Biomechanical Task-Based Gait Analysis Suggests ReWalk Gait Resembles Crutch Gait
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Gait Cycle Event Identification
2.3. Signal Processing
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perry, J. Gait Analysis: Normal and Pathological Function; SLACK Incorporated: Thorofare, NJ, USA, 1992; pp. 3–16. [Google Scholar]
- Queralt, A.; Valls-Solé, J.; Castellote, J.M. Speeding up Gait Initiation and Gait-Pattern with a Startling Stimulus. Gait Posture 2010, 31, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Swank, C.; Wang-Price, S.; Gao, F.; Almutairi, S. Walking with a Robotic Exoskeleton Does Not Mimic Natural Gait: A within-Subjects Study. JMIR Rehabil. Assist. Technol. 2019, 6, e11023. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Roemmich, R.T.; Elrod, J.M.; Hass, C.J.; Hsiao-Wecksler, E.T. Effects of Aging and Parkinson’s Disease on Joint Coupling, Symmetry, Complexity and Variability of Lower Limb Movements during Gait. Clin. Biomech. 2016, 33, 92–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scafetta, N.; Marchi, D.; West, B.J. Understanding the Complexity of Human Gait Dynamics. Chaos 2009, 19, 026108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggioni, M.A.; Veicsteinas, A.; Rampichini, S.; Cé, E.; Nemni, R.; Riboldazzi, G.; Merati, G. Energy Cost of Spontaneous Walking in Parkinson’s Disease Patients. Neurol. Sci. 2012, 33, 779–784. [Google Scholar] [CrossRef]
- Kharb, A.; Saini, V.; Jain, Y.; Dhiman, S. A review of gait cycle and its parameters. IJCEM Int. J. Comput. Eng. Manag. 2011, 13, 78–83. [Google Scholar]
- Chen, C.L.; Chen, H.C.; Tang, S.F.T.; Wu, C.Y.; Cheng, P.T.; Hong, W.H. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am. J. Phys. Med. Rehabil. 2003, 82, 925–935. [Google Scholar] [CrossRef]
- Van Peppen, R.P.S.; Kwakkel, G.; Wood-Dauphinee, S.; Hendriks, H.J.M.; van der Wees, P.J.; Dekker, J. The Impact of Physical Therapy on Functional Outcomes after Stroke: What’s the Evidence? Clin. Rehabil. 2004, 18, 833–862. [Google Scholar] [CrossRef]
