Corrosion Behavior in RC Member with Different Cover Depths under Cyclic Chloride Ingress Conditions for 2 Years
Abstract
:1. Introduction
2. Corrosion Mechanism and Its Detection Techniques
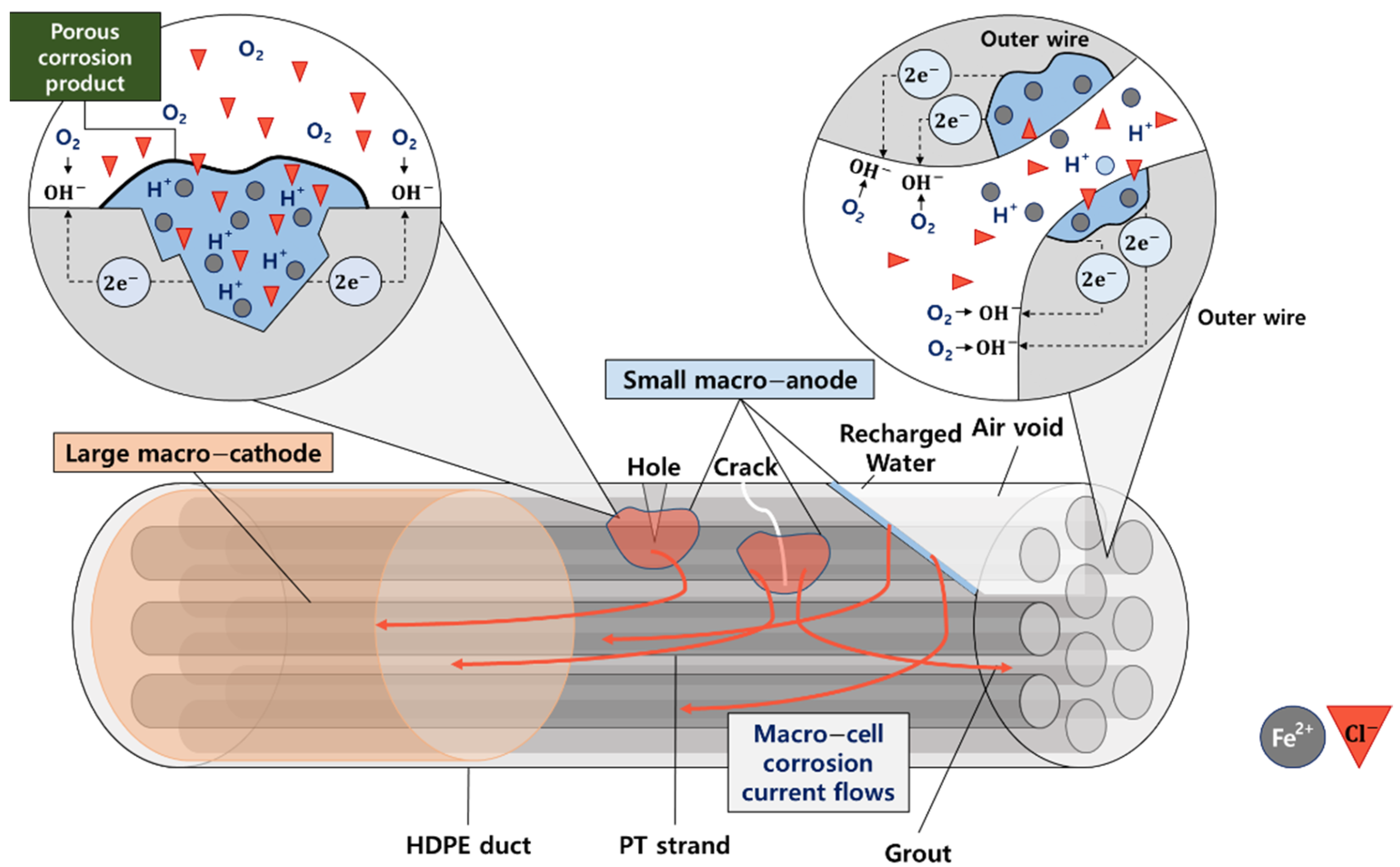
2.1. Corrosion Mechanism in Steel in RC and PSC
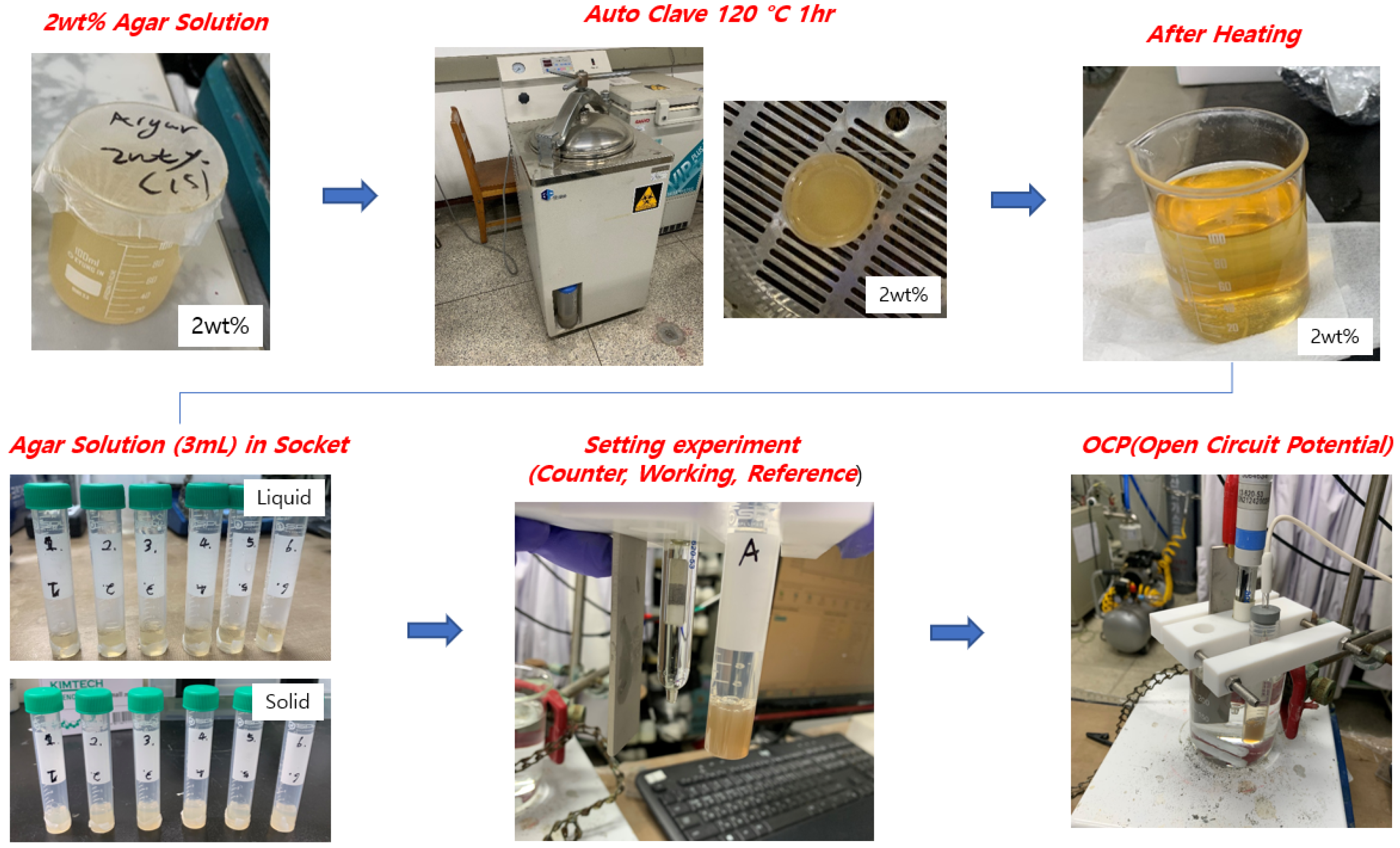
2.2. Corrosion Detection Sensor
3. Test Program for Accelerated Chloride Ingress and Potential Measurement
3.1. RC Samples and Used Material
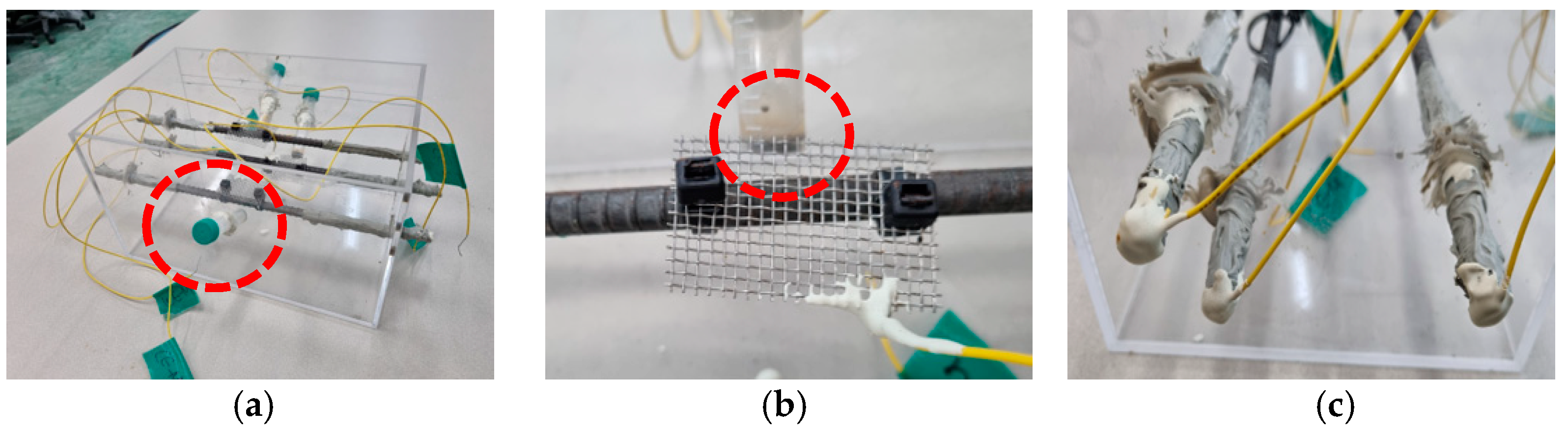
