Investigation of Antibacterial Activity of Carob-Mediated Calcium Hydroxide Nanoparticles against Different Aerobic and Anaerobic Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of C-CaOH2 NPs and Preparation of Suspensions
2.3. Preparation of Bacterial Suspensions
2.4. Agar Well Diffusion ZOI Test
2.5. Resazurin-Based Broth Microdilution MIC Test
2.6. Minimum Bactericidal Concentrations (MBC)
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciambelli, P.; La Guardia, G.; Vitale, L. Nanotechnology for green materials and processes. Stud. Surf. Sci. Catal. 2019, 179, 97–116. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Estrela, C.; Holland, R. Calcium hydroxide: Study based on scientific evidences. J. Appl. Oral Sci. 2003, 11, 269–282. [Google Scholar] [CrossRef] [Green Version]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 59–81. [Google Scholar] [CrossRef]
- Devatha, C.P.; Thalla, A.K. Chapter 7—Green Synthesis of Nanomaterials. In Synthesis of Inorganic Nanomaterials; Mohan Bhagyaraj, S., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 169–184. [Google Scholar] [CrossRef]
- Beğiç, N.; Bener, M.; Apak, R. Development of a green synthesized silver nanoparticle-based antioxidant capacity method using carob extract. J. Nanostruct. Chem. 2021, 11, 381–394. [Google Scholar] [CrossRef]
- Meziani, S.; Oomah, B.D.; Zaidi, F.; Simon-Levert, A.; Bertrand, C.; Zaidi-Yahiaoui, R. Antibacterial activity of carob (Ceratonia siliqua L.) extracts against phytopathogenic bacteria Pectobacterium atrosepticum. Microb. Pathog. 2015, 78, 95–102. [Google Scholar] [CrossRef]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2009, 27, 238–254. [Google Scholar] [CrossRef]
- Lewis, K.; Ausubel, F.M. Prospects for plant-derived antibacterials. Nat. Biotechnol. 2006, 24, 1504–1507. [Google Scholar] [CrossRef]
- Barabadi, H.; Mojab, F.; Vahidi, H.; Marashi, B.; Talank, N.; Hosseini, O.; Saravanan, M. Green synthesis, characterization, antibacterial and biofilm inhibitory activity of silver nanoparticles compared to commercial silver nanoparticles. Inorg. Chem. Commun. 2021, 129, 108647. [Google Scholar] [CrossRef]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Lukáčová Bujňáková, Z.; Balážová, L.; Tkáčiková, L. Green synthesis of silver nanoparticles with antibacterial activity using various medicinal plant extracts: Morphology and antibacterial efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Shahid, S.; Hanif, S.; Almoallim, H.S.; Alharbi, S.A.; Sellami, H. Green synthesis of chromium oxide nanoparticles for antibacterial, antioxidant anticancer, and biocompatibility activities. Int. J. Mol. Sci. 2021, 22, 502. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Mahadevan, S.; Arulmozhi, P.; Sriram, S.; Praseetha, P.K. Green synthesis of zinc oxide nanoparticles using Atalantia monophylla leaf extracts: Characterization and antimicrobial analysis. Mater. Sci. Semicond. Process. 2018, 82, 39–45. [Google Scholar] [CrossRef]
- Fidan, H.; Mihaylova, D.; Petkova, N.; Sapoundzhieva, T.; Slavov, A.; Krastev, L. Determination of chemical composition, antibacterial and antioxidant properties of products obtained from carob and honey locust. Turk. J. Biochem. 2019, 44, 316–322. [Google Scholar] [CrossRef]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional components of carob fruit: Linking the chemical and biological space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef]
- Abdulkareem, R.S.; Al-Hayali, W.R.Y.; Ibrahim, I.I. Antimicrobial activity of Ceratonia silique L. extract against diarrheagenic E-coli. Syst. Rev. Pharm. 2020, 11, 2139–2141. [Google Scholar] [CrossRef]
- Al-Seeni, M.N. The antimicrobial, antioxidant, and in vivo hypoglycemic activities of the of the carob extract. IOSR J. Environ. Sci. Toxicol. Food Technol. 2017, 11, 24–29. [Google Scholar] [CrossRef]