- Carr, J.H.; Shepherd, R.B. A Motor Learning Model for Stroke Rehabilitation. Physiotherapy 1989, 75, 372–380. [Google Scholar] [CrossRef]
- Bowles, A.O.; Kevorkian, C.G.; Rintala, D.H. Gender Differences Regarding Career Issues and Promotion in Academic Physical Medicine and Rehabilitation. Am. J. Phys. Med. Rehabil. 2007, 86, 918–925. [Google Scholar] [CrossRef]
- Daly, J.J.; Ruff, R.L. Construction of Efficacious Gait and Upper Limb Functional Interventions Based on Brain Plasticity Evidence and Model-Based Measures for Stroke Patients. Sci. World J. 2007, 7, 2031–2045. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.; Shepherd, R. Neurological Rehabilitation: Optimizing Motor Performance, 2nd ed.; Churchill Livingstone: Edinburgh, UK, 2010. [Google Scholar]
- Morone, G.; Paolucci, S.; Cherubini, A.; de Angelis, D.; Venturiero, V.; Coiro, P.; Iosa, M. Robot-Assisted Gait Training for Stroke Patients: Current State of the Art and Perspectives of Robotics. Neuropsychiatr. Dis. Treat. 2017, 13, 1303–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sale, P.; Franceschini, M.; Waldner, A.; Hesse, S. Use of the robot assisted gait therapy in rehabilitation of patients with stroke and spinal cord injury. Eur. J. Phys. Rehabil. Med. 2012, 48, 111–121. [Google Scholar] [PubMed]
- Calabrò, R.S.; Cacciola, A.; Bertè, F.; Manuli, A.; Leo, A.; Bramanti, A.; Naro, A.; Milardi, D.; Bramanti, P. Robotic Gait Rehabilitation and Substitution Devices in Neurological Disorders: Where Are We Now? Neurol. Sci. 2016, 37, 503–514. [Google Scholar] [CrossRef]
- Gassert, R.; Dietz, V. Rehabilitation Robots for the Treatment of Sensorimotor Deficits: A Neurophysiological Perspective. J. Neuroeng. Rehabil. 2018, 15, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poli, P.; Morone, G.; Rosati, G.; Masiero, S. Robotic Technologies and Rehabilitation: New Tools for Stroke Patients’ Therapy. BioMed Res. Int. 2013, 2013, 153872. [Google Scholar] [CrossRef] [PubMed]
