Abstract
Biodegradable membranes with innovative antifouling properties are emerging as possible substitutes for conventional membranes. These types of membranes have the potential to be applied in a wide range of applications, from water treatment to food packaging and energy production. Nevertheless, there are several existing challenges and limitations associated with the use of biodegradable membranes in large scale applications, and further studies are required to determine the degradation mechanisms and their scalability. Biodegradable membranes can be produced from either renewable natural resources or synthesized from low-molecular monomers that increase the number of possible structures and, as a result, greatly expand the membrane application possibilities. This study focused on bio-sourced and synthesized biodegradable polymers as green membrane materials. Moreover, the article highlighted the excellent antifouling properties of biodegradable membranes that assist in improving membrane lifetime during filtration processes, preventing chemical/biological disposal due to frequent cleaning processes and ultimately reducing the maintenance cost. The industrial and biomedical applications of biodegradable membranes were also summarized, along with their limitations. Finally, an overview of challenges and future trends regarding the use of biodegradable membranes in various industries was thoroughly analyzed.
1. Introduction
Membrane technology has been extensively used in various applications, including but not limited to waste/wastewater treatment, gas separation, hemodialysis, energy production, drug delivery, and the food industry. Polymeric membranes are mainly employed in all those separation processes [1,2]. The production and disposal processes of polymeric membranes result in unsustainable accumulations of waste and several environmental concerns [3,4,5,6,7]. Even though these types of membranes are more prevalent and dominate the membrane market, they are still subject to waste disposal challenges, chemical, mechanical, and thermal stability concerns, as well as the membrane fouling issue [7]. As an environmentally friendly and sustainable approach to solving these problems, biodegradable membranes have been introduced, especially to reduce the amount of waste disposal. Under favorable conditions in the environment in terms of pH, humidity, and temperature, biodegradable membranes are broken down into non-toxic compounds by enzymes and microorganisms in nature [1]. Biodegradable membranes proved to be potential substitutes for conventional membranes in various applications such as oil/water separation, dye removal, tissue engineering, food packaging, fuel cells, etc. [1,8,9,10,11].
2. Green Membrane Materials
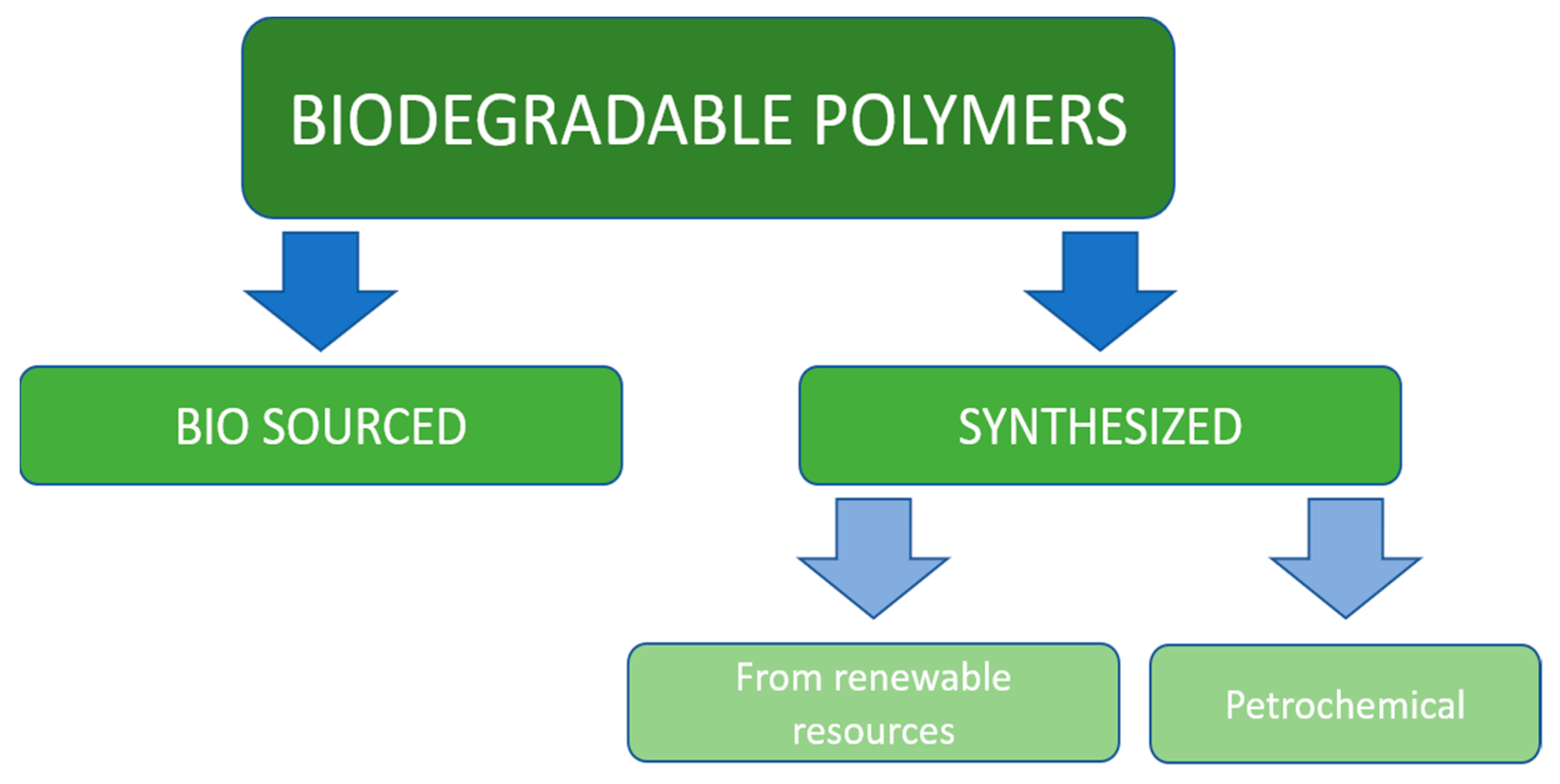
Synthetic polymer materials have been developed less than 100 years ago but because of their low ability to be utilized in nature are considered to have serious ecological impact on the environment. The interest in biodegradable plastics is constantly increasing, and their production is forecasted to reach more than 1.3 million tons in 2024 [12]. The main position on the biopolymers market belongs to the Asia-Pacific region, North America, and Europe [13]. All biodegradable polymeric materials considered in this chapter could be divided into two main classes: bio-sourced and synthetic. Synthetic materials, in turn, fall into two categories: petrochemical and made out of renewable sources, which is illustrated on Figure 1.
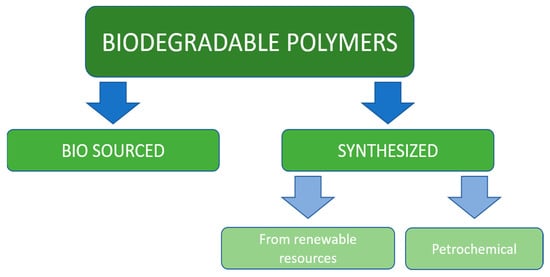
Figure 1.
Classification of biodegradable polymers.
2.1. Bio-Sourced Polymers for Membrane Fabrication
Bio-sourced materials have been used by humanity since ancient times as materials for construction (wood), tools (wood, bones), cloth (plants, leather, wool, fur), jewelry (bones, wood, amber), and other. Nowadays we also use bio-sourced crude to create new materials, including membranes. The majority of bio-sourced polymers used for membrane fabrication are carbohydrates/polysaccharides and their derivatives. The origin of bio-sourced materials possesses a high degree of biodegradability that makes them attractive as green materials to be used for reducing pollution of the environment with plastic wastes.
2.1.1. Cellulose and Its Derivatives
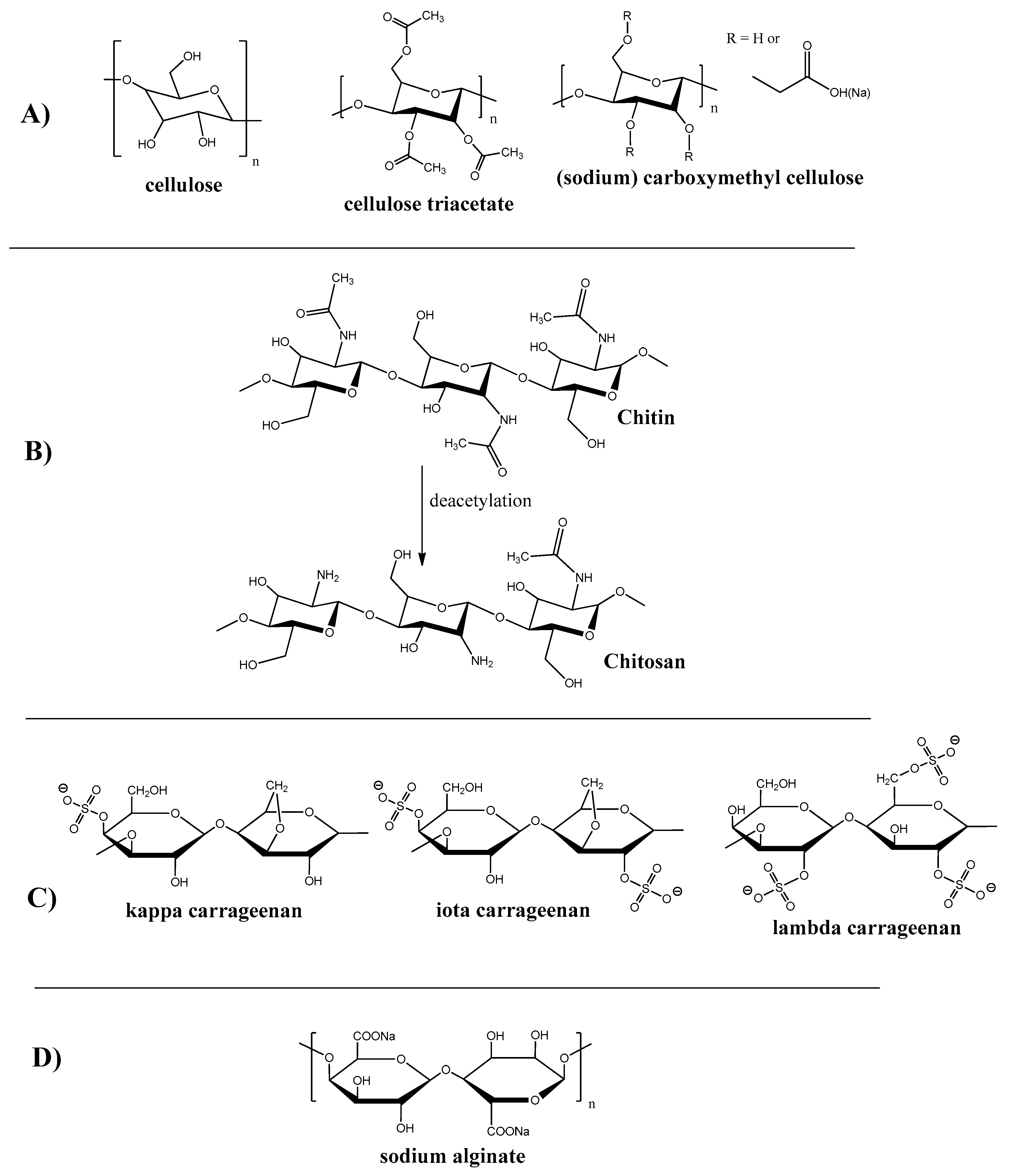
Cellulose is one of the first materials used for membrane production. Thus, the first membrane for hemodialysis was produced from cellophane, which is the product of cellulose treatment. Cellulose is one of the most prevalent polysaccharides that occurs in natural sources like trees and plants. Saccharide monomer units in cellulose (see Figure 2A) macromolecules are arranged predominately in a linear manner, which results in its fiber structure and high mechanical properties.
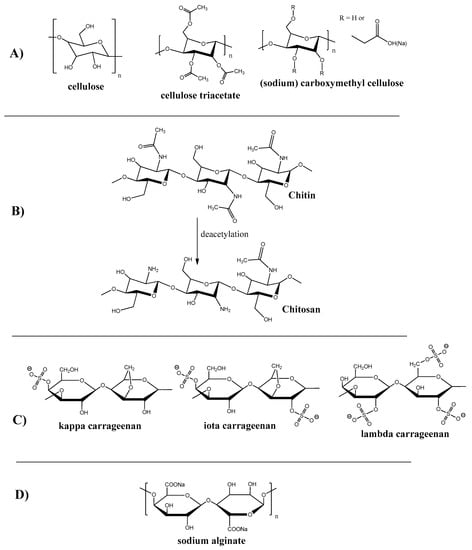
Figure 2.
Chemical structure of polysaccharides polymers and their derivatives. (A) Cellulose and Its Derivatives; (B) Chitin and Chitosan; (C) Carrageenan; (D) Alginate.
Due to the presence of active hydroxyl groups in the saccharide unit, cellulose is widely used for its modification to obtain new artificial materials with improved properties. Some of the structures that resulted from cellulose modification are presented on Figure 2A.
Thus, the most common cellulose derivative is cellulose triacetate, which is, along with other cellulose derivatives, used as the fabrication material for membranes applied in many industries such as oil-water separation [14,15,16], antifouling improvement [17], virus removal [18], heavy metal adsorption [19,20], desalination [21,22,23], hemodialysis [24], and gas separation [25,26,27].
2.1.2. Chitin and Chitosan
Chitosan is the second (after cellulose) most abundant biopolymer in nature that can be found in insects, shells, mollusks, shrimps, crabs, fungi etc. It also can be derived from chitin (the exoskeleton of many living creatures, insects in particular) by its deacetylation (see Figure 2B).
Though this biopolymer is widely spread in nature, the research of chitosan began only in the 1980s because of the structure’s features and insolubility in water [28].
Examples of chitin and chitosan membranes applications are ion exchange membranes [29,30,31], wastewater treatment [32,33], oil-water separation [34], dye removal [35], etc.
2.1.3. Carrageenan
Carrageenan is a naturally occurred polysaccharide containing sulphate groups. This polymer can be extracted from red algae, particularly from Rhodophyceae [36]. Depending on the degree of sulfanation, there are three classes of carrageenan: kappa, iota, and lambda. The structure of carrageenan is shown on Figure 2C. One carrageenan application example is the super-oleophobic membrane for the removal of dyes and heavy metals [37].
2.1.4. Other Polysaccharides and Biopolymers
Starch is another example of a polysaccharide. It is one of the most abundant biopolymers in nature and can be found in such common products as potato, corn, and rice. Due to its branched structure, resulting in poor mechanical properties, starch can be hardly used as the main material for membrane production [38]. Thus, starch was reported to be used as an additive to produce membranes for wastewater treatment [39,40,41].
Cyclodextrin (CD) can also be used as an additive for the improvement of hydrophilicity and permeability of membranes used for water treatment [42]. The functionalization of hydroxyl groups to amino, sulphonyl, and other groups resulted in the further improvement of membrane selectivity by enhancing porosity and hydrophilicity [43].
Alginate is a naturally occurring anionic water-soluble linear polysaccharide composed of α-L-guluronic acid and β-D-mannuronic acid (see Figure 2D), typically obtained from brown seaweed (Phaeophyceae), including Laminaria hyperborea, Laminaria digitata, Laminaria japonica, Ascophyllum nodosum, and Macrocystis pyrifera [44]. Alginate-containing membranes were reported to be used for dyes and heavy metal removal [45,46,47,48,49].
Silk fibroin is another natural biopolymer, extracted from insects’ cocoons when treating them with hot alkali solution [50]. This protein is added to membranes to improve their adsorption of heavy metal ions [51,52].
Similar to fibroin, collagen can be extracted from animal connective tissues [53]. Despite being sensitive to pH, temperature, and bacteria, collagen can be used for membrane fabrication, though its application is limited. Due to its protein nature, collagen is perfectly suitable to create fiber-type membranes for oil-water separation [54] and pervaporation [55,56].
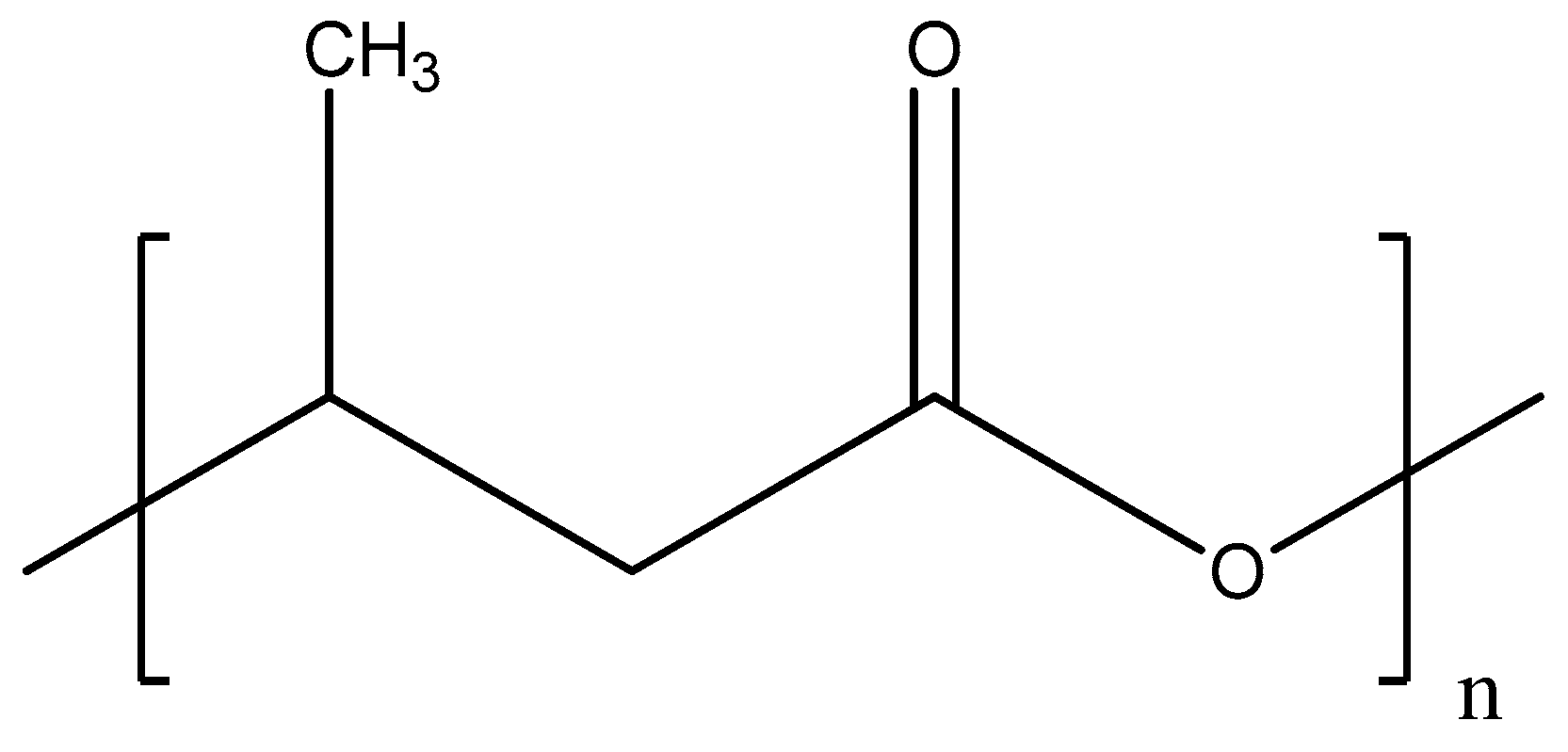
Polyhydroxybutyrate (PHB) is bio-derived polyester (see Figure 3) that is produced by some microorganisms such as Cupriavidus necator, Methylobacterium rhodesianum, or Bacillus megaterium during assimilation of glucose or starch [57].
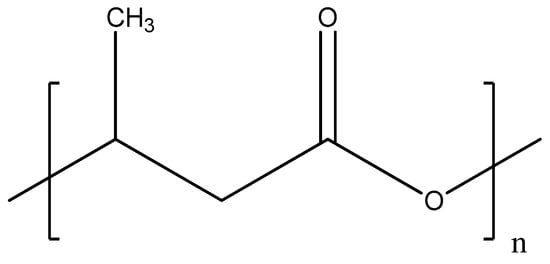
Figure 3.
Chemical structure of polyhydroxybutyrate.
This polymer has a tensile strength close to polypropylene, making it suitable to manufacture fibers. As a result, PHB has been used to prepare electrospun fibers-based membranes with improved antibacterial properties for medical applications, dye removal, and microfiltration [58,59,60,61].