- Abdul-Hussein, I.F. Study of the Effect of Carob (Ceratonia siliqua L.) Extract Activity as Antibiotic from UTI. Al-Qadisiyah J. Agric. Sci. 2018, 8, 6–12. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M.; Abdeen, A.O. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int. J. Ind. Chem. 2013, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Aksu Demirezen, D.; Yılmaz, Ş.; Demirezen, Y.D.; Yıldız, Y.Ş. Green Synthesis of Iron Oxide Nanoparticles Using Ceratonia siliqua L. Aqueous Extract: Optimization, Characterization, Stabilization and Evaluation of its Antibacterial Activity against Gram-Positive and Gram-Negative Bacteria. SSRN Electron. J. 2022, 113, 849–861. [Google Scholar] [CrossRef]
- Garrocho-Rangel, A.; Escobar-García, D.M.; Gutiérrez-Sánchez, M.; Herrera-Badillo, D.; Carranco-Rodríguez, F.; Flores-Arriaga, J.C.; Pozos-Guillén, A. Calcium hydroxide/iodoform nanoparticles as an intracanal filling medication: Synthesis, characterization, and in vitro study using a bovine primary tooth model. Odontology 2021, 109, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Zand, V.; Mokhtari, H.; Hasani, A.; Jabbari, G. Comparison of the Penetration Depth of Conventional and Nano-Particle Calcium Hydroxide into Dentinal Tubules. Iran. Endod. J. 2017, 12, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Moseke, C.; Braun, W.; Ewald, A. Electrochemically Deposited Ca(OH)2 Coatings as a Bactericidal and Osteointegrative Modification of Ti Implants. Adv. Eng. Mater. 2009, 11, B1–B6. [Google Scholar] [CrossRef]
- Dianat, O.; Saedi, S.; Kazem, M.; Alam, M. Antimicrobial Activity of Nanoparticle Calcium Hydroxide against Enterococcus Faecalis: An In Vitro Study. Iran. Endod. J. 2014, 10, 39–43. [Google Scholar] [CrossRef]
- Karthik, K.; Dhanuskodi, S.; Gobinath, C.; Prabukumar, S.; Sivaramakrishnan, S. Dielectric and antibacterial studies of microwave assisted calcium hydroxide nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 16509–16518. [Google Scholar] [CrossRef]
- Harish; Kumari, S.; Parihar, J.; Akash; Kumari, J.; Kumar, L.; Debnath, M.; Kumar, V.; Mishra, R.K.; Gwag, J.S.; et al. Synthesis, Characterization, and Antibacterial Activity of Calcium Hydroxide Nanoparticles Against Gram-Positive and Gram-Negative Bacteria. ChemistrySelect 2022, 7, e202203094. [Google Scholar] [CrossRef]
- Hegazi, M.S.M.F.A.; Ali, M.M.; Hassan, R.E.S. Evaluation of Antimicrobial Effect of Conventional Calcium Hydroxide, Calcium Hydroxide Nanoparticle and Combined Calcium Hydroxide with Silver Nanoparticle as Intracanal Medication against Enterococcus Faecalis. Indian J. Public Health Res. Dev. 2019, 10, 2107. [Google Scholar] [CrossRef]
- Alayed, H.S.; Devanesan, S.; AlSalhi, M.S.; Alkindi, M.G.; Alghamdi, O.G.; Alkhalaf, R.I. Green synthesis of calcium hydroxide nanoparticles using carob fruit extract and evaluation of their cytotoxic activity. Appl. Nanosci. 2022, 12, 2511–2521. [Google Scholar] [CrossRef]
- Topală, C.M.; Tătaru, L.D.; Ducu, C. ATR-FTIR spectra fingerprinting of medicinal herbs extracts prepared using microwave extraction. Arab. J. Med. Aromat. Plants 2017, 3, 1–9. [Google Scholar]
- Chen, P.; Wang, Y.; He, S.; Wang, P.; Xu, Y.; Zhang, L. Green synthesis of spherical calcium hydroxide nanoparticles in the presence of tannic acid. Adv. Mater. Sci. Eng. 2020, 2020, 9501897. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Alsalhi, M.S.; Devanesan, S.; Alkhalaf, R.I.S.; Allayed, H.A.; Alqhtani, N.R.R.; Alkindi, M.G.; Alghamdi, O.G.M. Calcium Hydroxide Nanoparticles Synthesized with Carob Pulp Extract. U.S. Patent US10780111B1, 22 September 2020. Available online: https://uspto.report/patent/grant/[10,780],111 (accessed on 22 September 2020).