- Dzahir, M.A.M.; Yamamoto, S.I. Recent Trends in Lower-Limb Robotic Rehabilitation Orthosis: Control Scheme and Strategy for Pneumatic Muscle Actuated Gait Trainers. Robotics 2014, 3, 120–148. [Google Scholar] [CrossRef] [Green Version]
- Goffredo, M.; Iacovelli, C.; Russo, E.; Pournajaf, S.; di Blasi, C.; Galafate, D.; Pellicciari, L.; Agosti, M.; Filoni, S.; Aprile, I.; et al. Stroke Gait Rehabilitation: A Comparison of End-Effector, Overground Exoskeleton, and Conventional Gait Training. Appl. Sci. 2019, 9, 2627. [Google Scholar] [CrossRef] [Green Version]
- Valè, N.; Gandolfi, M.; Vignoli, L.; Botticelli, A.; Posteraro, F.; Morone, G.; Dell’orco, A.; Dimitrova, E.; Gervasoni, E.; Goffredo, M.; et al. Electromechanical and Robotic Devices for Gait and Balance Rehabilitation of Children with Neurological Disability: A Systematic Review. Appl. Sci. 2021, 11, 12061. [Google Scholar] [CrossRef]
- Pennycott, A.; Wyss, D.; Vallery, H.; Klamroth-Marganska, V.; Riener, R. Towards More Effective Robotic Gait Training for Stroke Rehabilitation: A Review. J. Neuroeng. Rehabil. 2012, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Belda-Lois, J.M.; Mena-Del Horno, S.; Bermejo-Bosch, I.; Moreno, J.C.; Pons, J.L.; Farina, D.; Iosa, M.; Molinari, M.; Tamburella, F.; Ramos, A.; et al. Rehabilitation of Gait after Stroke: A Review towards a Top-down Approach. J. Neuroeng. Rehabil. 2011, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Israel, J.F.; Campbell, D.D.; Kahn, J.H.; Hornby, T.G. Metabolic Costs and Muscle Activity Patterns during Robotic- and Therapist-Assisted Treadmill Walking in Individuals with Incomplete Spinal Cord Injury. Phys. Ther. 2006, 86, 1466–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Androwis, G.J.; Pilkar, R.; Ramanujam, A.; Nolan, K.J. Electromyography Assessment during Gait in a Robotic Exoskeleton for Acute Stroke. Front. Neurol. 2018, 9, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sylos-Labini, F.; la Scaleia, V.; d’Avella, A.; Pisotta, I.; Tamburella, F.; Scivoletto, G.; Molinari, M.; Wang, S.; Wang, L.; van Asseldonk, E.; et al. EMG Patterns during Assisted Walking in the Exoskeleton. Front. Hum. Neurosci. 2014, 8, 423. [Google Scholar] [CrossRef]