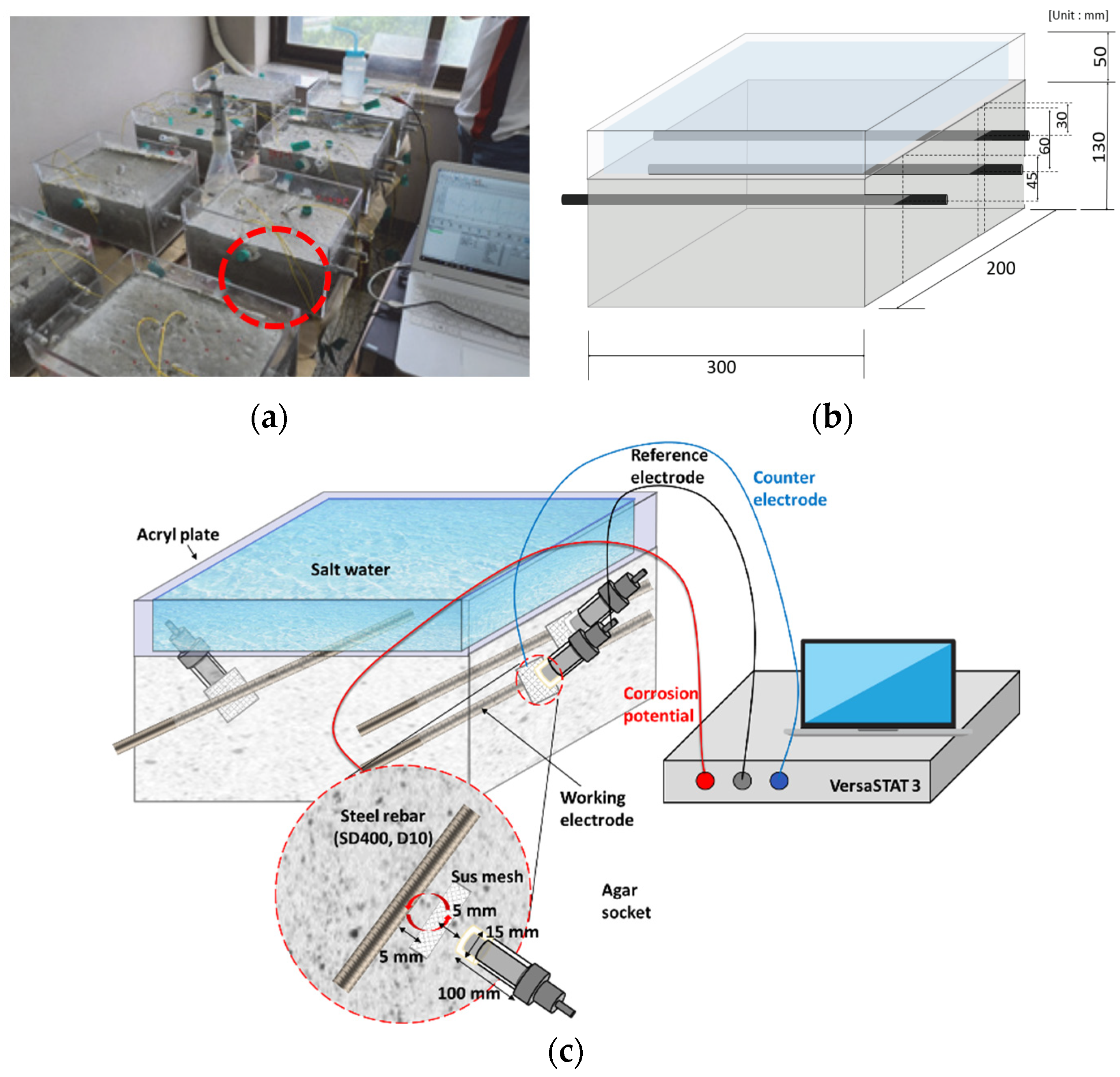
3.1.1. Fabricated RC Samples with Different Cover Depths and w/c Ratios
3.1.2. Mix Proportions and Materials
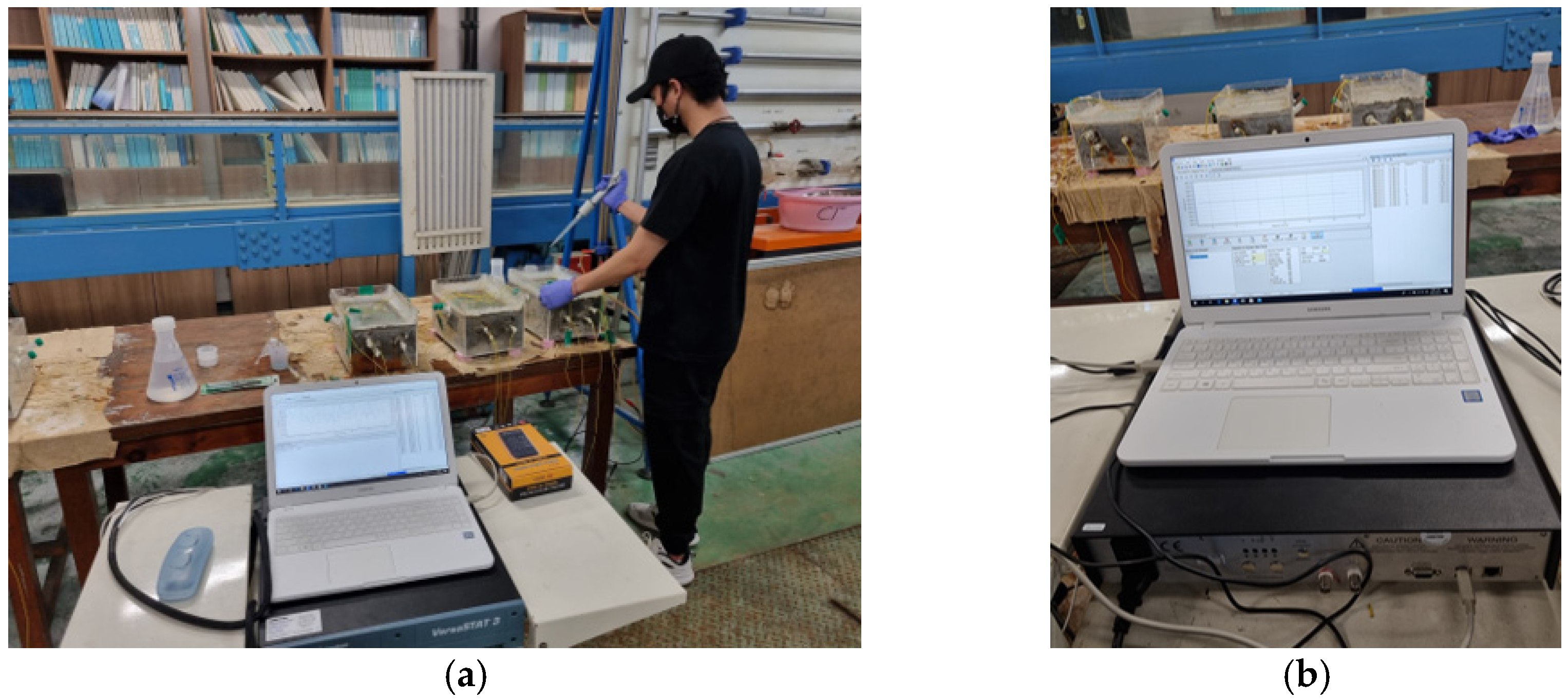
3.2. Accelerated Chloride Intrusion Test and OCP Measurement
4. Corrosion Potential Behavior with Test Parameters
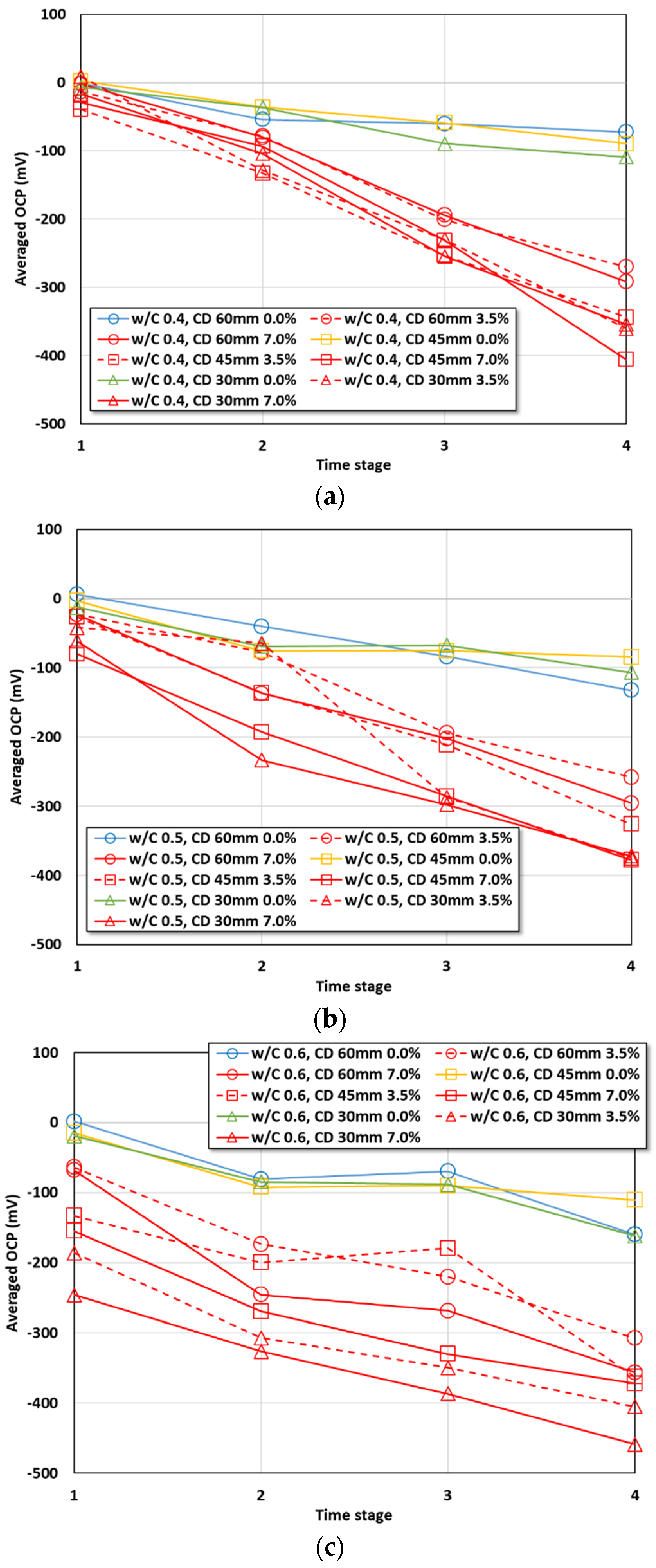
4.1. OCP Evaluation with the Averaged OCP
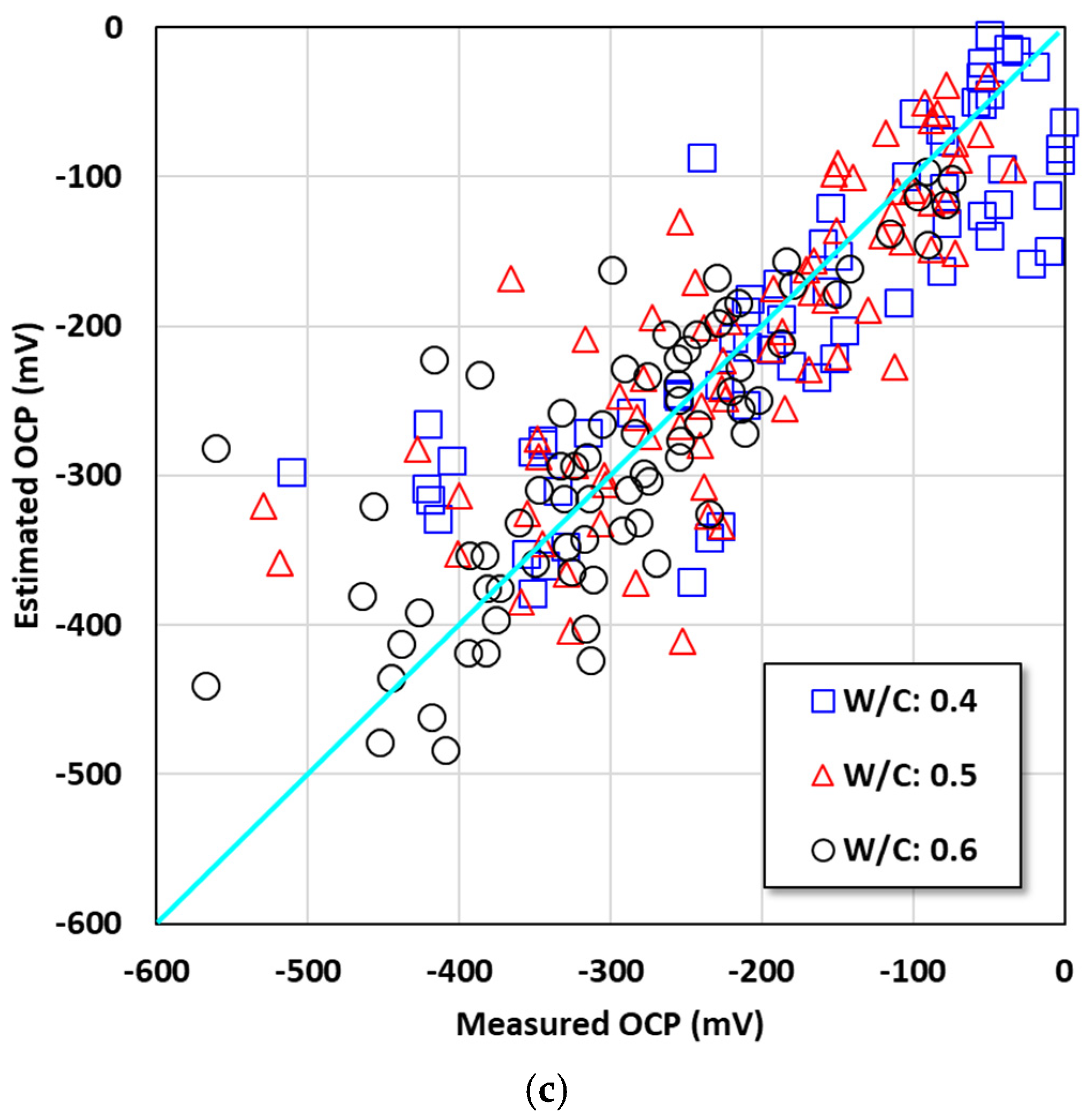
4.2. Relationship between OCP and the Parameters