- M02-A12; Performance Standards for Antimicrobial Disk Susceptibility Tests. Twelfth ed. Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2015.
- M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Eleventh ed. Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Barry, A.L.; Craig, W.A.; Nadler, H.; Reller, L.B.; Sanders, C.C.; Swenson, J.M. M26-A Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 1999; Volume 19.
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krein, S.L.; Kowalski, C.P.; Hofer, T.P.; Saint, S. Preventing Hospital-Acquired Infections: A National Survey of Practices Reported by U.S. Hospitals in 2005 and 2009. J. Gen. Intern. Med. 2011, 27, 773–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inweregbu, K.; Dave, J.; Pittard, A. Nosocomial infections. Contin. Educ. Anaesth. Crit. Care Pain 2005, 5, 14–17. [Google Scholar] [CrossRef]
- Sahrmann, P.; Gilli, F.; Wiedemeier, D.B.; Attin, T.; Schmidlin, P.R.; Karygianni, L. The Microbiome of Peri-Implantitis: A Systematic Review and Meta-Analysis. Microorganisms 2020, 8, 661. [Google Scholar] [CrossRef]
- Valgas, C.; De Souza, S.M.; Smânia, E.F.A.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, J.F.; Lopes, H.P. Mechanisms of antimicrobial activity of calcium hydroxide: A critical review. Int. Endod. J. 1999, 32, 361–369. [Google Scholar] [CrossRef] [Green Version]
- De Gomes, B.P.F.A.; Ferraz, C.C.R.; Garrido, F.D.; Rosalen, P.R.; Zaia, A.A.; Batista Teixeira, F.; De Souza-Filho, F.J. Microbial susceptibility to calcium hydroxide pastes and their vehicles. J. Endod. 2002, 28, 758–761. [Google Scholar] [CrossRef]
- Beeby, M.; Gumbart, J.C.; Roux, B.; Jensen, G.J. Architecture and assembly of the Gram-positive cell wall. Mol. Microbiol. 2013, 88, 664–672. [Google Scholar] [CrossRef]
