High-Efficiency Adsorption of SARS-CoV-2 Spike 1 Protein by Plasma-Modified Porous Polymers
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
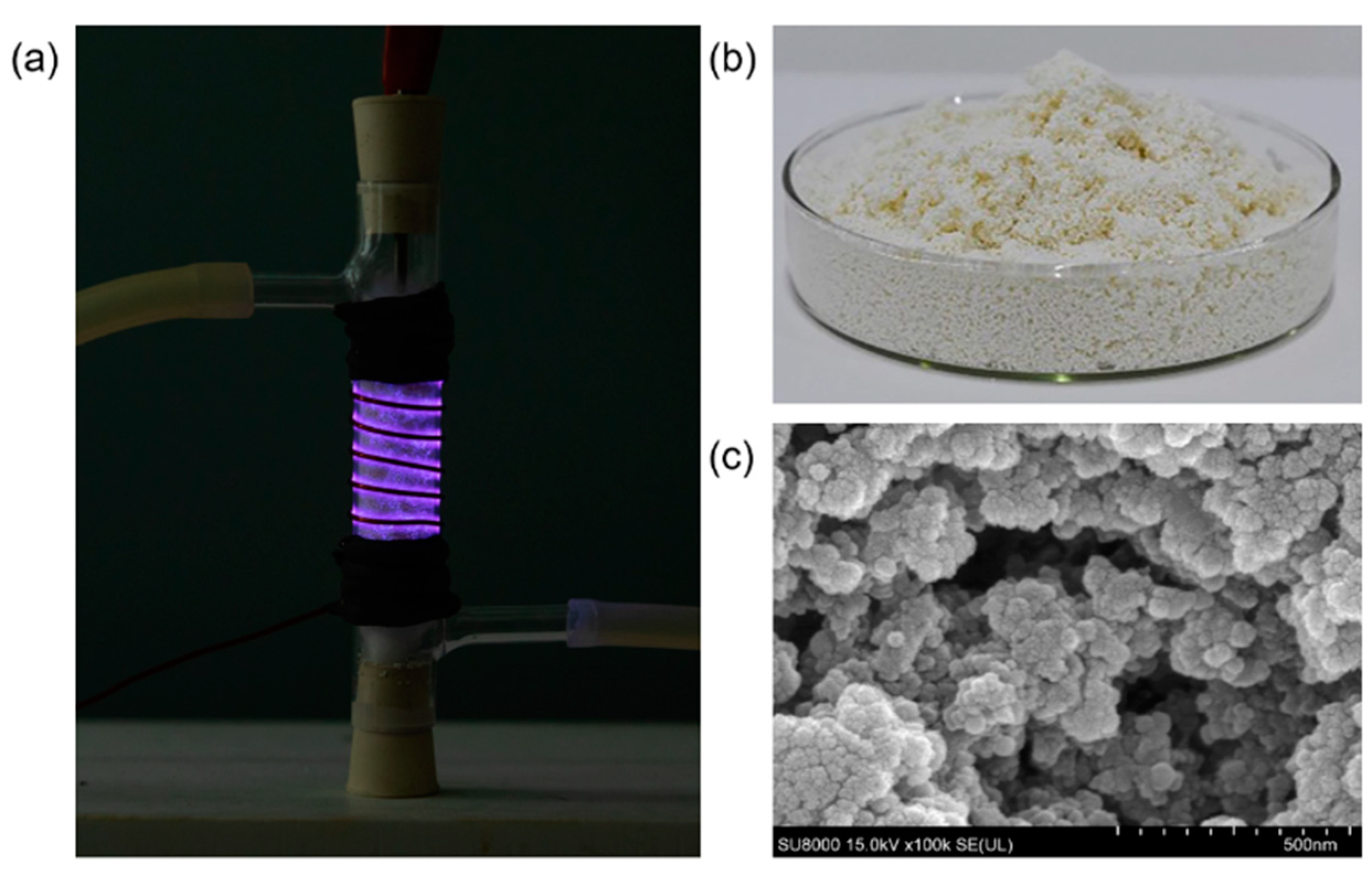
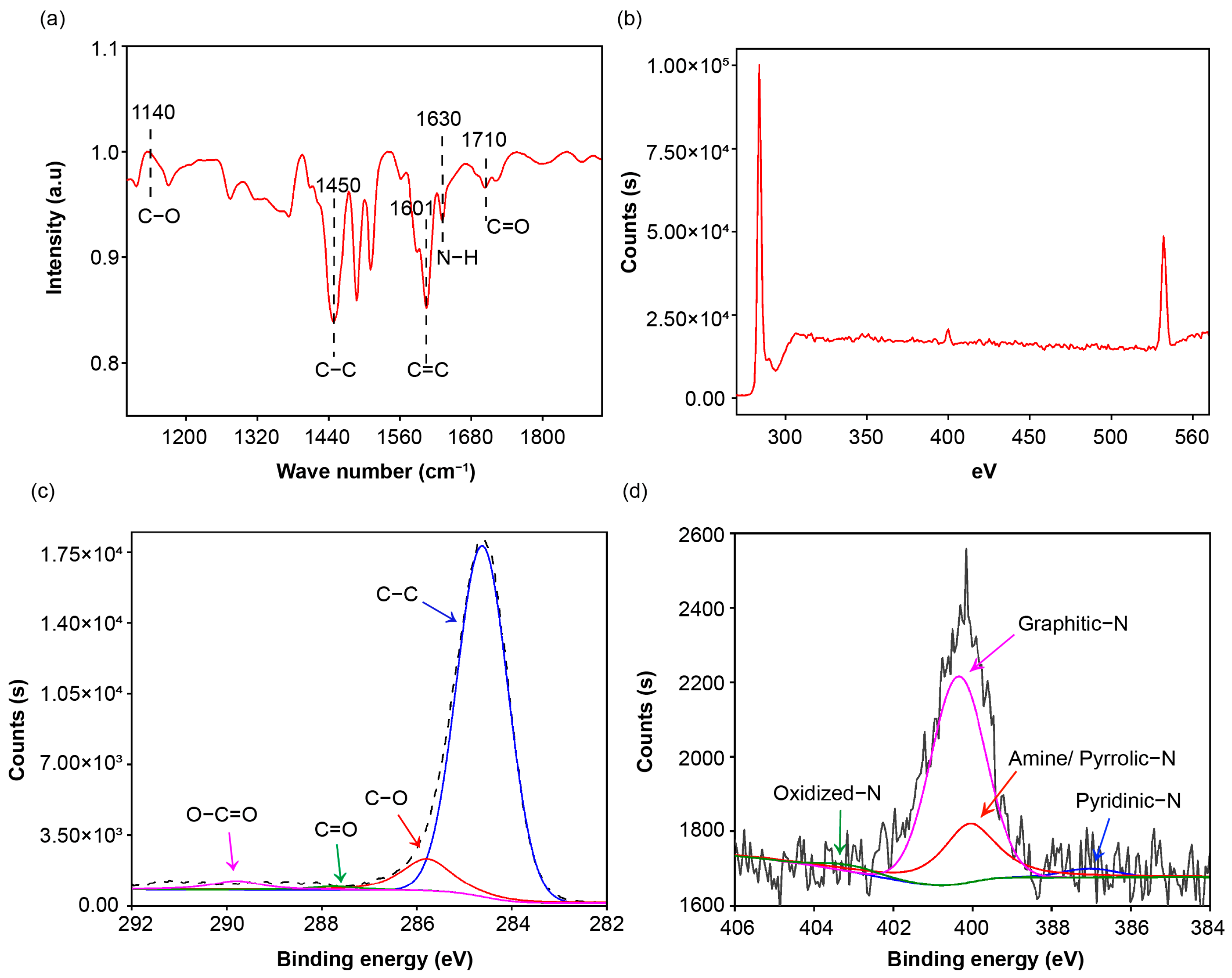