2.2. Synthetic Biodegradable Polymers
2.2.1. Polymers Synthesized from Renewable Sources
Polylactic Acid
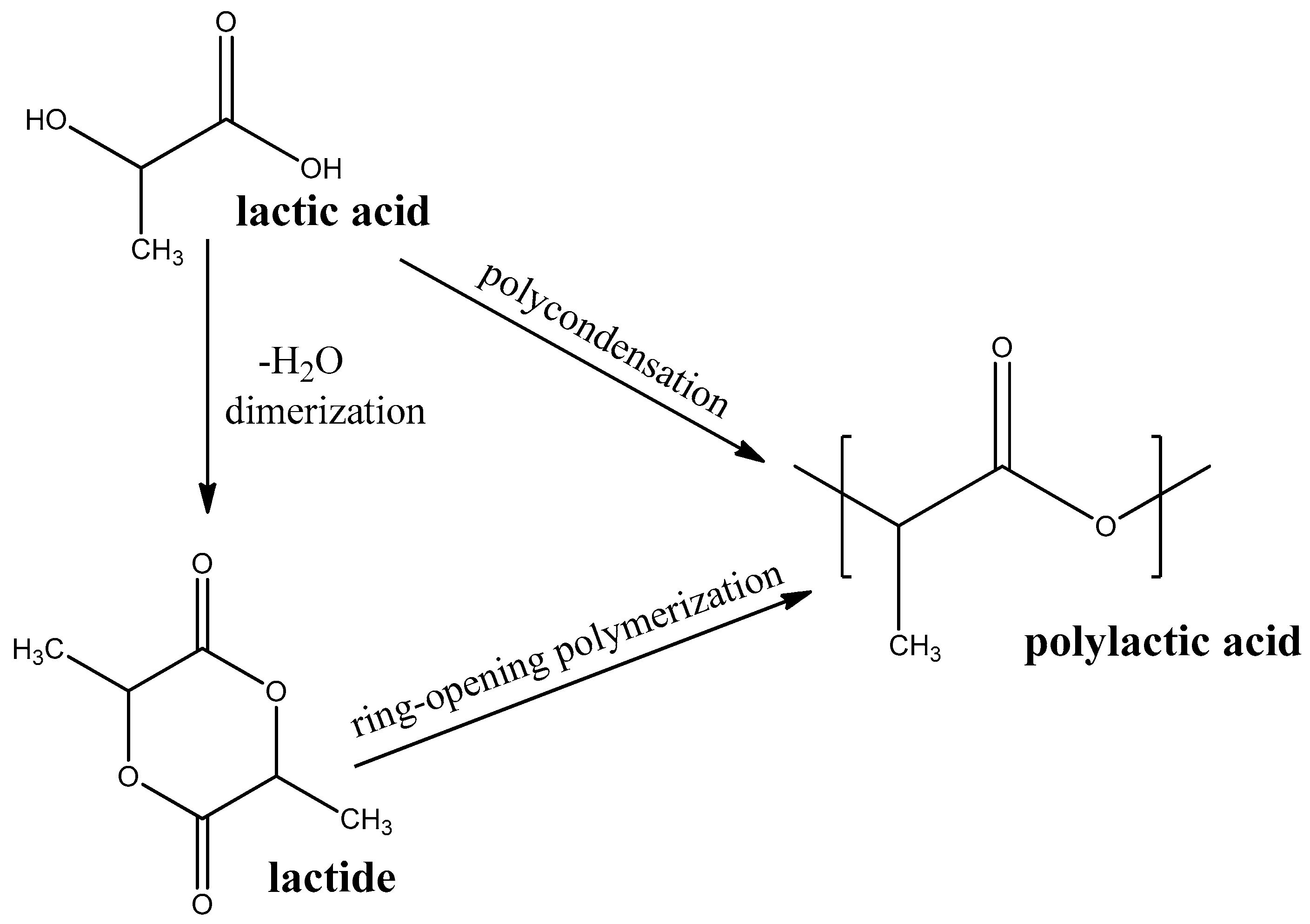
Polylactic acid (PLA) is a thermoplastic polyester that is produced by polycondensation of lactic acid or ring-opening polymerization of cyclic diester-lactide (see Figure 4). Lactic acid, in turn, is produced from a renewable bio-source, such as sugarcane or starch, [62] by aerobic fermentation [63]. PLA is one of the most researched and mass-produced biodegradable polymers.
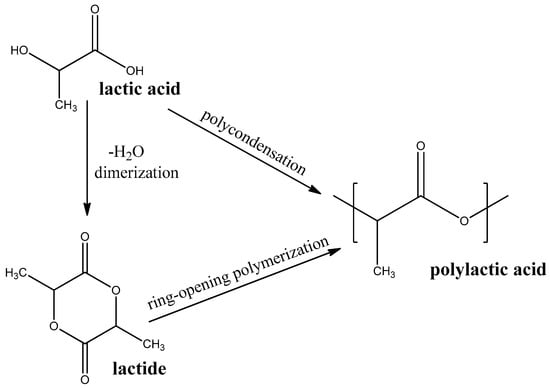
Figure 4.
Synthesis routes PLA.
PLA belongs to semicrystalline polymers. PLA-application temperatures are limited by its low glass-transition temperature (Tg = 55–60 °C) and melting temperature (Tm = 170–180 °C) [64]. PLA is suitable to prepare membranes based on electro-spun fibers for oil-water separation [65] and tissue regeneration [66]. PLA biodegradation proceeds quite fast to CO2 and water [67], which meets all the requirements of all standards for biodegradable plastics [68]. PLA is also suitable for decomposition as a part of compost [69].
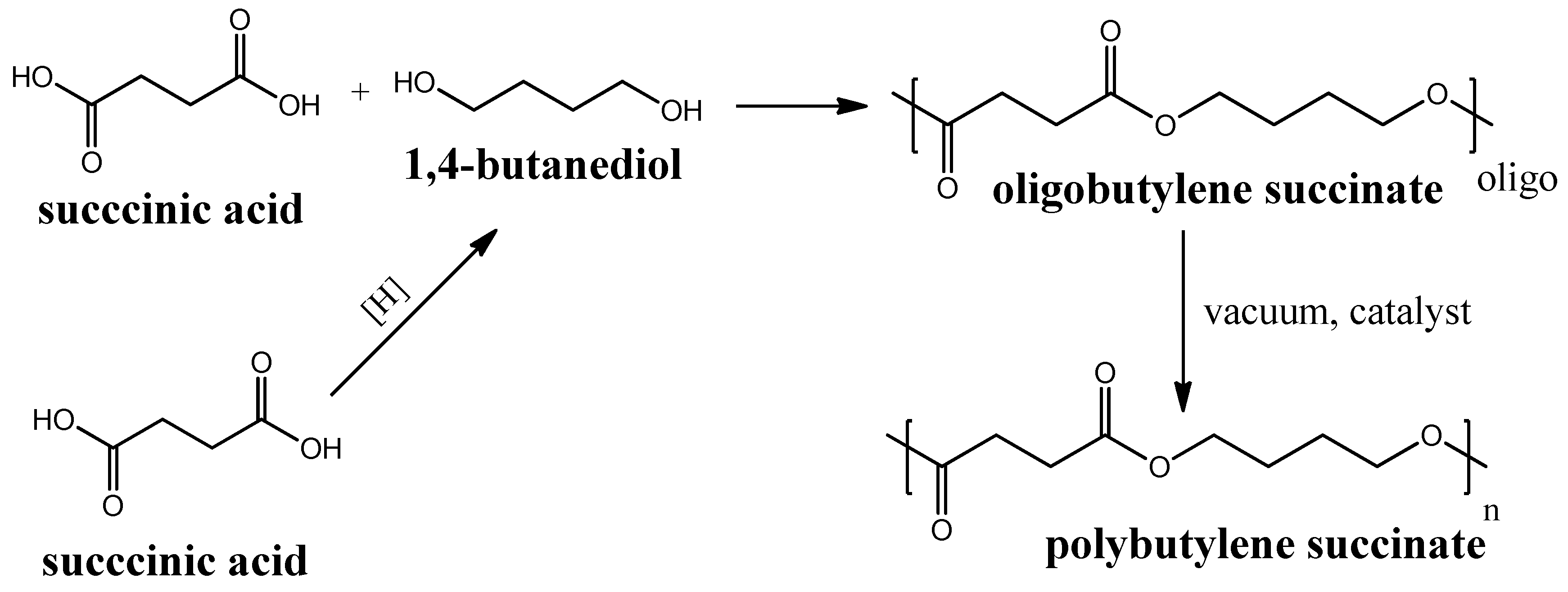
Polybutylene Succinate
Polybutylene succinate (PBS) is another (like PLA) example of a biodegradable polyester that is produced by polycondensation of succinic acid and 1,4-butanediol. Succinic acid can be produced through biomass fermentation [70] by further hydrogenation to 1,4-butanediol [71]. The further polycondensation process of succinic acid and 1,4-butanediol proceeds in two steps and requires catalysts to produce a polymeric PBS from its oligomers, obtained in the first step (see Figure 5). The first mass production of this polymer began in 1990s in Japan by the Showa Highpolymer company [72]. PBS are reported to be used for the fabrication of membranes for pervaporation and other applications [73,74,75]. PBS biodegradation in soil was reported to proceed with 65% carbon mineralization (conversion to CO2) for 425 days [76].
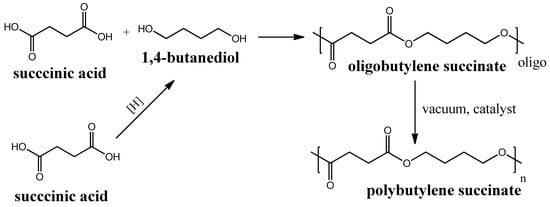
Figure 5.
Circuit diagram of polybutylene succinate synthesis.
2.2.2. Petrochemical Polymers
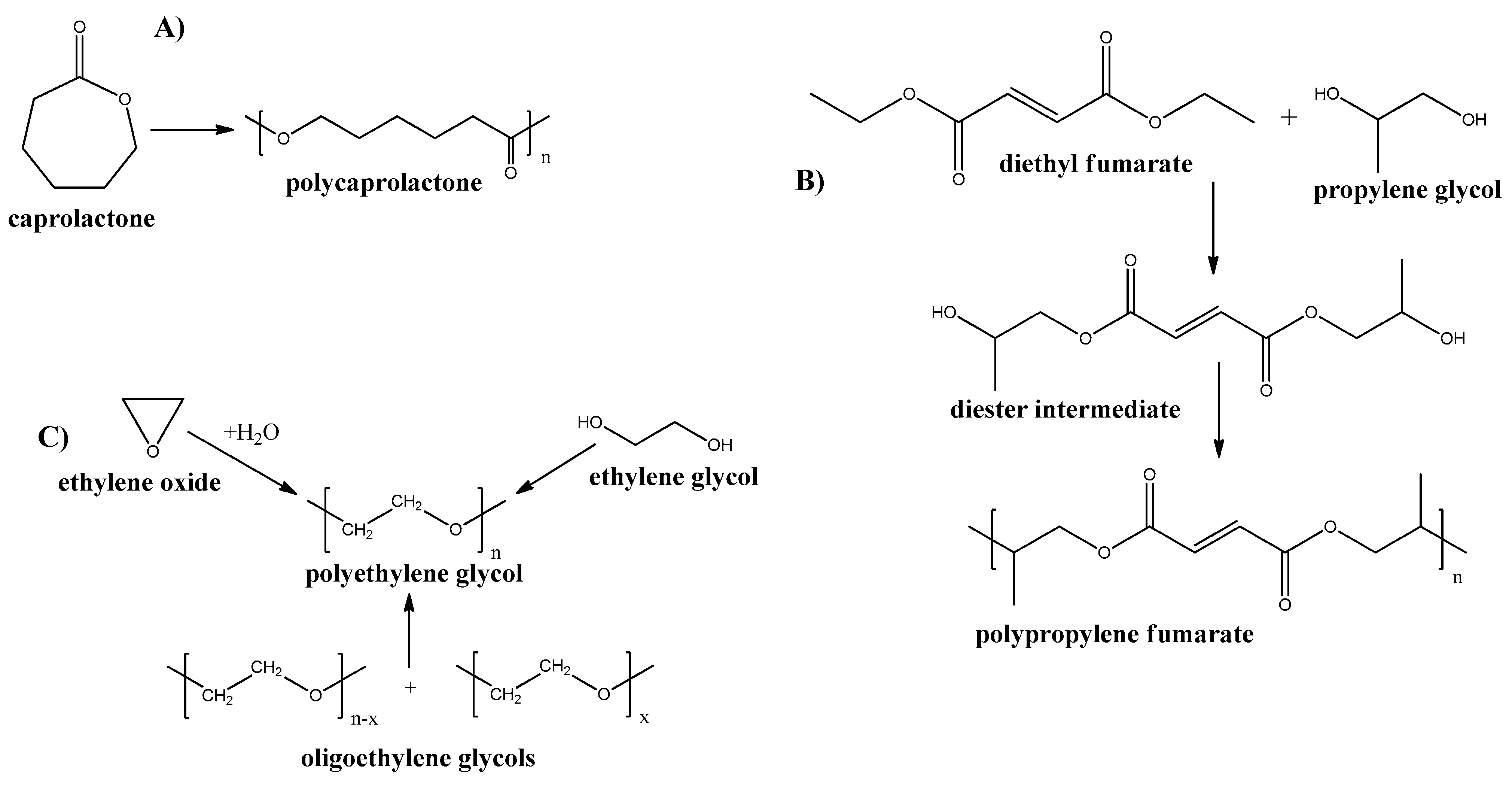
Poly-ε-caprolactone
Poly-ε-caprolactone is an aliphatic polyester, produced by ring-opening polymerization of ε-caprolactone (see Figure 6A) [77]. This polymer has a low Tg = −55–60 °C and low melting point of Tm = 60–65 °C and good resistance to oil and water. Examples of using poly-ε-caprolactone for membranes include dye removal [78], wound dressing [79], antifouling [80], treatment blood infections [81], and guided tissue regeneration [82] applications. Furthermore, poly-ε-caprolactone is highly degradable polymer. Thus, Pseudozyma japonica-Y7-09 enzyme, which is present in different microorganisms, results in Poly-ε-caprolactone degradation by 93.3% for 15 days for polymer film and 43.2% for 30 days when the polymer was made as foam plastic [83].
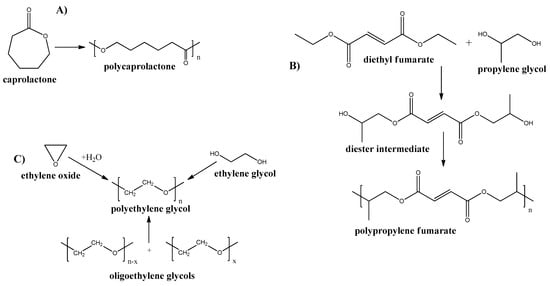
Figure 6.
Synthesis routes of some petrochemical derived biodegradable plastics. (A) Poly-ε-caprolactone; (B) Poly-propylene Fumarate; and (C) Poly-ethylene Glycol.
Poly-propylene Fumarate
Polypropylene fumarate (PPF) is an aliphatic polyester, containing a double bond in the main chain. This polymer can be produced by multistep synthesis (see Figure 6B) [84]. Because of the presence of double bonds, this polymer can be cross-linked by various methods which, results in the broad mechanical properties of the resultant structures [85]. Although, the mechanical properties are still low and limit the use of PPF for the membrane and other applications. However, some new approaches have been applied by the introduction of nanofillers into PPF to improve its properties [86,87,88]. The in vitro biodegradation of PPF scaffolds revealed nearly 17% mass loss at 6 weeks [89].
Poly-ethylene Glycol
Polyethylene glycol (PEG) is a synthetic polyether that can be produced by various ways: from ethylene oxide, ethylene glycol, or ethylene glycol oligomers (see Figure 6C).
PEG production has been reported in 1859 by both A. V. Lourenço and Charles Adolphe Wurtz, independently. PEG membranes are predominately used for drug release systems [90,91,92,93,94]. In addition, PEG is well known to possess sensitivity to oxidative degradation [95]; thus, this material is substantial to oxidative biological degradation by different microorganisms [96,97,98].
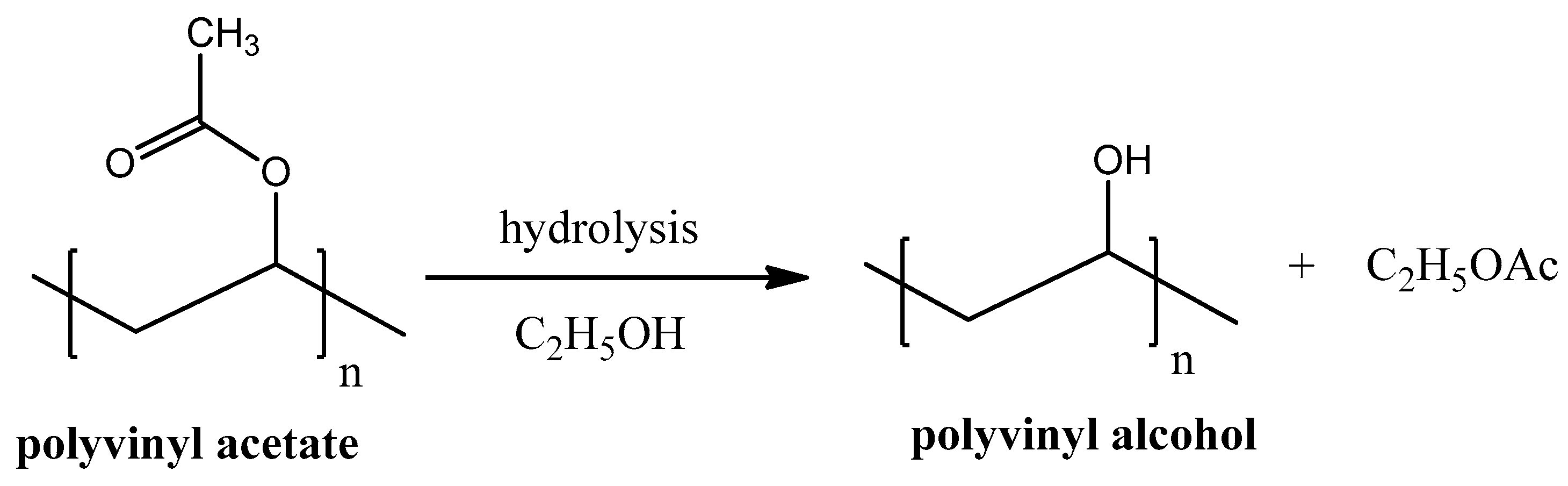
Poly-vinyl Alcohol
Polyvinyl alcohol (PVA), unlike most of other vinyl polymers, cannot be produced by polymerization of its monomer units because vinyl alcohol is thermodynamically unstable. PVA is predominately produced by hydrolysis of polyvinyl acetate (see Figure 7). Hydrolysis reaction is usually proceeded in the presence of ethanol but can be easily done without it.
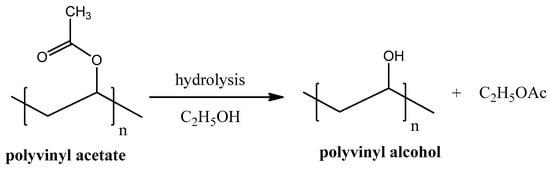
Figure 7.
Polyvinyl alcohol synthesis from polyvinyl acetate hydrolysis.
PVA has been reported to use for membrane preparation for wound dressing applications [99,100]. In addition, PVA is biodegradable in the presence of different microorganisms under two-step metabolic processes, including oxidation and hydrolysis [101].
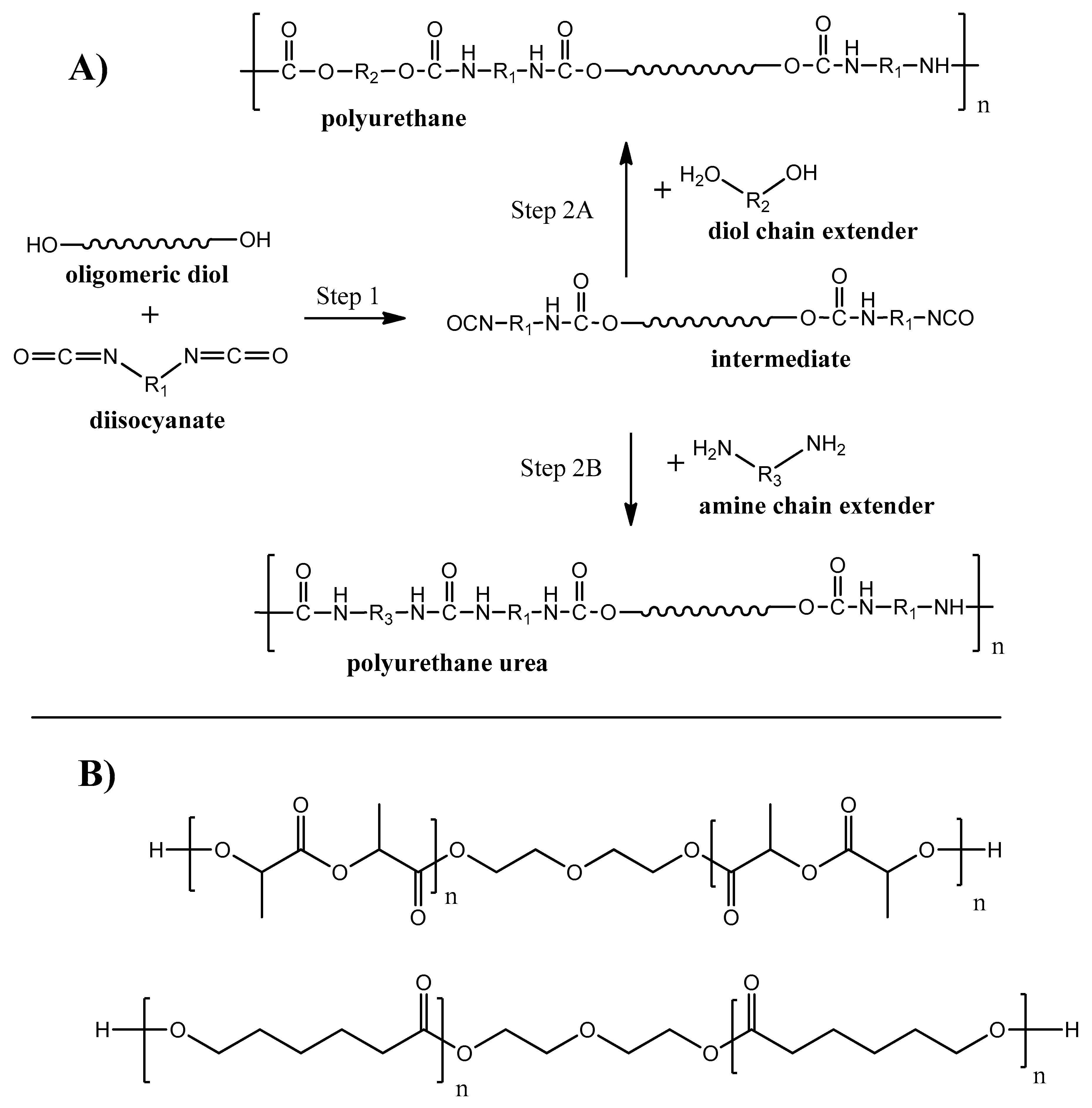
Polyurethane and Polyurethane Urea
Polyurethanes (PU) are one of the most produced polymers in the world. Around 25 million tons of this class of polymer was produced in 2019, which is about of 6% of all manufactured polymers [102]. The interest to polyurethanes is determined by the broad chemical variety of monomer units, resulting in a wide range of physical properties–from rigid and strong plastics to flexible elastomers [103]. Linear PU are basically produced during polycondensation reaction between diisocyanates and diols (see Figure 8A, step 2A) with the ability to produce polyurethane urea when a diamine chain extender is used on the second synthesis step (see Figure 8A, step 2B).