- Turner, R.D.; Vollmer, W.; Foster, S.J. Different walls for rods and balls: The diversity of peptidoglycan. Mol. Microbiol. 2014, 91, 862–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, S.; Singh, M.; Kim, S.J.; Schaefer, J. Staphylococcus aureus Peptidoglycan Tertiary Structure from Carbon-13 Spin Diffusion. J. Am. Chem. Soc. 2009, 131, 7023–7030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanyal, D.; Greenwood, D. An electronmicroscope study of glycopeptide antibiotic-resistant strains of Staphylococcus epidermidis. J. Med. Microbiol. 1993, 39, 204–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Teeseling, M.C.F.; de Pedro, M.A.; Cava, F. Determinants of bacterial morphology: From fundamentals to possibilities for antimicrobial targeting. Front. Microbiol. 2017, 8, 1264. [Google Scholar] [CrossRef] [Green Version]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of Specialized Biomolecules against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Barani, M.; Zeeshan, M.; Kalantar-Neyestanaki, D.; Farooq, M.A.; Rahdar, A.; Jha, N.K.; Sargazi, S.; Gupta, P.K.; Thakur, V.K. Nanomaterials in the Management of Gram-Negative Bacterial Infections. Nanomaterials 2021, 11, 2535. [Google Scholar] [CrossRef]
- Yang, T.; Moreira, W.; Nyantakyi, S.A.; Chen, H.; Aziz, D.B.; Go, M.-L.; Dick, T. Amphiphilic Indole Derivatives as Antimycobacterial Agents: Structure–Activity Relationships and Membrane Targeting Properties. J. Med. Chem. 2017, 60, 2745–2763. [Google Scholar] [CrossRef]
- Athanassiadis, B.; Abbott, P.V.; George, N.; Walsh, L.J. An in vitro study of the antimicrobial activity of some endodontic medicaments and their bases using an agar well diffusion assay. Aust. Dent. J. 2009, 54, 141–146. [Google Scholar] [CrossRef]
