Anti-Breast Cancer Activity of Essential Oil: A Systematic Review
Abstract
1. Introduction
2. Methodology
3. Result
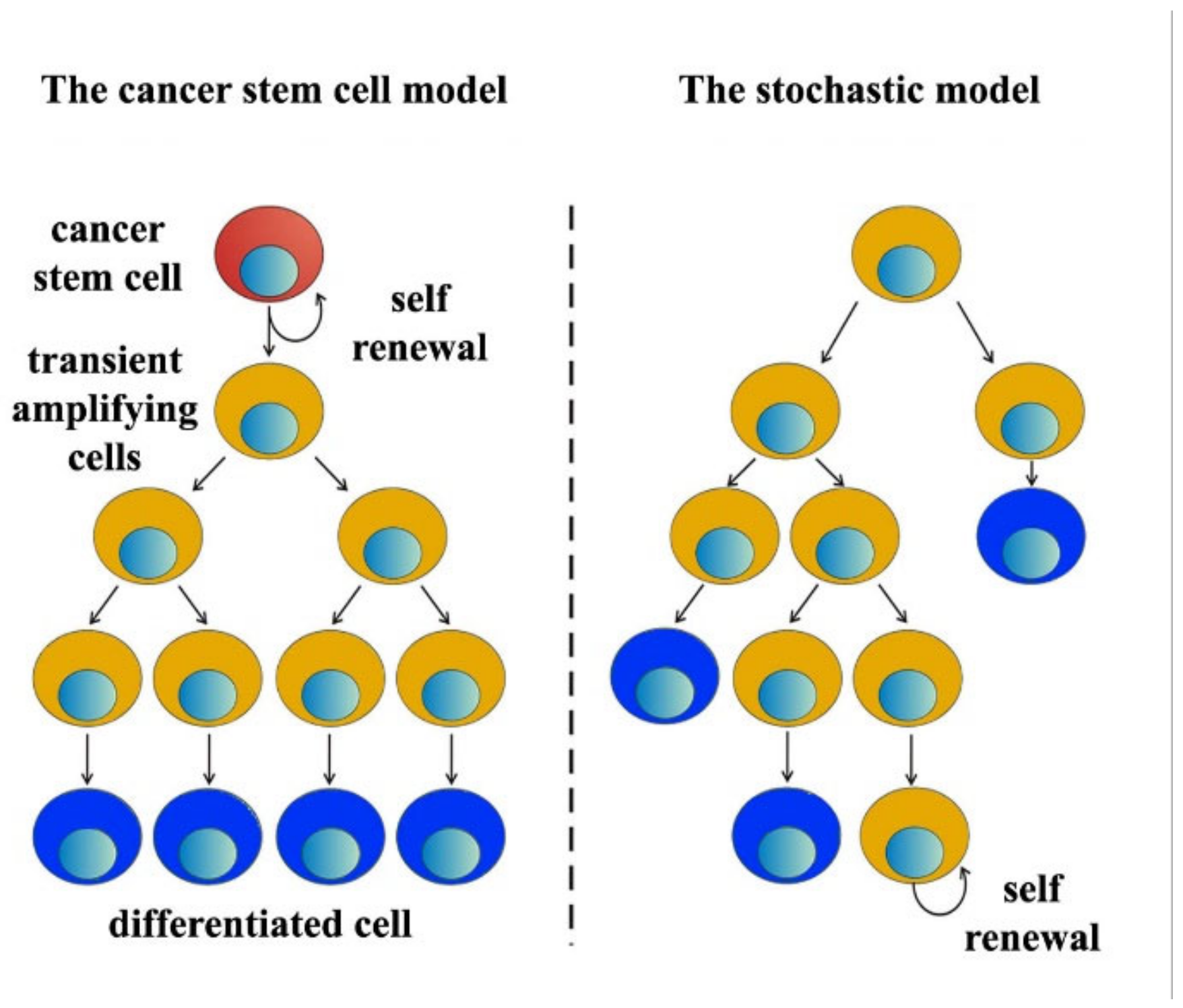
3.1. Breast Cancer
3.2. Essential Oil
3.2.1. Classification Based on the Method of Extraction
- a.
- Steam Distillation
- b.
- Cold Pressing Method
- c.
- Extraction Using Solvent
- d.
- Microwave-Assisted Hydrodistillation (MAHD)
- e.
- Ohmic-Assisted Hydrodistillation (OAHD)
3.2.2. Classification Based on Contents
- a.
- Terpenes
- b.
- Monoterpenes
- c.
- Sesquiterpene
3.3. Bioactivity of Essential Oils as Cancer Agents
3.3.1. Prostate Cancer
3.3.2. Glioblastoma
3.3.3. Colon Cancer
3.3.4. Liver Cancer
3.3.5. Uterus and Cervix Cancer
3.3.6. Lung Cancer
3.3.7. Leukimia
3.4. Bioactivity of Essential Oils as Anti-Breast Cancer Agents
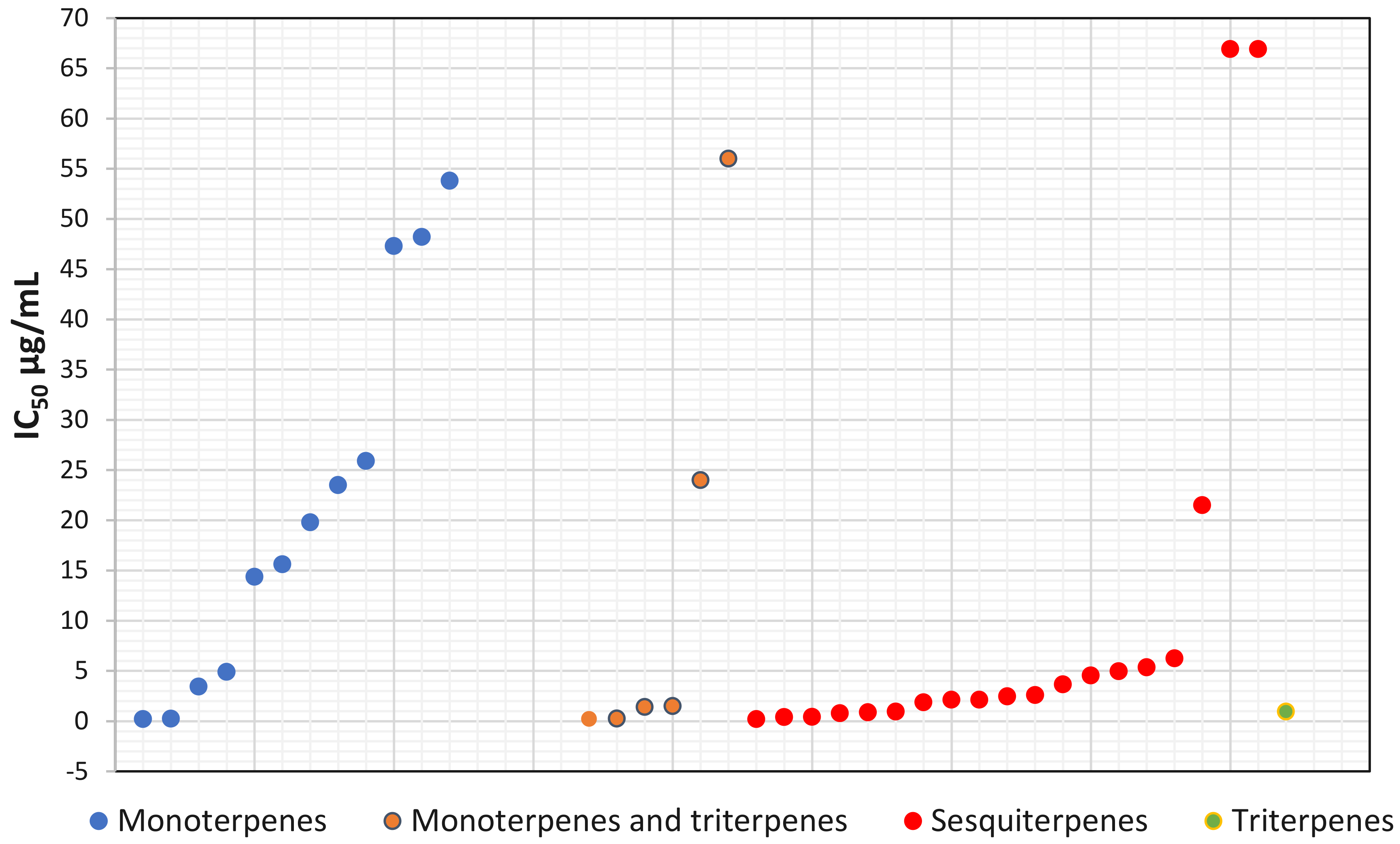
3.4.1. Monoterpenes
3.4.2. Sesquiterpene
3.4.3. Triterpenes
3.4.4. Diterpenes
3.4.5. Other Terpenes
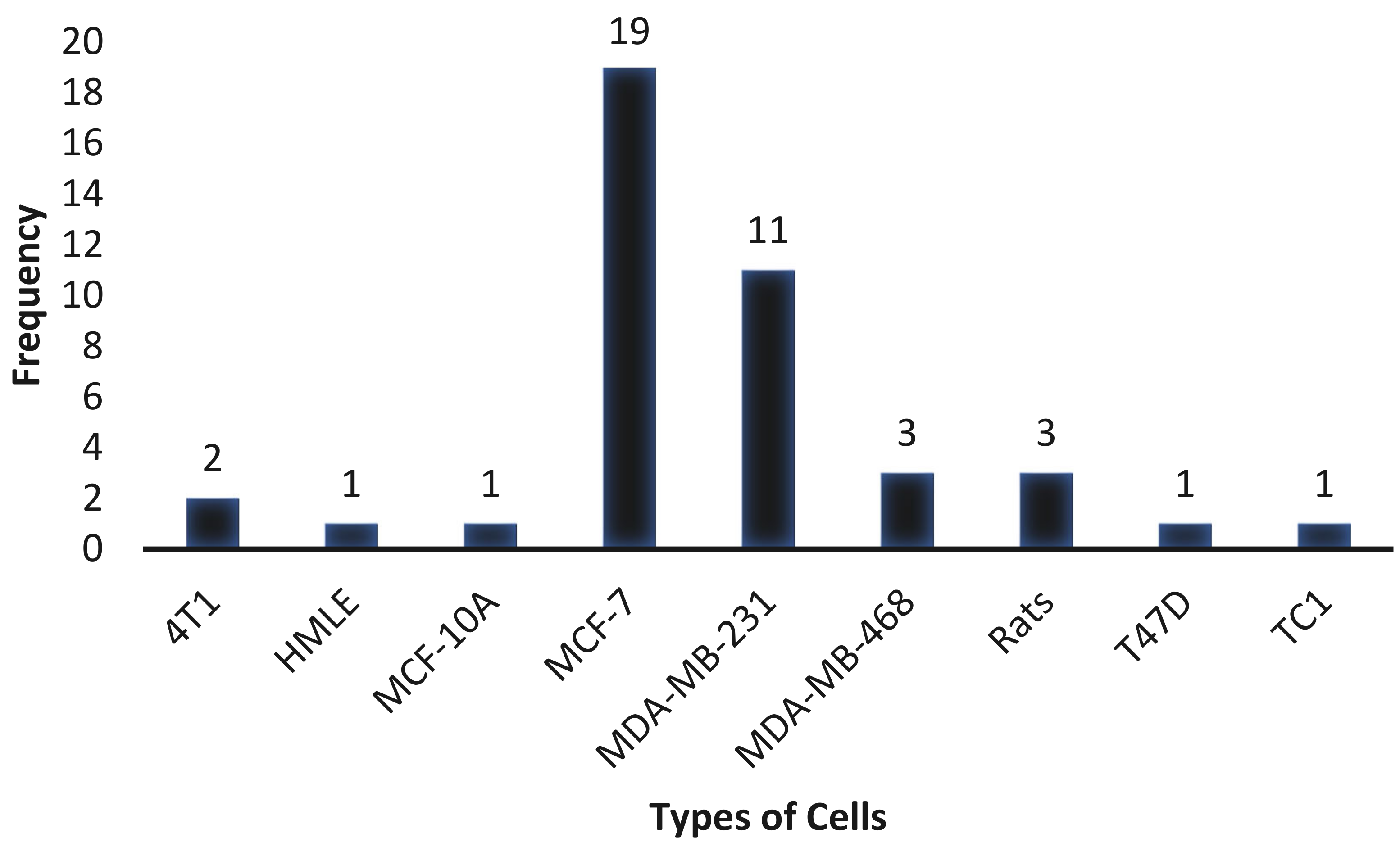
3.5. Types of Cells Used in Cytotoxic Studies
3.6. Clinical Trials of Essential Oils for Breast Cancer
3.7. Toxicity and Side Effect of Essential Oils
4. Author Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hortobagyi, G.N.; de la Garza Salazar, J.; Pritchard, K.; Amadori, D.; Haidinger, R.; Hudis, C.A.; Khaled, H.; Liu, M.-C.; Martin, M.; Namer, M. The global breast cancer burden: Variations in epidemiology and survival. Clin. Breast Cancer 2005, 6, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Zendehdel, M.; Niakan, B.; Keshtkar, A.; Rafiei, E.; Salamat, F. Subtypes of benign breast disease as a risk factor for breast cancer: A systematic review and meta-analysis protocol. Iran. J. Med. Sci. 2018, 43, 1. [Google Scholar] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide; IARC: Lyon, France, 2012. [Google Scholar]
- Fasching, P.A.; Ekici, A.B.; Adamietz, B.R.; Wachter, D.L.; Hein, A.; Bayer, C.M.; Häberle, L.; Loehberg, C.R.; Jud, S.M.; Heusinger, K.; et al. Breast Cancer Risk—Genes, Environment and Clinics. Geburtshilfe Frauenheilkd 2011, 71, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Youlden, D.R.; Cramb, S.M.; Yip, C.H.; Baade, P.D. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol. Med. 2014, 11, 101. [Google Scholar] [PubMed]
- Gnant, M.; Harbeck, N.; Thomssen, C. St. Gallen/Vienna 2017: A Brief Summary of the Consensus Discussion about Escalation and De-Escalation of Primary Breast Cancer Treatment. Breast Care 2017, 12, 102–107. [Google Scholar] [CrossRef]
- Han, S.-J.; Guo, Q.-Q.; Wang, T.; Wang, Y.-X.; Zhang, Y.-X.; Liu, F.; Luo, Y.-X.; Zhang, J.; Wang, Y.-L.; Yan, Y.-X. Prognostic significance of interactions between ER alpha and ER beta and lymph node status in breast cancer cases. Asian Pac. J. Cancer Prev. 2013, 14, 6081–6084. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Gautama, W. Breast Cancer in Indonesia in 2022: 30 Years of Marching in Place. Indones. J. Cancer 2022, 16, 2. [Google Scholar] [CrossRef]
- Sledge, G.W.; Mamounas, E.P.; Hortobagyi, G.N.; Burstein, H.J.; Goodwin, P.J.; Wolff, A.C. Past, present, and future challenges in breast cancer treatment. J. Clin. Oncol. 2014, 32, 1979–1986. [Google Scholar] [CrossRef]
- Jatoi, I.; Sung, H.; Jemal, A. The Emergence of the Racial Disparity in U.S. Breast-Cancer Mortality. N. Engl. J. Med. 2022, 386, 2349–2352. [Google Scholar] [CrossRef]
- Tran, N.; Pham, B.; Le, L. Bioactive compounds in anti-diabetic plants: From herbal medicine to modern drug discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef]
- Privitera, G.; Luca, T.; Castorina, S.; Passanisi, R.; Ruberto, G.; Napoli, E. Anticancer activity of Salvia officinalis essential oil and its principal constituents against hormone-dependent tumour cells. Asian Pac. J. Trop. Biomed. 2019, 9, 24–28. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Fitsiou, E.; Bouloukosta, E.; Tiptiri-Kourpeti, A.; Vamvakias, M.; Oreopoulou, A.; Papavassilopoulou, E.; Pappa, A.; Chlichlia, K. Extraction, Chemical Composition, and Anticancer Potential of Origanum onites L. Essential Oil. Molecules 2019, 24, 2612. [Google Scholar] [CrossRef]
- Rajivgandhi, G.; Saravanan, K.; Ramachandran, G.; Li, J.-L.; Yin, L.; Quero, F.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Manoharan, N. Enhanced anti-cancer activity of chitosan loaded Morinda citrifolia essential oil against A549 human lung cancer cells. Int. J. Biol. Macromol. 2020, 164, 4010–4021. [Google Scholar] [CrossRef]
- Asif, M.; Yehya, A.H.; Dahham, S.S.; Mohamed, S.K.; Shafaei, A.; Ezzat, M.O.; Majid, A.S.A.; Oon, C.E.; Majid, A.M.S.A. Establishment of in vitro and in vivo anti-colon cancer efficacy of essential oils containing oleo-gum resin extract of Mesua ferrea. Biomed. Pharmacother. 2019, 109, 1620–1629. [Google Scholar] [CrossRef]
- Rattanamaneerusmee, A.; Thirapanmethee, K.; Nakamura, Y.; Chomnawang, M. Differentiation-inducing effect in human colon cancer cells of essential oils. Pharm. Sci. Asia 2018, 45, 154–160. [Google Scholar] [CrossRef]
- Rezaie-Tavirani, M.; Fayazfar, S.; Heydari-Keshel, S.; Rezaee, M.B.; Zamanian-Azodi, M.; Rezaei-Tavirani, M.; Khodarahmi, R. Effect of essential oil of Rosa Damascena on human colon cancer cell line SW742. Gastroenterol. Hepatol. Bed. Bench. 2013, 6, 25–31. [Google Scholar]
- Zare, E.; Jamali, T.; Ardestani, S.K.; Kavoosi, G. Synergistic effect of Zataria Multiflora essential oil on doxorubicin-induced growth inhibition of PC3 cancer cells and apoptosis. Complement. Ther. Clin. Pract. 2021, 42, 101286. [Google Scholar] [CrossRef]
- Russo, A.; Cardile, V.; Graziano, A.C.E.; Avola, R.; Bruno, M.; Rigano, D. Involvement of Bax and Bcl-2 in Induction of Apoptosis by Essential Oils of Three Lebanese Salvia Species in Human Prostate Cancer Cells. Int. J. Mol. Sci. 2018, 19, 292. [Google Scholar] [CrossRef]
