Data Protection and Privacy of the Internet of Healthcare Things (IoHTs)
Abstract
:1. Introduction
- We present IoHT classification, identify the vulnerabilities in IoHT implementation and map the security problem on the defined five-layer IoHT architecture;
- We discuss major existing healthcare legislative and regulatory initiatives, compare various legislative approaches and identify the gaps and governance challenges.
- We conclude with the recommendations on both frontiers (i.e., technical and legislative).
| Papers with Authors | IoHT Architecture | Legislative and Regulatory | Communication Technologies | Standards | Security and Privacy | |
|---|---|---|---|---|---|---|
| S. Ketu et al., 2021 [16] | ✔ | ✔ | Challenges ✔ | Countermeasures ✔ | ||
| M. Mamdouh, 2021 [17] | ✔ | ✔ | ✔ | |||
| R. Somasundaram, 2021 [18] | ✔ | ✔ | ||||
| R. Sivan et al., 2021 [19] | ✔ | ✔ | ✔ | |||
| This Paper | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
2. Internet of Healthcare Things (IoHT)
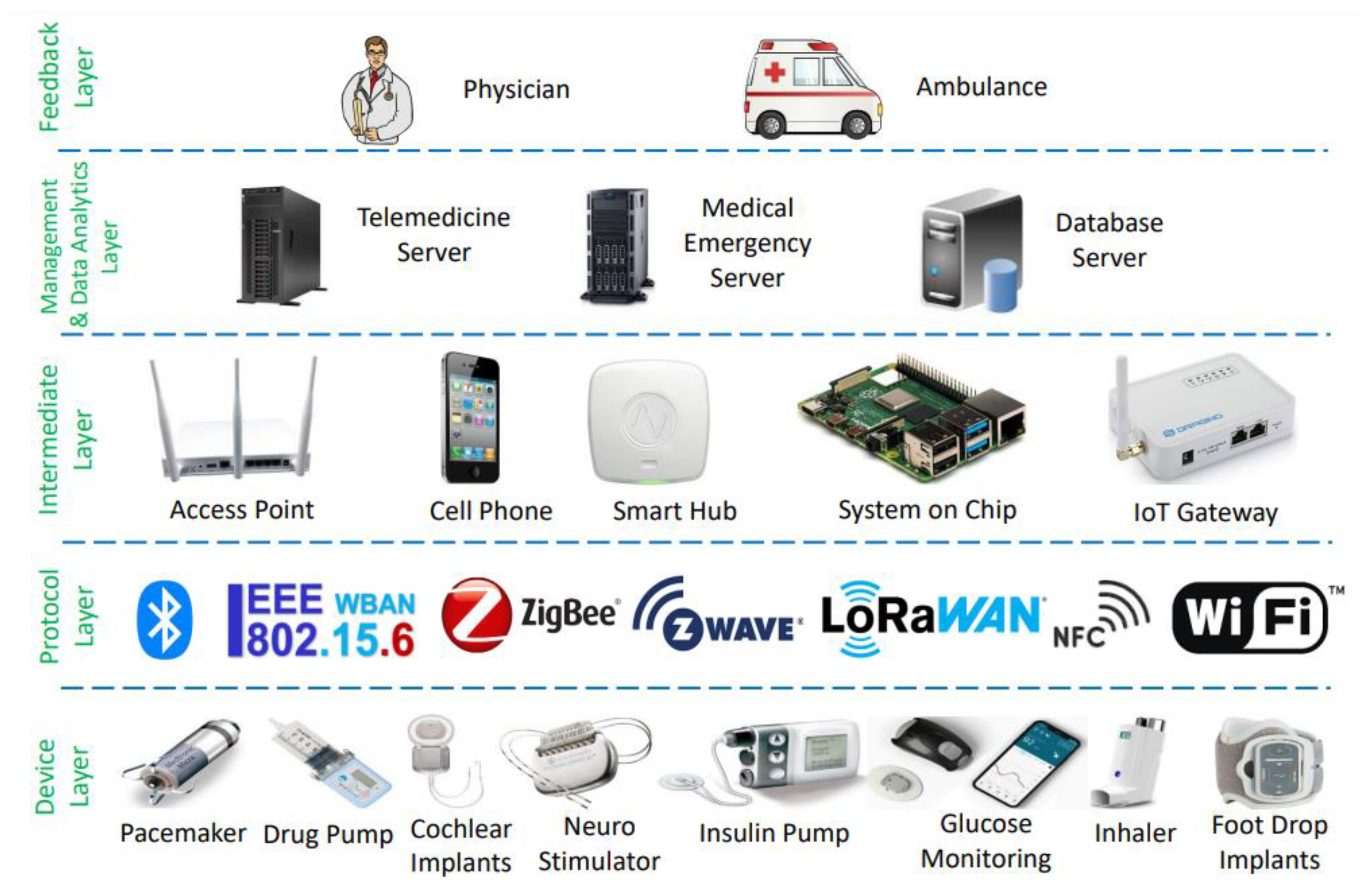
2.1. IoHT Classification Based on Architecture
2.2. IoHT Components
2.2.1. Device Layer
2.2.2. Protocol Layer
2.2.3. Intermediate Layer
2.2.4. Management and Data Link Layer
2.2.5. Feedback Layer
2.3. IoHT Working
3. IoHT Security Landscape
3.1. Security Vulnerabilities in IoHT Implementations
3.1.1. IoT Device’s Operating System
3.1.2. Communication Protocols
3.1.3. Insecure Middleware
3.2. Possible Points for Data Leakage
3.2.1. Persistent Data
3.2.2. Transient Data
3.3. Essential Security Features of IoHT
4. Healthcare Data Protection Legislation and Framework
4.1. Major Healthcare Initiative
4.1.1. Health Insurance Portability and Accountability Act (HIPAA)
- Common Identifier (e.g., name, address, birth date);
- Past, present, or future physical and mental health or condition;
- Past, present, or future payment provision for healthcare;
- Provision of healthcare to individuals.
