 ± 2.52 vs. 14.49 ± 2.58 with p = 0.1) -CAT: TG (24.23 ± 4.54 vs. 27.47 ± 4.12 with p = 0.004), CG (23.14 ± 3.52 vs. 22.72 ± 5.58 with p = 0.77) -GPX: TG (243.04 ± 68.37 vs. 262.04 ± 62.45 with p = 0.004), CG (231.18 ± 67.28 vs. 233.18 ± 62.66 with p = 0.71) -TAC: TG (0.289 ± 0.04 vs. 0.313 ± 0.05 with p = 0.02), CG (0.318 ± 0.06 vs. 0.327 ± 0.08 with p = 0.48) -MDA: TG (17.2 ± 1.82 vs. 16.13 ± 1.76 with p < 0.001), CG (17.49 ± 1.38 vs. 17.17 ± 1.39 with p = 0.1) -IL-1b: TG (2.41 ± 0.55 vs. 2.06 ± 0.48 with p = 0.008), CG (2.47 ± 0.48 vs. 2.33 ± 0.54 with p = 0.12). |
| Tinto et al., 2020 [34] | -Diagnosis of untreated severe stage III periodontitis (interdental CAL ≥ 5 mm, ≤4 teeth lost, maximum PD ≥ 6 mm) | -TG: NSPT + OHI+ 1 mg oral melatonin capsules for 30 days -CG: NSPT + OHI+ 1 mg oral placebo capsules for 30 days | Baseline Vs. After treatment -PD: TG (3.72 ± 0.90 vs. 2.45 ± 0.91 with p = 0.2); CG (3.40 ± 0.81 vs. 2.67 ± 0.85 with p = 0.2) -PD 4–5 mm: TG (1.86 (0.81)), CG (1.04 (0.69)), -PD ≥ 6 mm: TG (3.33 (1.43)), CG (2.11 (0.96)) -FMBS% and FMPS%: No differences were found |
| Bazyar et al., 2019 [32] | -Patients with type 2 diabetes and chronic periodontitis with mild and moderate periodontitis (PD ≥ 4 mm and CAL = 1–4 mm). -Body mass index of 18.5–30 kg/m2, confirmed DM2 (no more than 5 years since diagnosis) | -TG: NSPT + OHI+ 6 mg of melatonin (2 capsules) once daily. -CG: NSPT + OHI+ 6 mg placebo (2capsules) 1 a day. | Baseline vs. After treatment -PD: TG (4.45 ± 0.96 vs. 2.59 ± 1.04 with p < 0.001), CG (4.54 ± 1.01 vs. 4.36 ± 1.04 with p = 0.1) -CAL: TG (3.04 ± 0.78 vs. 1.59 ± 0.59 with p < 0.001), CG (3 ± 0.75 vs. 2.77 ± 0.68 with p = 0.021) -IL-6: TG (2 ± 0.92 vs. 1.42 ± 0.73 with p = 0.008), CG (2.16 ± 0.91 vs. 2.08 ± 0.87 with p = 0.58) -TNF-α: TG (9.05 ± 3.56 vs. 8.24 ± 3.45 with p = 0.1), CG (8.65 ± 3.87 vs. 8.5 ± 3.95 with p = 0.81) -MELATONIN: TG (4.52 ± 1.78 vs. 5.03 ± 1.68 with p = 0.005), CG (4.32 ± 1.93 vs. 4.07 ± 1.91 with p = 0.43) |
| El-Sharkawy et al., 2019 [stage II] [35] | -Patients with insomniac individuals with generalized chronic periodontitis and have at least 20 teeth, diagnosed moderate to severe periodontitis chronic (radiographic evidence of bone loss and presence of PD ≥ 5 mm and at least three sites in each quadrant with attachment loss ≥ 4 mm) | -TG: NSPT+ OHI+ 2-month regimen of 10 mg oral melatonin capsules 1 time daily -CG: NSPT + OHI + 2 months regiment of oral placebo capsules | Baseline vs. After treatment -PD: TG (4.3 ± 0.8 vs. 2.3 ± 0.9), CG (4.4 ± 0.7 vs. 3.0 ± 0.8) -PI: TG (2.35 ± 0.45 vs. 0.81 ± 0.23), CG (2.44 ± 0.67 vs. 0.95 ± 0.17) -GI: TG (2.14 ± 0.36 vs. 0.68 ± 0.17), CG (2.21 ± 0.24 vs. 0.69 ± 0.15) -BOP%: TG (63 ± 21 vs. 12 ± 2.1), CG (59 ± 19 vs. 18 ± 2.8) -CAL: TG (4.8 ± 0.9 vs. 2.6 ± 1.0), CG (4.7 ± 1.0 vs. 3.4 ± 1.2) |
| Authors (Year) | Clinical Parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PD mm (Baseline) (±SD) | PD mm (Final) (±SD) | p-Value (%) | CAL mm (Baseline) (±SD) | CAL mm (Final) (±SD) | p-Value (%) | PI (Baseline) (± SD) | PI (Final) (±SD) | p-Value (%) | GI (Baseline) (± SD) | GI (Final) (±SD) | p-Value (%) | |