- Khanavi, M.; Enayati, A.; Shams Ardekani, M.; Akbarzadeh, T.; Karimpour Razkenari, E.; Eftekhari, M. Cytotoxic activity of Juniperus excelsa M. Bieb. Leaves Essent. Oil Breast Cancer Cell Lines. Res. J. Pharm. 2019, 6, 1–7. [Google Scholar]
- Nguyen, T.K.; Le Nguyen, T.N.; Nguyen, K.; Nguyen, H.V.; Tran, L.T.; Ngo, T.X.; Pham, P.T.; Tran, M.H. Machine learning-based screening of MCF-7 human breast cancer cells and molecular docking analysis of essential oils from Ocimum basilicum against breast cancer. J. Mol. Struct. 2022, 1268, 133627. [Google Scholar] [CrossRef]
- Ruttanapattanakul, J.; Wikan, N.; Chinda, K.; Jearanaikulvanich, T.; Krisanuruks, N.; Muangcha, M.; Okonogi, S.; Potikanond, S.; Nimlamool, W. Essential Oil from Zingiber ottensii Induces Human Cervical Cancer Cell Apoptosis and Inhibits MAPK and PI3K/AKT Signaling Cascades. Plants 2021, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.A.; Avanço, G.B.; Nerilo, S.B.; Marcelino, R.I.; Janeiro, V.; Valadares, M.C.; Machinski, M. Assessment of Cytotoxic Activity of Rosemary (Rosmarinus officinalis L.), Turmeric (Curcuma longa L.), and Ginger (Zingiber officinale R.) Essential Oils in Cervical Cancer Cells (HeLa). Sci. World J. 2016, 2016, 9273078. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, L.H.; Kastan, M.B. Cell cycle control and cancer. Science 1994, 266, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Dumars, C.; Ngyuen, J.-M.; Gaultier, A.; Lanel, R.; Corradini, N.; Gouin, F.; Heymann, D.; Heymann, M.-F. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget 2016, 7, 78343. [Google Scholar] [CrossRef]
- Maffini, M.V.; Soto, A.M.; Calabro, J.M.; Ucci, A.A.; Sonnenschein, C. The stroma as a crucial target in rat mammary gland carcinogenesis. J. Cell Sci. 2004, 117, 1495–1502. [Google Scholar] [CrossRef]
- Sgroi, D.C. Preinvasive breast cancer. Annu. Rev. Pathol. 2010, 5, 193. [Google Scholar] [CrossRef]
- Harris, L.N.; Ismaila, N.; McShane, L.M.; Andre, F.; Collyar, D.E.; Gonzalez-Angulo, A.M.; Hammond, E.H.; Kuderer, N.M.; Liu, M.C.; Mennel, R.G. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 1134. [Google Scholar] [CrossRef]
- Ziperstein, M.J.; Guzman, A.; Kaufman, L.J. Evaluating breast cancer cell morphology as a predictor of invasive capacity. Biophys. J. 2016, 110, 621a. [Google Scholar] [CrossRef]
- West, A.-K.V.; Wullkopf, L.; Christensen, A.; Leijnse, N.; Tarp, J.M.; Mathiesen, J.; Erler, J.T.; Oddershede, L.B. Division induced dynamics in non-Invasive and invasive breast cancer. Biophys. J. 2017, 112, 123a. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Khan, G.S.; Mushtaq, Z.; Zubair, M. Essential Oils. In Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Malik, S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–17. [Google Scholar] [CrossRef]
- Shasby, G. Erratum. Inhibition of motility by NEO100 through the calpain-1/RhoA pathway. J. Neurosurg. 2019, 133, 1262. [Google Scholar] [CrossRef]
- Antonioli, G.; Fontanella, G.; Echeverrigaray, S.; Delamare, A.P.L.; Pauletti, G.F.; Barcellos, T. Poly (lactic acid) nanocapsules containing lemongrass essential oil for postharvest decay control: In vitro and in vivo evaluation against phytopathogenic fungi. Food Chem. 2020, 326, 126997. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, Y.; Liu, Y.; Cui, B. Physicochemical characterization and antibacterial activity assessment of lavender essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Ind. Crops Prod. 2019, 130, 104–110. [Google Scholar] [CrossRef]
- Lapkina, E.; Zaharova, T.; Tirranen, L. Component composition of essential oil of Artemisia salsoloides willd and its antimicrobial properties. Chem. Plant Raw Mater. 2017, 3, 157–162. [Google Scholar] [CrossRef]
- Jamali, T.; Kavoosi, G.; Ardestani, S.K. In-vitro and in-vivo anti-breast cancer activity of OEO (Oliveria decumbens vent essential oil) through promoting the apoptosis and immunomodulatory effects. J. Ethnopharmacol. 2020, 248, 112313. [Google Scholar] [CrossRef]
- Karakaya, S.; Koca, M.; Yılmaz, S.V.; Yıldırım, K.; Pınar, N.M.; Demirci, B.; Brestic, M.; Sytar, O. Molecular docking studies of coumarins isolated from extracts and essential oils of zosima absinthifolia link as potential inhibitors for Alzheimer’s disease. Molecules 2019, 24, 722. [Google Scholar] [CrossRef]
- Oboh, G.; Olasehinde, T.A.; Ademosun, A.O. Essential oil from lemon peels inhibit key enzymes linked to neurodegenerative conditions and pro-oxidant induced lipid peroxidation. J. Oleo Sci. 2014, 63, 373–381. [Google Scholar] [CrossRef]
- Cassel, E.; Vargas, R.M.F.; Martinez, N.; Lorenzo, D.; Dellacassa, E. Steam distillation modeling for essential oil extraction process. Ind. Crops Prod. 2009, 29, 171–176. [Google Scholar] [CrossRef]
- Božović, M.; Navarra, A.; Garzoli, S.; Pepi, F.; Ragno, R. Esential oils extraction: A 24-hour steam distillation systematic methodology. Nat. Prod. Res. 2017, 31, 2387–2396. [Google Scholar] [CrossRef]
- Masango, P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005, 13, 833–839. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Diantini, A.; Subarnas, A. Analysis of Indonesian Spice Essential Oil Compounds That Inhibit Locomotor Activity in Mice. Pharmaceuticals 2011, 4, 590–602. [Google Scholar] [CrossRef]
- Flodin, C.; Helidoniotis, F.; Whitfield, F.B. Seasonal variation in bromophenol content andbromoperoxidase activity in Ulva lactuca. Phytochemistry 1999, 51, 135–138. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Wang, W.; Zhao, X.; Zhang, J.; Ewart, S.H. Properties of polysaccharides in several seaweeds from Atlantic Canada and their potential anti-influenza viral activities. J. Ocean Univ. China 2012, 11, 205–212. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Meklati, B.Y.; Chemat, F. Comparison of different isolation methods of essential oil from Citrus fruits: Cold pressing, hydrodistillation and microwave ‘dry’ distillation. Flavour Fragr. J. 2007, 22, 494–504. [Google Scholar] [CrossRef]