4.2.1. Effects of the Cover Depth and w/c Ratio on the OCP with Increasing Chloride Content
4.2.2. Effects of the Cover Depth and w/c Ratio on the OCP with Increasing Period
5. Conclusions
- (1)
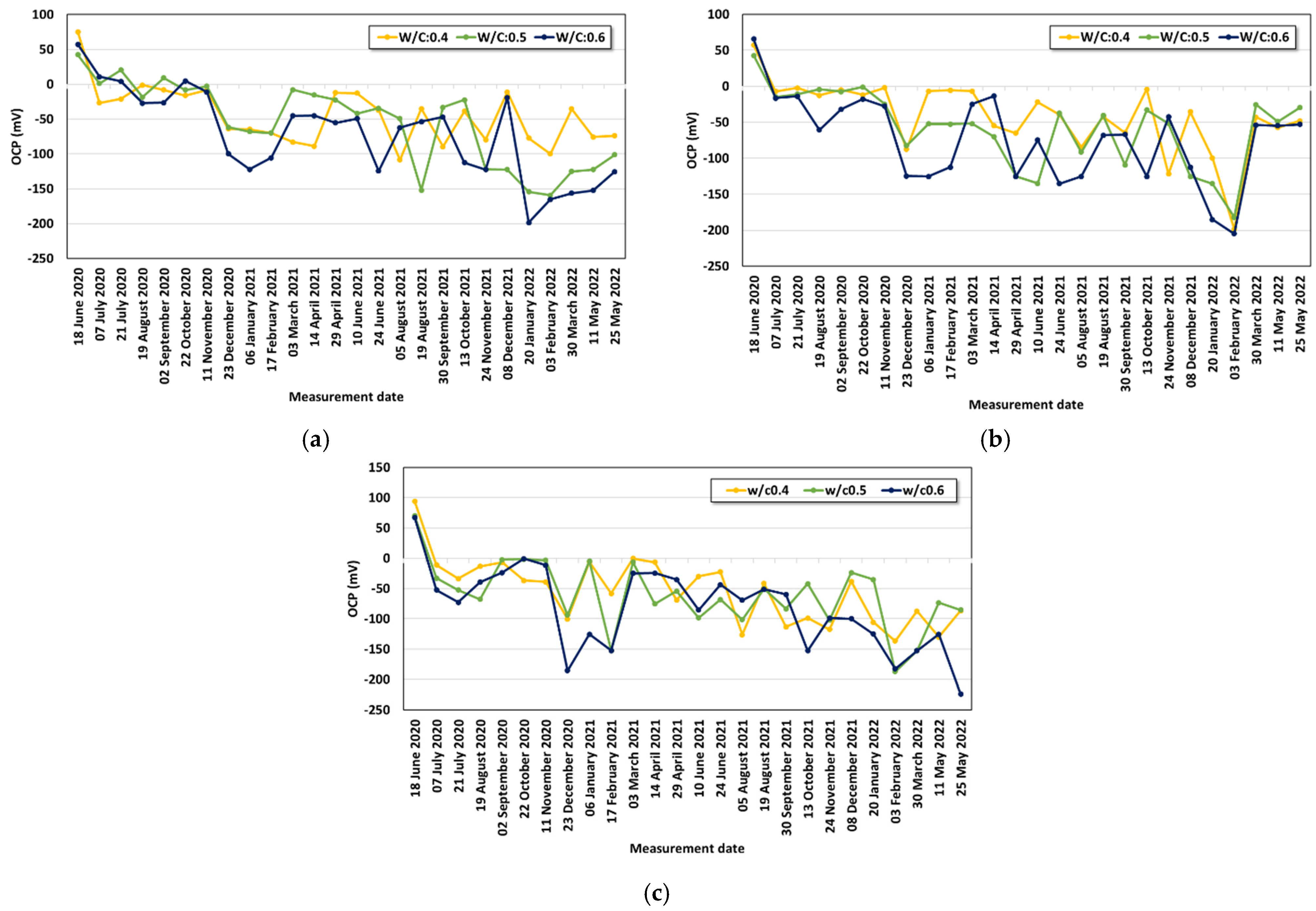
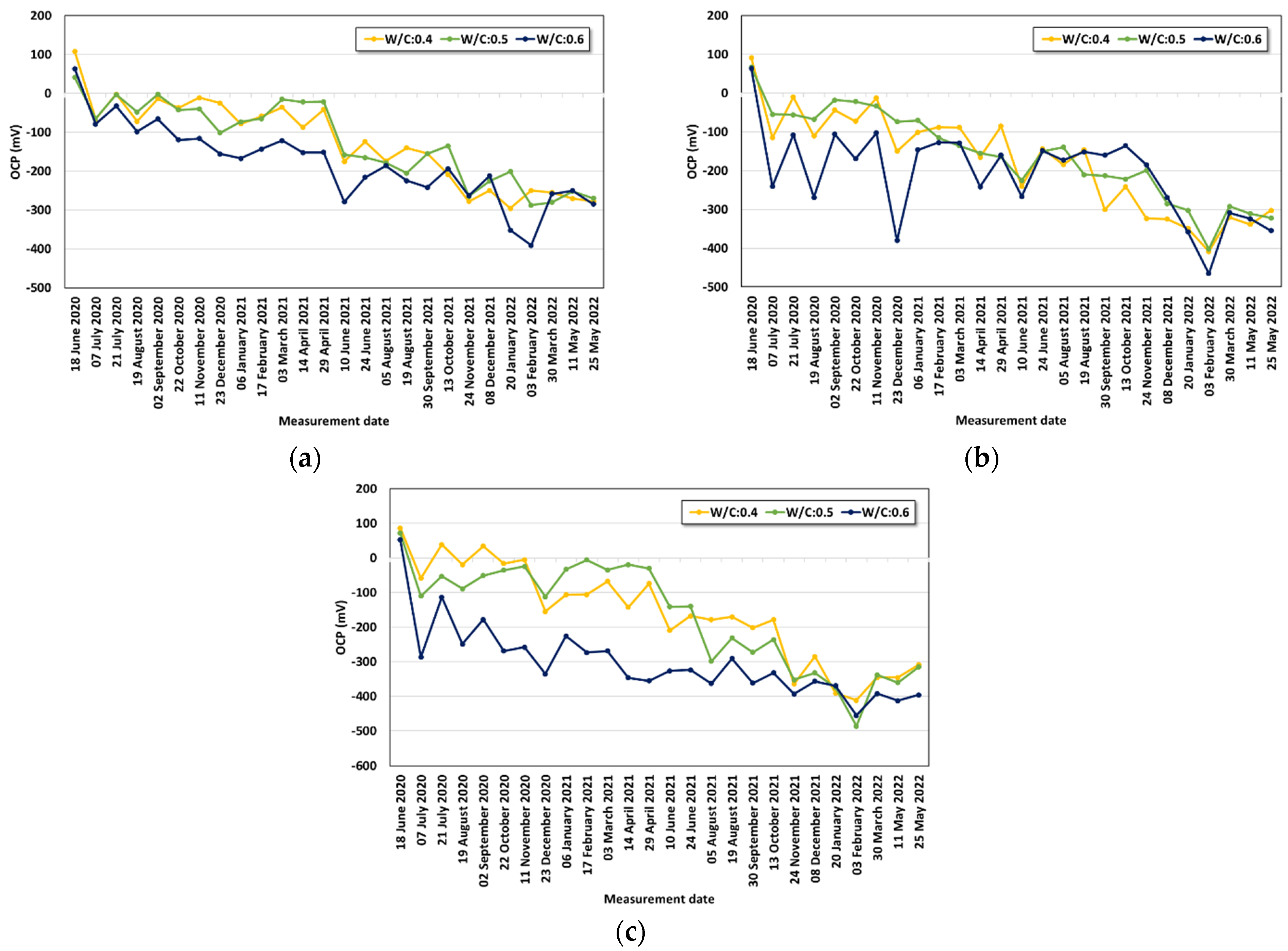
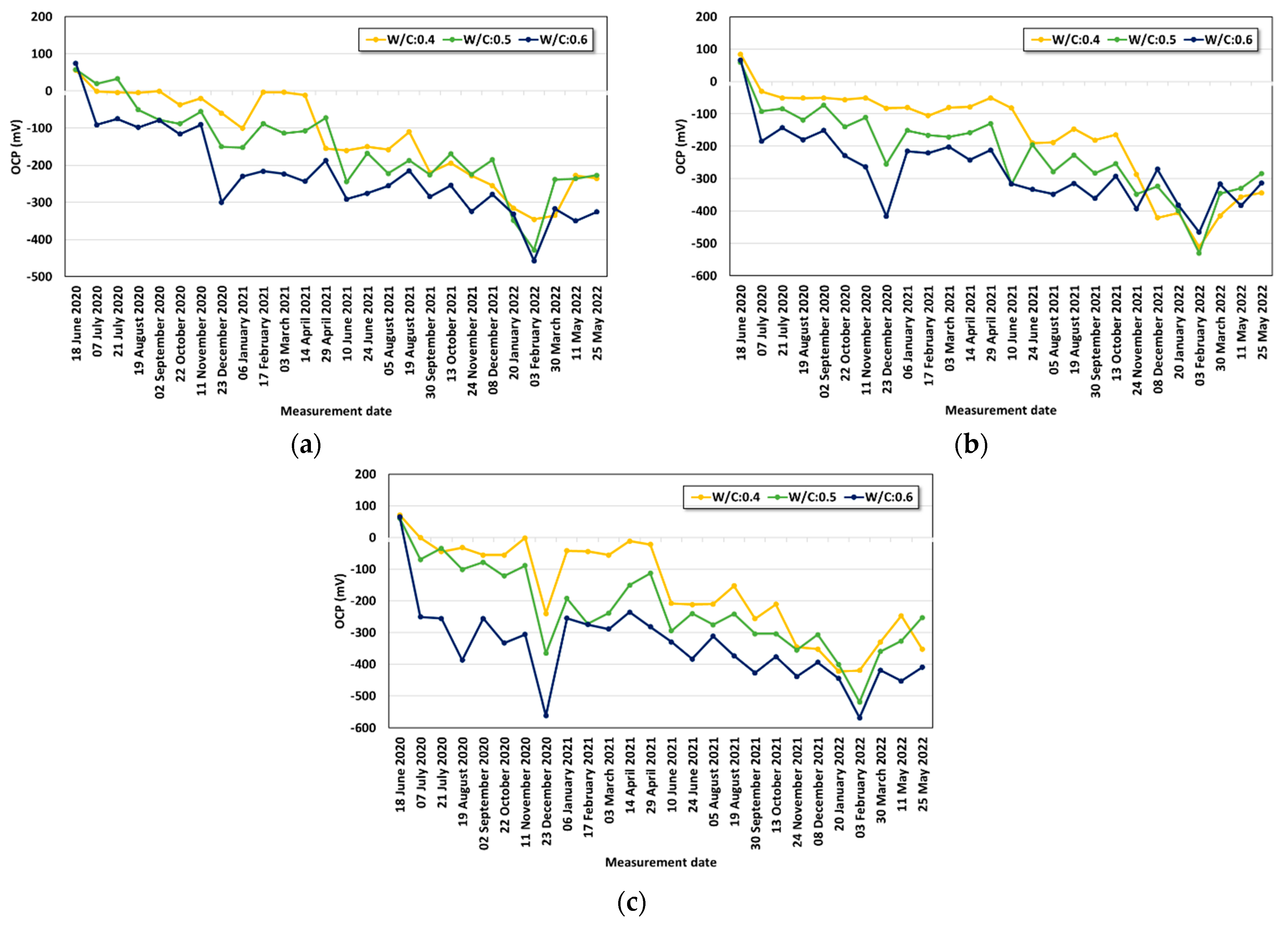
- Under the conditions that considered saltwater, the OCP was evaluated to be lower than the critical potential (−450 mV) regardless of the w/c ratio under the lowest cover depth condition (30 mm) after six months. As corrosion progressed, the OCP varied due to the partial saturation and rust product.
- (2)
- The averaged OCP measured for two years was derived, and each average value was analyzed with the cover depth. The corrosion potential decreased with increasing chloride exposure period and high chloride concentration. When the w/c ratio was 0.4, a significant drop in the OCP value began to be observed under the 3.5% and 7.0% conditions only after one year. Under the condition with a high w/c ratio (w/c: 0.6), values below −100 mV were observed regardless of the cover depth after six months. The averaged OCP increased linearly in the negative direction with decreasing cover depth, and they showed a clearer tendency with higher chloride concentration. Regression equations were evaluated for the OCP behavior for each exposure environment considering the exposure period and cover depth.
- (3)
- The measured OCP showed fluctuations with varying saturation and the corrosion pitting effect was not investigated. If the bearing capacity in the corroded RC beam with the mineral admixture such as fly ash and slag was evaluated through long-term accelerated conditions, the results from this work would show more reasonable ones that can be applied to a real RC member.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Broomfield, J.P. Corrosion of Steel in Concrete: Understanding Investigation and Repair; E & FN: London, UK, 1997; pp. 1–15.
