Treatment Possibilities in Mandibular Defect Reconstruction Based on Ameloblastic Fibro-Odontoma Treatment—Does Small Bone Defects Heal without Bone Grafting?
Abstract
:1. Introduction
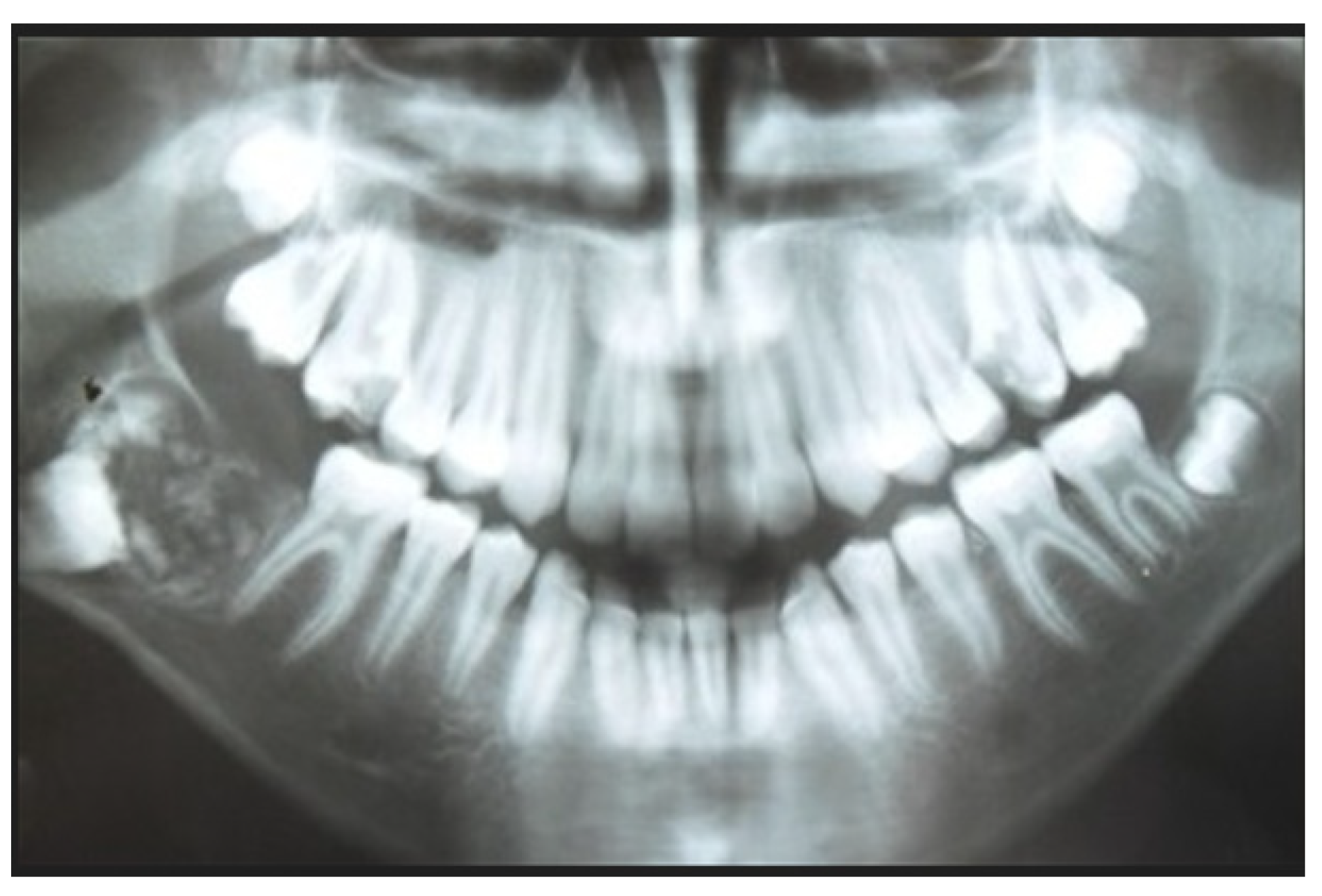
2. Case Report Presentation
3. Discussion
- -
- Conservative approach with enucleation alone
- -
- Conservative approach with enucleation and bone curettage
- -
- Surgical curettage with bony cavity ostectomy
- -
- Surgical marginectomy in bigger lesions with/without reconstruction;
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franco, A.; Riscala, S.; Kahoudji, M.; Croue, A. Endoscopic removal of a mandibular ameloblastic fibro-odontoma. Rev. Stomatol. Chir. Maxillofac. 2009, 110, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, A.S.; Anbinder, A.L.; Costa, N.C.; Lima, J.R.; Carvalho, Y.R. Ameloblastic fibro-odontoma: A case report. Med. Oral Patol. Oral Cir. Bucal 2009, 14, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Santos TD, S.; de Carvalho RW, F.; Avelar, R.L.; Frota, R.; Anjos, E.D. Ameloblastic fibro-odontoma in children: Report of 2 cases. J. Dent. Child. 2011, 78, 173–177. [Google Scholar]
- Nanda, K.D.; Marwaha, M. Ameloblastic fibro-odontoma masquerading as odontoma. Indian J. Dent. Res. 2011, 22, 616. [Google Scholar] [CrossRef] [PubMed]
- Boxberger, N.R.; Brannon, R.B.; Fowler, C.B. Ameloblastic fibro-odontoma: A clinicopathologic study of 12 cases. J. Clin. Pediatr. Dent. 2011, 35, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Flaitz, C.M.; Hicks, J. Delayed tooth eruption associated with an ameloblastic fibro- odontoma. Pediatr. Dent. 2001, 23, 253–254. [Google Scholar]
- Wright, J.M.; Odell, E.W.; Speight, P.M.; Takata, T. Odontogenic tumors, WHO 2005: Where do we go from here? Head Neck Pathol. 2014, 8, 373–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, L.; Eveson, J.W.; Reichart, P.; Sidransky, D. World Health Organization Classification of Tumours: Pathology and Genetics, Head and Neck Tumours; IARC Press: Lyon, France, 2005. [Google Scholar]
- Wright, J.M.; Soluk Tekkesin, M. Odontogenic tumors: Where are we in 2017? J. Istanb. Univ. Fac. Dent. 2017, 51, 10–30. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P. (Eds.) WHO Classification of Head and Neck Tumours. Chapter 8: Odontogenic and Maxilofacial Bone Tumours, 4th ed.; IARC: Lyon, France, 2017; pp. 205–260. [Google Scholar]
- Soluk-Tekkesin, M.; Vered, M. Ameloblastic Fibro-Odontoma: At the Crossroad between “Developing Odontoma” and True Odontogenic Tumour. Head Neck Pathol. 2021, 15, 1202–1211. [Google Scholar] [CrossRef]
- Okui, T.; Ibaragi, S.; Shimo, T.; Takabatake, K.; Fujita, M.; Hassan, N.M.M.; Sasaki, A. Ameloblastic fibro-odontoma in a 9-year-old boy. Stomatol. Dis. Sci. 2018, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Sanjai, K.; Reddy, L.P.; Roopavathi, K.; Muniswamy, H.K. Ameloblastic Fibro-Odontoma: A Journey of Progression? J. Oral Maxillofac. Pathol. 2022, 26 (Suppl. S1), 40–45. [Google Scholar] [CrossRef] [PubMed]