- Aladić, K.; Jokić, S.; Moslavac, T.; Tomas, S.; Vidović, S.; Vladić, J.; Šubarić, D. Cold pressing and supercritical CO2 extraction of hemp (Cannabis sativa) seed oil. Chem. Biochem. Eng. Q. 2014, 28, 481–490. [Google Scholar] [CrossRef]
- Ferhat, M.-A.; Boukhatem, M.N.; Hazzit, M.; Meklati, B.Y.; Chemat, F. Cold pressing, hydrodistillation and microwave dry distillation of citrus essential oil from Algeria: A comparative study. Electron. J. Biol. S 2016, 1, 30–41. [Google Scholar]
- Lu-Martínez, A.A.; Báez-González, J.G.; Castillo-Hernández, S.; Amaya-Guerra, C.; Rodríguez-Rodríguez, J.; García-Márquez, E. Studied of Prunus serotine oil extracted by cold pressing and antioxidant effect of P. longiflora essential oil. J. Food Sci. Technol. 2021, 58, 1420–1429. [Google Scholar] [CrossRef]
- Mehraban, M.S.A.; Shirzad, M.; Ahmadian-Attari, M.M.; Shakeri, R.; Kashani, L.M.T.; Tabarrai, M.; Shirbeigi, L. Effect of rose oil on gastroesophageal reflux disease in comparison with omeprazole: A double-blind controlled trial. Complement. Ther. Clin. Pract. 2021, 43, 101361. [Google Scholar] [CrossRef]
- Avila, R.; Santos, S.; Araujo, D.; Vidal, V.; Macêdo, J. Semantic Links Using SKOS Predicates. Procedia Comput. Sci. 2017, 112, 467–473. [Google Scholar] [CrossRef]
- Moradi, S.; Fazlali, A.; Hamedi, H. Microwave-Assisted Hydro-Distillation of Essential Oil from Rosemary: Comparison with Traditional Distillation. Avicenna J. Med. Biotechnol. 2018, 10, 22–28. [Google Scholar]
- Gavahian, M.; Farahnaky, A. Ohmic-assisted hydrodistillation technology: A review. Trends Food Sci. Technol. 2018, 72, 153–161. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. Does antioxidant properties of the main component of essential oil reflect its antioxidant properties? The comparison of antioxidant properties of essential oils and their main components. Nat. Prod. Res. 2014, 28, 1952–1963. [Google Scholar] [CrossRef]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and medicinal uses of terpenes. In Medicinal Plants 2019; Springer: Cham, Switzerland, 2019; pp. 333–359. [Google Scholar]
- Blank, P.N.; Shinsky, S.A.; Christianson, D.W. Structure of sesquisabinene synthase 1, a terpenoid cyclase that generates a strained [3.1. 0] bridged-bicyclic product. ACS Chem. Biol. 2019, 14, 1011–1019. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and their derivatives—Recent development in biological and medical applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Rostro-Alanis, M.d.J.; Báez-González, J.; Torres-Alvarez, C.; Parra-Saldívar, R.; Rodriguez-Rodriguez, J.; Castillo, S. Chemical composition and biological activities of oregano essential oil and its fractions obtained by vacuum distillation. Molecules 2019, 24, 1904. [Google Scholar] [CrossRef]
- Saleh, A.M.; Al-Qudah, M.A.; Nasr, A.; Rizvi, S.A.; Borai, A.; Daghistani, M. Comprehensive analysis of the chemical composition and in vitro cytotoxic mechanisms of Pallines spinosa flower and leaf essential oils against breast cancer cells. Cell. Physiol. Biochem. 2017, 42, 2043–2065. [Google Scholar] [CrossRef]
- Wu, M.; Li, T.; Chen, L.; Peng, S.; Liao, W.; Bai, R.; Zhao, X.; Yang, H.; Wu, C.; Zeng, H. Essential oils from Inula japonica and Angelicae dahuricae enhance sensitivity of MCF-7/ADR breast cancer cells to doxorubicin via multiple mechanisms. J. Ethnopharmacol. 2016, 180, 18–27. [Google Scholar] [CrossRef]
- Matejić, J.; Šarac, Z.; Ranđelović, V. Pharmacological activity of sesquiterpene lactones. Biotechnol. Biotechnol. Equip. 2010, 24, 95–100. [Google Scholar] [CrossRef]
- Yu, Y.P.; Landsittel, D.; Jing, L.; Nelson, J.; Ren, B.; Liu, L.; McDonald, C.; Thomas, R.; Dhir, R.; Finkelstein, S.; et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J. Clin. Oncol. 2004, 22, 2790–2799. [Google Scholar] [CrossRef]
- Yousuf Dar, M.; Shah, W.A.; Mubashir, S.; Rather, M.A. Chromatographic analysis, anti-proliferative and radical scavenging activity of Pinus wallichina essential oil growing in high altitude areas of Kashmir, India. Phytomedicine 2012, 19, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, E.H.; Ko, S.R.; Choi, K.J.; Park, J.H.; Im, D.S. Effects of ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer cells. Arch. Pharm. Res. 2004, 27, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Do N Fontes, J.E.; Ferraz, R.P.; Britto, A.C.; Carvalho, A.A.; Moraes, M.O.; Pessoa, C.; Costa, E.V.; Bezerra, D.P. Antitumor effect of the essential oil from leaves of Guatteria pogonopus (Annonaceae). Chem. Biodivers. 2013, 10, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Ashraf, M.; Gilani, A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 2010, 90, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Jena, L.; McErlean, E.; McCarthy, H. Delivery across the blood-brain barrier: Nanomedicine for glioblastoma multiforme. Drug Deliv. Transl. Res. 2020, 10, 304–318. [Google Scholar] [CrossRef]
- Buckle, J. Use of aromatherapy as a complementary treatment for chronic pain. Altern. Health Med. 1999, 5, 42–51. [Google Scholar]
- Quassinti, L.; Lupidi, G.; Maggi, F.; Sagratini, G.; Papa, F.; Vittori, S.; Bianco, A.; Bramucci, M. Antioxidant and antiproliferative activity of Hypericum hircinum L. subsp. majus (Aiton) N. Robson essential oil. Nat. Prod. Res. 2013, 27, 862–868. [Google Scholar] [CrossRef]