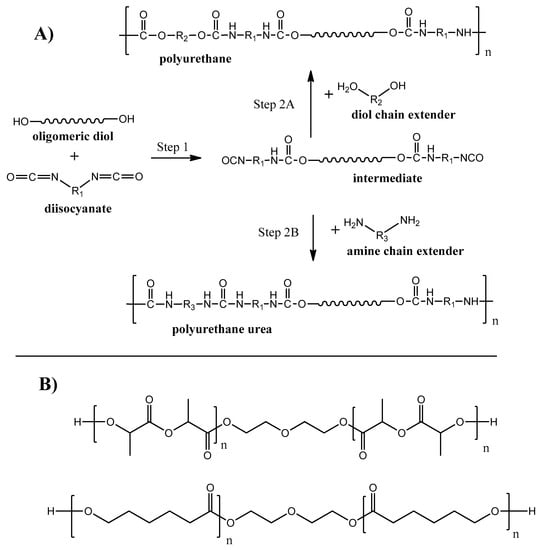
Figure 8.
Synthesis routes of biodegradable PU and PU urea.
Polyurethanes were known for a long time as non-degradable polymers. For the last decades, biodegradable PU were developed by using monomer units with hydrolysable bonds (e.g., ester, amide) groups [104,105]. Examples of oligomeric diols [106,107] used for biodegradable PU synthesis are given on Figure 8B. A broad opportunity to adjust and control PU chemistry and structure makes PU degradable by enzymes, fungi, and bacteria [108].
PU applications include sprayable membranes [109], tissue repair [110], and many other biomedical applications [110].
The biodegradable membranes synthesis routes and main applications are summarized in Table 1.

Table 1.
Green chemistry biodegradable membranes chemistry and applications.
3. Biodegradable Antifouling Membrane Properties
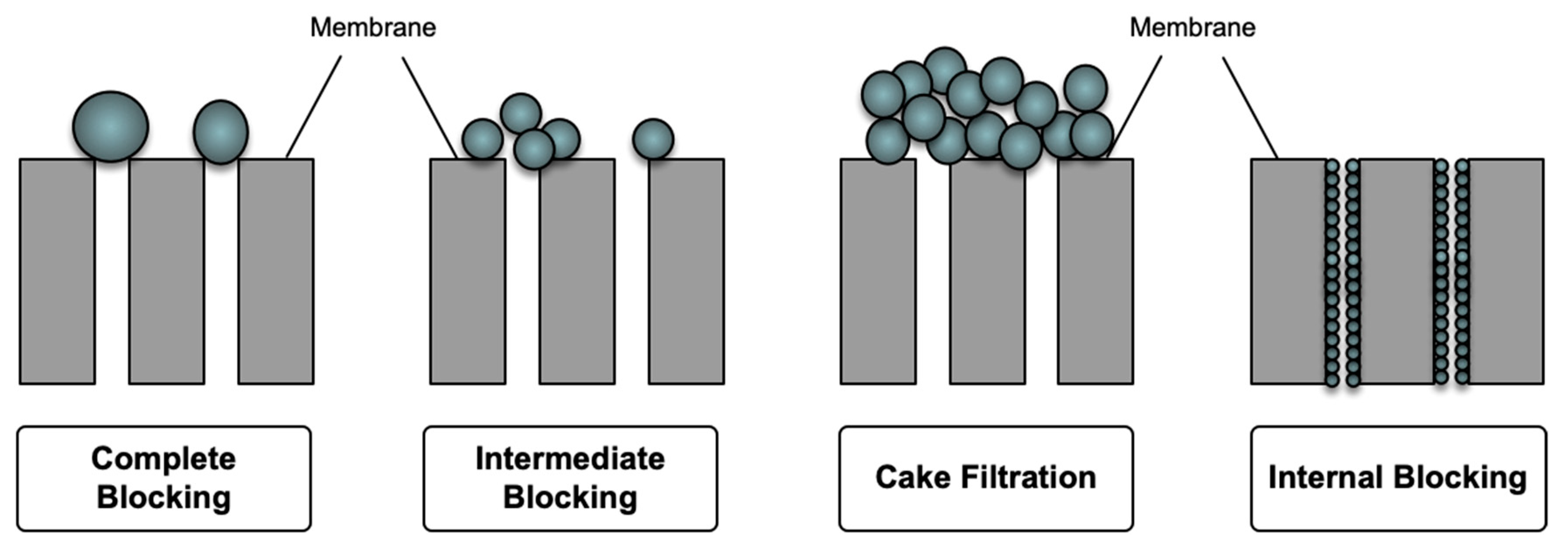
Fouling is a serious drawback of polymeric membranes used in membrane-based separation processes. This phenomenon occurs as a result of particle-membrane and particle-particle attachments on the membrane surface or within the pores, which is mostly due to the hydrophobicity of the membranes [111]. This phenomenon can be observed at the membrane surface as well as in the membrane pores as a result of sieving and adsorption. In the sieving mechanism, particles larger than the membrane pores agglomerate and partially/completely block the pores (intermediate/complete blockage). A further accumulation of particles on the membrane surface would lead to the formation of cake layer. In addition, small particles enter the pores and are adsorbed (intermolecular interaction) to the pore wall, causing an internal blockage (standard blockage) [112]. Figure 9 shows the schematic of membrane fouling mechanisms.
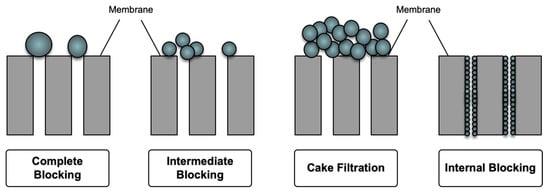
Figure 9.
Schematic of membrane fouling mechanisms.
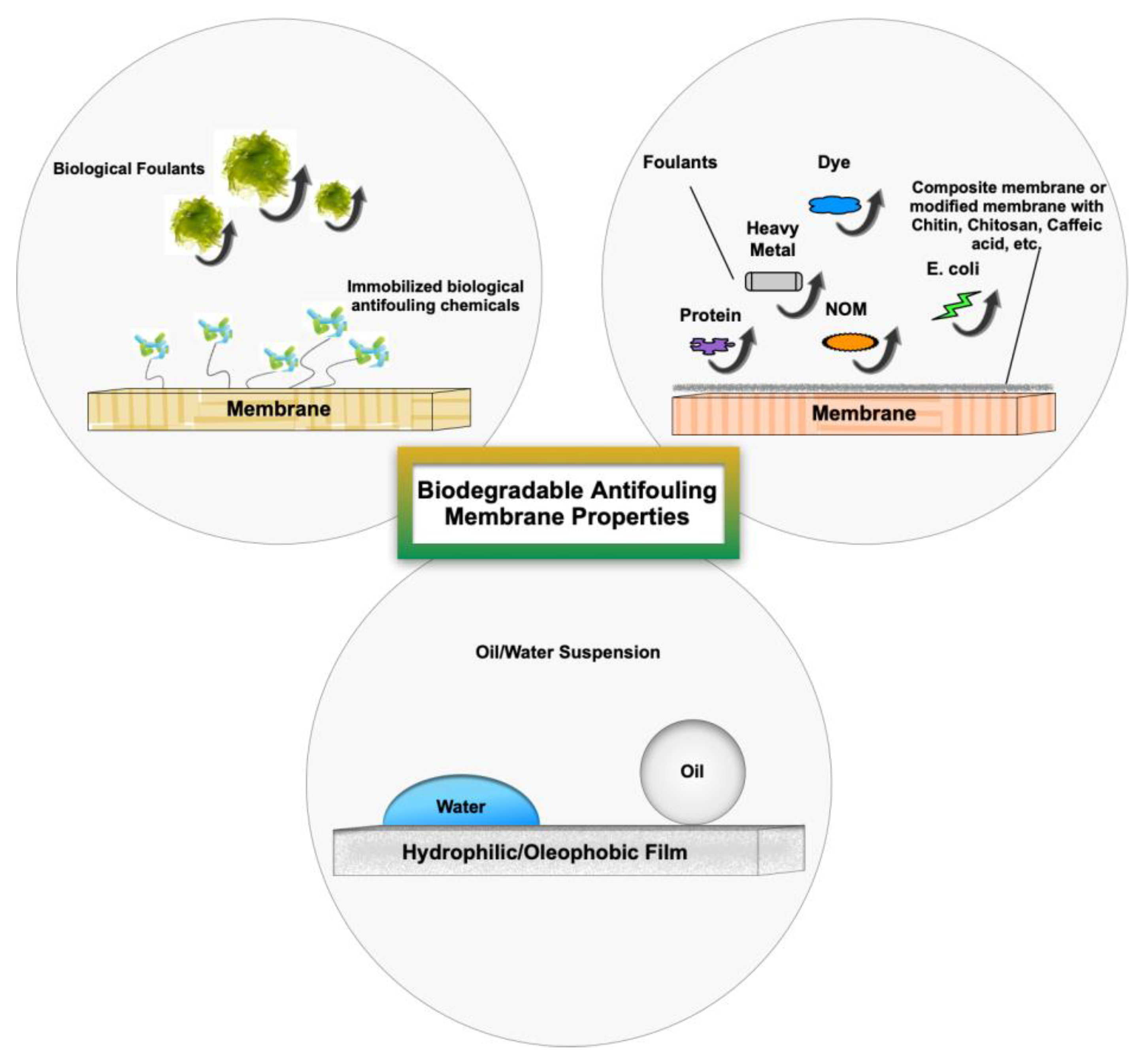
Several methods have been employed to prevent fouling formation (feed pre-treatment or membrane modification) or clean the membrane surface (chemical/biological cleaning, electrochemical, advanced oxidation, ultrasonic cleaning, forward/backflushing, pulsing mode, vibration, air sparging, and electrical field). Many of these methods suffer from limitations such as reduced membrane lifetime due to frequent cleaning, higher operating costs and energy consumption due to system downtime and interruption of filtering, and secondary pollutants as a result of the use of chemical/biological agents for surface cleaning [112,113]. The foulant-membrane interaction and fouling behavior are mainly controlled by the chemistry/surface properties of membranes. Therefore, there is a great demand for membranes with antifouling properties to reduce the cleaning costs and prevent membrane damage. Natural antifoulant materials have shown promising results and proved to be an environmentally friendly solution to membrane fouling [114,115,116,117]. Figure 10 shows the antifouling properties of various types of membranes.
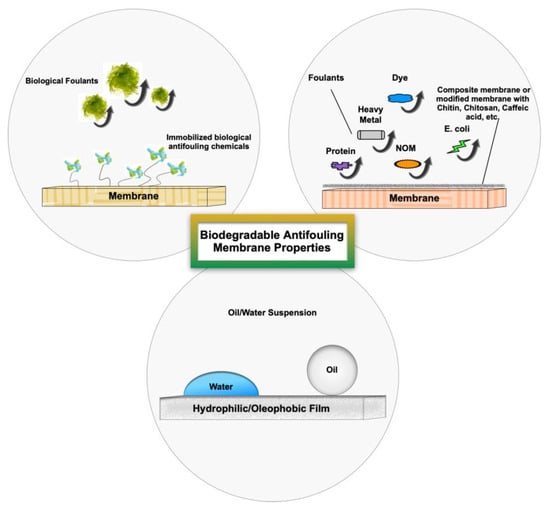
Figure 10.
Schematic of membranes’ antifouling properties.
3.1. Bio-Based Materials
In recent decades, bio-based materials have been widely used to enhance the antifouling property of the membranes [118,119,120,121,122]. According to Ye et al. [54], the collagen-based nanofibrous membranes incorporated with carbon nano-tubes (CNT) were capable of separating the viscous emulsions (up to 200.0 mPa.s) by more than 99%, whereas the conventional membranes, such as polytetrafluoroethylene (PTFE) and polyvinylidene fluoride (PVDF), failed to separate these types of emulsions. The fabricated membranes exhibited a high separation flux of 1582 Lm−2h−1 bar−1 and robust reusability after 10 filtration cycles, which prove their antifouling properties. Due to the presence of the mixture of -NH2 and -COOH functional groups in collagen/CNT nanofibrous membranes, these materials show amphiprotic properties and act as demulsifiers. They separate the emulsions by selectively ionizing, targeting, and capturing oppositely charged molecules. Biomimetic membranes are also among the potential candidates for wastewater treatment and seawater desalination, owing to their excellent antifouling, water permeability, and selectivity characteristics [111,117,123,124,125]. Generally, biological surfaces contain particular functional groups (like zwitterionic groups on lipid head groups or glycocalyx oligosaccharide chains) that inhibit the foulant adhesion. Zwitterionics prevent the protein adhesion to the exterior surface of the cells by causing electrostatic hydration and strong binding to water molecules. Glycocalyx chains also hinder foulant adhesion by electrostatic and entropic forces [117]. Biological cells also contain protein water channels called aquaporins. Aquaporins have excellent water permeability and high solute rejection [126]. Water flux and permeate flux recovery, which show the antifouling property, are improved by incorporating these water channels in membranes. The antifouling properties of cell membranes inspired the fabrication of biomimetic membranes for separation processes [127,128,129,130]. A summary of the antifouling properties of common biodegradable membranes from several studies is provided in the following.
3.2. Biodegradable Membranes for Oil Fouling
Oil fouling is one of the major challenges involved in oil/water separation owing to the low surface energy of oil droplets. In this regard, several bio-inspired materials have been employed to fabricate different filters with self-cleaning and antifouling properties [131]. Chitosan (CS) is among the bio-based polymers, containing hydroxyl and amino groups and showing hydrophilic behavior in air and oleophobic properties in water. In addition to those characteristics, its biocompatibility, non-toxicity, and low cost allow chitosan to be an excellent alternative to treat oily wastewater with a separation efficiency of more than 99% [113,132,133,134,135,136]. Chitosan can also be used as an adsorbent for removing dyes, macromolecules, and heavy metals from a system with a high permeate flux recovery rate [137,138]. Zhang et al. [113] used the chitosan-coated mesh to remove the oil from the hypersaline environments and solution at various pH levels. Their research on fabricating a bio-inspired filter with stable wettability in complex environments was inspired by shrimp shells. Shrimp shells are both hydrophilic and oleophilic in the air (contact angles of about 41° and 24.7° for water and oil, respectively). However, the shells exhibit a superoleophobic property once placed in seawater (oil contact angle > 150°). CS is the deacetylation form of chitin, which is the major component of shrimp shells. Despite chitin, CS can be dissolved in an acid solution and form filtration films. The spin coating technique was used to fabricate the CS film, and the result showed that the water contact angle of the film in the air and the oil contact angle in the water was about 7° and 155°, respectively. Krishnamoorthi et al. [116] also used chitosan (CS) and caffein acid (CAF) as the biodegradable coating agents on cotton fibres to prepare a superwettable filter for oil/water separation. The hydrophilicity (contact angle of almost 0°) and underwater superoleophobicity (oil contact angle ≥ 160°) of those polymers result in high permeate flux (up to 50,050 L h−1 m−2 bar−1) and separation efficiency (>99.9%) under low applied pressure (0.1 bar). Despite its inability to form a film, chitin can be used as an additive to the polymer solution or as a coating agent to modify the surface properties of membranes. In the research conducted by Goetz et al. [139], chitin nanocrystals were coated on electrospun cellulose acetate (CA) to improve its antifouling properties. The nanofibrous CA has hydrophobic surfaces with a water contact angle of about 132°. However, the results showed that the cellulose acetate/chitin membrane turned superhydrophilic characteristics (water contact angle of almost 0°). The modified membranes were found to be less prone to biofouling, especially toward the solutions which contain bovine serum albumin (BSA) and humic acid (HA), such that no flux decay was observed over a complete filtration cycle. In recent decades, chitosan-based composite membranes have gained increasing attention. The antifouling properties of chitosan have been found to be useful in developing membrane separation processes for water purification and wastewater treatment [138,140,141,142,143,144,145,146].
3.3. Polyvinyl Alcohol (PVA) Membranes
Polyvinyl alcohol (PVA) is another biodegradable, non-toxic polymer with inherent hydrophilicity and antifouling properties. PVA is one of the most favorable polymers that can be used for membrane fabrication and exhibits high mechanical, thermal, and pH stability [143,147,148,149,150]. PVA membranes are useful for wastewater treatment applications due to their oleophobic and highly polar nature, which minimizes foulant adsorption and the adhesion between the membrane surface and water contaminants [11,143,147,151,152]. Hydrophilic membranes appear to be less prone to fouling formation, especially toward organic matters like proteins in water, and it is much easier to clean the attached particles or recover the permeate flux [153]. Such membranes absorb water molecules much more easily, resulting in the formation of a hydration layer that protects the membrane from particle accumulation [114,117]. Amanda et al. [153] evaluated the protein fouling of PVA ultrafiltration membranes using diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS). They compared their results with fouling formation on polyethersulfone (PES) as one of the most commonly used ultrafiltration membranes. According to their findings, PVA membranes had a higher resistance to protein fouling. PVA membranes can also be used for oil/water separation. According to Wu et al. [154], the flux recovery ratio of ultrafiltration PVA membrane was about 95% after washing the fouled membrane with pure water, which demonstrates the excellent antifouling properties of the membrane toward the oily wastewater. Wang et al. [155] also fabricated a high performance PVA-based composite membrane for oil/water separation. As the support layer, they prepared a PVA nanofibrous scaffold and coated it with PVA hydrogel as the anti-fouling layer. The hydrophilic nanofibrous membrane was found to have a filtration efficiency of more than 99% with no flux decay over the filtration time, which was similar to the commercial UF membrane with Pebax 1074 coating. However, the permeate flux of the prepared membrane was more than twofold that of commercial UF membrane (about 130 LMH for electrospun composite PVA membrane and 60 LMH for commercial UF membrane). Similar to chitosan, PVA has been employed in several studies to fabricate biodegradable fouling-resistant composite membranes [128,156,157,158,159,160,161].
3.4. Cellulose Acetate (CA) Membranes
Cellulose acetate (CA) is among the natural polymers with excellent mechanical stability. In light of their high hydrophilicity, antifouling, and film-forming properties, cellulose and its derivatives have attracted considerable interest as potential membranes for water/wastewater filtration [127,162,163,164,165,166,167,168,169,170,171]. Several membrane filtration applications have been conducted with cellulose-based membranes, including separating bacteria, salt, dye, protein, oil, and heavy metal from the aqueous solutions [2,131,162,172,173,174,175,176]. Lv et al. [172] reported the antifouling property and filtration performance of the fabricated cellulose-based membranes for protein solution filtration. To improve the hydrophilicity and antifouling property of the flat sheet cellulose diacetate membrane (CDA), cellulose nanocrystals (CNCs) were added into the casting solution. The results of their research indicated that the protein adsorption (bovine serum albumin) decreased by about 48%, and the flux recovery ratio (FRR) after three complete filtration cycles improved by 50%. In another study by Kanagaraj et al. [177], hydrophilic CA ultrafiltration membranes modified with biodegradable materials, polyurethane, and lactic acid, were prepared for the removal of humic acid and copper ions. The prepared membrane exhibited enhanced antifouling and hydrophilicity properties. The flux recovery rate was 97.8% and 92% for humic acid and copper ultrafiltration, respectively. Additionally, low irreversible fouling was reported for both contaminants (2.2% for humic acid and 7.8% for copper), demonstrating the superior antifouling property of the prepared biodegradable membrane. Ye et al. [178] compared the adsorption of blood proteins by CA membranes and non-biodegradable polyethersulfone (PES) membranes. The adsorption of albumin protein by PES membrane was about 2.5 times that of CA membrane. Protein adsorption decreased by 50% (<0.5 μg.com−2) for the modified CA membrane with a biocompatible phospholipid polymer (poly(2-methacryloyloxyethyl phosphorylcholine (MPC)-co-n-butyl methacrylate (BMA))).