- Gantala, R.; Gotoor, S.G.; Kumar, R.V.; Munisekhar, M.S. Ameloblastic fibro-odontoma. BMJ Case Rep. 2015, 2015, 2015209739. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.; Moghe, S.; Guru, K.N.; Nair, P.P. Large ameloblastic fibro-odontoma in an 18-year-old girl and review of literature. BMJ Case Rep. 2012, 2012, 007160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.J.; Zhou, L.; Liao, Q.; Gong, H.H. A large ameloblastic fibro-odontoma in the mandible. Quant. Imaging Med. Surg. 2012, 2, 135–136. [Google Scholar] [PubMed]
- Lúcio, P.S.; Cavalcante, R.B.; Maia, R.N.; Santos, E.S.; Godoy, G.P. Aggressive ameloblastic fibro-odontoma assessment with CBCT and treatment. Eur. Arch. Paediatr. Dent. 2013, 14, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Hunter, A.K.; Muller, S.; Kalathingal, S.M.; Burnham, M.A.; Moore, W.S. Evaluation of an ameloblastic fibro-odontoma with cone beam computed tomography. Tex. Dent. J. 2012, 129, 619–624. [Google Scholar]
- Damm, D.D. Pericoronal radiolucency with radiopacities of posterior mandible. Ameloblastic fibro-odontoma. Gen. Dent. 2008, 56, 585–587. [Google Scholar]
- Buchner, A.; Kaffe, I.; Vered, M. Clinical and radiological profile of ameloblastic fibro-odontoma: An update on an uncommon odontogenic tumor based on a critical analysis of 114 cases. Head Neck Pathol. 2013, 7, 54–63. [Google Scholar] [CrossRef]
- Kämmerer, P.W.; Wriedt, S.; Al-Nawas, B. Re: “Pontes et al. Report of four cases of ameloblastic fibro-odontoma in mandible and discussion of the literature about the treatment. J. Craniomaxillofac. Surg. 2012, 40, 59–63”. J. Craniomaxillofac. Surg. 2012, 40, 509–610. [Google Scholar]
- Reibel, J.; Grønbaek, A.B.; Poulsen, S. Peripheral ameloblastic fibro-odontoma or peripheral developing complex odontoma: Report of a case. Int. J. Paediatr. Dent. 2011, 21, 468–470. [Google Scholar] [CrossRef]
- Phillips, M.D.; Closmann, J.J.; Baus, M.R.; Torske, K.R.; Williams, S.B. Hybrid odontogenic tumor with features of ameloblastic fibro-odontoma, calcifying odontogenic cyst, and adenomatoid odontogenic tumor: A case report and review of the literature. J. Oral Maxillofac. Surg. 2010, 68, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Govindrajan, S.; Muruganandhan, J.; Shamsudeen, S.; Kumar, N.; Ramasamy, M.; Prasad, S. Complex composite odontoma with characteristic histology. Case Rep. Dent. 2013, 2013, 157614. [Google Scholar] [CrossRef] [PubMed]
- Chrysomali, E.; Leventis, M.; Titsinides, S.; Kyriakopoulos, V.; Sklavounou, A. Odontogenic tumors. J. Craniofac. Surg. 2013, 24, 1521–1525. [Google Scholar] [CrossRef] [PubMed]
- Mummolo, S.; Marchetti, E.; Di Martino, S.; Scorzetti, L.; Marzo, G. Ameloblastic fibro-odontoma: A case report. Ann. Stomatol. 2010, 1, 11–13. [Google Scholar]
- Hegde, V.; Hemavathy, S. A massive ameloblastic fibro-odontoma of the maxilla. Indian J Dent Res. 2008, 19, 162–164. [Google Scholar] [CrossRef]