- Bayala, B.; Bassole, I.H.; Gnoula, C.; Nebie, R.; Yonli, A.; Morel, L.; Figueredo, G.; Nikiema, J.B.; Lobaccaro, J.M.; Simpore, J. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS ONE 2014, 9, e92122. [Google Scholar] [CrossRef]
- Tangrea, J.; Helzlsouer, K.; Pietinen, P.; Taylor, P.; Hollis, B.; Virtamo, J.; Albanes, D. Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes Control 1997, 8, 615–625. [Google Scholar] [CrossRef]
- Carnesecchi, S.; Schneider, Y.; Ceraline, J.; Duranton, B.; Gosse, F.; Seiler, N.; Raul, F. Geraniol, a component of plant essential oils, inhibits growth and polyamine biosynthesis in human colon cancer cells. J. Pharm. Exp. 2001, 298, 197–200. [Google Scholar]
- Rasoanaivo, P.; Fortuné Randriana, R.; Maggi, F.; Nicoletti, M.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Papa, F.; et al. Chemical composition and biological activities of the essential oil of Athanasia brownii Hochr. (Asteraceae) endemic to Madagascar. Chem. Biodivers. 2013, 10, 1876–1886. [Google Scholar] [CrossRef]
- Murata, S.; Shiragami, R.; Kosugi, C.; Tezuka, T.; Yamazaki, M.; Hirano, A.; Yoshimura, Y.; Suzuki, M.; Shuto, K.; Ohkohchi, N.; et al. Antitumor effect of 1, 8-cineole against colon cancer. Oncol. Rep. 2013, 30, 2647–2652. [Google Scholar] [CrossRef]
- Akrout, A.; Gonzalez, L.A.; El Jani, H.; Madrid, P.C. Antioxidant and antitumor activities of Artemisia campestris and Thymelaea hirsuta from southern Tunisia. Food Chem. Toxicol. 2011, 49, 342–347. [Google Scholar] [CrossRef]
- El-Najjar, N.; Chatila, M.; Moukadem, H.; Vuorela, H.; Ocker, M.; Gandesiri, M.; Schneider-Stock, R.; Gali-Muhtasib, H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis 2010, 15, 183–195. [Google Scholar] [CrossRef]
- Zhao, M.; Bu, Y.; Feng, J.; Zhang, H.; Chen, Y.; Yang, G.; Liu, Z.; Yuan, H.; Yuan, Y.; Liu, L.; et al. SPIN1 triggers abnormal lipid metabolism and enhances tumor growth in liver cancer. Cancer Lett. 2020, 470, 54–63. [Google Scholar] [CrossRef]
- Wu, S.; Wei, F.X.; Li, H.Z.; Liu, X.G.; Zhang, J.H.; Liu, J.X. Chemical composition of essential oil from Thymus citriodorus and its toxic effect on liver cancer cells. Zhong Yao Cai 2013, 36, 756–759. [Google Scholar]
- Paik, S.Y.; Koh, K.H.; Beak, S.M.; Paek, S.H.; Kim, J.A. The essential oils from Zanthoxylum schinifolium pericarp induce apoptosis of HepG2 human hepatoma cells through increased production of reactive oxygen species. Biol. Pharm. Bull. 2005, 28, 802–807. [Google Scholar] [CrossRef]
- Su, Y.C.; Hsu, K.P.; Wang, E.I.; Ho, C.L. Composition and in vitro anticancer activities of the leaf essential oil of Neolitsea variabillima from Taiwan. Nat. Prod. Commun. 2013, 8, 531–532. [Google Scholar] [CrossRef]
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. J. Natl. Cancer Inst. 2018, 110, 354–361. [Google Scholar] [CrossRef]
- Bou, D.D.; Lago, J.H.; Figueiredo, C.R.; Matsuo, A.L.; Guadagnin, R.C.; Soares, M.G.; Sartorelli, P. Chemical composition and cytotoxicity evaluation of essential oil from leaves of Casearia sylvestris, its main compound α-zingiberene and derivatives. Molecules 2013, 18, 9477–9487. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Eid, H.H.; Ashour, M.L.; Eid, S.Y.; Labib, R.M.; Sporer, F.; Wink, M. Variations of the chemical composition and bioactivity of essential oils from leaves and stems of Liquidambar styraciflua (Altingiaceae). J. Pharm. Pharm. 2013, 65, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Zheng, Y.P.; Lin, D.H.; Zhang, H.; Zhao, F.; Yuan, C.S. Potential anti-cancer activities of Furanodiene, a Sesquiterpene from Curcuma wenyujin. Am. J. Chin. Med. 2009, 37, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.P.; Cardoso, G.M.; Da Silva, T.B.; Fontes, J.E.; Prata, A.P.; Carvalho, A.A.; Moraes, M.O.; Pessoa, C.; Costa, E.V.; Bezerra, D.P. Antitumour properties of the leaf essential oil of Xylopia frutescens Aubl. (Annonaceae). Food Chem. 2013, 141, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Manjamalai, A.; Kumar, M.J.; Grace, V.M. Essential oil of Tridax procumbens L. induces apoptosis and suppresses angiogenesis and lung metastasis of the B16F-10 cell line in C57BL/6 mice. Asian Pac. J. Cancer Prev. 2012, 13, 5887–5895. [Google Scholar] [CrossRef] [PubMed]
- Seal, S.; Chatterjee, P.; Bhattacharya, S.; Pal, D.; Dasgupta, S.; Kundu, R.; Mukherjee, S.; Bhattacharya, S.; Bhuyan, M.; Bhattacharyya, P.R.; et al. Vapor of volatile oils from Litsea cubeba seed induces apoptosis and causes cell cycle arrest in lung cancer cells. PLoS ONE 2012, 7, e47014. [Google Scholar] [CrossRef]
- Keawsa-ard, S.; Liawruangrath, B.; Liawruangrath, S.; Teerawutgulrag, A.; Pyne, S.G. Chemical constituents and antioxidant and biological activities of the essential oil from leaves of Solanum spirale. Nat. Prod. Commun. 2012, 7, 955–958. [Google Scholar] [CrossRef]
- Jacob, E.A. Complete Blood Cell Count and Peripheral Blood Film, Its Significant in Laboratory Medicine: A Review Study. Am. J. Lab. Med. 2016, 1, 34–57. [Google Scholar]
- Rashid, S.; Rather, M.A.; Shah, W.A.; Bhat, B.A. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef]
- Saab, A.M.; Guerrini, A.; Sacchetti, G.; Maietti, S.; Zeino, M.; Arend, J.; Gambari, R.; Bernardi, F.; Efferth, T. Phytochemical analysis and cytotoxicity towards multidrug-resistant leukemia cells of essential oils derived from Lebanese medicinal plants. Planta Med. 2012, 78, 1927–1931. [Google Scholar] [CrossRef]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Incorporation of Zataria multiflora essential oil into chitosan biopolymer nanoparticles: A nanoemulsion based delivery system to improve the in-vitro efficacy, stability and anticancer activity of ZEO against breast cancer cells. Int. J. Biol. Macromol. 2020, 143, 382–392. [Google Scholar] [CrossRef]