- De Luca, A.; Bellitto, A.; Mandraccia, S.; Marchesi, G.; Pellegrino, L.; Coscia, M.; Leoncini, C.; Rossi, L.; Gamba, S.; Massone, A.; et al. Exoskeleton for Gait Rehabilitation: Effects of Assistance, Mechanical Structure, and Walking Aids on Muscle Activations. Appl. Sci. 2019, 9, 2868. [Google Scholar] [CrossRef] [Green Version]
- Van Nes, I.J.W.; van Dijsseldonk, R.B.; van Herpen, F.H.M.; Rijken, H.; Geurts, A.C.H.; Keijsers, N.L.W. Improvement of Quality of Life after 2-Month Exoskeleton Training in Patients with Chronic Spinal Cord Injury. J. Spinal Cord Med. 2022. [Google Scholar] [CrossRef]
- Gottlieb, U.; Balasukumaran, T.; Hoffman, J.R.; Springer, S. Agreement of Gait Events Detection during Treadmill Backward Walking by Kinematic Data and Inertial Motion Units. Sensors 2020, 20, 6331. [Google Scholar] [CrossRef]
- Jasiewicz, J.M.; Allum, J.H.J.; Middleton, J.W.; Barriskill, A.; Condie, P.; Purcell, B.; Li, R.C.T. Gait Event Detection Using Linear Accelerometers or Angular Velocity Transducers in Able-Bodied and Spinal-Cord Injured Individuals. Gait Posture 2006, 24, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Nandedkar, S.D.; Sanders, D.B. Sanders. Measurement of the amplitude of the EMG envelope. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 1990, 13, 933–938. [Google Scholar] [CrossRef]
- Kim, J.; Chung, S.H.; Choi, J.; Lee, J.M.; Kim, S.J. Practical Method for Predicting Intended Gait Speed via Soleus Surface EMG Signals. Electron. Lett. 2020, 56, 528–531. [Google Scholar] [CrossRef]