- Reller, L.B.; Weinstein, M.; Jorgensen, J.H.; Ferraro, M.J. Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef]
- Pham, X.N.; Nguyen, H.T.; Pham, N.T. Green Synthesis and Antibacterial Activity of HAp@Ag Nanocomposite Using Centella asiatica (L.) Urban Extract and Eggshell. Int. J. Biomater. 2020, 2020, 8841221. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2015, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putri, C.M.D.; Prisinda, D.; Malinda, Y. The MIC and MBC of calcium hydroxide medicament against bacteria that cause chronic periapical abscess in the vulnerable initial 7-days of endodontic treatment. Padjadjaran J. Dent. 2022, 34, 16–25. [Google Scholar] [CrossRef]
- Davis, J.L. Pharmacologic Principles. Equine Intern. Med. 2018, 4, 79–137. [Google Scholar] [CrossRef]
- Albukhaty, S.; Al-Bayati, L.; Al-Karagoly, H.; Al-Musawi, S. Preparation and characterization of titanium dioxide nanoparticles and in vitro investigation of their cytotoxicity and antibacterial activity against Staphylococcus aureus and Escherichia coli. Anim. Biotechnol. 2022, 33, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Alarfaj, A.A. Preparation, characterization and antibacterial effect of chitosan nanoparticles against food spoilage bacteria. J. Pure Appl. Microbiol. 2019, 13, 1273–1278. [Google Scholar] [CrossRef] [Green Version]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Manzoor, U.; Siddique, S.; Ahmed, R.; Noreen, Z.; Bokhari, H.; Ahmad, I. Antibacterial, structural and optical characterization of mechano-chemically prepared ZnO nanoparticles. PLoS ONE 2016, 11, e0154704. [Google Scholar] [CrossRef] [Green Version]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [Green