| Ahmed et al. (2021) [30] | Melatonin 4.3 ± 0.8 Placebo 4.0 ± 0.6 (p = 0.1485) | 2.9 ± 0.7 3.1 ± 0.7 (p = 0.3275) | <0.001, −32.56% <0.001, −22.5% | Melatonin 4.7 ± 0.9 Placebo 4.3 ± 0.6 (p = 0.07) | 3.5 ± 0.6 3.7 ± 0.6 (p = 0.254) | <0.001, −25.53% <0.001, −13.95% | Melatonin 2 ± 0.7 Placebo 1.96 ± 0.6 (p = 0.83) | 0.7 ± 0.6 0.7 ± 0.6 (p = 0.83) | <0.0001, −65% <0.0001, −64.29% | Melatonin 2.5 ± 0.5 Placebo 2.4 ± 0.5 (p = 0.5) | 0.6 ± 0.5 0.7 ± 0.5 (p = 0.5) | <0.001, −80% <0.001, −70.83% |
| Anton et al. (2021) [31] | Melatonin 4.65 ± 1.04 Placebo 4.53 ± 1.01 (p = 0.15) | 2.27 ± 0.7 4.40 ± 1.02 (p < 0.001) | <0.001 0.12 | Melatonin 3.05 ± 0.56 Placebo 3.02 ± 0.93 (p = 0.1) | 1.24 ± 0.45 2.98 ± 0.96 (p < 0.001) | <0.001 0.08 | Melatonin 100% Placebo 100% (p = 0.9) | 24% 48% (p = 0.07) | <0.001 <0.05 | NR | NR | - |
| Bazyar et al. (2019) [32] and Javid et al. (2020) [33] | Melatonin 4.45 ± 0.96 Placebo 4.54 ± 1.01 (p = 0.76) | 2.59 ± 1.04 4.36 ± 1.04 (p < 0.001) | < 0.001 0.1 | Melatonin 3.04 ± 0.78 Placebo 3 ± 0.75 (p = 0.84) | 1.59 ± 0.59 2.77 ± 0.68 (p < 0.001) | < 0.001 0.021 | Melatonin 100% Placebo 100% | 59.1% 81.8% | 0.09 | NR | NR | - |
| Tinto et al. (2020) [34] | Melatonin 3.72 ± 0.90 Placebo 3.40 ± 0.83 | 2.45 ± 0.91 2.67 ± 0.85 | 0.02 0.02 | NR | NR | NR | NR | NR | NR | - | ||
| El-Sharkawy et al. (2019) [35] | Melatonin 4.3 ± 0.8 Placebo 4.4 ± 0.7 (p = 0.679) | 3/6 months: 2.4 ± 1.0 2.3 ± 0.9 3.1 ± 0.9 3.0 ± 0.8 (p < 0.01) | p < 0.001 p < 0.001 | Melatonin 4.8 ± 0.9 Placebo 4.7 ± 1.0 (p = 0.538) | 3/6 months: 2.7 ± 1.1 2.6 ± 1.0 3.5 ± 0.9 3.4 ± 1.2 (p < 0.01) | p < 0.001 p < 0.001 | Melatonin 2.3 ± 0.5 Placebo 2. 4 ± 0.7 (p = 0.424) | 3/6 months: 0.84 ± 0.26 0.81 ± 0.23 0.92 ± 0.14 0.95 ± 0.17 | p < 0.001 p < 0.001 | Melatonin 2.14 ± 0.36 Placebo 2.21 ± 0.24 (p = 0.736) | 3/6 months: 0.73 ± 0.19 0.68 ± 0.17 0.67 ± 0.14 0.69 ± 0.15 | p < 0.001 p < 0.001 |
| Authors (Year) | Biochemical Parameters | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAC ± SD % (μmol/L) Baseline | TAC ± SD % (μmol/L) Final | p-Value (%) | MMP-9 ± SD (pg/μL) Baseline | MMP-9 ± SD (pg/μL) Final | p-Value (%) | IL-1b (pg/mL) ± SD Baseline | IL-1b (pg/mL) ± SD Final | p-Value | IL-6 (pg/mL) ± SD Baseline | IL-6 (pg/mL) ± SD Final | p-Value | TNF-α (pg/mL) Baseline | TNF-α (pg/mL) Final | p-Value | MDA (μM) Baseline | MDA (μM) Final | p-Value | SOD (U/mL) Baseline | SOD (U/mL) Final | p-Value | |
| Ahmed et al. (2021) [30] | Melatonin 284.5 ± 33.2 Placebo 283.2 ± 30.9 | 584.4±64.1 437.3 ± 60.4 | <0.001, 109.21 ± 41.79% <0.001, 56.25 ± 27.64% | Melatonin 77.71 ± 2.86 Placebo 77.53 ± 3.4 | 31.20 ± 2.3 47.1 ± 1.8 | <0.001, −59.57 ± 14.42% <0.001, −39.25 ± 16.46% | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - |
| Anton et al. (2021) [31] | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - |
| Bazyar et al. (2019) [32] and Javid et al. (2020) [33] | Melatonin 0.289 ± 0.04 Placebo 0.318 ± 0.06 (p = 0.09) | 0.313 ± 0.05 0.327 ± 0.08 (p = 0.53) | 0.02 0.48 | NR | NR | - | Melatonin 2.41 ± 0.55 Placebo 2.47 ± 0.48 (p = 0.67) | 2.06 ± 0.48 2.33 ± 0.54 (p = 0.1) | 0.008 0.12 | Melatonin 2 ± 0.92 Placebo 2.16 ± 0.91 (p = 0.58) | 1.42 ± 0.73 2.08 ± 0.87 (p = 0.01) | 0.008 0.58 | Melatonin 9.05 ± 3.56 Placebo 8.65 ± 3.87 (p = 0.72) | 8.24 ± 3.45 8.5 ± 3.95 (p = 0.81) | 0.1 0.81 | Melatonin 17.2 ± 1.82 Placebo 17.49 ± 1.38 (p = 0.56) | 16.13 ± 1.76 17.17 ± 1.39 (p = 0.03) | <0.001 0.1 | Melatonin 13.91 ± 2.75 Placebo 14.27 ± 2.52 (p = 0.65) | 15.53 ± 4.37 14.49 ± 2.58 (p = 0.34) | 0.008 0.1 |
| Tinto et al. (2020) [34] | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - |
| El-Sharkawy et al. (2019) [35] | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - | Melatonin 14Placebo 14(p>0.05) | 69.5(p<0.01) | - | NR | NR | - | NR | NR | - |
| Authors (Year) | CAT (U/mL) Baseline | CAT (U/mL) Final | p-Value | GPx (U/mL) Baseline | GPx (U/mL) Final | p-Value | Melatonin (pg/mL) Baseline | Melatonin (pg/mL) Final | p-Value | hc-CRP (mg/L) Baseline | hc-CRP (mg/L) Final | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmed et al. (2021) [30] | NR | NR | NR | NR | NR | NR | NR | NR | ||||
| Anton et al. (2021) [31] | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - |
| Bazyar et al. (2019) [32] and Javid et al. (2020) [33] | Melatonin 24.23 ± 4.54 Placebo 23.14 ± 3.52 (p = 0.37) | 27.47 ± 4.12 22.72 ± 5.58 (p = 0.003) | 0.004 0.77 | Melatonin 243.04 ± 68.37 Placebo 231.18 ± 67.28 (p = 0.56) | 262.04 ± 62.45 233.18 ± 62.66(p = 0.13) | 0.004 0.71 | Melatonin 4.52 ± 1.78 Placebo 4.32 ± 1.93 (p = 0.72) | 5.03 ± 1.68 4.07 ± 1.91 (p = 0.08) | 0.005 0.43 | Melatonin 2.53 ± 0.77 Placebo 2.31 ± 0.96 (p = 0.41) | 1.6 ± 0.91 2.4 ± 0.94 (p = 0.08) | 0.017 0.45 |
| Tinto et al. (2020) [34] | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - |
| El-Sharkawy et al. (2019) [35] | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purpura, S.; Fernandes, G.V.O.; Oliveira, F.P.; de Castro, F.C. Effects of Melatonin in the Non-Surgical Treatment of Periodontitis: A Systematic Review. Appl. Sci. 2022, 12, 11698. https://doi.org/10.3390/app122211698
Purpura S, Fernandes GVO, Oliveira FP, de Castro FC. Effects of Melatonin in the Non-Surgical Treatment of Periodontitis: A Systematic Review. Applied Sciences. 2022; 12(22):11698. https://doi.org/10.3390/app122211698
Chicago/Turabian StylePurpura, Simone, Gustavo Vicentis Oliveira Fernandes, Filipa Pinto Oliveira, and Filipe Correia de Castro. 2022. "Effects of Melatonin in the Non-Surgical Treatment of Periodontitis: A Systematic Review" Applied Sciences 12, no. 22: 11698. https://doi.org/10.3390/app122211698
APA StylePurpura, S., Fernandes, G. V. O., Oliveira, F. P., & de Castro, F. C. (2022). Effects of Melatonin in the Non-Surgical Treatment of Periodontitis: A Systematic Review. Applied Sciences, 12(22), 11698. https://doi.org/10.3390/app122211698