- Li, F.; Yuan, Y.; Li, C.Q. Corrosion propagation of prestressing steel strands in concrete subject to chloride attack. Constr. Build. Mater. 2011, 25, 3878–3885. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Corrosion rate monitoring in the laboratory and on-site. Constr. Build. Mater. 1996, 10, 315–328. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Ryu, H.S.; Lim, H.S.; Koh, K.T.; Kim, J.S.; Kwon, S.J. Effect of grout conditions and tendon location on corrosion pattern in PS tendon in grout, Constr. Build. Mater. 2018, 186, 1005–1015. [Google Scholar]
- Chung, L.; Kim, J.H.J.; Yi, S.T. Bond Strength Prediction for Reinforced Concrete Members with Highly Corroded Reinforcing Bars. Cem. Concr. Compos. 2008, 30, 603–611. [Google Scholar] [CrossRef]
- Kwon, S.J. Current Trends of Durability Design and Government Support in South Korea: Chloride Attack. Sustainability 2017, 9, 417. [Google Scholar] [CrossRef] [Green Version]
- Asiedu, Y.; Gu, P. Product lifecycle cost analysis: State of the art review. Int. J. Prod. Res. 1998, 36, 883–908. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Wang, J.; Chien, W.T.; Wei, C.Y.; Wang, X.; Hsieh, S.H. A BIM-based approach for predicting corrosion under insulation. Autom. Constr. 2019, 107, 102923. [Google Scholar] [CrossRef]
- Pack, S.W.; Jung, M.S.; Song, H.W.; Kim, S.H.; Ann, K.Y. Prediction of time dependent chloride transport in concrete structures exposed to a marine environment. Cem. Concr. Res. 2010, 40, 302–312. [Google Scholar] [CrossRef]
- Hüthwohl, P.; Brilakis, I.; Borrmann, A.; Sacks, R. Integrating RC bridge defect information into BIM models. J. Comput. Civ. Eng. 2018, 32. [Google Scholar] [CrossRef]
- Figueira, R.B. Electrochemical sensors for monitoring the corrosion conditions of reinforced concrete structures: A review. Appl. Sci. 2017, 7, 1157. [Google Scholar] [CrossRef] [Green Version]
- Karthick, S.P.; Muralidharan, S.; Saraswathy, V.; Thangavel, K. Long-term Relative Performance of Embedded Sensor and Surface Mounted Electrode for Corrosion Monitoring of Steel in Concrete Structures. Sens. Actuators B-Chem. 2014, 192, 303–309. [Google Scholar] [CrossRef]
- Karthick, S.P.; Muralidharan, S.; Lee, H.S.; Kwon, S.J.; Saraswathy, V. Reliability and long-term evaluation of GO-MnO2 nano material as a newer corrosion monitoring sensor for reinforced concrete structures. Cem. Concr. Compos. 2019, 100, 74–84. [Google Scholar] [CrossRef]
- Pereira, E.V.; Figueira, R.B.; Salta, M.M.; Fonseca, I.T.E. Embedded Sensors for Corrosion Monitoring of Existing Reinforced Concrete Structures. Mater. Sci. Forum 2008, 587, 677–681. [Google Scholar] [CrossRef]
- Yu, B.; Liu, J.; Chen, Z. Probabilistic evaluation method for corrosion risk of steel reinforcement based on concrete resistivity. Constr. Build. Mater. 2017, 138, 101–113. [Google Scholar] [CrossRef]
- Lim, Y.C.; Noguchi, T.; Cho, C.G. A quantitative analysis of the geometric effects of reinforcement in concrete resistivity measurement above reinforcement. Constr. Build. Mater. 2015, 83, 189–193. [Google Scholar] [CrossRef]
- Jaśniok, M. Studies on the Effect of a Limited Polarization Range of Reinforcement on Impedance Spectra Shapes of Steel in Concrete. Procedia Eng. 2015, 108, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Meira, G.R.; Andrade, C.; Vilar, E.O.; Nery, K.D. Analysis of chloride threshold from laboratory and field experiments in marine atmosphere zone. Constr. Build. Mater. 2014, 55, 289–298. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, H.; Chu, H.; Zhu, C.; Xiong, C.; You, L.; Xu, J.; Zhang, Y.; Qin, Y. Influence of compression fatigue on chloride threshold value for the corrosion of steels in simulated concrete pore. Constr. Build. Mater. 2014, 73, 699–704. [Google Scholar] [CrossRef]
- Behnood, A.; Van Tittelboom, K.; De Belie, N. Methods for measuring pH in concrete: A review. Constr. Build. Mater. 2016, 105, 176–188. [Google Scholar] [CrossRef]
- Gandía-Romero, J.M.; Bataller, R.; Monzón, P.; Campos, I.; García-Breijo, E.; Valcuende, M.; Soto, J. Characterization of embeddable potentiometric thick-film sensors for monitoring chloride penetration in concrete. Sens. Actuators B-Chem. 2016, 222, 407–418. [Google Scholar] [CrossRef]
- Jin, M.; Jiang, L.; Zhu, Q. Monitoring chloride ion penetration in concrete with different mineral admixtures based on embedded chloride ion selective electrodes. Constr. Build. Mater. 2017, 143, 1–15. [Google Scholar] [CrossRef]
- Lee, S.K.; Zielske, J. An FHWA Special Study: Post-Tensioning Tendon Grout Chloride Thresholds; Technical report (FHWA-HRT-14-039); Federal Highway Administration: McLean, VA, USA, 2014.
- Lau, K. Corrosion of Post-Tensioned Tendons with Deficient Grout; Final report (BDV29-977-04); Florida Department of Transportation Research Center: Tallahassee, FL, USA, 2016; p. 202.