3.1. Microstructure and Functionalization of the Modified Porous Polymer
3.2. Removal of SARS-CoV-2 S1 Protein
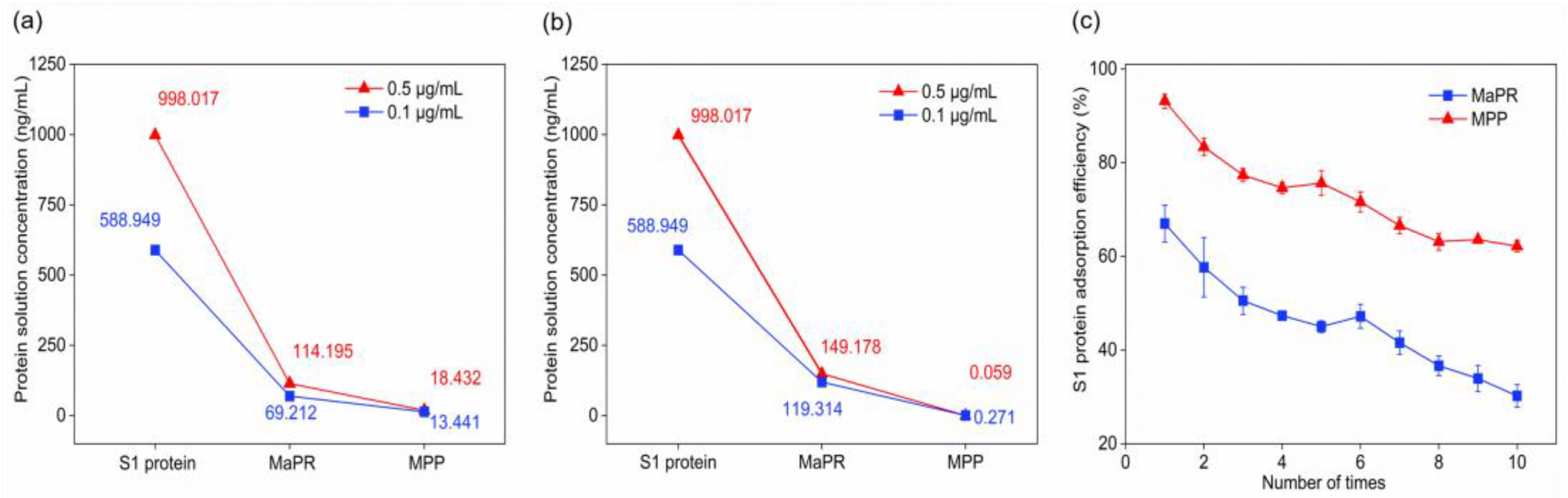
3.3. The Adsorption Efficiency of S1 Protein was Quantified by ELISA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Coronavirus (COVID-19) Dashboard [Internet]. 2022. Available online: https://covid19.who.int/ (accessed on 10 November 2022).