- Azadi, M.; Jamali, T.; Kianmehr, Z.; Kavoosi, G.; Ardestani, S.K. In-vitro (2D and 3D cultures) and in-vivo cytotoxic properties of Zataria multiflora essential oil (ZEO) emulsion in breast and cervical cancer cells along with the investigation of immunomodulatory potential. J. Ethnopharmacol. 2020, 257, 112865. [Google Scholar] [CrossRef]
- Rojas-Armas, J.P.; Arroyo-Acevedo, J.L.; Palomino-Pacheco, M.; Herrera-Calderón, O.; Ortiz-Sánchez, J.M.; Rojas-Armas, A.; Calva, J.; Castro-Luna, A.; Hilario-Vargas, J. The essential oil of Cymbopogon citratus stapt and carvacrol: An approach of the antitumor effect on 7, 12-dimethylbenz-[α]-anthracene (DMBA)-induced breast cancer in female rats. Molecules 2020, 25, 3284. [Google Scholar] [CrossRef]
- Namshir, J.; Shatar, A.; Khandaa, O.; Tserennadmid, R.; Shiretorova, V.G.; Nguyen, M.C. Antimicrobial, antioxidant and cytotoxic activity on human breast cancer cells of essential oil from Pinus sylvestris. var mongolica needle. Mong. J. Chem. 2020, 21, 19–26. [Google Scholar] [CrossRef]
- Xing, X.; Ma, J.-H.; Fu, Y.; Zhao, H.; Ye, X.-X.; Han, Z.; Jia, F.-J.; Li, X. Essential oil extracted from Erythrina corallodendron L. leaves inhibits the proliferation, migration, and invasion of breast cancer cells. Medicine 2019, 98, e17009. [Google Scholar] [CrossRef]
- Zito, P.; Labbozzetta, M.; Notarbartolo, M.; Sajeva, M.; Poma, P. Essential oil of Cyphostemma juttae (Vitaceae): Chemical composition and antitumor mechanism in triple negative breast cancer cells. PLoS ONE 2019, 14, e0214594. [Google Scholar] [CrossRef]
- El-Abid, H.; Amaral, C.; Cunha, S.C.; Augusto, T.V.; Fernandes, J.O.; Correia-da-Silva, G.; Teixeira, N.; Moumni, M. Chemical composition and anti-cancer properties of Juniperus oxycedrus L. essential oils on estrogen receptor-positive breast cancer cells. J. Funct. Foods 2019, 59, 261–271. [Google Scholar] [CrossRef]
- Bagheri, S.M.; Asl, A.A.; Shams, A.; Mirghanizadeh-Bafghi, S.A.; Hafizibarjin, Z. Evaluation of Cytotoxicity Effects of Oleo-Gum-Resin and Its Essential Oil of Ferula assa-foetida and Ferulic Acid on 4T1 Breast Cancer Cells. Indian J. Med. Paediatr. Oncol. 2017, 38, 116–120. [Google Scholar]
- Estanislao Gómez, C.; Aquino Carreño, A.; Pérez Ishiwara, D.; San Martín Martínez, E.; Morales López, J.; Pérez Hernández, N.; García, G. Decatropis bicolor (Zucc.) Radlk essential oil induces apoptosis of the MDA-MB-231 breast cancer cell line. BMC Complement. Altern. Med. 2016, 16, 266. [Google Scholar] [CrossRef]
- Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Anticancer activity of an ultrasonic nanoemulsion formulation of Nigella sativa L. essential oil on human breast cancer cells. Ultrason. Sonochem. 2016, 31, 449–455. [Google Scholar] [CrossRef]
- Yeo, S.K.; Ali, A.Y.; Hayward, O.A.; Turnham, D.; Jackson, T.; Bowen, I.D.; Clarkson, R. β-Bisabolene, a sesquiterpene from the essential oil extract of opoponax (Commiphora guidottii), exhibits cytotoxicity in breast cancer cell lines. Phytother. Res. 2016, 30, 418–425. [Google Scholar] [CrossRef]
- De Mel, Y.; Perera, S.; Ratnaweera, P.B.; Jayasinghe, C.D. Novel insights of toxicological evaluation of herbal medicine: Human based toxicological assays. Asian J. Pharm. Pharmacol. 2017, 3, 41–49. [Google Scholar]
- Lee, J.-H.; Lee, K.; Lee, D.H.; Shin, S.Y.; Yong, Y.; Lee, Y.H. Anti-invasive effect of β-myrcene, a component of the essential oil from Pinus koraiensis cones, in metastatic MDA-MB-231 human breast cancer cells. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 563–569. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Li, T.; Xu, J.; Ma, Y. A myrsinol diterpene isolated from a traditional herbal medicine, LANGDU reverses multidrug resistance in breast cancer cells. J. Ethnopharmacol. 2016, 194, 1–5. [Google Scholar] [CrossRef]
- Suhail, M.M.; Wu, W.; Cao, A.; Mondalek, F.G.; Fung, K.-M.; Shih, P.-T.; Fang, Y.-T.; Woolley, C.; Young, G.; Lin, H.-K. Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC Complement. Altern. Med. 2011, 11, 129. [Google Scholar] [CrossRef]
- Kumar, P.S.; Febriyanti, R.M.; Sofyan, F.F.; Luftimas, D.E.; Abdulah, R. Anticancer potential of Syzygium aromaticum L. in MCF-7 human breast cancer cell lines. Pharmacogn. Res. 2014, 6, 350. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Khaleghi, A.A.; Alipanah, H.; Zarenezhad, E.; Osanloo, M. Anticarcinogenic Effect of Chitosan Nanoparticles Containing Syzygium aromaticum Essential Oil or Eugenol toward Breast and Skin Cancer Cell Lines. BioNanoScience 2021, 11, 678–686. [Google Scholar] [CrossRef]
- Abu-Dahab, R.; Kasabri, V.; Afifi, F.U. Evaluation of the Volatile Oil Composition and Antiproliferative Activity of Laurus nobilis L. (Lauraceae) on Breast Cancer Cell Line Models. Rec. Nat. Prod. 2014, 8, 136–147. [Google Scholar]
- Yu, J.-Q.; Lei, J.-C.; Zhang, X.-Q.; Yu, H.-D.; Tian, D.-Z.; Liao, Z.-X.; Zou, G.-l. Anticancer, antioxidant and antimicrobial activities of the essential oil of Lycopus lucidus Turcz. var. hirtus Regel. Food Chem. 2011, 126, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Ashmawy, A.M.; Ayoub, I.M.; Eldahshan, O.A. Chemical composition, cytotoxicity and molecular profiling of Cordia africana Lam. on human breast cancer cell line. Nat. Prod. Res. 2021, 35, 4133–4138. [Google Scholar] [CrossRef]
- Ashmawy, A.; Mostafa, N.; Eldahshan, O. GC/MS analysis and molecular profiling of lemon volatile oil against breast cancer. J. Essent. Oil Bear. Plants 2019, 22, 903–916. [Google Scholar] [CrossRef]
- Nagappan, T.; Ramasamy, P.; Wahid, M.E.A.; Segaran, T.C.; Vairappan, C.S. Biological activity of carbazole alkaloids and essential oil of Murraya koenigii against antibiotic resistant microbes and cancer cell lines. Molecules 2011, 16, 9651–9664. [Google Scholar] [CrossRef]