- Shao, X.M.; Feldman, J.L. Micro-agar salt bridge in patch-clamp electrode holder stabilizes electrode potentials. J. Neurosci. Methods 2007, 159, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffó, G.S.; Farina, S.B.; Giordano, C.M. Characterization of solid embeddable reference electrodes for corrosion monitoring in reinforced concrete structures. Electrochim. Acta 2009, 54, 1010–1020. [Google Scholar] [CrossRef]
- Lee, S.S.; Kwon, S.J. Characteristics of OCP of Reinforced Concrete Using Socket-type Electrodes during Periodic Salt Damage Test. J. Korea Inst. Struct. Maint. Inspect. 2021, 25, 28–36. [Google Scholar]
- Hassel, A.W.; Fushimi, K.; Seo, M. An agar-based silver| silver chloride reference electrode for use in micro-electrochemistry. Electrochem. Commun. 1999, 1, 180–183. [Google Scholar] [CrossRef]
- Kakiuchi, T. Salt bridge in electroanalytical chemistry: Past, present, and future. J. Solid State Electrochem. 2011, 15, 1661–1671. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Wang, Y.; Xu, S.; Fan, M. Stray current distributing model in the subway system: A review and outlook. Int. J. Electrochem. Sci. 2018, 13, 1700–1727. [Google Scholar] [CrossRef]
- Elsener, B. Corrosion Rate of Steel in Concrete Measurements beyond the Tafel law. Corros. Sci. 2008, 47, 3019–3033. [Google Scholar] [CrossRef]
- Hussain, R.R.; Singh, J.K.; Alhozaimy, A.; Al-Negheimish, A.; Bhattacharya, C.; Pathania, R.S.; Singh, D.D.N. Effect of Reinforcing Bar Microstructure on Passive Film Exposed to Simulated Concrete Pore Solution. ACI Mater. J 2018, 115, 181–190. [Google Scholar] [CrossRef]
- Pargar, F.; Koleva, D. Polarization behaviour of silver in model solutions. Int. J. Struct. Civ. Eng. Res. 2017, 6, 172–176. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Kim, J.M.; Bang, J.W.; Kwon, S.J. Effect of cover depth, w/c ratio, and crack width on half cell potential in cracked concrete exposed to salt sprayed condition. Constr. Build. Mater. 2014, 54, 636–645. [Google Scholar] [CrossRef]
- Kioumarsi, M.; Markeset, G.; Hooshmandi, S. Effect of pit distance on failure probability of a corroded RC beam. Procedia Eng. 2017, 171, 526–533. [Google Scholar] [CrossRef]
- Kioumarsi, M.M.; Hendriks, M.A.; Kohler, J.; Geiker, M.R. The effect of interference of corrosion pits on the failure probability of a reinforced concrete beam. Eng. Struct. 2016, 114, 113–121. [Google Scholar] [CrossRef]
- Kwon, S.J.; Na, U.J.; Park, S.S.; Jung, S.H. Service life prediction of concrete wharves with early-aged crack: Probabilistic approach for chloride diffusion. Struct. Saf. 2009, 31, 75–83. [Google Scholar] [CrossRef]
- Ye, H.; Jin, X.; Fu, C.; Jin, N.; Xu, Y.; Huang, T. Chloride penetration in concrete exposed to cyclic drying-wetting and carbonation. Constr. Build. Mater. 2016, 112, 457–463. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Bamforth, P.B. Modeling chloride diffusion in concrete: Effect of fly ash and slag. Cem. Concr. Res. 1999, 29, 487–495. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Bentz, E.C. Computer Program for Predicting the Service Life and Life-Cycle Costs of Reinforced Concrete Exposed to Chlorides, Life365 Manual; Silica Fume Association (SFA): Lovettsville, VA, USA, 2002. [Google Scholar]






| No. | W/C | S/a (%) | Unit Weight (kg/m3) | ||||
|---|---|---|---|---|---|---|---|
| Water | Cement | Sand | Gravel | Admixtures | |||
| 1 | 0.4 | 43.0 | 180 | 450 | 712 | 966 | 3.15 |
| 2 | 0.5 | 45.0 | 180 | 360 | 779 | 974 | 2.52 |
| 3 | 0.6 | 47.0 | 180 | 300 | 837 | 966 | 2.10 |
| Items | Chemical Compositions (%) | Physical Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Ig. Loss | Specific Gravity | Blaine (cm2/g) | |
| OPC | 21.96 | 5.27 | 3.44 | 63.41 | 2.13 | 1.96 | 0.79 | 3.16 | 3214 | |
| Items | Gmax (mm) | Specific Gravity (g/cm3) | Absorption (%) | F.M | |
|---|---|---|---|---|---|
| Type | |||||
| Fine aggregate | - | 2.62 | 1.01 | 2.90 | |
| Coarse aggregate | 20 | 2.68 | 0.82 | 6.87 | |
| Items | Diameter (mm) | Yield Stength (MPa) | Steel Ratio | Spacing (mm) | |
|---|---|---|---|---|---|
| Type | |||||
| Steel rebar | 10 | 400 | 0.90 | 41.5 | |
| Averaged OCP = a × Cover Depth + b | ||||
|---|---|---|---|---|
| a | b | R2 | ||
| WC-0.4 (0.0%) | 0.3297 | −61.669 | 0.5075 | |
| WC-0.5 (0.0%) | 0.1771 | −65.936 | 0.9926 | |
| WC-0.6 (0.0%) | 0.384 | −93.324 | 0.9377 | |
| WC-0.4 (3.5%) | 1.1218 | −204.33 | 0.4354 | |
| WC-0.5 (3.5%) | 1.5005 | −218.96 | 0.8921 | |
| WC-0.6 (3.5%) | 4.0884 | −414.6 | 0.9099 | |
| WC-0.4 (7.0%) | 1.2439 | −208.4 | 0.6597 | |
| WC-0.5 (7.0%) | 2.5515 | −314.69 | 0.8382 | |
| WC-0.6 (7.0%) | 3.9919 | −459.86 | 0.987 | |
| Condition | W/C Ratio | OCP = A(Time) + B(Cover Depth) + C | ||||
|---|---|---|---|---|---|---|
| Multiple Correlation | Determination Coefficient | A | B | C | ||
| Wet Condition | 0.4 | 0.634 | 0.402 | −0.140 | 0.330 | −14.392 |
| 0.5 | 0.627 | 0.393 | −0.159 | 0.177 | −12.385 | |
| 0.6 | 0.629 | 0.395 | −0.183 | 0.384 | −31.730 | |
| 3.5% Concentration | 0.4 | 0.905 | 0.819 | −0.528 | 1.122 | −26.206 |
| 0.5 | 0.887 | 0.787 | −0.501 | 1.501 | −49.861 | |
| 0.6 | 0.789 | 0.622 | −0.357 | 4.088 | −294.175 | |
| 7.0% Concentration | 0.4 | 0.878 | 0.771 | −0.564 | 1.244 | −18.187 |
| 0.5 | 0.838 | 0.702 | −0.468 | 2.552 | −156.681 | |
| 0.6 | 0.783 | 0.614 | −0.390 | 3.992 | −328.259 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-M.; Yoon, Y.-S.; Yang, K.-H.; Yoo, B.-Y.; Kwon, S.-J. Corrosion Behavior in RC Member with Different Cover Depths under Cyclic Chloride Ingress Conditions for 2 Years. Appl. Sci. 2022, 12, 13002. https://doi.org/10.3390/app122413002
Lee K-M, Yoon Y-S, Yang K-H, Yoo B-Y, Kwon S-J. Corrosion Behavior in RC Member with Different Cover Depths under Cyclic Chloride Ingress Conditions for 2 Years. Applied Sciences. 2022; 12(24):13002. https://doi.org/10.3390/app122413002
Chicago/Turabian StyleLee, Kwang-Myong, Yong-Sik Yoon, Keun-Hyeok Yang, Bong-Young Yoo, and Seung-Jun Kwon. 2022. "Corrosion Behavior in RC Member with Different Cover Depths under Cyclic Chloride Ingress Conditions for 2 Years" Applied Sciences 12, no. 24: 13002. https://doi.org/10.3390/app122413002
APA StyleLee, K.-M., Yoon, Y.-S., Yang, K.-H., Yoo, B.-Y., & Kwon, S.-J. (2022). Corrosion Behavior in RC Member with Different Cover Depths under Cyclic Chloride Ingress Conditions for 2 Years. Applied Sciences, 12(24), 13002. https://doi.org/10.3390/app122413002