- Dolanmaz, D.; Pampu, A.A.; Kalayci, A.; Etöz, O.A.; Atici, S. An unusual size of ameloblastic fibro-odontoma. Dentomaxillofac. Radiol. 2008, 37, 179–182. [Google Scholar] [CrossRef]
- Oghli, A.A.; Scuto, I.; Ziegler, C.; Flechtenmacher, C.; Hofele, C. A large ameloblastic fibro-odontoma of the right mandible. Med. Oral Patol. Oral Cir. Bucal. 2007, 12, 34–37. [Google Scholar]
- Friedrich, R.E.; Siegert, J.; Donath, K.; Jäkel, K.T. Recurrent ameloblastic fibro-odontoma in a 10-year-old boy. J. Oral Maxillofac. Surg. 2001, 59, 1362–1366. [Google Scholar] [CrossRef]
- Furst, I.; Pharoah, M.; Phillips, J. Recurrence of an ameloblastic fibro-odontoma in a 9-year-old boy. J. Oral Maxillofac. Surg. 1999, 57, 620–623. [Google Scholar] [CrossRef]
- Gururaju, C.R.; Rathva, V.J.; Usha, C.; Sundaresh, K.J. A compound odontoma in the path of an erupting incisor. BMJ Case Rep. 2013, 2013, 2013200825. [Google Scholar] [CrossRef] [Green Version]
- D’Cruz, A.M.; Hegde, S.; Shetty, U.A. Large Complex Odontoma: A report of a rare entity. Sultan Qaboos Univ. Med. J. 2013, 13, 342–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, J.E.; Araújo, L.D.; Almeida, L.Y.; De Paula, A.M.; Bonan, P.R. Ameloblastic fibro-odontoma: A conservative surgical approach. Med. Oral Patol. Oral Cir. Bucal 2009, 14, 654–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zehani, A.; Kourda, N.; Landolsi, A.; Adouani, A.; Zermani, R.; Ben Jilani, S. Pediatric mandibular ameloblastic fibro-odontoma. Rev. Stomatol. Chir. Maxillofac. 2011, 112, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, L.F.; Amaral, J.B.D.; Santos, É.B.D.; Martinez, E.F.; Montalli, V.A.M.; Junqueira, J.L.C.; Araújo, V.C.; Napimoga, M.H. Presence of Cells in Fresh-Frozen Allogeneic Bone Grafts from Different Tissue Banks. Braz. Dent. J. 2017, 28, 152–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, X.; Shi, P.; Zhang, P.; Shen, J.; Kang, J. Impact of platelet-rich fibrin on mandibular third molar surgery recovery: A systematic review and meta-analysis. BMC Oral Health 2019, 19, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Li, X.; Sun, X.; Qi, M.; Chi, M.; Yin, L.; Zhou, Y. Bone regeneration around immediate placed implant of molar teeth with autologous platelet-rich fibrin: Two case reports. Medicine 2018, 97, 13058. [Google Scholar] [CrossRef] [PubMed]
- Pietruszka, P.; Chruścicka, I.; Duś-Ilnicka, I.; Paradowska-Stolarz, A. PRP and PRF-Subgroups and Divisions When Used in Dentistry. J. Pers. Med. 2021, 11, 944. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, P.; Sharma, S.D.; Chhabra, N.; Gupta, A.; Shukla, D. Platelet-Rich Fibrin as an Aid to Soft- and Hard-Tissue Healing. J. Maxillofac. Oral Surg. 2021, 20, 496–501. [Google Scholar] [CrossRef]
- Bernabeu-Mira, J.C.; Soto-Peñaloza, D.; Peñarrocha-Oltra, S.; Diago, M.P. Regenerated Traumatic Bone Cyst with Platelet-Rich Fibrin in the Mandible: A Case Report. Clin. Adv. Periodontics 2021, 11, 33–38. [Google Scholar] [CrossRef]