- Afoulous, S.; Ferhout, H.; Raoelison, E.G.; Valentin, A.; Moukarzel, B.; Couderc, F.; Bouajila, J. Chemical composition and anticancer, antiinflammatory, antioxidant and antimalarial activities of leaves essential oil of Cedrelopsis grevei. Food Chem. Toxicol. 2013, 56, 352–362. [Google Scholar] [CrossRef]
- Hamzeloo-Moghadam, M.; Aghaei, M.; Fallahian, F.; Jafari, S.M.; Dolati, M.; Abdolmohammadi, M.H.; Hajiahmadi, S.; Esmaeili, S. Britannin, a sesquiterpene lactone, inhibits proliferation and induces apoptosis through the mitochondrial signaling pathway in human breast cancer cells. Tumor Biol. 2015, 36, 1191–1198. [Google Scholar] [CrossRef]
- Karimian, H.; Fadaeinasab, M.; Moghadamtousi, S.Z.; Hajrezaei, M.; Zahedifard, M.; Razavi, M.; Safi, S.Z.; Mohan, S.; Khalifa, S.A.; El-Seedi, H.R. The chemopreventive effect of Tanacetum polycephalum against LA7-induced breast cancer in rats and the apoptotic effect of a cytotoxic sesquiterpene lactone in MCF7 cells: A bioassay-guided approach. Cell. Physiol. Biochem. 2015, 36, 988–1003. [Google Scholar] [CrossRef]
- Fallahian, F.; Aghaei, M.; Abdolmohammadi, M.H.; Hamzeloo-Moghadam, M. Molecular mechanism of apoptosis induction by Gaillardin, a sesquiterpene lactone, in breast cancer cell lines. Cell Biol. Toxicol. 2015, 31, 295–305. [Google Scholar] [CrossRef]
- Nakagawa-Goto, K.; Chen, J.-Y.; Cheng, Y.-T.; Lee, W.-L.; Takeya, M.; Saito, Y.; Lee, K.-H.; Shyur, L.-F. Novel sesquiterpene lactone analogues as potent anti-breast cancer agents. Mol. Oncol. 2016, 10, 921–937. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, Y.; Lou, C.; Zhao, H. Eupalinolide O, a novel sesquiterpene lactone from Eupatorium lindleyanum DC., induces cell cycle arrest and apoptosis in human MDA-MB-468 breast cancer cells. Oncol. Rep. 2016, 36, 2807–2813. [Google Scholar] [CrossRef]
- Pragna Lakshmi, T.; Vajravijayan, S.; Moumita, M.; Sakthivel, N.; Gunasekaran, K.; Krishna, R. A novel guaiane sesquiterpene derivative, guai-2-en-10α-ol, from Ulva fasciata Delile inhibits EGFR/PI3K/Akt signaling and induces cytotoxicity in triple-negative breast cancer cells. Mol. Cell. Biochem. 2018, 438, 123–139. [Google Scholar] [CrossRef]
- Salehi, F.; Jamali, T.; Kavoosi, G.; Ardestani, S.K.; Vahdati, S.N. Stabilization of Zataria essential oil with pectin-based nanoemulsion for enhanced cytotoxicity in monolayer and spheroid drug-resistant breast cancer cell cultures and deciphering its binding mode with gDNA. Int. J. Biol. Macromol. 2020, 164, 3645–3655. [Google Scholar] [CrossRef]
- Abedinpour, N.; Ghanbariasad, A.; Taghinezhad, A.; Osanloo, M. Preparation of nanoemulsions of mentha piperita essential oil and investigation of their cytotoxic effect on human breast cancer lines. BioNanoScience 2021, 11, 428–436. [Google Scholar] [CrossRef]
- El Gaafary, M.; Hafner, S.; Lang, S.J.; Jin, L.; Sabry, O.M.; Vogel, C.V.; Vanderwal, C.D.; Syrovets, T.; Simmet, T. A novel polyhalogenated monoterpene induces cell cycle arrest and apoptosis in breast cancer cells. Mar. Drugs 2019, 17, 437. [Google Scholar] [CrossRef]
- Duran, G.G.; Duran, N.; Ay, E.; Kaya, D.A.; Kaya, M.G.A. Synergistic activities of the essential oils Hypericum perforatum with methotrexate on human breast cancer cell line MCF-7. In Proceedings of the International Conference on Advanced Materials and Systems (ICAMS), Qingdao, China, 26–27 March 2016; pp. 245–250. [Google Scholar]
- Fu, J.; Yu, J.; Chen, J.; Xu, H.; Luo, Y.; Lu, H. In vitro inhibitory properties of sesquiterpenes from Chloranthus serratus on cell motility via down-regulation of LIMK1 activation in human breast cancer. Phytomedicine 2018, 49, 23–31. [Google Scholar] [CrossRef]
- Aghaei, M.; Ghanadian, M.; Sajjadi, S.E.; Saghafian, R. Pimpinelol, a novel atypical sesquiterpene lactone from Pimpinella haussknechtii fruits with evaluation of endoplasmic reticulum stress in breast cancer cells. Fitoterapia 2018, 129, 198–202. [Google Scholar] [CrossRef]
- Liu, Y.R.; Cai, Q.Y.; Gao, Y.G.; Luan, X.; Guan, Y.Y.; Lu, Q.; Sun, P.; Zhao, M.; Fang, C. Alantolactone, a sesquiterpene lactone, inhibits breast cancer growth by antiangiogenic activity via blocking VEGFR2 signaling. Phytother. Res. 2018, 32, 643–650. [Google Scholar] [CrossRef]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as potentially cytotoxic compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef]
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci. 2011, 16, 980–996. [Google Scholar] [CrossRef]
- Xue, Z.; Li, J.; Cheng, A.; Yu, W.; Zhang, Z.; Kou, X.; Zhou, F. Structure identification of triterpene from the mushroom Pleurotus eryngii with inhibitory effects against breast cancer. Plant Foods Hum. Nutr. 2015, 70, 291–296. [Google Scholar] [CrossRef]
- Subash-Babu, P.; Alshammari, G.M.; Ignacimuthu, S.; Alshatwi, A.A. Epoxy clerodane diterpene inhibits MCF-7 human breast cancer cell growth by regulating the expression of the functional apoptotic genes Cdkn2A, Rb1, mdm2 and p53. Biomed. Pharmacother. 2017, 87, 388–396. [Google Scholar] [CrossRef]
- Shadi, S.; Saeidi, H.; Ghanadian, M.; Rahimnejad, M.R.; Aghaei, M.; Ayatollahi, S.M.; Iqbal Choudhary, M. New macrocyclic diterpenes from Euphorbia connata Boiss. with cytotoxic activities on human breast cancer cell lines. Nat. Prod. Res. 2015, 29, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.A.; Asili, J.; HosseinNia, S.; Yazdian-Robati, R.; Sahranavard, M.; Tayarani-Najaran, Z. Growth inhibition and apoptosis induction of essential oils and extracts of Nepeta cataria L. on human prostatic and breast cancer cell lines. Asian Pac. J. Cancer Prev. 2016, 17, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Mouchira, A.C. Chemical composition and anticancer activity of Achillea fragrantissima (Forssk.) Sch. Bip.(Asteraceae) essential oil from Egypt. J. Pharmacogn. Phytother. 2017, 9, 1–5. [Google Scholar] [CrossRef]