3.5. Polylactic Acid (PLA) Membranes
Recent studies have shown that polylactic acid (PLA) biopolymers can be an excellent alternative to conventional thermoplastic membranes used in separation applications [179,180,181,182]. The polar oxygen linkage in PLA membranes contacts the water molecules over the separation of aqueous solutions and results in a higher permeate flux and decreased foulant attachment [183]. Khalil et al. [180] prepared an ultrafiltration PLA membrane through the phase inversion method and evaluated its antifouling property using the BSA solution. After one filtration cycle, the flux recovery ratio was about 57% for membranes containing 15 wt% PLA polymer, and it reached 93% as the PLA concentration increased to 22 wt%. For membranes containing 22 wt% of PLA, the permeate flux recovery ratio after the second cycle was about 85% (decreased only 8%), indicating high fouling resistance of the fabricated membranes. Although PLA membranes demonstrated good antifouling properties, their hydrophobic characteristic and brittleness at room temperature may hinder their use in various applications. Therefore, a number of techniques, including blending, self-assembly, and surface grafting and coating have been used to enhance the hydrophilicity, mechanical stability, and fouling resistance of those membranes [1,184]. In terms of fouling resistance and hydrophilicity, Ouda et al. [7] proposed hydroxyapatite (Hap) as a filler and polydopamine (PDA) as the surface coating for PLA membranes. It was reported that the water permeability of PLA membrane improved by 120% when only 2 wt% Hap/1-h PDA was employed in the system. After three complete filtration cycles, the flux recovery ratio (FRR) improved from 20% for pristine PLA membranes to 54% for composite membranes. The irreversible fouling of non-organic material removal was also decreased from 79.7% to 70.7%. In fact, all of those promising findings were the result of improvements in membrane contact angle, porosity, and mean pore size. In another research, the bio-based β-cyclodextrin (β-CD) was also employed as the pore foaming to improve water permeability in the PLA membrane due to its hydrophilic exterior [185]. The SEM images of the surface and cross-section of the PLA membrane show that it was composed of a dense skin layer with interconnected internal pores. However, the addition of 5 wt% of β-CD to PLA solution led to the fabrication of membranes with homogenous pores on the surface (mean size of about 1 µm) and some finger-shaped pores in cross-section. As a result of changes in membrane morphology, the pure water flux and the flux recovery ratio improved by about 230% and 50%, respectively.
4. Green Membrane Technology Applications
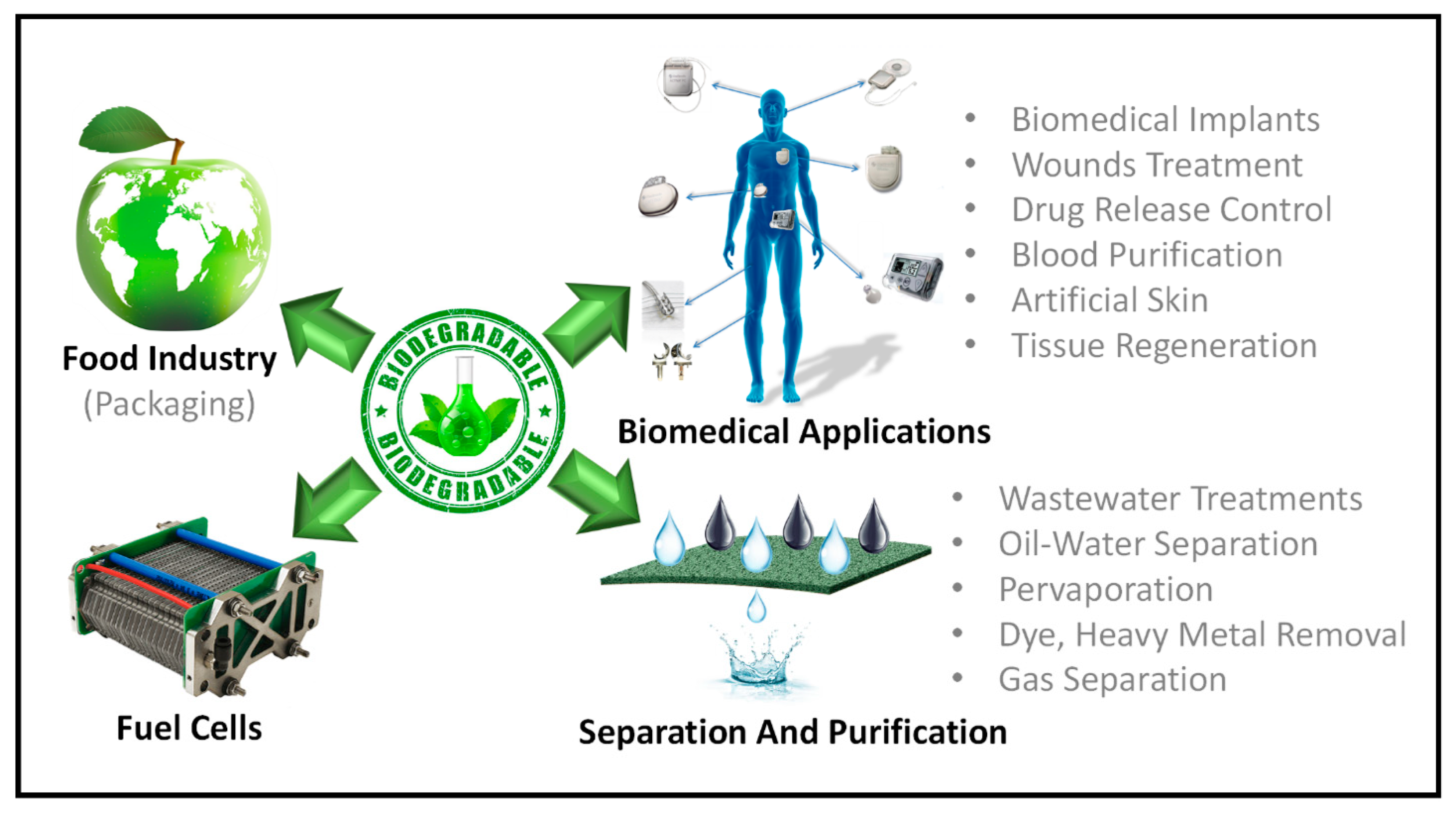
Biodegradable membranes have been employed in a variety of industrial and biomedical applications. Figure 11 summarized the applications of biodegradable membrane. In Table 1, various applications of green membranes and their advantages/disadvantages are discussed. Following are some of the most prevalent applications of these membranes.
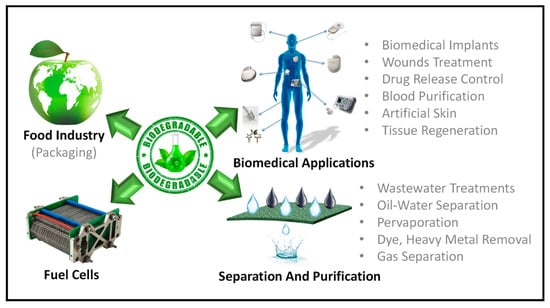
Figure 11.
Applications of biodegradable polymers-based membranes.
4.1. Wastewater Treatment
Membrane technology has been extensively used for water purification, wastewater treatment, and seawater desalination due to its superior separation performance, ease of operation, and reasonable energy consumption [2]. One of the most common applications of biodegradable membranes is wastewater treatment, including but not limited to oil separation and removing heavy metals, dye, pathogens, and proteins from aqueous solutions. Biodegradable membranes are strong candidates for wastewater treatment owing to their excellent stability, high durability, energy efficiency, high permeability, and selectivity [2]. Depending on the industry that releases wastewater and the pollution type, various membranes can be used in filtration processes. For instance, in oil absorption (oil/water separation), membranes with underwater superoleophilic/superhydrophobic characteristics are more desirable. On the other hand, composite membranes, containing hydrophilic materials such as PVA, CS, CA, etc., have been widely used to remove dyes from industrial wastewater [8,78,186,187,188].
PLA is among the biodegradable materials that can be used to prepare a porous superhydrophobic membrane and replace the non-biodegradable commercial membranes such as polyvinylidene fluoride (PVDF), polytetrafluoroethylene (PTFE), polypropylene (PP), etc. [113,116,179,189,190,191,192,193]. Su et al. [179] prepared the superhydrophobic PLA membrane with excellent oil adsorption capacity using non-solvent induced phase separation. The preparation method may affect the surface roughness, porosity, and free energy of the membrane and its hydrophobicity. In their study, the prepared membranes displayed a high water contact angle of about 152°, and they were capable of absorbing oils by 4–5 times their weight.
Colorant effluent is one source of severe water pollution that can cause serious concerns for human and marine lives. The colorant effluent, mainly from the textile industry, may have low molecular concentrations and inert properties, which makes it difficult to remove from wastewater streams [143]. Adsorption techniques using bridgeable materials are innovative methods for removing dyes from industrial effluents. Incorporating nature-based nanoparticles or polymers with high adsorption capacities would also improve the separation efficiency of the filtration membranes and at the same time maintain their biodegradability [194]. In this regard, Tahazadeh et al. [176] employed porous carbon nanoparticles to improve the adsorption capacity of biodegradable CA membrane for Methylene Blue (MB) removal. The prepared membrane showed excellent reusability with 98.2% of MB removal and 76.03 LMH of water flux. Aside from dye removal, biodegradable composite membranes have been employed in several applications for heavy metal, pathogen organic, and inorganic matter removal [7,138,182,195,196,197,198,199]. Table 1 represents various studies that utilized biodegradable membranes in wastewater treatment applications.
4.2. Gas Separation
The separation of pure gases from gas mixtures is another critical application of biodegradable membranes. Gas separation in membrane-based applications mostly relies on the solution-diffusion mechanisms. Thus, the permeability and selectivity of each component in membranes play a crucial role in the separation process [2]. A common gas separation process is removing CO2 and H2S from natural gas or to recover N2, H2, and O2 from gas mixtures [2,200]. Some functional groups in the membrane structure could facilitate gas transportation and improve the separation efficiency. For instance, amine groups in biodegradable membranes like chitosan and PVA could enhance the CO2 separation from a gas mixture [200,201]. In fact, amine groups act as carriers for Co2 molecules and improve the gas transport through the reversible reactions presented in Equation (1) [201].
CO2 + NH2 + H2O ↔ HCO3− + NH3+
Upon exposure to gas mixture, amino groups and water in the film react with carbon dioxide to form bicarbonate ions. As the bicarbonate ions passed through the film, they were converted back into carbon dioxide at the permeate side [201].
Furthermore, the morphology and structure of polymers influence gas selectivity and separation efficiency such that glassy polymers pose the biggest challenge to gas separation processes because of non-selective voids in their structure. Therefore, rubbery polymers are more favorable for light gas separation [200]. Biodegradable rubbery polymers like polyurethanes have been widely used in gas separation applications, owing to their film-forming property, excellent selectivity, and permeability [200,202,203]. Considering the effect of polymer structure, the free volume, packing density, and chain mobility would also affect the separation efficiency [204]. To address this challenge, biodegradable fillers with desirable selectivity would be incorporated into the polymeric membranes (mix matrix membranes) to modify the polymer structure and improve the gas permeability. In order to robust the permeability and selectivity or even mechanical and thermal stability of the membrane, a composition of biodegradable polymers (mix matrix membranes) would also be used in the separation process [200,201,204,205,206]. A number of studies have examined the use of biodegradable membranes in gas separation, as shown in Table 1.
4.3. Pervaporation
As one of the emerging technologies, Pervaporation has experienced rapid development in recent decades. This technology involves the membrane separation and liquid-to-vapor phase transition through the pressure difference between the feed and permeate sides (as the driving force) and works based on the difference between the diffusion/sorption of the components in the mixtures [207,208]. In order to create the pressure difference, a vacuum pump is employed at the permeate side, resulting in the vaporization and transport of the components with a higher affinity. Pervaporation is an easily performed, which is an environmentally friendly and energy-efficient process used to separate an organic compound from a dilute liquid mixture or an organic mixture with a close boiling point and dehydration of azeotropic- aqueous organic mixtures [208,209,210,211]. Biodegradable membranes have been increasingly used in pervaporation systems to improve the sustainability of the process. However, the efficiency and performance of this process are highly dependent upon the membrane’s selectivity and its ability to separate the particular components from liquid mixtures [207,209]. Membranes with polar characteristics and aromatic groups such as CS, PVA, agarose, alginate, and fluorinated polymers are favorable for organic dehydration due to their hydrophilic property and high water selectivity [211]. Conversely, hydrophobic membranes like poly-dimethyl siloxane [211] polyurethane urea [212] are more suitable to remove non-polar components (mostly organic matters) from dilute solutions. PLA, PHA, CS-based, PVA-based, and mixed matrix membranes are the most common biodegradable membranes for boiling azeotrope mixtures like methanol-methyl tert-butyl ether or methanol-toluene [210,213,214,215]. Table 2 summarizes the use of biodegradable membranes in various pervaporation processes.
4.4. Biomedical Applications
Bio-inspired and biodegradable membranes have been gaining increasing attention for their potential applications in biomedical fields, including tissue engineering (dental implants, rebuilding of bone, artificial skin, liver, nerve, etc.), drug delivery, and hemodialysis/hemodiafiltration due to their low toxicity, osteoconductivity, antibacterial properties, physical/mechanical properties, formability, versatility with multiple functions, and sterilization capability [9,216,217,218,219,220,221]. The main purpose of tissue engineering is to recover or replace damaged body tissues and organs [217]. A variety of bio-inspired materials are being investigated for use in tissue engineering. Biodegradability, bioadhesivity, and bioactivity of chitosan make it a highly biocompatible biopolymer suitable for use in a variety of biomedical applications [222]. Chitosan and chitin-based materials are commonly used in tissue engineering in a variety of physical forms since they are capable of accelerating the wound healing by providing the tissue cells with an extracellular microenvironment [9,223,224,225]. Vivcharenko et al. [225] prepared a biodegradable chitosan-based film and used it to stimulate the wound healing process and repair the injured skin. The results of their research demonstrated that the prepared chitosan/agarose film has all the required characteristics to be served as an artificial skin substitute. Biodegradable membranes have also exhibited to be promising candidates for drug delivery systems and improving the treatment efficiency [216,217,226]. Obtaining therapeutic effects in host cells requires control of the dose and localization of drug release by preparing the porous materials with relevant size scales (nanometer for small molecules to a few tens of nanometer for macromolecules’ delivery). In this regard, Bernards and Desai [226] used the template etching method to prepare a nanoporous membrane for drug delivery purposes using the poly(caprolactone) (PCL) biodegradable polymer. For the diffusion experiment, they employed sodium fluorescein (SFC) as the small molecule (1 nm) and fluorescein isothiocyanate-labelled bovine serum albumin (FITC-BSA) as the macromolecule (7 nm). FC and FITC-BSA released at a normal first-order diffusion (exponential release) and zero-order (constant release), respectively. The thin film porous PCL membrane fabricated in this study was found to be useful in flexible therapeutic devices. Hemodialysis is another process in which membranes are used to remove blood toxins. Conventional membranes have been widely used for hemodialysis; however, their hydrophobic nature could negatively affect their biocompatibility and cause blood clotting [178,227]. Cellulose based membranes with high hydrophilicity are still used for HD membranes production, despite of development of other types of polymeric materials for biomedical applications [168,228]. PES is still the most spread polymer for HM membrane fabrication, though its surface modification is required to improve its hemocompatibility [229,230]. Table 1 presents further studies on biomedical applications of biodegradable membranes.
4.5. Food Packaging
In recent decades, a number of studies have been conducted on the development and application of biodegradable membranes/films in food packaging due to their antimicrobial, non-toxicity, antifungal, controlled permeability, good flexibility, lightness, and transparency [10,145,223,231,232,233,234,235,236,237]. Antimicrobial packaging is a promising method to improve food safety by preventing the growth of pathogenic, spoilage microorganisms, and certain bacteria [231,238]. Among the biodegradable materials, chitosan and bacterial cellulose have shown excellent antimicrobial properties and have been extensively used in food packaging [223,232,235,237]. The chitosan membranes also proved to have a low permeability to O2, N2, and CO2 and moderate permeability to water [223]. PLA is the most used biodegradable material for food packaging applications [239]. However, there are some limitations to using PLA films in the food industry, including its mechanical stability, antimicrobial, and barrier performance. In order to overcome those limitations, several techniques have been implemented, and PLA films have been modified with other biodegradable materials such as PHB, tea polyphenols (TP), and limonene [239,240,241,242,243]. PVA is another biodegradable polymer, commonly used with CS to fabricate the composite film for food packages owing to its good film-forming property [145,244,245]. Water and water vapor resistance property is a key factor for membranes/films employed in food packaging applications. Incorporating nanocellulose into polymeric membranes could improve the water barrier feature of biodegradable films by the creation of nanocrystal networks that prevent swelling and water absorption [234]. According to Chen et al. [246], the nanocellulose filled PVA films (1% nanocellulose loading) showed a 9.5% improvement in water vapor barrier property after 166 h of testing. An overview of biodegradable membranes in food packaging is presented in Table 1.
4.6. Fuel Cell
Fuel cells (FC) are a sustainable energy source with high efficiency that converts the chemical energy of the organic matters (fuels) into electricity and can replace fossil fuels [247]. This technology has a low environmental impact and can be employed in various devices to reduce global pollutions [2]. A fuel cell consists of an anodic and cathodic chamber as well as an electrolyte that separates the two chambers and facilitates the transfer of ions. In FC, membranes can be used as electrolytes, known as proton exchange membranes [248,249]. The most common membrane used in FC is Nafion, which has a high production cost and environmental impact [250]. Therefore, using the biodegradable membranes could be a promising alternative to Nafion. González-Pabón et al. [251] prepared biodegradable membranes using PVA, CS, and their compositions, and they compared their performance in a microbial fuel cell (MFC) to Nafion commercial membrane. According to their findings, PVA/CS membranes had a lower O2 permeability compared to Nafion, resulting in maintaining the anaerobic condition in MFC. They also reported that the PVA/CS composite membrane led to higher power production and lower cost, as well as less environmental impact. Aside from their high cost and environmental impact, Nafion membranes have shown a high fuel crossover (methanol or hydrogen leakage) and low ion conductivity in electrolyte membrane FC. Chitosan and alginate-based bio-membranes are strong candidates to tackle this issue [30,250,252,253]. Smitha et al. [254] prepared a chitosan/sodium alginate composite membrane and used that for the methanol fuel cell. The composite membrane exhibited an excellent mechanical property with low methanol permeability (4.4 × 10−8 cm2/s for CS/SA and 27.6 × 10−8 cm2/s for Nafion117) and favorable proton conductivity. In addition, they noted that the membrane was fabricated using a simple and cost-effective technique. In another study [253], they prepared PVA/CA and PVA/CS composite membranes and evaluated their performance in methanol fuel cell. Similar to previous research, the composite membranes demonstrated lower methanol permeabilities in comparison with Nafion117. More researches on using biodegradable membranes in proton exchange membrane fuel cells are presented in Table 2.

Table 2.
An overview of the applications of biodegradable membranes.
Table 2.
An overview of the applications of biodegradable membranes.