- Making sure of the integrity and availability of personal healthcare information;
- Detects and protects against known threats to confidentiality, integrity, and availability;
- Protect against processes not permitted and the disclosure of information.
4.1.2. The Health Information Technology for Economic and Clinical Health Act (HITECH)
4.1.3. Personal Information Protection and Electronic Document Act (PIPEDA)
4.1.4. EUROHEALTH
4.1.5. General Data Protection Regulation (GDPR)
4.1.6. The Privacy Act (Australia)
4.1.7. Saudi Health Information Exchange Policies (SHIEP)
4.2. Data Protection Issues/Aspects Not Covered in Healthcare Laws
4.3. IoHT Governance Challenges
4.3.1. Conflicts in Laws
4.3.2. Data Protection Issues in Healthcare Systems
4.3.3. Absence of Conflict of Laws for Healthcare Data Protection
4.3.4. Non-Standard Healthcare Devices and Protocols
4.3.5. Other Considerations
- Awareness: There is a lack of awareness among the users of healthcare systems about the importance of the security of healthcare data;
- System Management Staff: Most of the time healthcare data is processed and forwarded by system management staff in healthcare institutes. They work on intermediate devices like data servers and gateway devices. The staff is not qualified enough to understand the complexities of data privacy and legal aspects. They are unaware of the latest threats because their primary tasks are network configurations and the availability of data at the endpoints. Mostly, they do not update healthcare systems and leave them unpatched until an issue is raised. They have little exposure to awareness programs and practices for data security and privacy;
- Doctors and Healthcare staff: One of the critical facts is not knowing much about the security and privacy laws of healthcare data by the healthcare staff and emergency response teams in hospitals. Specifically, the doctor uses personal devices such as cell phones or laptops to view the data, therefore, these personal devices also need to be secure. Moreover, they are not even familiar with the consequences of healthcare data leakage. There is no proper framework implemented that enforces healthcare staff to follow rules and policies to share and process patients’ healthcare data legally and securely;
- Patients: These are the central entities in the healthcare system. However, they are less attractive to attackers due to having less information i.e., about themselves only. They are conscious about their privacy and all these legalities are there to protect their healthcare privacy. However, they do not have any idea about how their data is shared with other organizations and what their rights are regarding their healthcare data. If their data is shared without conferring with them, it begs the question of what the legal liberties are that can be taken by concerned organizations about data sharing;
- Enforcement Difficulties: There is no enforcement authority or body established that helps to enforce data privacy laws in the healthcare sector. All the healthcare institutes should be obliged to follow instructions by some authority. The authority should implement healthcare laws;
- Low Budget: In developing countries, the medical budget is very limited. The trend of using IoHT devices is emerging in big cities. Mostly, there is no central system or facility provided to facilitate data privacy in developing countries. A very low or limited budget is allocated for new and innovative technologies in the healthcare sector. Therefore, the authorities consider that there is no need to enforce data privacy policies if the usage of such devices is limited;
- Lack of Qualified Staff: The IT staff does not configure/enable security functions in IoHT due to inadequate qualifications and expertise. The main reason behind it is the lack of security training programs for medical staff. They are only interested in the functional requirements of medical devices but do not take care of non-functional requirements of a medical device like communication security and data privacy;
- No Internal Auditing: There is no IoHT audit like IT audit, conducted in healthcare organizations and hospitals. If any organization is processing healthcare data, then it is unlikely to make checks and balance the healthcare data. The internal audit ensures what data is being processed by which organization for what purposes. The auditing activities reduce the risk of data leakage and unauthorized usage;
- No Special Enforcement Authority: One of the difficulties in the enforcement of data privacy laws and regulations is the absence of special enforcement authorities in the healthcare sector. Formation and implementation of data privacy laws should be the primary responsibilities of the authority. It can also ensure the compliance of state-level or national-level policies with international policies.