- Barzilay, O.; Wolf, A. A Fast Implementation for EMG Signal Linear Envelope Computation. J. Electromyogr. Kinesiol. 2011, 21, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Englehart, K.; Hudgins, B. A Robust, Real-Time Control Scheme for Multifunction Myoelectric Control. IEEE Trans. Biomed. Eng. 2003, 50, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Kizawa, S.; Kobayashi, Y.; Miyawaki, K. Pose Estimation by Extended Kalman Filter Using Noise Covariance Matrices Based on Sensor Output. ROBOMECH J. 2020, 7, 36. [Google Scholar] [CrossRef]
- Boudarham, J.; Hameau, S.; Zory, R.; Hardy, A.; Bensmail, D.; Roche, N. Coactivation of Lower Limb Muscles during Gait in Patients with Multiple Sclerosis. PLoS ONE 2016, 11, e0158267. [Google Scholar] [CrossRef] [PubMed]
- Platz, T.; Gillner, A.; Borgwaldt, N.; Kroll, S.; Roschka, S. Device-Training for Individuals with Thoracic and Lumbar Spinal Cord Injury Using a Powered Exoskeleton for Technically Assisted Mobility: Achievements and User Satisfaction. BioMed Res. Int. 2016, 2016, 8459018. [Google Scholar] [CrossRef] [Green Version]
- Lajeunesse, V.; Vincent, C.; Routhier, F.; Careau, E.; Michaud, F. Exoskeletons’ Design and Usefulness Evidence According to a Systematic Review of Lower Limb Exoskeletons Used for Functional Mobility by People with Spinal Cord Injury. Disabil. Rehabil. Assist. Technol. 2016, 11, 535–547. [Google Scholar] [CrossRef]
- Tamburella, F.; Lorusso, M.; Tramontano, M.; Fadlun, S.; Masciullo, M.; Scivoletto, G. Overground Robotic Training Effects on Walking and Secondary Health Conditions in Individuals with Spinal Cord Injury: Systematic Review. J. Neuroeng. Rehabil. 2022, 19, 27. [Google Scholar] [CrossRef]
- Fritz, H.; Patzer, D.; Galen, S.S. Robotic Exoskeletons for Reengaging in Everyday Activities: Promises, Pitfalls, and Opportunities. Disabil. Rehabil. 2019, 41, 560–563. [Google Scholar] [CrossRef]
- Iosa, M.; Cereatti, A.; Merlo, A.; Campanini, I.; Paolucci, S.; Cappozzo, A. Assessment of Waveform Similarity in Clinical Gait Data: The Linear Fit Method. BioMed Res. Int. 2014, 2014, 214156. [Google Scholar] [CrossRef]