| Polymer | Application | Advantage | Disadvantage | Ref |
|---|---|---|---|---|
| PLA/PBS/SNPs PLA/PPC/SNPs PLA/PHB/SNPs | Oil/water separation | Favourable thermal stability, high separation efficiency and turbidity removal (>98% and >89%, respectively) | Low metal ion and dissolved solid removal | [1] |
| CS | Oil/water separation | Excellent underwater superoleophobicity, stable wettability in various pH, >99% oil separation efficiency | Low diesel removal in saline solutions | [113] |
| CAF/CS/CF | Oil/water separation | Superhydrophilicity and underwater superoleophobicity property, >99% separation efficiency, good reusability, high recyclability | [116] | |
| PLA | Oil/water separation | Superhydrophobic properties, excellent oil absorption capacity, antifouling property, not complicated preparation method | [179] | |
| PLA | Oil/water separation | Superoleophilic and superhydrophobic property, excellent reusability | Low oil adsorption capacity | [189] |
| PLA/TiO2 | Oil/water separation | Superhydrophilicity and underwater superoleophobicity, long-term behavior, excellent separation efficiency (>99%), high permeate flux, antifouling property | Low BSA adsorption capacity | [190] |
| PLA | Oil/water separation | Robust recyclability, excellent adsorption capacity | [191] | |
| CA/PVP | Oil, dye, metal removal | Excellent removal efficiency for oil, dye and metal ions (>99%), long-term reusability, super hydrophilicity and underwater superoleophobicity | [192] | |
| CA | Oil/water separation | Robust recyclability, antifouling property, high infuse flux, enhanced adsorption capacity | lower infused flux for solutions, containing crude oil | [193] |
| CA/MOFDPC | Dye removal | Excellent reusability, High MB removal, long term behaviour, high water flux | Lower tensile strength and flexibility compared to CA membrane | [176] |
| CA/Cotton | Dye removal | MB rejection of 98% over 8 filtration cycle, high durability, low fouling, high mechanical stability | Tested for low volume wastewater (5–10 mL, dead end filtration setup) | [186] |
| CS/PVA | Dye removal | Strong interaction between CS and PVA, higher tensile strength, stability in DI, acidic and alkaline medium | Lower thermal stability compared to pristine membranes | [8] |
| CS/GO | Dye removal | At least 95% of cationic MB removal | GO size affected the anionic MB removal such that the maximum removal efficiency was 64% for nanoscale GO | [187] |
| PLA/CS | Dye removal | Large porous framework though a nanofibrous structure, High removal capacity of 86.43 and 82.37 mg/g for rhodamine B and MB | Adsorption capacity reduction after fourth cycle | [255] |
| PCL/MXene | Dye removal | High hydrophilicity and water permeability by adding only 4 wt% of MXene, more than 99% or crystal violet removal | Lower CV rejection after adding MXene nanoparticles | [78] |
| CS/PVA/MMT | Heavy metal removal | High hydrophilicity and water flux, antibiofouling property, 84–88.34% of Chromium removal efficiency | Low removal efficacy after 1 h and at high pH (pH of 9) | [138] |
| PLA/PBS | Heavy metal removal | Heat resistance improvement, enhanced hydrophilicity and mechanical stability, about 83% of ion removal efficiency (cobalt and nickel), antifouling property | [182] | |
| CS/PVA | Heavy metal removal | Improved chemical stability in acidic medium, enhanced hydrophilicity and anti-swelling, good adsorption recovery | Adsorption capacity decay by increasing the Cd (II) concentration (about 95% at 40 mg/L to 75% at 50 mg/L) | [195] |
| CS/Cellulose | Heavy metal removal | High removal efficiency of 85% and 94% for Pb and Cd, respectively, Excellent adsorption capacity for Pb, Cd | Low removal efficacy and adsorption capacity for Cr. | [256] |
| SCS/PVA | Heavy metal removal | Up to 90% of CU ion removal after 3 h, improved areal swelling | Low Ni ion removal | [196] |
| PLA-HAp/PDA | NOM removal | Antifouling properties, Flux improvement, thermal stability, more resistance to damage in harsh environment | Reversible fouling increased from 3.6% to 10.5 % | [7] |
| PLA | NOM removal | Enhanced BSA removal up to 92%, improved antifouling property, increased FRR from 57% to 93% | Lower water flux by increasing the PLA concentration | [180] |
| PCL/PBS | Wastewater treatment | Enhanced hydrophilicity and biodegradability, improved water flux and FRR, and pollution rejection | Lower mechanical properties compared to neat PCL | [197] |
| PLLA/PDLA/AlCl3 | Pathogen removal | Excellent filtration efficiency, high porosity, small pore size, low pressure drops | [198] | |
| PBS/CA/DEX | Dairy wastewater treatment | Enhanced porosity and hydrophilicity, robust permeate flux, antifouling property, >99% turbidity removal | Foulant rejection decreased, decreased mechanical property, low TDS removal | [199] |
| PU/PVA | Gas separation | Enhanced CO2 solubility, high CO2/N2 and CO2/CH4 selectivity | Low permeability for pure gases such as N2 and O2 | [200] |
| CS/DS-PVA | Gas separation | Robust self-healing efficiency, enhanced CO2/N2 selectivity, amino groups increase the CO2 permeance | Lower CO2 and N2 permeability by increasing the PVA concentration | [201] |
| PU | Gas separation | Improved mechanical and chemical stability, high CO2 separation | Low CO2/H2 selectivity | [203] |
| PLA/PBS/MWCNT | Gas separation | Improved hydrophilicity, tensile strength, porosity, and crystallinity, enhanced pure gas permeability (Ar, CO2, H2, and N2), H2/N2 selectivity improvement | Low selectivity and no improvement for CO2/N2, Ar/N2, and CO2/Ar | [204] |
| PLA/layer silicate | Gas separation | Improved thermal and mechanical stability, enhanced pure gas permeability by increasing silicate concentration | Decreased CO2, N2 and O2 permeability by increasing clay content | [205] |
| CS/PVA | Gas separation | Improved specific surface area, high CO2 adsorption capacity | [206] | |
| PVA/CNC | Gas separation | Increased CO2 permeability and CO2/N2 separation factor | [257] | |
| CMS | Gas separation | High specific area, pore volume, and CO2 absorption capacity, improved N2 absorption, | [258] | |
| CS/PHB/MWCNT | Pervaporation (1,4-dioxane/water) | Low swelling degree compared to pristine CS, improved mechanical properties, improved water selectivity by increasing the 1,4-dioxane concentration in feed | Not significant water selectivity improvement for mixed matrix membrane compared to pristine CS | [207] |
| SA/PCL/GO | Pervaporation (Alcohol/water) | Excellent membrane hydrophilicity and dehydration performance for alcohol-water, water flux and separation factor improvement | Increased swelling degree by increasing GO content | [208] |
| CS/FGS | Pervaporation (isopropanol/water and ethanol-water) | Good isopropanol and ethanol barrier, good water selectivity | Low water permeability | [209] |
| PLA | Pervaporation (MeOH/MTBE) | Good mechanical properties, good methanol selectivity | Enrichment factor dropped drastically by 75% as the methanol concentration changed from 1 wt% to 10 wt% | [210] |
| PUU | Pervaporation (phenol/water) | Low swelling degree, good separation factor and flux for phenolic components | Separation factor dropped by 60% as the phenol concentration changed from 0.1% to 0.4% | [212] |
| PHA/PHBHV | Pervaporation (MeOH/MTBE) | Good mechanical properties, favourable methanol selectivity | Reduced methanol selectivity by adding the additives (PEG, EBO) to the polymer solution | [215] |
| CS/PVA/NH2-MWCNT | Pervaporation (Isopropyl alcohol/water) | Improved mechanical property and separation factor and reduced swelling degree by adding NH2-MWCNT, excellent water flux and PSI | Lower separation factor compared to similar studies | [259] |
| PLA | Pervaporation (MeOH/MTBE) | Good mechanical and chemical stability, good methanol selectivity | Low permeate flux compared to similar researches | [260] |
| PVA/APS/MBA | Pervaporation (Ethanol/water) | Reduced swelling rate, high separation efficiency (95%), water flux, and membrane durability | Reduced selectivity by increasing the feed temperature (from 40 °C to 70 °C) | [261] |
| PGS APS | Pervaporation (Organic solvents/water) | Higher separation factor and PSI compared to commercial membrane (PDMS), good stability | [262] | |
| PLA/Fe-MOF | Pervaporation (MeOH/MTBE) | Improved methanol selectivity by adding Fe-MO and pressure increase (from 0 to 7.5 mbar) | Lower flexibility and mechanical strength by adding 0.5 wt% of Fe-MO, reduced selectivity by increasing the feed temperature (from 25 °C to 45 °C) | [263] |
| Agarose | Pervaporation (Organic solvents/water) | High water flux and permselectivity | [264] | |
| CS/Agarose | Artificial skin | Nontoxicity, high exudate absorption capacity, high elastic deformations, extracellular matrix similarity, support reproduction of skin fibroblast | [225] | |
| ALG/GC | Hepatocyte attachment | Increase spheroid formation, higher viability and mechanical property rather than alginate sponge | [265] | |
| PLLA/CS | Periodontitis treatment | Higher hydrophilicity, biocompatibility and bioactivity rather than electrospun PLLA membrane, higher cell reproduction, fibroblast barrier (mitigate the destructive effect of fibroblast on tissue recovery) | Lower degradation rate due to the presence of electrospun PLLA | [266] |
| CS/PEEK | Culturing the liver cells | Favourable microenvironment for liver cells, higher cell proliferation in 8–11 days, higher level of specific functions for longer time (compared to former substrates like collagen and PSCD) | [267] | |
| PCE | Biomedical implants | Elastomeric behavior, antibacterial activity, biomimetic mechanical property, photoluminescent capacity (favourable for real-time monitoring), high cytocompatibility and hemocompatibility, low inflammatory response | [268] | |
| GEL | Tissue regeneration | High tensile strength, improved water resistance, good biocompatibility | Low elastic and ductile characteristic in dry state | [269] |
| PU/PGSAP | Nerve tissue | Increasing the Schwann cells’ (SCs) myelin gene expressions, higher neurotrophin secretion, inducing neurite growth and elongation of PC12 cells, reducing the intracellular Ca+2 level | [270] | |
| PCL | Drug delivery | Constrained diffusion for drug release control, no negative effect on cells’ growth, cellular compatibility | Low diffusivity of water-soluble components like FS and FITC-BSA due to the hydrophobic nature of PCL | [226] |
| CA/Phospholipid | Blood purification | Good water and solute permeability, lower protein adsorption (compared to CA and commercial PES membranes), sharp molecule weight cut-off, permselectivity and antifouling property over a long-term filtration, good hemocompatibility | Lower dye and protein retention (compare to pristine CA membrane) | [178] |
| PLA (blended with three different copolymers: PA, PD, and PH) | Hemodiafiltration | Desirable protein and urea retention, improved hydrophilicity and permeate flux (compared to PLA), antifouling property, hemocompatibility | Relatively low lysozyme retention (17.4%) for PLA/PA | [184] |
| PLA/DA/HEP | Hemodialysis | Improved hemocompatibility, low platelet adhesion and hemolysis ratio, reasonable urea and BSA separation | Relatively low lysozyme retention (18%), it was bioincompatible with human blood. | [227] |
| PLA/ DA/GOC | Hemodialysis | High hydrophilicity and electronegativity, improved hemocompatibility, low platelet adhesion and hemolysis ratio, longer plasma recalcification time, high BSA separation | Lower lysozyme retention compared to pristine PLA, the hemocompatibility was not proved for human serum. | [228] |
| CS/PVA | Food packaging | Good antimicrobial property, molecular miscibility between PVA and CS, improved crystallinity | [238] | |
| PLA/limonene | Food packaging | Improved water barrier ability, excellent flexibility | Reduced oxygen barrier (still acceptable for food packaging) | [240] |
| PLA/TP | Food packaging | Improved antioxidant and antimicrobial activity | Reduced tensile strength and breakage elongation | [241] |
| PLA/PHB/limonene | Food packaging | Robust elongation at break, improved oxygen barrier, increased hydrophobicity | [242] | |
| PLA/ATBC/CS | Food packaging | Improved thermal and mechanical properties, reduced brittleness, increased transparency, good antifungal and antibacterial activity | Low water vapour barrier | [243] |
| PVA/CS | Food packaging | Good antimicrobial activity, improved thermal property | Reduced film stretchability, reduced water vapour barrier | [244] |
| PVA/CS/Silica | Food packaging | Improved tensile strength, increased oxygen and moisture barrier | Deferred resolving time | [245] |
| PVA/CS | Microbial fuel cell | Compared to Nafion: Reduced O2 permeability, lower cost, higher power generation, higher water uptake capacity environmentally friendly | Low proton conductivity | [251] |
| CSS | Methanol fuel cell | Much lower methanol cross over compared to Nafion 112, improved mechanical strength | Low breaking elongation compared to Nafion112 | [30] |
| PVA/CS/SA | Methanol fuel cell | Low methanol permeability, high ion exchange capacity, excellent thermal and mechanical stability | Low proton conductivity compared to Nafion117 | [253] |
| CS/SA | Methanol fuel cell | Low methanol permeability, excellent mechanical property, low cost | Low proton conductivity compared to Nafion117 | [254] |
| Graphene-PVA/CS | Methanol fuel cell | Improved methanol barrier, conductivity and ion selectivity compared to Nafion117 | Lower elongation break by adding graphene | [271] |
| CCS | Direct borohydride fuel cell | Higher ionic conductivity and power performance compared to Nafion212, cost effective, stable performance for >100 h | Higher borohydride crossover compared to Nafion212 | [272] |
| CS/PEO | Fuel cell | Improved conductivity, reduced swelling, low production cost | [273] | |
| PVA/GO | Ethanol fuel cell | Reduced water uptake and ethanol permeability, and improved proton conductivity by adding GO, higher power density compared to Nafion117 | Reduced elongation at break ratio | [274] |
| CS/PMC | Microbial fuel cell | Lower bioelectricity start-up time compared to Nafion117 and Agar salt bridge, acceptable power density, improved retention time | Antifouling property was not investigated | [275] |
5. Green Membranes Challenges and Limitations
Despite their wide range of potential applications, biodegradable membranes pose a number of challenges and limitations. The biocompatibility, mechanical properties, and degradability of the membranes, in terms of degradation behavior and degradation rate, must be thoroughly evaluated to determine their suitability for the desired applications [276]. Degradation rate/time is directly related to the type of the materials, the concentration of enzymes and microorganisms, pH, humidity, oxygen, and light levels in the surrounding environment. In other words, the degradability of different materials might vary depending on their surrounding environment [277]. The long-term biodegradation of some membranes would cause serious environmental problems owing to the increased waste disposal in nature. On the other hand, in biomedical applications where drugs should be slowly released into the environment or membranes should not degrade prior to tissue regeneration, rapid membrane degradation would have negative impacts. Thus, a thorough understanding of the membrane degradation mechanisms is necessary to determine the membrane’s performance in a particular application.
Another challenge is incorporating non-biodegradable or toxic materials into the biodegradable membranes (especially bio-based materials) through the blending, crosslinking, and copolymerization processes to improve their mechanical stability, thermal resistance, or to gain structural diversity and specific properties. The composition of green membranes with non-environment friendly materials results in secondary pollution since the biodegradable part degrades and the non-biodegradable material converts into small molecules that would persist in the environment for a very long time [2,10,231]. In addition, some biodegradable membranes are water-soluble. Therefore, degraded materials dissolve in water (ground/surface water) due to membrane breakdown and negatively affect the water quality and plant growth [2].
The economic performance of bio-based biodegradable membrane production has been evaluated and compared with conventional membrane production in several studies. Although it is essential to develop viable biodegradable alternatives in order to overcome the challenges associated with conventional membranes, bio-based biodegradable polymers have not been accounted for more than 1% of the total plastics production (335 million tons) in 2019. This is mainly due to their uneconomical production processes and expensive raw materials compared to low-cost fossil-fuel-based plastics [278,279,280,281].
6. Future Trends
Biodegradable polymers have been considered sustainable materials and proved to be promising alternatives for conventional membranes. As mentioned earlier, the biodegradability and degradation rate of the green membranes depend on the environment in which they are used. Biodegradable membranes may also cause waste disposal once the required conditions for their degradation are not provided. Thus, it is necessary to investigate the presence of various enzymes and microorganisms in different environments, their operating mechanisms, and required physical conditions to design and prepare a suitable membrane with enhanced biodegradation ability in nature. Over the past decade, society has increasingly moved toward environment-friendly materials that minimize the reliance on single-use polymeric membranes. Therefore, besides their biodegradability, the reusability of the membranes also plays a crucial role in preventing increased waste disposal. It is important to note that thermal and mechanical stability and swelling rates of biodegradable membranes should be thoroughly investigated. In order to improve the performance of the biodegradable membranes and non-toxic and biodegradable additives, nanoparticles and cross-linking agents are required to be incorporated into the membranes. These environmentally responsible materials preserve membrane sustainability and improve its biodegradation rate in nature.
Biodegradable membranes should also compete with conventional membranes in terms of production cost. In some cases, biodegradable membranes (especially biomimetic membranes) are manufactured on a pilot-scale due to their unique raw materials, resulting in high production costs. However, in order to run a sustainable experimental application, tons of those membranes must be produced. Thus, efficient scale-up models are required to fully address the production cost of biodegradable membranes [117]. In addition to existing biodegradable membranes, designing a new sustainable membrane with competitive performance and scalability needs to be explored in future studies.
7. Conclusions
Biodegradable membranes have proven to be an alternative to conventional membranes, especially in terms of permeate flux and antifouling properties. It has been successfully employed in various applications including wastewater treatment, gas separation, pervaporation, tissue engineering, food packaging, and fuel cell. In many cases, biodegradable membranes have shown comparable results to the commercial membranes such as PES, PVDF, and PTFE. The possibility to synthesize biodegradable membranes as well as to modify natural materials opens a wide opportunity to adjust and control the properties of resulted membranes, which significantly enlarges their application. One of the directions to create biodegradable materials is to incorporate biodegradable bonds into the initial chemical structure to retain the physical properties close to the properties of original material. Nevertheless, there are still a number of challenges and limitations associated with biodegradable membranes, including their degradation rate and its composition with non-biodegradable materials, which results in the waste disposal and secondary pollutions. Further studies are needed to determine the degradation mechanisms and behavior of microorganisms and enzymes under different environmental conditions. As biodegradable membranes are typically produced on pilot scales, efficient scale-up models are also needed to scale up the production process.
Author Contributions
Conceptualization: A.A.; Writing-Original Draft: M.E. and D.K.; Visualization: D.K.; Writing-Review & Editing: H.D., A.L. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (DG).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is available from the corresponding author (Amira Abdelrasoul; A.A.) on reasonable request.
Acknowledgments
The authors are thankful for Natural Sciences and Engineering Research Council of Canada (NSERC), University of Saskatchewan, and Toronto Metropolitan University for the support.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| ALG | Alginate |
| APS | Poly(1,3-diamino-2-hydroxypropane-co-polyol sebacinate) |
| ATBC | Tributyl o-acetyl citrate |
| BMA | Butyl methacrylate |
| BSA | Bovine serum albumin |
| CA | Cellulose acetate |
| CAF | Caffeic acid |
| CCS | Cross-linked chitosan |
| CD | Cyclodextrin |
| CDA | Cellulose diacetate |
| CF | Cotton Fiber |
| CMS | Carboxymethyl starch |
| CNC | Cellulose nanocrystals |
| CNT | Carbon nano-tubes |
| CS | Chitosan |
| CSS | Chitosan sulfate |
| DA | Dopamine |
| DEX | Dextran |
| DS-PVA | Dialdehyde starch-polyvinyl alcohol |
| FC | Fuel cell |
| Fe-MOF | Iron metal-organic Framework |
| FGS | Functionalized graphene sheets |
| FITC-BSA | Fluorescein isothiocyanate-labelled bovine serum albumin |
| FO | Forward osmosis |
| GC | Galactosylated chitosan |
| GO | Graphene oxide |
| GOC | Carboxylated graphene oxide |
| GEL | Gelatin |
| GO | Graphene oxide |
| HA | Humic acid |
| HAp | Hydroxyapatite |
| HEP | Heparin |
| MB | Methylene Blue |
| MBA | N,N’-methylene bisacrylamide |
| MeOH | Methanol |
| MFC | Microbial fuel cell |
| MMT | Montmorillonite |
| MOFDPC | Metal-organic framework derived porous carbon |
| MPC | Poly(2-methacryloyloxyethyl phosphorylcholine) |
| MTBE | Methyl tert-butyl ether |
| MWCNT | Multiwalled carbon nano tube |
| SCS | Sulfated chitosan |
| SFC | Sodium fluorescein |
| SNPs | Silica nanoparticles |
| PA | Poly(methyl methacrylate)-b-poly(2-acryloamido-2-methyl-1-propanesulfonic acid) |
| PBS | Polybutylene Succinate |
| PCE | Polycitrate-(ε-polypeptide) |
| PCL | Poly(caprolactone) |
| PD | Poly(methyl methacrylate)-b-poly(2-dimethylamino ethyl methacrylate) |
| PDA | Polydopamine |
| PDLA | Poly(D-lactic acid) |
| PDMS | Polydimethyl-siloxane |
| PEEK | Polyetheretherketone |
| PEO | Poly(ethylene oxide) |
| PES | Polyethersulfone |
| PEG | Polyethylene glycol |
| PGS | Poly(glycerol sebacate) |
| PGSAP | Poly(glycerol sebacate)-co-aniline pentamer |
| PH | Poly(methyl methacrylate)-b-poly(2-hydroxyethyl methacrylate) |
| PHB | Polyhydroxy butyrate |
| PHBHV | Poly(hydroxybutyrate-co- hydroxyvalerate) |
| PLA | Polylactic acid |
| PLLA | Poly(L-lactic acid) |
| PMC | Poly(malic acid-citric acid) |
| PP | Polypropylene |
| PPC | Polypropylene carbonate |
| PPF | Polypropylene fumarate |
| PSI | Pervaporation separation index |
| PTFE | Polytetraflouroethylene |
| PVA | Polyvinyl alcohol |
| PVDF | Polyvinylidene fluoride |
| PVP | Polyvinyl pyrrolidone |
| PU | Polyurethan |
| PUU | Polyurethan urea |
| SA | Sodium alginate |
| TDS | Total dissolved solid |
| TP | Tea polyphenol |
References
- Ghorbani, M.; Vakili, M.H.; Ameri, E. Fabrication and evaluation of a biopolymer-based nanocomposite membrane for oily wastewater treatment. Mater. Today Commun. 2021, 28, 102560. [Google Scholar] [CrossRef]
- Bandehali, S.; Sanaeepur, H.; Amooghin, A.E.; Shirazian, S.; Ramakrishna, S. Biodegradable polymers for membrane separation. Sep. Purif. Technol. 2021, 269, 118731. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Al Sharabati, M.; Abokwiek, R.; Al-Othman, A.; Tawalbeh, M.; Karaman, C.; Orooji, Y.; Karimi, F. Biodegradable polymers and their nano-composites for the removal of endocrine-disrupting chemicals (EDCs) from wastewater: A review. Environ. Res. 2021, 202, 111694. [Google Scholar] [CrossRef]
- Agarwal, S. Biodegradable Polymers: Present Opportunities and Challenges in Providing a Microplastic-Free Environment. Macromol. Chem. Phys. 2020, 221, 2000017. [Google Scholar] [CrossRef]
- Ouda, M.; Ibrahim, Y.; Kallem, P.; Govindan, B.; Banat, F.; Hasan, S.W. Highly permeable, environmentally-friendly, antifouling polylactic ultrafiltration membranes. J. Clean. Prod. 2022, 330, 129871. [Google Scholar] [CrossRef]
- Habiba, U.; Siddique, T.A.; Talebian, S.; Lee, J.J.L.; Salleh, A.; Ang, B.C.; Afifi, A.M. Effect of deacetylation on property of electrospun chitosan/PVA nanofibrous membrane and removal of methyl orange, Fe(III) and Cr(VI) ions. Carbohydr. Polym. 2017, 177, 32–39. [Google Scholar] [CrossRef]
- Suh, J.-K.F.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Choudhury, R.R.; Gohil, J.M.; Dutta, K. Poly(vinyl alcohol)-based membranes for fuel cell and water treatment applications: A review on recent advancements. Polym. Adv. Technol. 2021, 32, 4175–4203. [Google Scholar] [CrossRef]
- European Bioplastics, Bioplastics Market Data. Available online: https://www.european-bioplastics.org/market-update-2020-bioplastics-continue-to-become-mainstream-as-the-global-bioplastics-market-is-set-to-grow-by-36-percent-over-the-next-5-years/ (accessed on 23 July 2021).
- Bioplastics & Biopolymers Market by Type (Non-Biodegradable/Bio-Based, Biodegradable), End-Use Industry (Packaging, Consumer Goods, Automotive & Transportation, Textiles, Agriculture & Horticulture), Region—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/market-reports/biopolymersbioplastics-market-88795240.html (accessed on 23 July 2021).