- Tian, D.; Sun, Y.; Xu, H.; Ye, Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J. Med. Virol. 2022, 94, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Sellaoui, L.; Badawi, M.; Monari, A.; Tatarchuk, T.; Jemli, S.; Dotto, G.L.; Bonilla-Petriciolet, A.; Chen, Z. Make it clean, make it safe: A review on virus elimination via adsorption. Chem. Eng. J. 2021, 412, 128682. [Google Scholar] [CrossRef]
- Wolski, K.; Nowakowska, M.; Pyrc, K.; Szczubiałka, K.; Ciejka, J. Biopolymeric nano/microspheres for selective and reversible adsorption of coronaviruses. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 76, 735–742. [Google Scholar]
- Park, J.A.; Kang, J.K.; Kim, J.H.; Kim, S.B.; Yu, S.; Kim, T.H. Bacteriophage removal in various clay minerals and clay-amended soils. Environ. Eng. Res. 2015, 20, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Ultra-fine cellulose nanofibers: New nano-scale materials for water purification. J. Mater. Chem. 2011, 21, 7507–7510. [Google Scholar] [CrossRef]
- Junter, G.A.; Lebrun, L. Polysaccharide-based chromatographic adsorbents for virus purification and viral clearance. J. Pharm. Anal. 2020, 10, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Imbert-Laurenceau, E.; Crepinior, J.; Crance, J.M.; Jouan, A.; Migonney, V. Polystyrene derivatives substituted with arginine interact with babanki (togaviridae) and kedougou (flaviviridae) viruses. J. Med. Virol. 2003, 69, 503–509. [Google Scholar] [CrossRef]
- Ogura, F.; Hayashi, K.; Lee, J.B.; Kanekiyo, K.; Hayashi, T. Evaluation of an edible blue-green alga, Aphanothece sacrum, for its inhibitory effect on replication of herpes simplex virus type 2 and influenza virus type A. Biosci. Biotechnol. Biochem. 2010, 74, 1687–1690. [Google Scholar] [CrossRef] [Green Version]
- Preeprame, S.; Hayashi, K.; Lee, J.B.; Sankawa, U.; Hayashi, T. A novel antivirally active fucan sulfate derived from an edible brown alga, Sargassum horneri. Chem. Pharm. Bull. 2001, 49, 484–485. [Google Scholar] [CrossRef] [Green Version]
- Pisharody, L.; Suresh, S.; Mukherji, S. Evaluation of adsorbents and eluents for application in virus concentration and adsorption-desorption isotherms for coliphages. Chem. Eng. J. 2021, 403, 126267. [Google Scholar] [CrossRef]
- De Luca, G.; Petrosino, F.; Luque Di Salvo, J.; Chakraborty, S.; Curcio, S. Advanced descriptors for long-range noncovalent interactions between SARS-CoV-2 spikes and polymer surfaces. Sep. Purif. Technol. 2021, 11, 120125. [Google Scholar] [CrossRef] [PubMed]
- Curcio, S.; Petrosino, F.; Morrone, M.; de Luca, G. Interactions between Proteins and the Membrane Surface in Multiscale Modeling of Organic Fouling. J. Chem. Inf. Model. 2018, 58, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Sahihi, M.; Faraudo, J. Molecular Dynamics Simulations of Adsorption of SARS-CoV-2 Spike Protein on Polystyrene Surface. J. Chem. Inf. Model. 2022, 62, 3814–3824. [Google Scholar] [CrossRef]
- Xin, Y.; Grundmeier, G.; Keller, A. Adsorption of SARS-CoV-2 Spike Protein S1 at Oxide Surfaces Studied by High-Speed Atomic Force Microscopy. Adv. Nanobiomed. Res. 2021, 1, 2000024. [Google Scholar] [CrossRef]
- Freitas, P.A.M.; Iha, K.; Felinto, M.C.F.C.; Súarez-Iha, M.E.V. Adsorption of di-2-pyridyl ketone salicyloylhydrazone on Amberlite XAD-2 and XAD-7 resins: Characteristics and isotherms. J. Colloid Interface Sci. 2008, 323, 1–5. [Google Scholar] [CrossRef]