- Iosa, M.; Peppe, A.; Morone, G.; Bottino, S.; Bini, F.; Marinozzi, F.; Paolucci, S. Assessment of Waveform Similarity in Electromyographical Clinical Gait Data: The Linear Fit Method. J. Med. Biol. Eng. 2018, 38, 774–781. [Google Scholar] [CrossRef]
- Wren, T.A.L.; Patrick Do, K.; Rethlefsen, S.A.; Healy, B. Cross-Correlation as a Method for Comparing Dynamic Electromyography Signals during Gait. J. Biomech. 2006, 39, 2714–2718. [Google Scholar] [CrossRef]

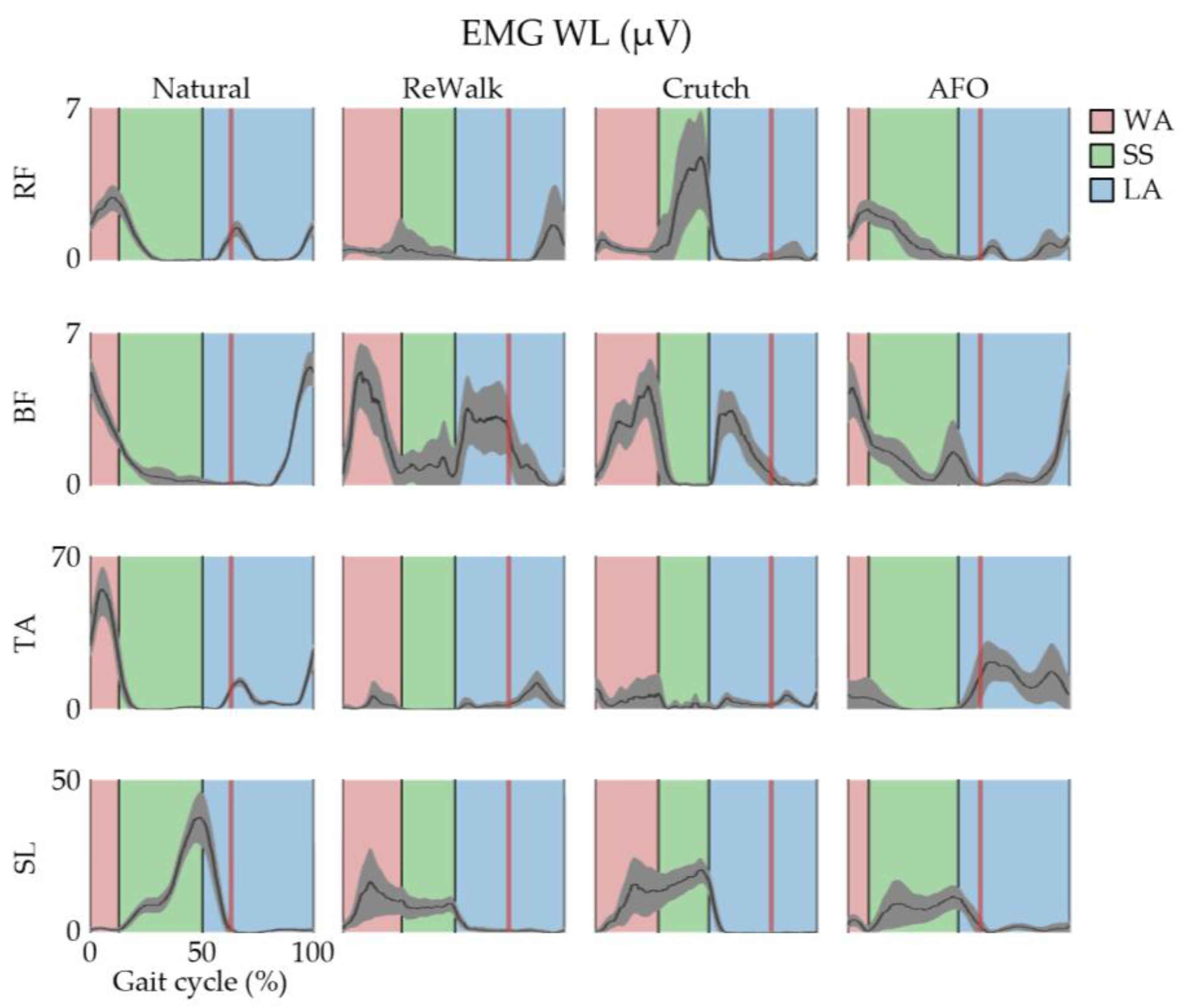
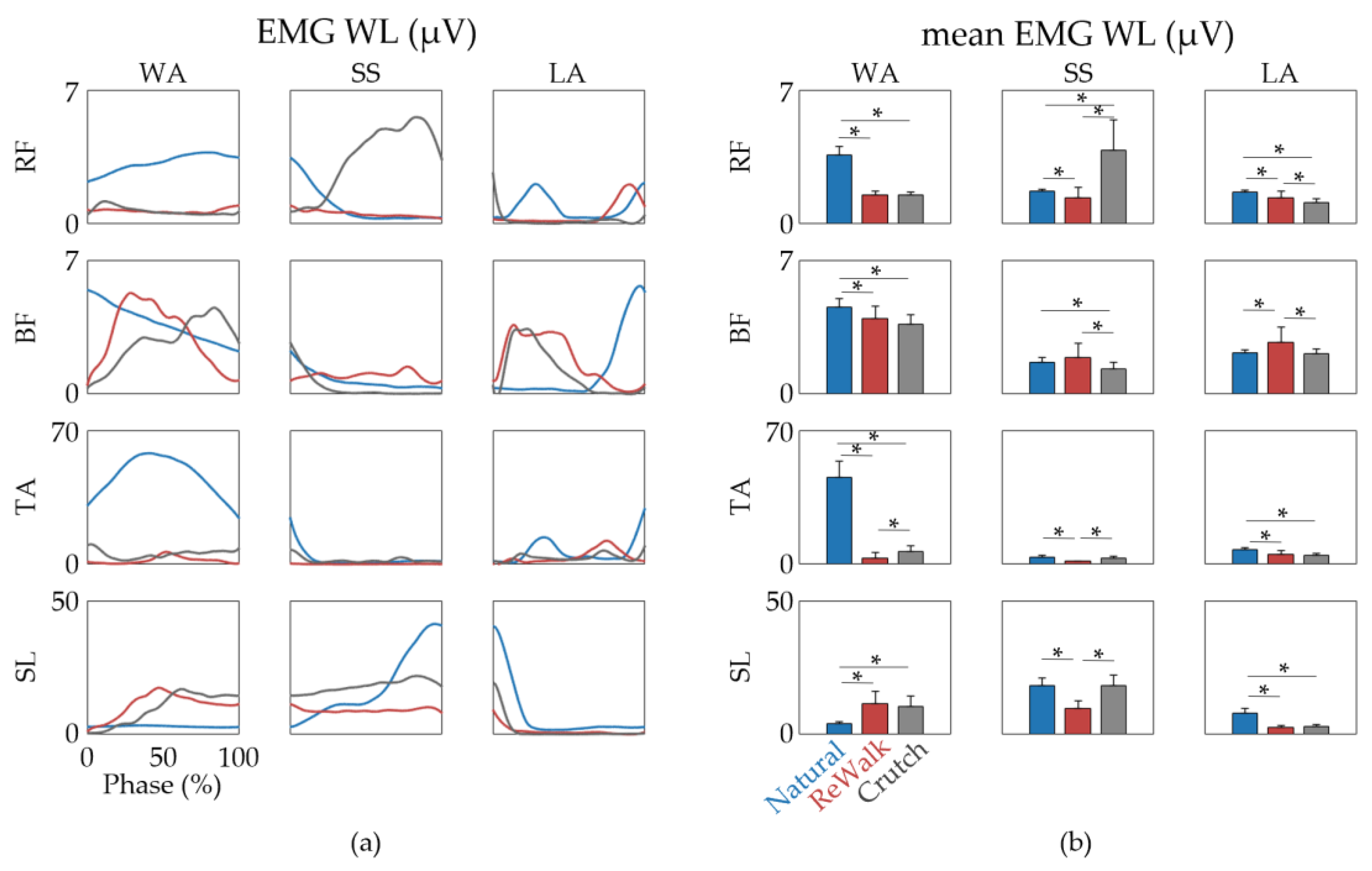
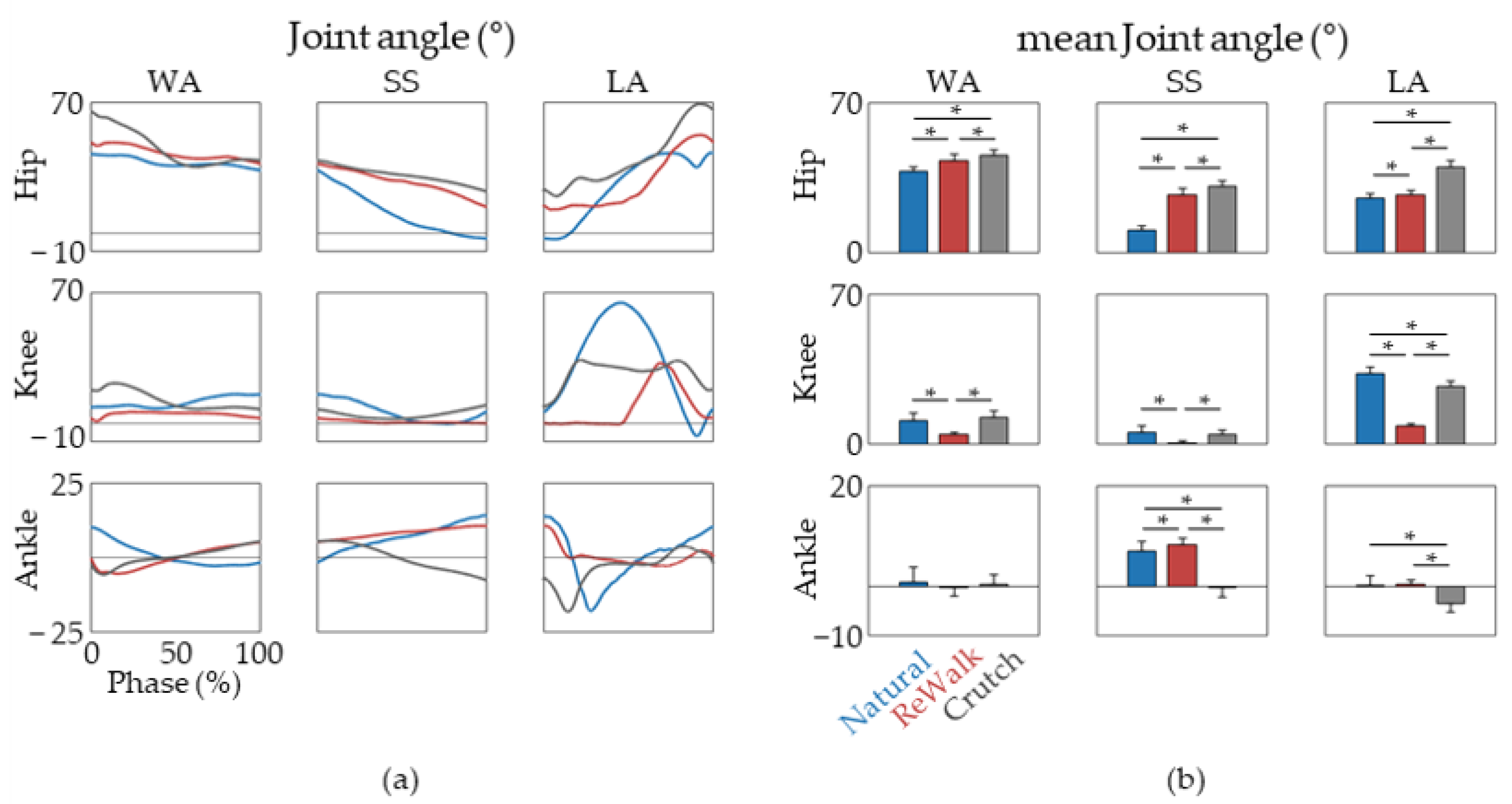
| Phase | Discrepancies | Subjects | |||||
|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | ||
| WA | RF has diminished from the previous LA phase | o | o | o | o | o | |
| BF is activated after the WA phase has started | o | o | o | o | o | ||
| SL starts to increase | o | o | o | o | o | ||
| TA remains inactive | o | o | o | o | o | o | |
| Increased hip flexion | o | o | o | o | o | o | |
| SS | No marked increase in SL | o | o | o | o | ||
| Increased hip flexion | o | o | o | o | o | o | |
| LA | Significant increase in BF | o | o | o | |||
| Decreased knee flexion | o | o | o | o | o | o | |
| Subject | Natural | ReWalk | Crutch | ReWalk- Natural | Crutch- Natural | Crutch- ReWalk |
|---|---|---|---|---|---|---|
| Mean ± SD (μV) | Mean ± SD (μV) | Mean ± SD (μV) | Difference (%) | Difference (%) | Difference (%) | |
| #1 | 3.61 ± 0.45 | 1.51 ± 0.22 | 1.51 ± 0.15 | −58.10 *** | −58.05 *** | 0.11 |
| #2 | 2.03 ± 0.15 | 1.23 ± 0.08 | 1.30 ± 0.08 | −39.65 *** | −35.94 *** | 6.14 *** |
| #3 | 2.12 ± 0.18 | 1.78 ± 0.48 | 1.59 ± 0.19 | −15.95 ** | −24.90 *** | −10.65 |
| #4 | 2.74 ± 0.13 | 1.13 ± 0.04 | 1.36 ± 0.08 | −58.69 *** | −50.43 *** | 19.97 *** |
| #5 | 2.39 ± 0.20 | 1.20 ± 0.06 | 1.29 ± 0.06 | −49.81 *** | −46.02 *** | 7.55 *** |
| #6 | 4.55 ± 0.40 | 1.75 ± 0.36 | 1.92 ± 0.11 | −61.43 *** | −57.86 *** | 9.25 |
| Subject | Natural | ReWalk | Crutch | ReWalk- Natural | Crutch- Natural | Crutch- ReWalk |
|---|---|---|---|---|---|---|