- Elsalanty, M.E.; Genecov, D.G. Bone grafts in craniofacial surgery. Craniomaxillofac. Trauma Reconstr. 2009, 2, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Sittitavornwong, S.; Gutta, R. Bone graft harvesting from regional sites. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Wewel, J.; Narayana, N. Ameloblastic fibro-odontoma of the anterior mandible in a 22-month-old boy. Indian J. Dent. Res. 2010, 21, 618–620. [Google Scholar] [PubMed]
- de Souza Tolentino, E.; Centurion, B.S.; Lima, M.C.; Freitas-Faria, P.; Consolaro, A.; Sant’ana, E. Ameloblastic fibro-odontoma: A diagnostic challenge. Int. J. Dent. 2010, 2010, 104630. [Google Scholar] [PubMed]
- Sivapathasundharam, B.; Manikandhan, R.; Sivakumar, G.; George, T. Ameloblastic fibro odontoma. Indian J. Dent. Res. 2005, 16, 19–21. [Google Scholar] [PubMed]
- Gilani, S.M.; Raza, A.; Al-Khafaji, B.M. Ameloblastic fibrosarcoma: A rare malignant odontogenic tumor. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2014, 131, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Khalili, M.; Shakib, P.A. Ameloblastic fibrosarcoma of the upper jaw: Report of a rare case with long-term follow-up. Dent. Res. J. 2013, 10, 112–115. [Google Scholar]
- Demoor-Goldschmidt, C.; Minard-Colin, V.; Cassagneau, E.; Supiot, S.; Oberlin, O.; D’hautuille, C.; Corradini, N. Ameloblastic fibrosarcoma of the mandible: Report of 2 chemosensitive pediatric cases. J. Pediatr. Hematol. Oncol. 2012, 34, 72–76. [Google Scholar] [CrossRef]
- Gupta, N.; Barwad, A.; Kumar, R.; Rijuneeta Vaiphei, K. Ameloblastic fibrosarcoma: A cytologist’s perspective. Diagn. Cytopathol. 2011, 39, 598–602. [Google Scholar] [CrossRef]
- Soluk-Tekkesin, M.; Wright, J.M. The World Health Organization Classification of Odontogenic Lesions: A Summary of the Changes of the 2022 (5th) Edition. Turk Patoloji Derg 2022, 38, 168–184. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelke, K.; Pawlak, W.; Łukaszewski, M.; Janeczek, M.; Pasicka, E.; Barnaś, S.; Morawska-Kochman, M.; Dobrzyński, M. Treatment Possibilities in Mandibular Defect Reconstruction Based on Ameloblastic Fibro-Odontoma Treatment—Does Small Bone Defects Heal without Bone Grafting? Appl. Sci. 2022, 12, 12963. https://doi.org/10.3390/app122412963
Nelke K, Pawlak W, Łukaszewski M, Janeczek M, Pasicka E, Barnaś S, Morawska-Kochman M, Dobrzyński M. Treatment Possibilities in Mandibular Defect Reconstruction Based on Ameloblastic Fibro-Odontoma Treatment—Does Small Bone Defects Heal without Bone Grafting? Applied Sciences. 2022; 12(24):12963. https://doi.org/10.3390/app122412963
Chicago/Turabian StyleNelke, Kamil, Wojciech Pawlak, Marceli Łukaszewski, Maciej Janeczek, Edyta Pasicka, Szczepan Barnaś, Monika Morawska-Kochman, and Maciej Dobrzyński. 2022. "Treatment Possibilities in Mandibular Defect Reconstruction Based on Ameloblastic Fibro-Odontoma Treatment—Does Small Bone Defects Heal without Bone Grafting?" Applied Sciences 12, no. 24: 12963. https://doi.org/10.3390/app122412963