- Eghbali, M.; Varaei, S.; Hosseini, M.; Yekaninejad, S.; Shahi, F. The Effect of Aromatherapy with Peppermint Essential Oil on Nausea and Vomiting in the Acute Phase of Chemotherapy in Patients with Breast Cancer. J. Babol Univ. Med. Sci. 2018, 20, 66–71. [Google Scholar] [CrossRef]
- Lua, P.L.; Salihah, N.; Mazlan, N. Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complement. Med. 2015, 23, 396–404. [Google Scholar] [CrossRef]
- Singh, S.; Chaurasia, P.K.; Bharati, S.L. A mini-review on the safety profile of essential oils. MOJ Biol. Med. 2022, 7, 33–36. [Google Scholar]
- Castilhos, R.V.; Grützmacher, A.D.; Coats, J.R. Acute Toxicity and Sublethal Effects of Terpenoids and Essential Oils on the Predator Chrysoperla externa (Neuroptera: Chrysopidae). Neotrop. Entomol. 2018, 47, 311–317. [Google Scholar] [CrossRef]
- Alipanah, H.; Farjam, M.; Zarenezhad, E.; Roozitalab, G.; Osanloo, M. Chitosan nanoparticles containing limonene and limonene-rich essential oils: Potential phytotherapy agents for the treatment of melanoma and breast cancers. BMC Complement. Med. 2021, 21, 186. [Google Scholar] [CrossRef]
- Lis-Balchin, M. Aromatherapy Science: A Guide for Healthcare Professionals; Pharmaceutical Press: London, UK, 2005; Volume 1. [Google Scholar]
- Tisserand, R.; Young, R. 3—Toxicity. In Essential Oil Safety, 2nd ed.; Tisserand, R., Young, R., Eds.; Churchill Livingstone: St. Louis, MI, USA, 2014; pp. 23–38. [Google Scholar]
- Ferreira, P.; Cardoso, T.; Ferreira, F.; Fernandes-Ferreira, M.; Piper, P.; Sousa, M.J. Mentha piperita essential oil induces apoptosis in yeast associated with both cytosolic and mitochondrial ROS-mediated damage. FEMS Yeast Res. 2014, 14, 1006–1014. [Google Scholar] [CrossRef]
- Jo, J.-R.; Park, J.S.; Park, Y.-K.; Chae, Y.Z.; Lee, G.-H.; Park, G.-Y.; Jang, B.-C. Pinus densiflora leaf essential oil induces apoptosis via ROS generation and activation of caspases in YD-8 human oral cancer cells. Int. J. Oncol. 2012, 40, 1238–1245. [Google Scholar] [CrossRef]

| No | Plant Name | Compounds | Methods | Activities | Ref. |
|---|---|---|---|---|---|
| 1 | Zataria multiflora | Monoterpenes and triterpenes | MCF-7 and MDA-MB-231 cells | Triggers apoptosis by inducing ROS, mitochondrial membrane potential (MMP) loss, DNA damage, G2 and S-phase arrest in MDA-MB-231 cells and spheroids. | [93] |
| 2 | Zataria multiflora | Monoterpenes | In vitro using 4T1 cells | Inhibition of proliferation and apoptosis of 4T1 and TC1 cells. | [94] |
| 3 | Cymbopogon citratus | Monoterpenes | In vivo using 54 Holtzman female rats | Shows tumor reduction as well as necrosis and mitosis. | [95] |
| 4 | Oliveria decumbens | Monoterpenes | In vitro using 4T1 cells | Induces apoptosis through ROS generation, mitochondrial membrane potential disruption, caspase-3 activation, and DNA damage. | [38] |
| 5 | Pinus sylvetris | Monoterpenes | In vitro using MCF-7 cells | Inhibits the growth of MCF-7 cells by 45.3% and 99.7%. | [96] |
| 6 | Erythrina corallodendron L. | MonotMonoterpenes erpen | In vitro using MDA-MB-231, MCF-7 and HMLE cells | Inhibits the proliferation, migration, and invasion of breast cancer cells in a dose-dependent manner. | [97] |
| 7 | Cyphostemma juttae | Diterpenes | In vitro using MDA-MB 231 and SUM 149 cells | C. juttae oil substantially reduces the activation of NF-κB transcription factors, resulting in a significant decrease in several NF-κB target genes. | [98] |
| 8 | Juniperus oxycedrus L. | Monoterpenes | In vitro using MCF-7 cells | ALEO shows an IC50 value of 31% (v/v) against MCF-7 cells after 36 h of treatment. | [99] |
| 9 | Pallines spinosa | Monoterpenes and triterpenes | In vitro using MCF-7 and MDA-MB-231 cells | Induces apoptosis in MCF-7 and MDA-MB-231 cell lines, and alters Bcl-2 and Bax protein levels. | [60] |
| 10 | oleo-gum-resin and its essential oil of Ferula assa-foetida and ferulic acid | Monoterpenes | In vitro 4T1 cells | The results show that the three constituents can inhibit the proliferation of 4T1 cells. Our MTT assay results demonstrated a significant cytotoxicity effect in a time and concentration-dependent manner. | [100] |
| 11 | Decatropis bicolor (Zucc.) | Monoterpenes | In vitro using MDA MB 231 cells | Cytotoxic effect on MDA-MB-231 cells in a dose- and time-dependent manner with an IC50 of 53.81 ± 1.691 µg/mL, but independent of the breast epithelial cell line MCF10A (207.51 ± 3.26 µg/mL). | [101] |
| 12 | Cinnamomum longepaniculatum | Monoterpenes | A549 and MCf-7 cells | The essential oil derived from C. longepaniculatum with the main compounds of terpinen-4-ol, α-terpineol, and safrole induces apoptosis or substantial necrosis in human A549 lung cancer and MCF-7 breast cancer cells. | [61] |
| 13 | Nigela sativa | Thymoquinone (p-benzoquinones) | In vitro using MCF-7 cells | Nigela sativa essential oil significantly reduced breast cancer cell survival (MCF-7). The nucleo-cytoplasmic morphological features of NSEO-NE-treated cells were cell membrane blistering, cytoplasmic vacuolation, chromatin marginalization, and nuclear fragmentation. The results demonstrated that NSEO-NE induces apoptosis in MCF-7 cells. | [102] |
| 14 | Opoponax (Commiphora guidottii) | Sesquiterpenes | MCF-7 cells | The loss of viability was due to the induction of apoptosis as demonstrated by the Annexin V-propidium iodide and caspase-3/7 activity assays/test. | [103] |
| 15 | Hypericum perforatum | Sesquiterpenes | MCF-7 cells | The essential oil of Hypericum perforatum also showed anticancer activity against MCF-7 cells. The IC50 values of essential oil, MTX, and MTX essential oil were 0.78, 6.25, and 0.195 µg/mL, respectively. However, Hypericum perforatum essential oil was found to be non-cytotoxic for MDBK cells. | [104] |