Version]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; De Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Custódio, L.; Escapa, A.L.; Fernandes, E.; Fajardo, A.; Aligué, R.; Alberício, F.; Neng, N.; Nogueira, J.M.F.; Romano, A. Phytochemical Profile, Antioxidant and Cytotoxic Activities of the Carob Tree (Ceratonia siliqua L.) Germ Flour Extracts. Mater. Veg. 2011, 66, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Ben Hsouna, A.; Trigui, M.; Ben Mansour, R.; Jarraya, R.M.; Damak, M.; Jaoua, S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int. J. Food Microbiol. 2011, 148, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytoher. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousif, A. Processing and characterization of carob powder. Food Chem. 2000, 69, 283–287. [Google Scholar] [CrossRef]
- Fulzele, P.; Baliga, S.; Thosar, N.; Pradhan, D. Evaluation of calcium ion, hydroxyl ion release and pH levels in various calcium hydroxide based intracanal medicaments: An in vitro study. Contemp. Clin. Dent. 2011, 2, 291–295. [Google Scholar] [CrossRef]
- Ben Ayache, S.; Reis, F.S.; Inês Dias, M.; Pereira, C.; Glamočlija, J.; Soković, M.; Saafi, E.B.; Ferreira, I.C.F.R.; Barros, L.; Achour, L. Chemical characterization of carob seeds (Ceratonia siliqua L.) and use of different extraction techniques to promote its bioactivity. Food Chem. 2021, 351, 129263. [Google Scholar] [CrossRef]
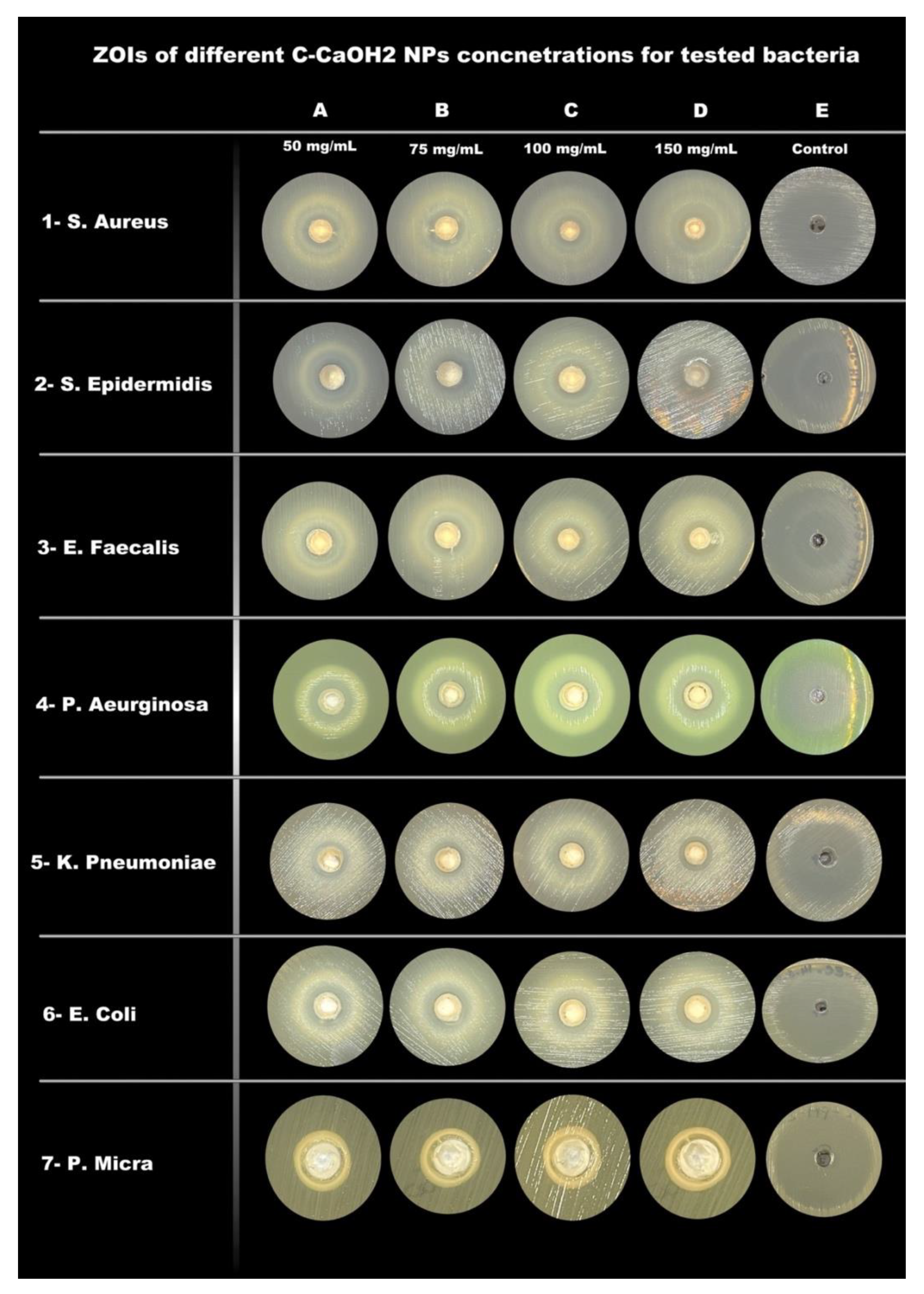


| Mean ± Standard Deviation of Zones of Inhibition (ZOI) in mm | |||||
|---|---|---|---|---|---|
| Bacterial Strains | 50 mg/mL | 75 mg/mL | 100 mg/mL | 150 mg/mL | Control |
| S. Aureus | 7.3 ± 0.6 | 9 ± 1 | 9.3 ± 0.6 | 10.8 ± 0.3 | 26.7 ± 0.6 |
| S. Epidermidis | 8.3 ± 0.6 | 9 ± 0 | 9.5 ± 0.5 | 9.7 ± 0.6 | 40.3 ± 0.6 |