- Lv, C.; Yang, J.; Liu, R.; Lu, Q.; Ding, Y.; Zhang, J.; Deng, J. A comparative study on the adsorption and desorption characteristics of flavonoids from honey by six resins. Food Chem. 2018, 268, 424–430. [Google Scholar] [CrossRef]
- Nassar, R.A.; Browne, E.P.; Chen, J.; Kibonov, A.M. Removing human immunodeficiency virus (HIV) from human blood using immobilized heparin. Biotechnol. Lett. 2012, 34, 853–856. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.N.; Wang, H.L.; Zhou, X.F.; Liang, J.P.; Yuan, H.; Xu, Y.; Yang, D.Z. Micro-mesoporous polystyrene resins prepared by nanosecond pulsed discharge plasma for the efficient adsorption of PAHs. Sep. Purif. Technol. 2022, 301, 121829. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, F.; Wang, N.; Hao, S.; Chu, W. Plasma-treated bimetallic Ni-Pt catalysts derived from hydrotalcites for the carbon dioxide reforming of methane. Catal. Lett. 2014, 144, 293–300. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Q.; Zhou, X.; Liang, J.; Yuan, H.; Yang, D.; Wang, W. Highly efficient adsorptive removal of persistent organic pollutants using NDP-acid combined modified NaY zeolites. Chem. Eng. J. 2022, 431, 133858. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, G.; Bao, Y.; Zeng, D.; Chen, Y. Effect of oxidative modification of coal tar pitch-based mesoporous activated carbon on the adsorption of benzothiophene and dibenzothiophene. Fuel Process. Technol. 2015, 129, 85–90. [Google Scholar] [CrossRef]
- Dou, S.; Tao, L.; Wang, R.L.; El Hankari, S.; Chen, R.; Wang, S.Y. Plasma-assisted synthesis and surface modification of electrode materials for renewable energy. Adv. Mater. 2018, 30, 1705850. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Liu, X.; Wang, Y.; Meng, Y.; Alsaedi, A.; Hayat, T.; Li, J. Plasma surface modification of materials and their entrapment of water contaminant: A review. Plasma Processes Polym 2017, 14, 1600218. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.Z.; Qiao, J.J.; Zhang, L.; Wang, W.Z.; Zhao, Z.L.; Zhou, X.F.; Yuan, H.; Wang, W.C. The dynamic evolution and interaction with dielectric material of the discharge in packed bed reactor. Plasma Sources Sci. Technol. 2020, 29, 055004. [Google Scholar] [CrossRef]
- Wu, L.; Wan, W.; Shang, Z.; Gao, X.; Kobayashi, N.; Luo, G.; Li, Z. Surface modification of phosphoric acid activated carbon by using non-thermal plasma for enhancement of Cu(II) adsorption from aqueous solutions. Sep. Purif. Technol. 2018, 197, 156–169. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Zheng, H.; Xiong, Z.; Zhao, R.; Liu, Y.; Zhao, C.; Zheng, C. Novel anionic polyacrylamide-modify-chitosan magnetic composite nanoparticles with excellent adsorption capacity for cationic dyes and pH-independent adsorption capability for metal ions. Chem. Eng. J. 2020, 392, 123706. [Google Scholar] [CrossRef]
- Xu, Q.N.; Yang, D.Z.; Wang, H.L.; Yuan, H.; Jia, Z.X.; Yang, H.; Zhou, X.F.; Wang, W.C.; Xu, Y.; Yan, E.Y. Enhancing the adsorption property of macroporous XAD-2 resin by nanosecond-pulsed discharge plasma modification. Plasma Processes Polym. 2021, 18, e2000117. [Google Scholar] [CrossRef]
- El Didamony, A.M.; Youssef, W.M.; Abdou, A.A. Modification of amberlite XAD-2 resin for U(VI) adsorption from egyptian crude phosphoric acid. Egypt. J. Pet. 2019, 28, 71–76. [Google Scholar] [CrossRef]
- Gradinaru, L.M.; Barbalata-Mandru, M.; Drobota, M.; Aflori, M.; Spiridon, M.; Gradisteanu Pircalabioru, G.; Bleotu, C.; Butnaru, M.; Vlad, S. Preparation and evaluation of nanofibrous hydroxypropyl cellulose and β-cyclodextrin polyurethane composite mats. Nanomaterials 2020, 10, 754. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Guo, M.; Yi, X.; Zhang, Z. Preparation and characterization of a naphthalenemodified poly (aryl ether ketone) and its phase separation morphology with bismaleimide resin. Polym. Bull. 2017, 74, 1519–1533. [Google Scholar] [CrossRef]