- Rohrbach, K.; Li, Y.; Zhu, H.; Liu, Z.; Dai, J.; Andreasen, J.; Hu, L. A cellulose based hydrophilic, oleophobic hydrated filter for water/oil separation. Chem. Commun. 2014, 50, 13296–13299. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shan, H.; Zhang, W.; Li, B. 3D printed robust superhydrophilic and underwater superoleophobic composite membrane for high efficient oil/water separation. Sep. Purif. Technol. 2020, 237, 116324. [Google Scholar] [CrossRef]
- Xu, X.; Long, Y.; Li, Q.; Li, D.; Mao, D.; Chen, X.; Chen, Y. Modified cellulose membrane with good durability for effective oil-in-water emulsion treatment. J. Clean. Prod. 2019, 211, 1463–1470. [Google Scholar] [CrossRef]
- Yao, A.; Yan, Y.; Tan, L.; Shi, Y.; Zhou, M.; Zhang, Y.; Zhu, P.; Huang, S. Improvement of filtration and antifouling performance of cellulose acetate membrane reinforced by dopamine modified cellulose nanocrystals. J. Membr. Sci. 2021, 637, 119621. [Google Scholar] [CrossRef]
- Szekeres, G.P.; Németh, Z.; Schrantz, K.; Németh, K.; Schabikowski, M.; Traber, J.; Pronk, W.; Hernádi, K.; Graule, T. Copper-Coated Cellulose-Based Water Filters for Virus Retention. ACS Omega 2018, 3, 446–454. [Google Scholar] [CrossRef]
- Pei, X.; Gan, L.; Tong, Z.; Gao, H.; Meng, S.; Zhang, W.; Wang, P.; Chen, Y. Robust cellulose-based composite adsorption membrane for heavy metal removal. J. Hazard. Mater. 2021, 406, 124746. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Heydaripour, S.; Abdouss, M. Novel carboxymethyl cellulose based nanocomposite membrane: Synthesis, characterization and application in water treatment. J. Environ. Manag. 2016, 166, 457–465. [Google Scholar] [CrossRef]
- Li, S.; Wang, D.; Xiao, H.; Zhang, H.; Cao, S.; Chen, L.; Ni, Y.; Huang, L. Ultra-low pressure cellulose-based nanofiltration membrane fabricated on layer-by-layer assembly for efficient sodium chloride removal. Carbohydr. Polym. 2021, 255, 117352. [Google Scholar] [CrossRef]
- Zheng, K.; Zhou, S.; Cheng, Z.; Huang, G. Thin-film composite forward osmosis membrane prepared from polyvinyl chloride/cellulose carbamate substrate and its potential application in brackish water desalination. J. Appl. Polym. Sci. 2021, 138, 49939. [Google Scholar] [CrossRef]
- Elkony, Y.; Mansour, E.; Elhusseiny, A.; Ebrahim, S. Effect of cellulose acetate/cellulose triacetate ratio on reverse osmosis blend membrane performance. Polym. Eng. Sci. 2020, 60, 2852–2863. [Google Scholar] [CrossRef]
- Hoseinpour, V.; Ghaee, A.; Vatanpour, V.; Ghaemi, N. Surface modification of PES membrane via aminolysis and immobilization of carboxymethylcellulose and sulphated carboxymethylcellulose for hemodialysis. Carbohydr. Polym. 2018, 188, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Mubashir, M.; Dumee, L.F.; Fong, Y.Y.; Jusoh, N.; Lukose, J.; Chai, W.S.; Show, P.L. Cellulose acetate-based membranes by interfacial engineering and integration of ZIF-62 glass nanoparticles for CO2 separation. J. Hazard. Mater. 2021, 415, 125639. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, D.; Azcune, I.; Tanczyk, M.; Warmuzinski, K.; Jaschik, M.; Sandru, M.; Vankelecom, I.F.J. The performance of affordable and stable cellulosebased poly-ionic membranes in CO2/N2 and CO2/CH4 gas separation. J. Membr. Sci. 2018, 564, 552–561. [Google Scholar] [CrossRef]
- Liu, L.; Doherty, C.M.; Ricci, E.; Chen, G.Q.; De Angelis, M.G.; Kentish, S.E. The influence of propane and n-butane on the structure and separation performance of cellulose acetate membranes. J. Membr. Sci. 2021, 638, 119677. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Ali, Q.; Taweepreda, W.; Techato, K. Preparation and characterization of polymer electrolyte membrane from chloroacetate chitosan/chitosan blended with epoxidized natural rubber. Polym. Test. 2020, 82, 106294. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, M.; Guo, Z.; Cui, Z. Alternatively chitosan sulfate blending membrane as methanol-blocking polymer electrolyte membrane for direct methanol fuel cell. J. Membr. Sci. 2009, 337, 318–323. [Google Scholar] [CrossRef]
- Zhang, H.-P.; Gandhi, N.S.; Gu, Y.; Zhang, Y.; Tang, Y. Chitosan/graphene complex membrane for polymer electrolyte membrane fuel cell: A molecular dynamics simulation study. Int. J. Hydrogen Energy 2020, 45, 25960–25969. [Google Scholar] [CrossRef]
- Tang, C.C.H.; Zhang, L. Efficient adsorption of Hg2+ ions on chitin/cellulose composite membranes prepared via environmentally friendly pathway. Chem. Eng. J. 2011, 173, 689–697. [Google Scholar] [CrossRef]
- Shen, S.S.; Yang, J.J.; Liu, C.X.; Bai, R.B. Immobilization of copper ions on chitosan/cellulose acetate blend hollow fiber membrane for protein adsorption. RSC Adv. 2017, 7, 10424–10431. [Google Scholar] [CrossRef]
- Wu, J.X.; Zhang, J.; Kang, Y.L.; Wu, G.; Chen, S.C.; Wang, Y.Z. Reusable and recyclable superhydrophilic electrospun nanofibrous membranes with in situ cocross-linked polymer—Chitin nanowhisker network for obust oil-in-water emulsion separation. ACS Sustain. Chem. Eng. 2017, 6, 1753–1762. [Google Scholar] [CrossRef]
- Zhijiang, C.; Ping, X.; Cong, Z.; Tingting, Z.; Jie, G.; Kongyin, Z. Preparation and characterization of a bi-layered nano-filtration membrane from a chitosan hydrogel and bacterial cellulose nanofiber for dye removal. Cellulose 2018, 25, 5123–5137. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Solanki, H.K. RETRACTED: Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014, 105, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Prasannan, A.; Udomsin, J.; Tsai, H.-C.; Wang, C.-F.; Lai, J.-Y. Robust underwater superoleophobic membranes with bio-inspired carrageenan/laponite multilayers for the effective removal of emulsions, metal ions, and organic dyes from wastewater. Chem. Eng. J. 2020, 391, 123585. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Zarei, V.G.A. Preparation and characterization of biodegradable cellulose acetate-starch membrane. Polym.-Plast. Technol. Eng. 2013, 52, 387–392. [Google Scholar] [CrossRef]
- Woranuch, S.; Pangon, A.; Puagsuntia, K.; Subjalearndee, N.; Intasanta, V. Starch-based and multi-purpose nanofibrous membrane for high efficiency nanofiltration. RSC Adv. 2017, 7, 35368–35375. [Google Scholar] [CrossRef]
- Nabavi, M.M.K.; Ghaffarian, V.; Zabihi, E. Polyacrylonitrile/starch semibiodegradable blend membrane: Preparation, morphology and performance. Desalin. Water Treat. 2016, 57, 495–504. [Google Scholar] [CrossRef]
- Wu, B.T.H.; Wu, P. Preparation and characterization of anti-fouling β-cyclodextrin/polyester thin film nanofiltration composite membrane. J. Membr. Sci. 2013, 428, 301–308. [Google Scholar] [CrossRef]
- Adams, E.N.F.; Krause, R.; Hoek, E.; Mamba, B. Application of polysulfone/cyclodextrin mixed-matrix membranes in the removal of natural organic matter from wate. Phys. Chem. Earth Parts A/B/C 2014, 67, 71–78. [Google Scholar] [CrossRef]
- Smidsrød, G.S.O. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.L.Y.S.; Kim, J.-H. Dye adsorption characteristics of alginate/polyaspartate hydrogels. J. Ind. Eng. Chem. 2008, 14, 726–731. [Google Scholar] [CrossRef]
- Abdel-Halim, S.S.A.-D.E. Removal of heavy metals from their aqueous solutions through adsorption onto natural polymers. Carbohydr. Polym. 2011, 84, 454–458. [Google Scholar] [CrossRef]
- Lim, J.P.C.S. Synthesis of an innovative calcium-alginate magnetic sorbent for removal of multiple contaminants. Appl. Surf. Sci. 2007, 253, 5772–5775. [Google Scholar] [CrossRef]
- Chen, J.H.; Xing, H.T.; Guo, H.X.; Li, G.P.; Weng, W.; Hu, S.R. Preparation, characterization and adsorption properties of a novel 3-aminopropyltriethoxysilane functionalized sodium alginate porous membrane adsorbent for Cr(III) ions. J. Hazard. Mater. 2013, 248–249, 285–294. [Google Scholar] [CrossRef]
- Wang, Q.; Ju, J.; Tan, Y.; Hao, L.; Ma, Y.; Wu, Y.; Zhang, H.; Xia, Y.; Sui, K. Controlled synthesis of sodium alginate electrospun nanofiber membranes for multi-occasion adsorption and separation of methylene blue. Carbohydr. Polym. 2019, 205, 125–134. [Google Scholar] [CrossRef]
- Costa, R.S.F.; Boccaccini, A. Fibrous Protein-Based Biomaterials (Silk, Keratin, Elastin, and Resilin Proteins) for Tissue Regeneration and Repair Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Elsevier: Amsterdam, The Netherlands, 2018; pp. 175–204. [Google Scholar]
- Khosa, S.S.S.M.A.; Feng, X. Metal sericin complexation and ultrafiltration of heavy metals from aqueous solution. Chem. Eng. J. 2014, 244, 446–456. [Google Scholar] [CrossRef]
- Gao, A.; Xie, K.; Song, X.; Zhang, K.; Hou, A. Removal of the heavy metal ions from aqueous solution using modified natural biomaterial membrane based on silk fibroin. Ecol. Eng. 2017, 99, 343–348. [Google Scholar] [CrossRef]
- Shoulders, R.T.R.M.D. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, Y.; Hao, B.; Cao, Y.; Cui, Y.; Bi Shi, X. Collagen Fiber-Based Advanced Separation Materials: Recent Developments and Future Perspectives. Adv. Mater. 2022, 34, 2107891. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.K.F.; Shibue, T. Formation having a tanning gradient structure of collagen membrane by the pervaporation technique. J. Membr. Sci. 2000, 165, 169–175. [Google Scholar] [CrossRef]
- Maser, F.; Ströher-Glowienka, C.; Kimmerle, K.; Gudernatsch, W. Collagen film as a new pervaporation membrane. J. Membr. Sci. 1991, 61, 269–278. [Google Scholar] [CrossRef]
- Ackermann, J.U.; Müller, S.; Lösche, A.; Bley, T. Wolfgang Babel Methylobacterium rhodesianum cells tend to double the DNA content under growth limitations and accumulate PHB. J. Biotechnol. 1995, 39, 9–20. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; Wang, Y.; Yang, X.; Jung, J.; Li, Z.; Ren, X.; Sun, Y. Fabrication of pH-responsive hydrophobic/hydrophilic antibacterial polyhydroxybutyrate/poly-ε-caprolactone fibrous membranes for biomedical application. Mater. Chem. Phys. 2021, 260, 124087. [Google Scholar] [CrossRef]
- Lin, X.; Yin, M.; Liu, Y.; Li, L.; Ren, X.; Sun, Y.; Huang, T.-S. Biodegradable polyhydroxybutyrate/poly-ε-caprolactone fibrous membranes modified by silica composite hydrol for super hydrophobic and outstanding antibacterial application. J. Ind. Eng. Chem. 2018, 63, 303–311. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Q.; Cai, Z.; Zhao, K. Preparation and dye filtration property of electrospun polyhydroxybutyrate–calcium alginate/carbon nanotubes composite nanofibrous filtration membrane. Sep. Purif. Technol. 2016, 161, 69–79. [Google Scholar] [CrossRef]
- Tomietto, P.; Carré, M.; Loulergue, P.; Paugam, L.; Audic, J.-L. Polyhydroxyalkanoate (PHA) based microfiltration membranes: Tailoring the structure by the non-solvent induced phase separation (NIPS) process. Polymer 2020, 204, 122813. [Google Scholar] [CrossRef]
- Leja, G.L.K. Polymer biodegradation and biodegradable polymers—A review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Ummartyotin, C.P.S. Strategies for development and implementation of bio-based materials as effective renewable resources of energy: A comprehensive review on adsorbent technology. Renew. Sustain. Energy Rev. 2016, 62, 654–664. [Google Scholar] [CrossRef]
- Singhvi, D.G.M. Biomass to biodegradable polymer (PLA). RSC Adv. 2013, 3, 13558–13568. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, L.; Huang, R.; Bai, D.; Bai, H.; Zhang, Q.; Fu, Q. Ultrahigh-performance electrospun polylactide membranes with excellent oil/water separation ability via interfacial stereocomplex crystallization. J. Mater. Chem. A 2017, 5, 19729–19737. [Google Scholar] [CrossRef]
- Liu, X.; He, X.; Jin, D.; Wu, S.; Wang, H.; Yin, M.; Aldalbahi, A.; El-Newehy, M.; Mo, X.; Wu, J. A biodegradable multifunctional nanofibrous membrane for periodontal tissue regeneration. Acta Biomater. 2020, 108, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Leejarkpai, T.; Suwanmanee, U.; Rudeekit, Y.; Mungcharoen, T. Biodegradable kinetics of plastics under controlled composting conditions. Waste Manag. 2011, 31, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M. Properties and application of aliphatic polyester products. Biopolymers 2005, 20. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation Assessment of Poly (Lactic Acid) Filled with Functionalized Titania Nanoparticles (PLA/TiO2) under Compost Conditions. Nanoscale Res. Lett. 2019, 14, 56. [Google Scholar] [CrossRef]
- Lee, J.-S.; Lin, C.-J.; Lee, W.-C.; Teng, H.-Y.; Chuang, M.-H. Production of succinic acid through the fermentation of Actinobacillus succinogenes on the hydrolysate of Napier grass. Biotechnol. Biofuels Bioprod. 2022, 15, 9. [Google Scholar] [CrossRef]
- Le, S.D.; Nishimura, S. Highly Selective Synthesis of 1,4-Butanediol via Hydrogenation of Succinic Acid with Supported Cu–Pd Alloy Nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 18483–18492. [Google Scholar] [CrossRef]
- Guo, J.X.A.B.-H. Plastics from Bacteria: Natural Functions and Applications. Microbiol. Monogr. 2010, 14, 347–385. [Google Scholar]
- Mizuno, S.; Maeda, T.; Kanemura, C.; Hotta, A. Biodegradability, reprocessability, and mechanical properties of polybutylene succinate (PBS) photografted by hydrophilic or hydrophobic membranes. Polym. Degrad. Stab. 2015, 117, 58–65. [Google Scholar] [CrossRef]
- Almwli, H.H.A.; Mousavi, S.M.; Kiani, S. Preparation of poly(butylene succinate)/polyvinylpyrrolidone blend membrane for pervaporation dehydration of acetone. Chem. Eng. Res. Des. 2021, 165, 361–373. [Google Scholar] [CrossRef]
- Ghaffarian, V.; Mousavi, S.M.; Bahreini, M.; Jalaei, H. Polyethersulfone/poly (butylene succinate) membrane: Effect of preparation conditions on properties and performance. J. Ind. Eng. Chem. 2014, 20, 1359–1366. [Google Scholar] [CrossRef]
- Nelson, T.F.; Baumgartner, R.; Jaggi, M.; Bernasconi, S.M.; Battagliarin, G.; Sinkel, C.; Künkel, A.; Kohler, H.-P.E.; McNeill, K.; Sander, M. Biodegradation of poly(butylene succinate) in soil laboratory incubations assessed by stable carbon isotope labelling. Nat. Commun. 2022, 13, 5691. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Geravand, M.H.A.; Saljoughi, E.; Mousavi, S.M.; Kiani, S. Biodegradable polycaprolactone/MXene nanocomposite nanofiltration membranes for the treatment of dye solutions. J. Taiwan Inst. Chem. Eng. 2021, 128, 124–139. [Google Scholar] [CrossRef]
- Ho, T.T.-P.; Doan, V.K.; Tran, N.M.-P.; Nguyen, L.K.-K.; Le, A.N.-M.; Ho, M.H.; Trinh, N.-T.; Van Vo, T.; Tran, L.D.; Nguyen, T.-H. Fabrication of chitosan oligomer-coated electrospun polycaprolactone membrane for wound dressing application. Mater. Sci. Eng. C 2021, 120, 111724. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zong, Y.; Liu, Q.; Sun, Y.; Li, Z.; Wang, H.; Li, Z. A highly stretchable and biodegradable superamphiphobic fluorinated polycaprolactone nanofibrous membrane for antifouling. Prog. Org. Coat. 2020, 147, 105776. [Google Scholar] [CrossRef]
- Zafari, M.; Aghajani, S.; Boroujeni, M.M.; Nosrati, H. Vancomycin-loaded electrospun polycaprolactone/nano-hydroxyapatite membrane for the treatment of blood infections. Med. Hypotheses 2020, 144, 109992. [Google Scholar] [CrossRef]
- Nowwarote, N.; Chanjavanakul, P.; Kongdecha, P.; Clayhan, P.; Chumprasert, S.; Manokawinchoke, J.; Egusa, H.; Pavasant, P.; Osathanon, T. Characterization of a bioactive Jagged1-coated polycaprolactone-based membrane for guided tissue regeneration. Arch. Oral Biol. 2018, 88, 24–33. [Google Scholar] [CrossRef]
- Abdel-Motaal, F.F.; El-Sayed, M.A.; El-Zayat, S.A.; Ito, S.I. Biodegradation of poly (epsilon-caprolactone) (PCL) film and foam plastic by Pseudozyma japonica sp. nov., a novel cutinolytic ustilaginomycetous yeast species. 3 Biotech 2014, 4, 507–512. [Google Scholar] [CrossRef]
- MShung, A.K.; Timmer, M.D.; Jo, S.; Engel, P.S.; Mikos, A.G. Kinetics of poly(propylene fumarate) synthesis by step polymerization of diethyl fumarate and propylene glycol using zinc chloride as a catalyst. J. Biomater. Sci. Polym. Ed. 2002, 13, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Frazier, V.K.L.D.D.; Gerhart, T.N.; Altobelli, D.E.; Hayes, W.C. In-Vivo Degradation Of A Poly(Propylene-Fumarate) Biodegradable, Particulate Composite Bone Cement. MRS Online Proc. Library 1995, 394, 15–19. [Google Scholar] [CrossRef]
- Diez-Pascual, M.A. Mechanical properties of poly(propylene fumarate)/nanotube composites: Experimental results and theoretical predictions. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bangkok, Thailand, 24–26 February 2018; Volume 383. [Google Scholar]
- Díez-Pascual, A.L.D.-V.A.M. Multifunctional poly (glycolic acid-copropylene fumarate) electrospun fibers reinforced with graphene oxide and hydroxyapatite nanorods. J. Mater. Chem. B 2017, 5, 4084–4096. [Google Scholar] [CrossRef]
- Zhang, P.; Tian, R.; Lv, R.; Na, B.; Liu, Q. Water-permeable polylactide blend membranes for hydrophilicity-based separation. Chem. Eng. J. 2015, 269, 180–185. [Google Scholar] [CrossRef]
- Wolfe, M.S.; Dean, D.; Chen, J.E.; Fisher, J.P.; Han, S.; Rimnac, C.M.; Mikos, A.G. In vitro degradation and fracture toughness of multilayered porous poly(propylene fumarate)/beta-tricalcium phosphate scaffolds. J. Biomed. Mater. Res. 2002, 61, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, K.; Ohta, S.; Ito, T. Analysis of model drug permeation through highly crosslinked and biodegradable polyethylene glycol membranes. J. Membr. Sci. 2022, 645, 120218. [Google Scholar] [CrossRef]
- Cavallo, A.; Madaghiele, M.; Masullo, U.; Lionetto, M.G.; Sannino, A. Photo-crosslinked poly(ethylene glycol) diacrylate (PEGDA) hydrogels from low molecular weight prepolymer: Swelling and permeation studies. J. Appl. Polym. Sci. 2017, 134, 2. [Google Scholar] [CrossRef]
- He, X.C.H.; Lee, L.J. Design of a novel hydrogel-based intelligent system for controlled drug release. J. Control. Release 2004, 95, 391–402. [Google Scholar] [CrossRef]
- Tokuyama, Y.N.H.; Ban, T. Diffusion coefficient of solute in heterogeneous and macroporous hydrogels and its correlation with the effective crosslinking density. J. Membr. Sci. 2020, 595, 117533. [Google Scholar] [CrossRef]
- Asai, H.; bin Miswan, M.H.; Shimada, N.; Nakane, K.; Sakai, T.; Ogata, N. Preparation and characterization of a nanofiber mat consisting of Tetra-PEG prepolymers. J. Appl. Polym. Sci. 2014, 132, 41353. [Google Scholar] [CrossRef]
- Ulbricht, J.; Jordan, R.; Luxenhofer, R. On the biodegradability of polyethylene glycol, polypeptoids and poly(2-oxazoline)s. Biomaterials 2014, 35, 4848–4861. [Google Scholar] [CrossRef] [PubMed]
- Kawai, F. Microbial degradation of polyethers. Appl. Microbiol. Biotechnol. 2002, 58, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, F.K.a.H. Biodegradation of polyethylene glycol by symbiotic mixed culture (obligate mutualism). Arch. Microbiol. 1986, 146, 125–129. [Google Scholar]
- Ulrike Kohlweyer, B.T.; Schrader, T.; Andreesen, J.R. Tetrahydrofuran degradation by a newly isolated culture of Pseudonocardia sp. strain K1. FEMS Microbiol. Lett. 2000, 186, 301–306. [Google Scholar] [CrossRef]
- Fahmy, A.; Kamoun, E.A.; El-Eisawy, R.; El-Fakharany, E.M.; Taha, T.H.; El-Damhougy, B.K.; Abdelhai, F. Poly(vinyl alcohol)-hyaluronic Acid Membranes for Wound Dressing Applications: Synthesis and in vitro Bio-Evaluations. J. Braz. Chem. Soc. 2015, 26, 1466–1474. [Google Scholar] [CrossRef]
- Yang, C.C.H.J.M. Preparation and characterizaton of poly(dimethyl amino ethyl methacrylate) modified poly(vinyl alcohol) membrane by UV radiation for the permeation of 5-fluorouracil. J. Appl. Polym. Sci. 2012, 123, 3182–3188. [Google Scholar] [CrossRef]
- Ben Halima, N. Poly(vinyl alcohol): Review of its promising applications and insights into biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Market Volume of Polyurethane Worldwide from 2015 to 2025, with a Forecast for 2022 to 2029, Polyurethane Global Market Volume 2015–2029. Statista. Available online: https://www.statista.com/statistics/720341/global-polyurethane-market-size-forecast/ (accessed on 23 July 2021).