| E. Faecalis | 0 ± 0 | 0 ± 0 | 7.3 ± 0.6 | 8.7 ± 0.6 | 19.3 ± 0.6 |
| P. Aeruginosa | 10 ± 0 | 11 ± 0 | 11.2 ± 0.3 | 11.7 ± 0.6 | 31.7 ± 0.6 |
| K. Pneumoniae | 9 ± 0 | 9 ± 0 | 9.2 ± 0.3 | 9.7 ± 0.6 | 30.7 ± 0.6 |
| E. Coli | 10 ± 0 | 10 ± 0 | 10.2 ± 0.3 | 10.8 ± 0.3 | 40.3 ± 0.6 |
| P. Micra | 5.7 ± 0.6 | 6.3 ± 0.6 | 7.5 ± 0.5 | 7.7 ± 0.6 | 41 ± 1.0 |
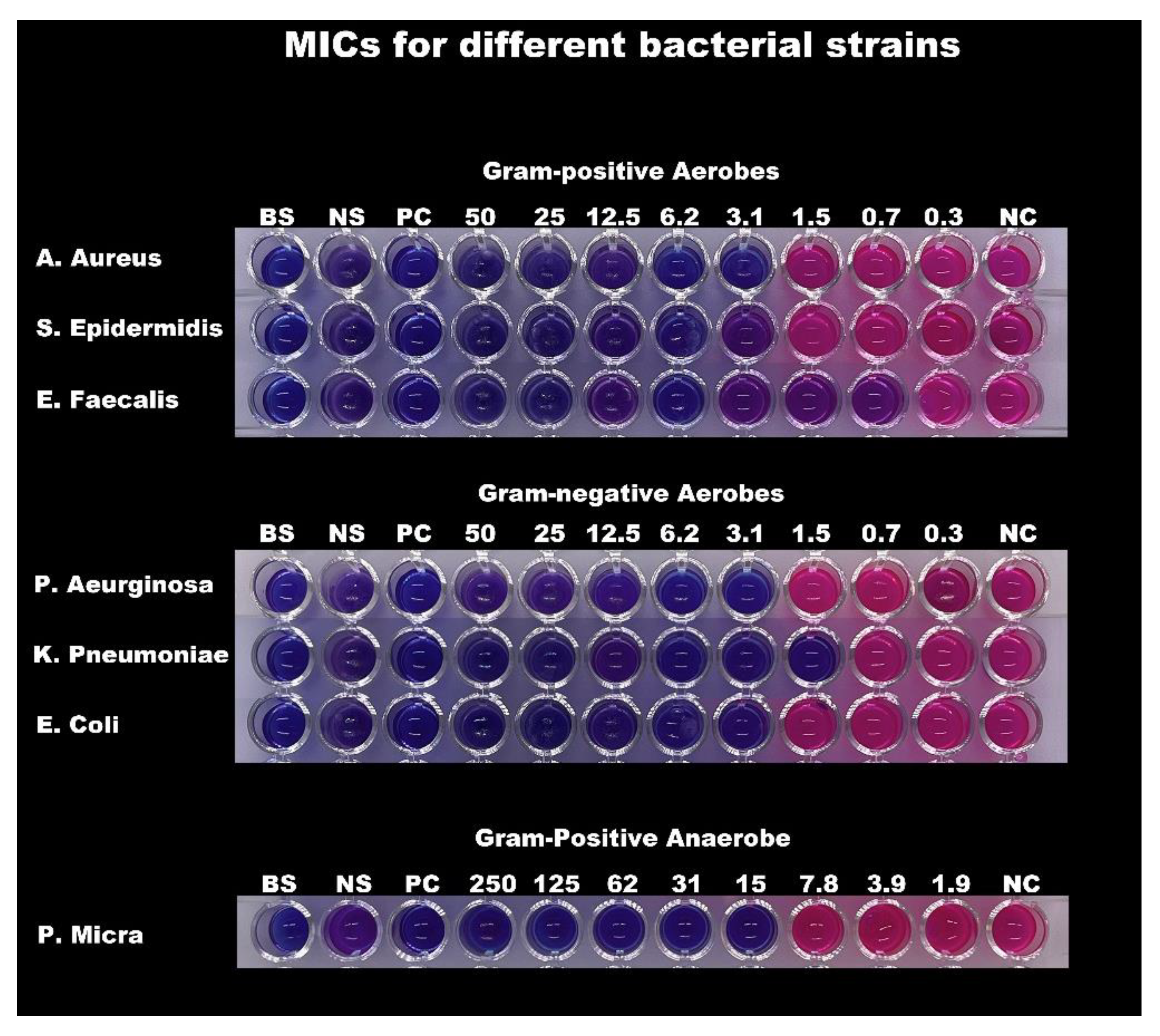
| Minimal Inhibitory concentrations (MIC) and Minimal Bactericidal Concentrations (MBC) | ||
|---|---|---|
| Bacterial Strains | MIC (mg/mL) | MBC (mg/mL) |
| S. Aureus | 3.12 | 12.5 |
| S. Epidermidis | 3.12 | 12.5 |
| E. Faecalis | 0.78 | 12.5 |
| P. Aeruginosa | 3.12 | 3.12 |
| K. Pneumoniae | 1.56 | 6.25 |
| E. Coli | 3.12 | 12.5 |
| P. Micra | 15.62 | 31.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alayed, H.S.; Devanesan, S.; AlSalhi, M.S.; Alkindi, M.G.; Alghamdi, O.G.; Alqhtani, N.R. Investigation of Antibacterial Activity of Carob-Mediated Calcium Hydroxide Nanoparticles against Different Aerobic and Anaerobic Bacteria. Appl. Sci. 2022, 12, 12624. https://doi.org/10.3390/app122412624
Alayed HS, Devanesan S, AlSalhi MS, Alkindi MG, Alghamdi OG, Alqhtani NR. Investigation of Antibacterial Activity of Carob-Mediated Calcium Hydroxide Nanoparticles against Different Aerobic and Anaerobic Bacteria. Applied Sciences. 2022; 12(24):12624. https://doi.org/10.3390/app122412624
Chicago/Turabian StyleAlayed, Hajar S., Sandhanasamy Devanesan, Mohamad S. AlSalhi, Mohammed G. Alkindi, Osama G. Alghamdi, and Nasser R. Alqhtani. 2022. "Investigation of Antibacterial Activity of Carob-Mediated Calcium Hydroxide Nanoparticles against Different Aerobic and Anaerobic Bacteria" Applied Sciences 12, no. 24: 12624. https://doi.org/10.3390/app122412624