- Kong, X.; Liu, Q.; Chen, D.; Chen, G. Identifying the active sites on N-doped graphene toward oxygen evolution reaction. Chem. Cat. Chem. 2017, 9, 846–852. [Google Scholar] [CrossRef]
- Mei, S.; Gu, J.; Ma, T.; Li, X.; Hu, Y.; Li, W.; Zhang, J.; Han, Y. N-doped activated carbon from used dyeing wastewater adsorbent as a metal-free catalyst for acetylene hydrochlorination. Chem. Eng. J. 2019, 371, 118–129. [Google Scholar] [CrossRef]
- Liu, L.; Yan, F.; Li, K.; Zhu, C.; Xie, Y.; Zhang, X.; Chen, Y. Ultrasmall FeNi3N particles with an exposed active (110) surface anchored on nitrogen-doped graphene for multifunctional electrocatalysts. J. Mater. Chem. A 2019, 7, 1083–1091. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, P.; Qiu, Y.; Yu, Q.; Ma, J.; Wu, H.; Luo, G.; Xu, M.; Yao, H. Increasing oxygen functional groups of activated carbon with non-thermal plasma to enhance mercury removal efficiency for flue gases. Chem. Eng. J. 2015, 263, 1–8. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Dobrynin, D.; Polishchuk, I.; Portal, L.; Zlotver, I.; Sosnik, A.; Pokroy, B. Adsorption of SARS CoV-2 spike proteins on various functionalized surfaces correlates with the high transmissibility of Delta and Omicron variants. Mater. Today Bio. 2022, 14, 100265. [Google Scholar] [CrossRef]
- Krähling, V.; Halwe, S.; Rohde, C.; Becker, D.; Berghöfer, S.; Dahlke, C.; Eickmann, M.; Ercanoglu, M.S.; Gieselmann, L.; Herwig, A.; et al. Development and characterization of an indirect ELISA to detect SARS-CoV-2 spike protein-specific antibodies. J. Immunol. Methods 2021, 490, 112958. [Google Scholar] [CrossRef]
- Horn, M.P.; Jonsdottir, H.R.; Brigger, D.; Damonti, L.; Suter-Riniker, F.; Endrich, O.; Froehlich, T.K.; Fiedler, M.; Largiadèr, C.R.; Marschall, J.; et al. Serological testing for SARS-CoV-2 antibodies in clinical practice: A comparative diagnostic accuracy study. Allergy 2022, 77, 2090–2103. [Google Scholar] [CrossRef]
- Zeng, X.; Li, B.; Liu, R.; Li, X.; Zhu, T. Investigation of promotion effect of Cu doped MnO2 catalysts on ketone-type VOCs degradation in a one-stage plasma-catalysis system. Chem. Eng. J. 2020, 384, 123362. [Google Scholar] [CrossRef]

| Chemical Contents | Binding Energy (eV) | MPP (%) |
|---|---|---|
| C (at. %) | 284.6 | 83.97% |
| O (at. %) | 532.64 | 12.81% |
| N (at. %) | 400.19 | 3.22% |
| O/C (%) | − | 3.98 |
| C−C | 284.6 | 85.18% |
| C−O | 285.7 | 10.25% |
| O−C=O | 289.8 | 3.20% |
| C=O | 287.8 | 1.37% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aikeremu, N.; Li, S.; Xu, Q.; Yuan, H.; Lu, K.; Si, J.; Yang, D. High-Efficiency Adsorption of SARS-CoV-2 Spike 1 Protein by Plasma-Modified Porous Polymers. Appl. Sci. 2022, 12, 12628. https://doi.org/10.3390/app122412628
Aikeremu N, Li S, Xu Q, Yuan H, Lu K, Si J, Yang D. High-Efficiency Adsorption of SARS-CoV-2 Spike 1 Protein by Plasma-Modified Porous Polymers. Applied Sciences. 2022; 12(24):12628. https://doi.org/10.3390/app122412628
Chicago/Turabian StyleAikeremu, Nigala, Sisi Li, Qingnan Xu, Hao Yuan, Ke Lu, Junqiang Si, and Dezheng Yang. 2022. "High-Efficiency Adsorption of SARS-CoV-2 Spike 1 Protein by Plasma-Modified Porous Polymers" Applied Sciences 12, no. 24: 12628. https://doi.org/10.3390/app122412628