| Mean ± SD (μV) | Mean ± SD (μV) | Mean ± SD (μV) | Difference (%) | Difference (%) | Difference (%) | |
| #1 | 45.34 ± 8.69 | 3.40 ± 2.62 | 6.58 ± 3.08 | −92.49 *** | −85.48 *** | 93.38 *** |
| #2 | 24.54 ± 3.74 | 6.89 ± 6.54 | 4.44 ± 1.86 | −71.94 *** | −81.91 *** | −35.54 |
| #3 | 26.82 ± 3.29 | 4.00 ± 1.42 | 2.27 ± 1.52 | −85.09 *** | −91.52 *** | −43.11 *** |
| #4 | 29.34 ± 7.51 | 5.99 ± 4.54 | 13.51 ± 8.79 | −79.57 *** | −53.94 ** | 125.40 ** |
| #5 | 11.51 ± 1.80 | 2.84 ± 1.43 | 3.95 ± 1.17 | −75.30 *** | −65.64 *** | 39.13 ** |
| #6 | 30.99 ± 7.37 | 4.46 ± 1.63 | 5.41 ± 3.91 | −85.60 *** | −82.56 *** | 21.16 |
| Subject | Natural | ReWalk | Crutch | ReWalk- Natural | Crutch- Natural | Crutch- ReWalk |
|---|---|---|---|---|---|---|
| Mean ± SD (μV) | Mean ± SD (μV) | Mean ± SD (μV) | Difference (%) | Difference (%) | Difference (%) | |
| #1 | 4.09 ± 0.54 | 11.64 ± 4.61 | 10.43 ± 4.04 | 184.46 *** | 154.83 *** | −10.42 |
| #2 | 3.82 ± 0.67 | 9.80 ± 2.11 | 12.40 ± 2.82 | 156.85 *** | 224.99 *** | 26.53 ** |
| #3 | 3.89 ± 0.45 | 10.80 ± 3.60 | 8.49 ± 2.60 | 177.39 *** | 118.11 *** | −21.37 * |
| #4 | 4.80 ± 2.83 | 13.21 ± 7.21 | 21.42 ± 13.21 | 175.18 *** | 346.31 *** | 62.19 |
| #5 | 5.66 ± 1.87 | 6.41 ± 1.75 | 4.20 ± 1.33 | 13.27 | −25.84 * | −34.53 *** |
| #6 | 3.39 ± 0.66 | 12.93 ± 3.61 | 4.87 ± 3.10 | 281.16 *** | 43.73 | −62.29 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, Y.; Kim, S.-J. Biomechanical Task-Based Gait Analysis Suggests ReWalk Gait Resembles Crutch Gait. Appl. Sci. 2022, 12, 12574. https://doi.org/10.3390/app122412574
Kim J, Kim Y, Kim S-J. Biomechanical Task-Based Gait Analysis Suggests ReWalk Gait Resembles Crutch Gait. Applied Sciences. 2022; 12(24):12574. https://doi.org/10.3390/app122412574
Chicago/Turabian StyleKim, Jaewook, Yekwang Kim, and Seung-Jong Kim. 2022. "Biomechanical Task-Based Gait Analysis Suggests ReWalk Gait Resembles Crutch Gait" Applied Sciences 12, no. 24: 12574. https://doi.org/10.3390/app122412574