| 16 | Pinus corainensis | Monoterpens | MDA-MB-231 cells (TNBC) | Inhibits TNF α-induced MDA-MB-231 cell invasion as determined by a three-dimensional spheroid invasion assay. | [105] |
| 17 | Chenopodium ambrosioides L. | Monoterpenes | MCF-7 cells | Inhibits the growth of MCF-7 cells within 24 h (p < 0.05), which is consistent with the results of fluorescent staining of live/dead cells. | [106] |
| 18 | Boswelia sacra | Triterpenes | MDA-MB-231 cells (TNBC) | Induces cancer cell death, prevent the formation of cellular tissue (MDA-MB-231) cells in Matrigel, and cause multicellular tumor spheroid damage (T47D cells), and regulates molecules involved in apoptosis, signal transduction, and cell cycle development. | [107] |
| 20 | Syzigium aromaticum | Diterpenes | BSLT (brine shrimp lethality test) method | Tests on BSLT and MTT showed essential oils had the highest cytotoxic effect, followed by ethanol and water extracts. The LD50 concentration of essential oil in 24 h of BSLT was 37 µg/mL. | [108,109] |
| 21 | Laurus nobilis L. | Monoterpenes | MCF-7 and T47D cells | Shows a strong antiproliferative activity for both leaves and fruit; however, the fruit remained more potent against both breast cancer cell models (MCF7 and T47D). | [110] |
| 22 | Lycopus lucidus Turcz. var. hirtus Regel | Sesquiterpenes | MCF-7 cells | The essential oil can induce apoptosis of carcinoma cell lines and decrease the level of intracellular GSH. | [111] |
| 23 | Cordia africana Lam. | Sesquiterpenes | MCF-7 cells | Has an apoptotic mechanism with IC50 inhibition of 4.55 µg/mL. | [112] |
| 24 | Lemon volatile oil | Monoterpenes | MCF-7 cells | Oil derived from lime leaves shows cytotoxic activity against breast cancer cells (MCF-7) at IC50 10% (v/v). | [113] |
| 25 | Murraya koenigii | Sesquiterpenes | MCF-7 cells | Essential oil in particular exhibits strong antibacterial and cytotoxic effects with a dose-dependent trend (≤5.0 µg/mL). | [114] |
| 26 | Cedrelopsis grevei | Sesquiterpenes | MCF-7 cells | The main constituents are (E)-β-farnesene (27.61%), δ-cadinene (14.48%), α-copaene (7.65%), and β-elemene (6.96%). Grevei essential oil is active against MCF-7 cells (IC50 = 21.5 mg/L). | [115] |
| 27 | Asteraceae family | Sesquiterpenes | MCF-7 and MDA-MB-468 cells | Britannin can induce apoptosis in MCF-7 and MDA-MB-468 cells. Western blot analysis shows that Bcl-2 expression markedly decreased in response to Britannin treatment, whereas Bax protein expression increased, which is positively correlated with increased p53 expression. | [116] |
| 28 | Tanacetum polycephalum L. Schultz-Bip | Sesquiterpenes | In vivo (white rats) | Histopathological examination showed that TPHE significantly suppressed the carcinogenic effect of LA7 tumor cells. Tumor sections from TPHE-treated mice showed a significantly decreased expression of Ki67 and PCNA compared to the control group. | [117] |
| 29 | Gaillardin, was isolated from the chloroform extract of Inula oculus-christi aerial | Sesquiterpenes | MCF-7 cells | Gaillardin is able to induce apoptosis in the breast cancer cell lines of MCF-7 and MDA-MB-468 and determine the mechanism underlying their anticancer effects. Induction of apoptosis with Gaillardin treatment is confirmed by annexin V-FITC/PI staining, and activation of caspase-3, -6, and -9. | [118] |
| 30 | Opoponax (Commiphora guidottii) | Sesquiterpenes | In vivo (white rats) | Opoponax essential oil shows specific cytotoxicity for mammary tumor cells of humans and mice in vitro and in vivo, and this warrants further investigation into the use of β-bisabolene in medicine. | [103] |
| 31 | Eupatorium lindleyanum DC. | Sesquiterpenes | MDA-MB-231 cells (TNBC) | Inhibits migration, invasion, and motility of MDA-MB-231 cells in a concentration-dependent manner by invasion and apoptosis. | [119] |
| 32 | Eupatorium lindleyanum DC. | Sesquiterpenes | MDA-MB-468 cells (TNBC) | EO-induced cytotoxicity is mediated by the induction of apoptosis. | [120] |
| 33 | Ulva fasciata | Sesquiterpenes | MDA-MB 231cells (TNBC) | Computational study shows the interaction of guai-2-en-10α-ol with the Asp855 residue of the EGFR kinase domain in the active conformation. All these results suggest the anticancer potential of guai-2-en-10α-ol via the EGFR/PI3K/Akt pathway. | [121] |
| 34 | Plants such as Inula helenium | Sesquiterpenes | MDA-MB 231cells (TNBC) | ALA can inhibit the proliferation, motility, migration, and tube formation of human umbilical vein endothelial cells. ALA also inhibits angiogenesis at the chorioallantoic membrane of chicken embryos and slows the growth of the xenograft of human breast cancer of MDA-MB-231. | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustapa, M.A.; Guswenrivo, I.; Zuhrotun, A.; Ikram, N.K.K.; Muchtaridi, M. Anti-Breast Cancer Activity of Essential Oil: A Systematic Review. Appl. Sci. 2022, 12, 12738. https://doi.org/10.3390/app122412738
Mustapa MA, Guswenrivo I, Zuhrotun A, Ikram NKK, Muchtaridi M. Anti-Breast Cancer Activity of Essential Oil: A Systematic Review. Applied Sciences. 2022; 12(24):12738. https://doi.org/10.3390/app122412738
Chicago/Turabian StyleMustapa, Mohammad Adam, Ikhsan Guswenrivo, Ade Zuhrotun, Nur Kusaira Khairul Ikram, and Muchtaridi Muchtaridi. 2022. "Anti-Breast Cancer Activity of Essential Oil: A Systematic Review" Applied Sciences 12, no. 24: 12738. https://doi.org/10.3390/app122412738
APA StyleMustapa, M. A., Guswenrivo, I., Zuhrotun, A., Ikram, N. K. K., & Muchtaridi, M. (2022). Anti-Breast Cancer Activity of Essential Oil: A Systematic Review. Applied Sciences, 12(24), 12738. https://doi.org/10.3390/app122412738