- Cooper, J.G.S.L. Advances in Polyurethane Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Zhang, C.; Wen, X.; Vyavahare, N.R.; Boland, T. Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet technique. Biomaterials 2008, 29, 3781–3791. [Google Scholar] [CrossRef]
- Pavlova, M.; Draganova, M. Biocompatible and biodegradable polyurethane polymers. Biomaterials 1993, 14, 1024–1029. [Google Scholar] [CrossRef]
- Wong, C.S.; Liu, X.; Xu, Z.; Lin, T.; Wang, X. Elastin and collagen enhances electrospun aligned polyurethane as scaffolds for vascular graft. J. Mater. Sci. Mater. Med. 2013, 24, 1865–1874. [Google Scholar] [CrossRef]
- Xu, C.; Huang, Y.; Wu, J.; Tang, L.; Hong, Y. Triggerable Degradation of Polyurethanes for Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2015, 7, 20377–20388. [Google Scholar] [CrossRef] [PubMed]
- Howard, G. Biodegradation of polyurethane: A review. Int. Biodeterior. Biodegrad. 2002, 49, 245–252. [Google Scholar] [CrossRef]
- Braunack, M.V.; Zaja, A.; Tam, K.; Filipović, L.; Filipović, V.; Wang, Y.; Bristow, K.L. A Sprayable Biodegradable Polymer Membrane (SBPM) technology: Effect of band width and application rate on water conservation and seedling emergence. Agric. Water Manag. 2020, 230, 105900. [Google Scholar] [CrossRef]
- Xu, C.; Hong, Y. Rational design of biodegradable thermoplastic polyurethanes for tissue repair. Bioact. Mater. 2021, 15, 250–271. [Google Scholar] [CrossRef]
- Fuwad, A.; Ryu, H.; Malmstadt, N.; Kim, S.M.; Jeon, T.J. Biomimetic membranes as potential tools for water purification: Preceding and future avenues. Desalination 2019, 458, 97–115. [Google Scholar] [CrossRef]
- Ehsani, M.; Doan, H.; Lohi, A. A comprehensive review of membrane fouling and cleaning methods with emphasis on ultrasound-assisted fouling control processes. Korean J. Chem. Eng. 2021, 38, 1531–1555. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, F.; Tao, L.; Liu, N.; Gao, C.; Feng, L.; Wei, Y. Bio-Inspired Anti-Oil-Fouling Chitosan-Coated Mesh for Oil/Water Separation Suitable for Broad pH Range and Hyper-Saline Environments. ACS Appl. Mater. Interfaces 2013, 5, 11971–11976. [Google Scholar] [CrossRef]
- Hou, D.; Wang, Z.; Wang, K.; Wang, J.; Lin, S. Composite membrane with electrospun multiscale-textured surface for robust oil-fouling resistance in membrane distillation. J. Membr. Sci. 2018, 546, 179–187. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; Zhang, G.; Qian, P.Y. Environmentally Friendly Antifouling Coatings Based on Biodegradable Polymer and Natural Antifoulant. ACS Sustain. Chem. Eng. 2017, 5, 6304–6309. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Anbazhagan, R.; Tsai, H.-C.; Wang, C.-F.; Lai, J.-Y. Biodegradable, superwettable caffeic acid/chitosan polymer coated cotton fibers for the simultaneous removal of oils, dyes, and metal ions from water. Chem. Eng. J. 2021, 427, 131920. [Google Scholar] [CrossRef]
- Shen, Y.x.; Saboe, P.O.; Sines, I.T.; Erbakan, M.; Kumar, M. Biomimetic membranes: A review. J. Membr. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Dendritic Saccharide Surfactant Polymers as Antifouling Interface Materials to Reduce Platelet Adhesion. Biomacromolecules 2006, 7, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, C.M.; Brennan, A.B. Bio-inspired antifouling strategies. Annu. Rev. Mater. Res. 2012, 42, 211–229. [Google Scholar] [CrossRef]
- Dalsin, J.L.; Messersmith, P.B. Bioinspired antifouling polymers. Mater. Today 2005, 8, 38–46. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coatings 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Tian, M.; Xu, H.; Yao, L.; Wang, R. A biomimetic antimicrobial surface for membrane fouling control in reverse osmosis for seawater desalination. Desalination 2021, 503, 114954. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Wicaksana, F.; Tang, C.; Torres, J.; Fane, A.G. Preparation of high performance nanofiltration (NF) membranes incorporated with aquaporin Z. J. Membr. Sci. 2014, 450, 181–188. [Google Scholar] [CrossRef]
- Chun, Y.; Qing, L.; Sun, G.; Bilad, M.R.; Fane, A.G.; Chong, T.H. Prototype aquaporin-based forward osmosis membrane: Filtration properties and fouling resistance. Desalination 2018, 445, 75–84. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, Y.; Wang, R.; Hélix-Nielsen, C.; Fane, A. Desalination by biomimetic aquaporin membranes: Review of status and prospects. Desalination 2013, 308, 34–40. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.; Huo, P.; Gu, J. Exploration of zwitterionic cellulose acetate antifouling ultrafiltration membrane for bovine serum albumin (BSA) separation. Carbohydr. Polym. 2017, 165, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gu, J.; Liu, X.; Wei, D.; Zhou, H.; Xiao, H.; Zhang, Z.; Yu, H.; Chen, S. Bactericidal and antifouling electrospun PVA nanofibers modified with a quaternary ammonium salt and zwitterionic sulfopropylbetaine. Mater. Sci. Eng. C 2020, 111, 110855. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Z.; Xie, M.; Zhang, B.; Li, G.; Luo, W. Resource recovery from digested manure centrate: Comparison between conventional and aquaporin thin-film composite forward osmosis membranes. J. Membr. Sci. 2020, 593, 117436. [Google Scholar] [CrossRef]
- Valverde-Pérez, B.; Pape, M.L.; Kjeldgaard, A.F.; Zachariae, A.A.; Schneider, C.; Hélix-Nielsen, C.; Zarebska, A.; Smets, B.F. Dewatering methanotrophic enrichments intended for single cell protein production using biomimetic aquaporin forward osmosis membranes. Sep. Purif. Technol. 2020, 235, 116133. [Google Scholar] [CrossRef]
- Liu, K.; Tian, Y.; Jiang, L. Bio-inspired superoleophobic and smart materials: Design, fabrication, and application. Prog. Mater. Sci. 2013, 58, 503–564. [Google Scholar] [CrossRef]
- Hegab, H.M.; Wimalasiri, Y.; Ginic-Markovic, M.; Zou, L. Improving the fouling resistance of brackish water membranes via surface modification with graphene oxide functionalized chitosan. Desalination 2015, 365, 99–107. [Google Scholar] [CrossRef]
- Su, C.; Yang, H.; Zhao, H.; Liu, Y.; Chen, R. Recyclable and biodegradable superhydrophobic and superoleophilic chitosan sponge for the effective removal of oily pollutants from water. Chem. Eng. J. 2017, 330, 423–432. [Google Scholar] [CrossRef]
- Chaudhary, J.P.; Vadodariya, N.; Nataraj, S.K.; Meena, R. Chitosan-Based Aerogel Membrane for Robust Oil-in-Water Emulsion Separation. ACS Appl. Mater. Interfaces 2015, 7, 24957–24962. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Zhang, Y.; Wang, L.; Lv, L.; Ma, X.; Zeng, S.; Wang, H. Biodegradable functional chitosan membrane for enhancement of artemisinin purification. Carbohydr. Polym. 2020, 246, 116590. [Google Scholar] [CrossRef]
- Salehi, E.; Daraei, P.; Shamsabadi, A.A. A review on chitosan-based adsorptive membranes. Carbohydr. Polym. 2016, 152, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, K.; Sudha, P.N.; Sukumaran, A. Novel chitosan based thin sheet nanofiltration membrane for rejection of heavy metal chromium. Int. J. Biol. Macromol. 2019, 132, 939–953. [Google Scholar]
- Goetz, L.A.; Jalvo, B.; Rosal, R.; Mathew, A.P. Superhydrophilic anti-fouling electrospun cellulose acetate membranes coated with chitin nanocrystals for water filtration. J. Membr. Sci. 2016, 510, 238–248. [Google Scholar] [CrossRef]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, J.; Huang, J.; Zhang, H.; Lin, S.; Chen, L.; Ni, Y.; Huang, L. A chitosan/dopamine-TiO2 composite nanofiltration membrane for antifouling in water purification. Cellulose 2021, 28, 4959–4973. [Google Scholar] [CrossRef]
- Wang, C.; Yang, F.; Meng, F.; Zhang, H.; Xue, Y.; Fu, G. High flux and antifouling filtration membrane based on non-woven fabric with chitosan coating for membrane bioreactors. Bioresour. Technol. 2010, 101, 5469–5474. [Google Scholar] [CrossRef]
- Ngah, W.W.; Teong, L.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Liu, R.; Xu, X.; Zhuang, X.; Cheng, B. Solution blowing of chitosan/PVA hydrogel nanofiber mats. Carbohydr. Polym. 2014, 101, 1116–1121. [Google Scholar] [CrossRef]
- Rafique, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Rehman, S. Chitosan functionalized poly(vinyl alcohol) for prospects biomedical and industrial applications: A review. Int. J. Biol. Macromol. 2016, 87, 141–154. [Google Scholar] [CrossRef]
- Cao, N.; Lyu, Q.; Li, J.; Wang, Y.; Yang, B.; Szunerits, S.; Boukherroub, R. Facile synthesis of fluorinated polydopamine/chitosan/reduced graphene oxide composite aerogel for efficient oil/water separation. Chem. Eng. J. 2017, 326, 17–28. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Gholap, S.G.; Badiger, M.V.; Gopinath, C.S. Molecular Origins of Wettability of Hydrophobic Poly(vinylidene fluoride) Microporous Membranes on Poly(vinyl alcohol) Adsorption: Surface and Interface Analysis by XPS. J. Phys. Chem. B 2005, 109, 13941–13947. [Google Scholar] [CrossRef] [PubMed]
- Anis, S.F.; Lalia, B.S.; Hashaikeh, R. Controlling swelling behavior of poly (vinyl) alcohol via networked cellulose and its application as a reverse osmosis membrane. Desalination 2014, 336, 138–145. [Google Scholar] [CrossRef]
- Sapalidis, A.A. Porous Polyvinyl Alcohol Membranes: Preparation Methods and Applications. Symmetry 2020, 12, 960. [Google Scholar] [CrossRef]
- Na, L.; Zhongzhou, L.; Shuguang, X. Dynamically formed poly (vinyl alcohol) ultrafiltration membranes with good anti-fouling characteristics. J. Membr. Sci. 2000, 169, 17–28. [Google Scholar] [CrossRef]
- Razmgar, M.N. Polyvinyl alcohol-based membranes for filtration of aqueous solutions: A comprehensive review. Polym. Eng. Sci. 2021, 62, 25–43. [Google Scholar] [CrossRef]
- Amanda, A.; Mallapragada, S. Comparison of Protein Fouling on Heat-Treated Poly(vinyl alcohol), Poly(ether sulfone) and Regenerated Cellulose Membranes Using Diffuse Reflectance Infrared Fourier Transform Spectroscopy. Biotechnol. Prog. 2001, 17, 917–923. [Google Scholar] [CrossRef]
- Wu, C.; Li, A.; Li, L.; Zhang, L.; Wang, H.; Qi, X.; Zhang, Q. Treatment of oily water by a poly(vinyl alcohol) ultrafiltration membrane. Desalination 2008, 225, 312–321. [Google Scholar] [CrossRef]
- Wang, X.; Fang, D.; Yoon, K.; Hsiao, B.S.; Chu, B. High performance ultrafiltration composite membranes based on poly(vinyl alcohol) hydrogel coating on crosslinked nanofibrous poly(vinyl alcohol) scaffold. J. Membr. Sci. 2006, 278, 261–268. [Google Scholar] [CrossRef]
- Falath, W.; Sabir, A.; Jacob, K.I. Novel reverse osmosis membranes composed of modified PVA/Gum Arabic conjugates: Biofouling mitigation and chlorine resistance enhancement. Carbohydr. Polym. 2017, 155, 28–39. [Google Scholar] [CrossRef]
- Falath, W.; Sabir, A.; Jacob, K.I. Highly improved reverse osmosis performance of novel PVA/DGEBA cross-linked membranes by incorporation of Pluronic F-127 and MWCNTs for water desalination. Desalination 2016, 397, 53–66. [Google Scholar] [CrossRef]
- An, Q.; Li, F.; Ji, Y.; Chen, H. Influence of polyvinyl alcohol on the surface morphology, separation and anti-fouling performance of the composite polyamide nanofiltration membranes. J. Membr. Sci. 2011, 367, 158–165. [Google Scholar] [CrossRef]
- Akther, N.; Ali, S.M.; Phuntsho, S.; Shon, H. Surface modification of thin-film composite forward osmosis membranes with polyvinyl alcohol–graphene oxide composite hydrogels for antifouling properties. Desalination 2020, 491, 114591. [Google Scholar] [CrossRef]
- Ahmed, A.; Niazi, M.B.K.; Jahan, Z.; Samin, G.; Pervaiz, E.; Hussain, A.; Mehran, M.T. Enhancing the Thermal, Mechanical and Swelling Properties of PVA/Starch Nanocomposite Membranes Incorporating g-C3N4. J. Polym. Environ. 2020, 28, 100–115. [Google Scholar] [CrossRef]
- Qing, W.; Li, X.; Wu, Y.; Shao, S.; Guo, H.; Yao, Z.; Chen, Y.; Zhang, W.; Tang, C.Y. In situ silica growth for superhydrophilic-underwater superoleophobic Silica/PVA nanofibrous membrane for gravity-driven oil-in-water emulsion separation. J. Membr. Sci. 2020, 612, 118476. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thanikaivelan, P. Fabrication of cellulose acetate-zirconia hybrid membranes for ultrafiltration applications: Performance, structure and fouling analysis. Sep. Purif. Technol. 2010, 74, 230–235. [Google Scholar] [CrossRef]
- Vetrivel, S.; Saraswathi, M.S.A.; Rana, D.; Nagendran, A. Fabrication of cellulose acetate nanocomposite membranes using 2D layered nanomaterials for macromolecular separation. Int. J. Biol. Macromol. 2018, 107, 1607–1612. [Google Scholar] [CrossRef]
- Ye, X.; Xiao, H.; Wang, Y.; Ke, L.; Luo, W.; Huang, X.; Shi, B. Efficient separation of viscous emulsion through amphiprotic collagen nanofibers-based membrane. J. Membr. Sci. 2019, 588, 117209. [Google Scholar] [CrossRef]
- Pandey, R.P.; Kallem, P.; Rasheed, P.A.; Mahmoud, K.A.; Banat, F.; Lau, W.J.; Hasan, S.W. Enhanced water flux and bacterial resistance in cellulose acetate membranes with quaternary ammoniumpropylated polysilsesquioxane. Chemosphere 2022, 289, 133144. [Google Scholar] [CrossRef]
- Ghaseminezhad, S.M.; Barikani, M.; Salehirad, M. Development of graphene oxide-cellulose acetate nanocomposite reverse osmosis membrane for seawater desalination. Compos. Part B Eng. 2019, 161, 320–327. [Google Scholar] [CrossRef]
- El-Din, L.N.; El-Gendi, A.; Ismail, N.; Abed, K.; Ahmed, A.I. Evaluation of cellulose acetate membrane with carbon nanotubes additives. J. Ind. Eng. Chem. 2015, 26, 259–264. [Google Scholar] [CrossRef]
- Janeca, A.; Rodrigues, F.S.C.; Gonçalves, M.C.; Faria, M. Novel Cellulose Acetate-Based Monophasic Hybrid Membranes for Improved Blood Purification Devices: Characterization under Dynamic Conditions. Membranes 2021, 11, 825. [Google Scholar] [CrossRef]
- Rajesh, S.; Jayalakshmi, A.; Senthilkumar, S.; Sankar, H.S.H.; Mohan, D.R. Performance Evaluation of Poly(amide-imide) Incorporated Cellulose Acetate Ultrafiltration Membranes in the Separation of Proteins and Its Fouling Propensity by AFM Imaging. Ind. Eng. Chem. Res. 2011, 50, 14016–14029. [Google Scholar] [CrossRef]
- Yin, J.; Fan, H.; Zhou, J. Cellulose acetate/poly(vinyl alcohol) and cellulose acetate/crosslinked poly(vinyl alcohol) blend membranes: Preparation, characterization, and antifouling properties. Desalination Water Treat. 2016, 57, 10572–10584. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Macni, C.R.M.; Caparanga, A.R.; Huang, S.-H.; Tsai, H.-A.; Lee, K.-R.; Lai, J.-Y. Mitigating the fouling of mixed-matrix cellulose acetate membranes for oil–water separation through modification with polydopamine particles. Chem. Eng. Res. Des. 2020, 159, 195–204. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, G.; Zhang, H.; Yang, F. Exploration of permeability and antifouling performance on modified cellulose acetate ultrafiltration membrane with cellulose nanocrystals. Carbohydr. Polym. 2017, 174, 190–199. [Google Scholar] [CrossRef]
- Su, J.; Yang, Q.; Teo, J.F.; Chung, T.-S. Cellulose acetate nanofiltration hollow fiber membranes for forward osmosis processes. J. Membr. Sci. 2010, 355, 36–44. [Google Scholar] [CrossRef]
- Shang, M.; Shi, B. Study on preparation and performances of cellulose acetate forward osmosis membrane. Chem. Pap. 2018, 72, 3159–3167. [Google Scholar] [CrossRef]
- Li, F.; Fei, P.; Cheng, B.; Meng, J.; Liao, L. Synthesis, characterization and excellent antibacterial property of cellulose acetate reverse osmosis membrane via a two-step reaction. Carbohydr. Polym. 2019, 216, 312–321. [Google Scholar] [CrossRef]
- Tahazadeh, S.; Mohammadi, T.; Tofighy, M.A.; Khanlari, S.; Karimi, H.; Emrooz, H.B.M. Development of cellulose acetate/metal-organic framework derived porous carbon adsorptive membrane for dye removal applications. J. Membr. Sci. 2021, 638, 119692. [Google Scholar] [CrossRef]
- Kanagaraj, P.; Mohamed, I.M.; Huang, W.; Liu, C. Membrane fouling mitigation for enhanced water flux and high separation of humic acid and copper ion using hydrophilic polyurethane modified cellulose acetate ultrafiltration membranes. React. Funct. Polym. 2020, 150, 104538. [Google Scholar] [CrossRef]
- Ye, S.H.; Watanabe, J.; Iwasaki, Y.; Ishihara, K. Antifouling blood purification membrane composed of cellulose acetate and phospholipid polymer. Biomaterials 2003, 24, 4143–4152. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhao, Y.; Zheng, W.; Yu, H.; Liu, Y.; Xu, L. Asymmetric Sc-PLA Membrane with Multi-scale Microstructures: Wettability, Antifouling, and Oil–Water Separation. ACS Appl. Mater. Interfaces 2020, 12, 55520–55526. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Hegab, H.M.; Nassar, L.; Wadi, V.S.; Naddeo, V.; Yousef, A.F.; Banat, F.; Hasan, S.W. Asymmetrical ultrafiltration membranes based on polylactic acid for the removal of organic substances from wastewater. J. Water Process Eng. 2022, 45, 102510. [Google Scholar] [CrossRef]
- Alam, A.K.M.M.; Ewaldz, E.; Xiang, C.; Qu, W.; Bai, X. Tunable Wettability of Biodegradable Multilayer Sandwich-Structured Electrospun Nanofibrous Membranes. Polymers 2020, 12, 2092. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Nasef, M.M.; Fouad, H.; Karim, Z. Poly(lactic acid)/poly(butylene succinate) dual-layer membranes with cellulose nanowhisker for heavy metal ion separation. Int. J. Biol. Macromol. 2021, 192, 654–664. [Google Scholar] [CrossRef]
- Dasari, A.; Quirós, J.; Herrero, B.; Boltes, K.; García-Calvo, E.; Rosal, R. Antifouling membranes prepared by electrospinning polylactic acid containing biocidal nanoparticles. J. Membr. Sci. 2012, 405, 134–140. [Google Scholar] [CrossRef]
- Xix-Rodriguez, C.; Varguez-Catzim, P.; Alonzo-García, A.; Rodriguez-Fuentes, N.; Vázquez-Torres, H.; González-Diaz, A.; Aguilar-Vega, M.; González-Díaz, M.O. Amphiphilic poly(lactic acid) membranes with low fouling and enhanced hemodiafiltration. Sep. Purif. Technol. 2021, 259, 118124–118135. [Google Scholar] [CrossRef]
- Xiong, Z.; Lin, H.; Liu, F.; Yu, X.; Wang, Y.; Wang, Y. A new strategy to simultaneously improve the permeability, heat-deformation resistance and antifouling properties of polylactide membrane via bio-based β-cyclodextrin and surface crosslinking. J. Membr. Sci. 2016, 513, 166–176. [Google Scholar] [CrossRef]
- Ao, C.; Zhao, J.; Li, Q.; Zhang, J.; Huang, B.; Wang, Q.; Gai, J.; Chen, Z.; Zhang, W.; Lu, C. Biodegradable all-cellulose composite membranes for simultaneous oil/water separation and dye removal from water. Carbohydr. Polym. 2020, 250, 116872. [Google Scholar] [CrossRef]
- Abolhassani, M.; Griggs, C.S.; Gurtowski, L.A.; Mattei-Sosa, J.A.; Nevins, M.; Medina, V.F.; Morgan, T.A.; Greenlee, L.F. Scalable Chitosan-Graphene Oxide Membranes: The Effect of GO Size on Properties and Cross-Flow Filtration Performance. ACS Omega 2017, 2, 8751–8759. [Google Scholar] [CrossRef] [PubMed]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumar, K.Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Xiao, P.; Chen, P.; Zhang, L.; Wang, H.; Dai, L.; Song, L.; Huang, Y.; Zhang, J.; Chen, T. Functionalization of Biodegradable PLA Nonwoven Fabric as Superoleophilic and Superhydrophobic Material for Efficient Oil Absorption and Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 5968–5973. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Lin, H.; Zhong, Y.; Qin, Y.; Li, T.; Liu, F. Robust superhydrophilic polylactide (PLA) membranes with a TiO2 nano-particle inlaid surface for oil/water separation. J. Mater. Chem. A 2017, 5, 6538–6545. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Yuan, H.; Su, M.; Shao, C.; Liu, C.; Guo, Z.; Shen, C.; Liu, X. Simple fabrication of superhydrophobic PLA with honeycomb-like structures for high-efficiency oil-water separation. Chin. Chem. Lett. 2020, 31, 365–368. [Google Scholar] [CrossRef]
- Lu, W.; Duan, C.; Zhang, Y.; Gao, K.; Dai, L.; Shen, M.; Wang, W.; Wang, J.; Ni, Y. Cellulose-based electrospun nanofiber membrane with core-sheath structure and robust photocatalytic activity for simultaneous and efficient oil emulsions separation, dye degradation and Cr(VI) reduction. Carbohydr. Polym. 2021, 258, 117676. [Google Scholar] [CrossRef]
- Kollarigowda, R.H.; Abraham, S.; Montemagno, C.D. Antifouling Cellulose Hybrid Biomembrane for Effective Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 29812–29819. [Google Scholar] [CrossRef]
- Kian, L.; Jawaid, M.; Alamery, S.; Vaseashta, A. Fabrication and Characterization of Novel Poly(D-lactic acid) Nanocomposite Membrane for Water Filtration Purpose. Nanomaterials 2021, 11, 255. [Google Scholar] [CrossRef]
- Kumar, M.; Tripathi, B.P.; Shahi, V.K. Crosslinked chitosan/polyvinyl alcohol blend beads for removal and recovery of Cd(II) from wastewater. J. Hazard. Mater. 2009, 172, 1041–1048. [Google Scholar] [CrossRef]
- Abu-Saied, M.; Wycisk, R.; Abbassy, M.M.; El-Naim, G.A.; El-Demerdash, F.; Youssef, M.; Bassuony, H.; Pintauro, P.N. Sulfated chitosan/PVA absorbent membrane for removal of copper and nickel ions from aqueous solutions—Fabrication and sorption studies. Carbohydr. Polym. 2017, 165, 149–158. [Google Scholar] [CrossRef]
- Sadeghi, A.; Mousavi, S.M.; Saljoughi, E.; Kiani, S. Biodegradable membrane based on polycaprolactone/polybutylene succinate: Characterization and performance evaluation in wastewater treatment. J. Appl. Polym. Sci. 2021, 138, e50332–e50345. [Google Scholar] [CrossRef]
- Gao, D.; Zhao, R.; Yang, X.; Chen, F.; Ning, X. Bicomponent PLA Nanofiber Nonwovens as Highly Efficient Filtration Media for Particulate Pollutants and Pathogens. Membranes 2021, 11, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Bahremand, A.H.; Mousavi, S.M.; Ahmadpour, A.; Taherian, M. Biodegradable blend membranes of poly (butylene succinate)/cellulose acetate/dextran: Preparation, characterization and performance. Carbohydr. Polym. 2017, 173, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, H.; Maghami, S.; Isfahani, A.P.; Sadeghi, M. Influence of Blend Composition and Silica Nanoparticles on the Morphology and Gas Separation Performance of PU/PVA Blend Membranes. Membranes 2019, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xuan, H.; Ge, L. Double network self-healing chitosan/dialdehyde starch-polyvinyl alcohol film for gas separation. Appl. Surf. Sci. 2019, 469, 213–219. [Google Scholar] [CrossRef]
- Tirouni, I.; Sadeghi, M.; Pakizeh, M. Separation of C3H8 and C2H6 from CH4 in polyurethane–zeolite 4Å and ZSM-5 mixed matrix membranes. Sep. Purif. Technol. 2015, 141, 394–402. [Google Scholar] [CrossRef]
- Isfahani, A.P.; Ghalei, B.; Wakimoto, K.; Bagheri, R.; Sivaniah, E.; Sadeghi, M. Plasticization resistant crosslinked polyurethane gas separation membranes. J. Mater. Chem. A 2016, 4, 17431–17439. [Google Scholar] [CrossRef]
- AlruwailI, B.M.; Saeed, U.; Ahmad, I.; Al-Turaif, H.; Aboalkhair, H.; AlsaiarI, A.O. Development of Multiwalled Carbon Nanotube-Reinforced Biodegradable Polylactic Acid/Polybutylene Succinate Blend Membrane. Membranes 2021, 11, 760. [Google Scholar] [CrossRef]
- Koh, H.C.; Park, J.S.; Jeong, M.A.; Hwang, H.Y.; Hong, Y.T.; Ha, S.Y.; Nam, S.Y. Preparation and gas permeation properties of biodegradable polymer/layered silicate nanocomposite membranes. Desalination 2008, 233, 201–209. [Google Scholar] [CrossRef]
- Jiamjirangkul, P.; Inprasit, T.; Intasanta, V.; Pangon, A. Metal organic framework-integrated chitosan/poly(vinyl alcohol) (PVA) nanofibrous membrane hybrids from green process for selective CO2 capture and filtration. Chem. Eng. Sci. 2020, 221, 115650. [Google Scholar] [CrossRef]
- Ong, Y.T.; Ahmad, A.L.; Zein, S.H.S.; Sudesh, K.; Tan, S.H. Poly(3-hydroxybutyrate)-functionalised multi-walled carbon nanotubes/chitosan green nanocomposite membranes and their application in pervaporation. Sep. Purif. Technol. 2011, 76, 419–427. [Google Scholar] [CrossRef]
- Mokhtarzadeh, S.; Hakimpour, F.; Sarvari, R.; Agbolaghi, S.; Mansourpanah, Y. Nanocomposite membranes based on sodium alginate/poly(ε-caprolactone)/graphene oxide for methanol, ethanol and isopropanol dehydration via pervaporation. Polym. Bull. 2020, 77, 3367–3387. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Anjanapura, R.V.; Han, J.M.; Aminabhavi, T.M. Functionalized Graphene Sheets Embedded in Chitosan Nanocomposite Membranes for Ethanol and Isopropanol Dehydration via Pervaporation. Ind. Eng. Chem. Res. 2014, 53, 14474–14484. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Baldasso, C.; Tessaro, I.C. Potential of chitosan-based membranes for the separation of essential oil components by target-organophilic pervaporation. Carbohydr. Polym. 2020, 247, 116676. [Google Scholar] [CrossRef]
- Das, S.; Banthia, A.; Adhikari, B. Porous polyurethane urea membranes for pervaporation separation of phenol and chlorophenols from water. Chem. Eng. J. 2008, 138, 215–223. [Google Scholar] [CrossRef]
- Additive, O.; Methyl, M. Application of Polymer Membranes for a Purification. Polymer 2020, 12, 2218. [Google Scholar]
- Moulik, S.; Bukke, V.; Sajja, S.C.; Sridhar, S. Chitosan-polytetrafluoroethylene composite membranes for separation of methanol and toluene by pervaporation. Carbohydr. Polym. 2018, 193, 28–38. [Google Scholar] [CrossRef]
- Tomietto, P.; Russo, F.; Galiano, F.; Loulergue, P.; Salerno, S.; Paugam, L.; Audic, J.-L.; De Bartolo, L.; Figoli, A. Sustainable fabrication and pervaporation application of bio-based membranes: Combining a polyhydroxyalkanoate (PHA) as biopolymer and Cyrene™ as green solvent. J. Membr. Sci. 2022, 643, 120061. [Google Scholar] [CrossRef]
- Chen, X.; Li, J. Bioinspired by cell membranes: Functional polymeric materials for biomedical applications. Mater. Chem. Front. 2020, 4, 750–774. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.S.; Deepthi, S.; Chennazhi, K.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable Polymers—A Review on Recent Trends and Emerging Perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yu, X.; Wan, P.; Yang, K. Biodegradable Materials for Bone Repairs: A Review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Krajewska, B. Membrane-based processes performed with use of chitin/chitosan materials. Sep. Purif. Technol. 2005, 41, 305–312. [Google Scholar] [CrossRef]
- Muzzarelli, C.; Muzzarelli, R.A. Natural and artificial chitosan–inorganic composites. J. Inorg. Biochem. 2002, 92, 89–94. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Benko, A.; Palka, K.; Wojcik, M.; Przekora, A. Elastic and biodegradable chitosan/agarose film revealing slightly acidic pH for potential applications in regenerative medicine as artificial skin graft. Int. J. Biol. Macromol. 2020, 164, 172–183. [Google Scholar] [CrossRef]
- Bernards, D.A.; Desai, T.A. Nanotemplating of Biodegradable Polymer Membranes for Constant-Rate Drug Delivery. Adv. Mater. 2010, 22, 2358–2362. [Google Scholar] [CrossRef]
- Gao, A.; Liu, F.; Xue, L. Preparation and evaluation of heparin-immobilized poly (lactic acid) (PLA) membrane for hemodialysis. J. Membr. Sci. 2014, 452, 390–399. [Google Scholar] [CrossRef]
- Ma, L.; Huang, L.; Zhang, Y.; Zhao, L.; Xin, Q.; Ye, H.; Li, H. Hemocompatible poly(lactic acid) membranes prepared by immobilizing carboxylated graphene oxide via mussel-inspired method for hemodialysis. RSC Adv. 2018, 8, 153–161. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone functionalized membranes: Properties and challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Song, X.; Ji, H.; Zhao, W.; Sun, S.; Zhao, C. Hemocompatibility enhancement of polyethersulfone membranes: Strategies and challenges. Adv. Membr. 2021, 1, 100013. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods 2021, 9, 1438. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2020, 113, 106530. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Eldin, R.E.S.; Serea, E.S.A.; Gomaa, N.M.; Aboelmagd, G.M.; Salem, S.A.; Elsayed, Z.A.; Edrees, A.; Shams-Eldin, E.; Shalan, A.E. Advances in nanotechnology and antibacterial properties of biodegradable food packaging materials. RSC Adv. 2020, 10, 20467–20484. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Ishak, M.R. Water barrier properties of biodegradable films reinforced with nanocellulose for food packaging application: A review. In Proceedings of the 6th Postgraduate Seminar on Natural Fiber Reinforced Polymer Composites, Selangor, Malaysia, 4 December 2018; pp. 55–59. [Google Scholar]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Banerjee, A.; Saurabh, C.K.; Tye, Y.Y.; Suriani, A.B.; Mohamed, A.; Karim, A.A.; Rizal, S.; Paridah, M.T. Biodegradable Films for Fruits and Vegetables Packaging Application: Preparation and Properties. Food Eng. Rev. 2018, 10, 139–153. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849–104860. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.; Dutta, P. Physicochemical and bioactivity of cross-linked chitosan–PVA film for food packaging applications. Int. J. Biol. Macromol. 2009, 45, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-limonene blends for food packaging applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Wang, S.; Qin, W.; Zhang, Q. Electrospun Antimicrobial Polylactic Acid/Tea Polyphenol Nanofibers for Food-Packaging Applications. Polymers 2018, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA-PHB-Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Râpă, M.; Miteluţ, A.C.; Tănase, E.E.; Grosu, E.; Popescu, P.; Popa, M.E.; Rosnes, J.T.; Sivertsvik, M.; Darie-Niţă, R.N.; Vasile, C. Influence of chitosan on mechanical, thermal, barrier and antimicrobial properties of PLA-biocomposites for food packaging. Compos. Part B Eng. 2016, 102, 112–121. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol-chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in situ enhanced PVA/chitosan biodegradable films for food packages. Carbohydr. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef]
- Chen, D.; Lawton, D.; Thompson, M.; Liu, Q. Biocomposites reinforced with cellulose nanocrystals derived from potato peel waste. Carbohydr. Polym. 2012, 90, 709–716. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Khalid, M.; Walvekar, R. Development of Poly(Vinyl Alcohol)-Based Polymers as Proton Exchange Membranes and Challenges in Fuel Cell Application: A Review. Polym. Rev. 2020, 60, 171–202. [Google Scholar] [CrossRef]
- Modi, J.; Choumal, A.; Vyas, D.; Shah, D.; Joshi, K.; Patel, K.; Iyer, K. Materials Today: Proceedings Sustainable technology for modern era effluent treatment: Microbial fuel cell. Mater. Today: Proc. 2022, 57, 1781–1788. [Google Scholar]
- Liu, Q.; Lan, F.; Chen, J.; Zeng, C.; Wang, J. A review of proton exchange membrane fuel cell water management: Membrane electrode assembly. J. Power Sources 2022, 517, 230723. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S. Chitosan and alginate types of bio-membrane in fuel cell application: An overview. J. Power Sources 2015, 289, 71–80. [Google Scholar] [CrossRef]
- González-Pabón, M.J.; Figueredo, F.; Martínez-Casillas, D.C.; Cortón, E. High-performance biodegradable membrane for point of need paper-based micro-scale microbial fuel cell analytical devices. bioRxiv 2018, 351890. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y. Chitosan biopolymer for fuel cell applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef] [PubMed]
- Smitha, B.; Sridhar, S.; Khan, A.A. Synthesis and characterization of poly(vinyl alcohol)-based membranes for direct methanol fuel cell. J. Appl. Polym. Sci. 2005, 95, 1154–1163. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A. Chitosan–sodium alginate polyion complexes as fuel cell membranes. Eur. Polym. J. 2005, 41, 1859–1866. [Google Scholar] [CrossRef]
- Zia, Q.; Tabassum, M.; Umar, M.; Nawaz, H.; Gong, H.; Li, J. Cross-linked chitosan coated biodegradable porous electrospun membranes for the removal of synthetic dyes. React. Funct. Polym. 2021, 166, 104995. [Google Scholar] [CrossRef]
- Rahaman, H.; Islam, A.; Islam, M.; Rahman, A.; Alam, S.N. Biodegradable composite adsorbent of modified cellulose and chitosan to remove heavy metal ions from aqueous solution. Curr. Res. Green Sustain. Chem. 2021, 4, 100119. [Google Scholar] [CrossRef]
- Torstensen, J.; Helberg, R.M.; Deng, L.; Gregersen, W.; Syverud, K. PVA/nanocellulose nanocomposite membranes for CO2 separation from flue gas. Int. J. Greenh. Gas Control 2019, 81, 93–102. [Google Scholar] [CrossRef]
- Serafin, J.; Antosik, A.; Wilpiszewska, K.; Czech, Z. Preparation of Activated Carbon from the Biodegradable film for CO2 Capture Applications. Pol. J. Chem. Technol. 2018, 20, 75–80. [Google Scholar] [CrossRef]
- Langari, S.; Saljoughi, E.; Mousavi, S.M. Chitosan/polyvinyl alcohol/amino functionalized multiwalled carbon nanotube pervaporation membranes: Synthesis, characterization, and performance. Polym. Adv. Technol. 2018, 29, 84–94. [Google Scholar] [CrossRef]
- Galiano, F.; Ghanim, A.H.; Rashid, K.T.; Marino, T.; Simone, S.; Alsalhy, Q.F.; Figoli, A. Preparation and characterization of green polylactic acid (PLA) membranes for organic/organic separation by pervaporation. Clean Technol. Environ. Policy 2019, 21, 109–120. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Gu, J.; Sun, Y.; Ji, X. Cross-linking of poly(vinyl alcohol) with N,N′-methylene bisacrylamide via a radical reaction to prepare pervaporation membranes. RSC Adv. R. Soc. Chem. 2015, 5, 19859–19864. [Google Scholar] [CrossRef]
- Chang, P.-Y.; Wang, J.; Li, S.-Y.; Suen, S.-Y. Biodegradable Polymeric Membranes for Organic Solvent/Water Pervaporation Applications. Membranes 2021, 11, 970. [Google Scholar] [CrossRef]
- Msahel, A.; Galiano, F.; Pilloni, M.; Russo, F.; Hafiane, A.; Castro-Muñoz, R.; Kumar, V.; Gedanken, A.; Ennas, G.; Porat, Z.; et al. Exploring the Effect of Iron Metal-Organic Framework Particles in Polylactic Acid Membranes for the Azeotropic Separation of Organic/Organic Mixtures by Pervaporation. Membranes 2021, 11, 65. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Masaki, K.; Ishikawa, M. Pervaporation separation of aqueous organic mixtures through agarose membranes. J. Membr. Sci. 2002, 205, 293–300. [Google Scholar] [CrossRef]
- Chung, T.W.; Yang, J.; Akaike, T.; Cho, K.Y.; Nah, J.W.; Kim, S.I.; Cho, C.S. Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials 2002, 23, 2827–2834. [Google Scholar] [CrossRef]
- Chen, S.; Hao, Y.; Cui, W.; Chang, J.; Zhou, Y. Biodegradable electrospun PLLA/chitosan membrane as guided tissue regeneration membrane for treating periodontitis. J. Mater. Sci. 2013, 48, 6567–6577. [Google Scholar] [CrossRef]
- Piscioneri, A.; Campana, C.; Salerno, S.; Morelli, S.; Bader, A.; Giordano, F.; Drioli, E.; De Bartolo, L. Biodegradable and synthetic membranes for the expansion and functional differentiation of rat embryonic liver cells. Acta Biomater. 2011, 7, 171–179. [Google Scholar] [CrossRef]
- Li, F.; Su, Y.; Pi, G.; Ma, P.X.; Lei, B. Biodegradable, Biomimetic Elastomeric, Photoluminescent, and Broad-Spectrum Antibacterial Polycitrate-Polypeptide-based Membrane toward Multifunctional Biomedical Implants. ACS Biomater. Sci. Eng. 2018, 4, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, Y.; Yang, X.; Mei, F.; Ma, Q.; Chen, G.; Ryu, S.; Deng, X. Gelatin nanofibrous membrane fabricated by electrospinning of aqueous gelatin solution for guided tissue regeneration. J. Biomed. Mater. Res. Part A 2009, 90A, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Guo, B.; Shao, Y.; Ma, P.X. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials 2016, 87, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Wang, S.A. Preparation of graphene-based poly(vinyl alcohol)/chitosan nanocomposites membrane for alkaline solid electrolytes membrane. J. Membr. Sci. 2015, 477, 49–57. [Google Scholar] [CrossRef]
- Ma, J.; Choudhury, N.A.; Sahai, Y.; Buchheit, R.G. A high performance direct borohydride fuel cell employing cross-linked chitosan membrane. J. Power Sources 2011, 196, 8257–8264. [Google Scholar] [CrossRef]
- Kalaiselvimary, J.; Prabhu, M.R. Fabrications and investigation of physicochemical and electrochemical properties of heteropoly acid-doped sulfonated chitosan-based polymer electrolyte membranes for fuel cell applications. Polym. Bull. 2019, 76, 1401–1422. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K.; Timmiati, S.N.; Masdar, M.S. New composite membrane poly(vinyl alcohol)/graphene oxide for direct ethanol–proton exchange membrane fuel cell. J. Appl. Polym. Sci. 2019, 136, 46928. [Google Scholar] [CrossRef]
- Harewood, A.J.T.; Popuri, S.R.; Cadogan, E.I.; Lee, C.-H.; Wang, C.-C. Bioelectricity generation from brewery wastewater in a microbial fuel cell using chitosan/biodegradable copolymer membrane. Int. J. Environ. Sci. Technol. 2017, 14, 1535–1550. [Google Scholar] [CrossRef]
- Azevedo, H.; Reis, R. Understanding the Enzymatic Degradation of Biodegradable Polymers and Strategies to Control Their Degradation Rate. In Biodegradable Systems in Tissue Engineering and Regenerative Medicine; CRC Press: Boca Raton, FL, USA, 2004; pp. 177–202. [Google Scholar]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef]
- Amulya, K.; Katakojwala, R.; Ramakrishna, S.; Mohan, S.V. Low carbon biodegradable polymer matrices for sustainable future. Compos. Part C Open Access 2021, 4, 100111. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing Biobased Recyclable Polymers for Plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Nikhil, G.; Chiranjeevi, P.; Reddy, C.N.; Rohit, M.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Patel, F.M.-W.M.; Schleich, J.; Hüsing, B.; Angerer, G.; Wolf, O.; Crank, M. Techno-economic Feasibility of Large-scale Production of Bio-based Polymers in Europe, Institute for Prospective Technological Studies. Teh. Rep. EUR 2005, 22103. Available online: https://bioplastique-gmat.weebly.com/uploads/1/4/2/0/14200175/feasibility_of_production_of_bio-based_polymers_in_europe.pdf (accessed on 23 July 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).