5. Recommendations
- Fines and Penalties: IoT devices gather a huge amount of information and there are several privacy risks associated with the usage and access of the data. Specifically, individual identification and behavior monitoring are major concerns. As the usage of IoT devices is increasing in the healthcare sector, a huge amount of private data is processed and stored. There is a need to introduce new privacy safeguards. The health information collected from devices like Fitbit/Jawbone [82] can be used to detect disease correlations with new treatment options with remote monitoring;
- Data Anonymization: IoT devices gather most of the data aggregated from the environment and forward it via a router or intermediate device for processing. During this process, several protocols and compression schemes are used as the storage space on the devices is limited and cannot handle big headers like that used for Internet Protocol IPv6. This data is sanitized as closely as possible to the device that created it since this communication avoids safety risks;
- Healthcare System Design: The healthcare system should be designed in such a way that it provides the controls in a user-friendly manner. An end-user must have full control over his/her collected data at any moment i.e., to whom it can be or cannot be shared. At any moment, the user should be given the possibility to know and control who has his data, what data have been collected, and for what purposes they will be used for the legitimate initial purpose;
- Privacy by Design: Privacy embedded into the design is an essential component integrated into the whole IoHT core system. The privacy safeguard framework must be implemented from the beginning of the system engineering process. The healthcare devices operate with user interactions or web interfaces. There are no privacy protection guidelines available while designing device interfaces. There are several vulnerabilities in web-based interfaces that are prone to data leakage and information leakage attacks. Most of the devices do not have authentication features or have default passwords that are difficult to enter due to their small size interfaces;
- Communication Security: There are several communication protocols used in IoT healthcare devices. There are no specific guidelines provided in data privacy laws about protocol security or what type of encryption or anonymity standards should be adopted for IoT devices, which operate on low memory and computation resources. These privacy laws should provide transparent policies about the communication security of these devices, especially for use in hospitals;
- Dispute Resolution: There is a need to resolve regional and international disputes regarding data protection. There are different versions of healthcare data privacy laws enforced regionally and internationally. If the healthcare data of a citizen is processed in a different country or state where different data privacy laws are enforced, then what are the possible legal issues that should apply to that person’s processed data? These types of disputes should be resolved in the national healthcare policies;
- Awareness Programs: Awareness programs are very significant to highlight the importance of data privacy, especially in the healthcare sector. IT staff, management staff, and other related staff of a healthcare facility should be aware and carry out the practices of secure processing of healthcare data. They must be aware of the consequences in the case of data leakage and what penalties they would be charged in the case of carelessness. Doctor and emergency response teams should be trained for the secure usage of their devices (i.e., laptops and cellphones, etc.) linked to healthcare systems, and they should share their experiences and difficulties while using these devices securely with healthcare organizations.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Telecommunication Union Yearbook of Statistics, 1991–2000; ITU: Geneva, Switzerland, 2001.
- Ahmad, T.; Ranise, S. Validating Requirements of Access Control for Cloud-Edge IoT Solutions (Short Paper). In International Symposium on Foundations and Practice of Security; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Culler, D.; Chakrabarti, S.; Infusion, I.P. 6LoWPAN: Incorporating IEEE 802.15. 4 into the IP Architecture, IPSO Alliance; White Paper. 2009. Available online: https://www.omaspecworks.org/wp-content/uploads/2018/03/6lowpan.pdf (accessed on 1 October 2021).
- Al Alkeem, E.; Yeun, C.Y.; Zemerly, M.J. Security and privacy framework for ubiquitous healthcare IoT devices. In Proceedings of the 10th IEEE International Conference for Internet Technology and Secured Transactions (ICITST), London, UK, 8–10 December 2015; pp. 70–75. [Google Scholar]
- Miorandi, D.; Sicari, S.; de Pellegrini, F.; Chlamtac, I. Internet of things: Vision, applications and research challenges. Ad. Hoc. Netw. 2012, 10, 1497–1516. [Google Scholar] [CrossRef] [Green Version]
- Porambage, P.; Ylianttila, M.; Schmitt, C.; Kumar, P.; Gurtov, A.; Vasilakos, A.V. The quest for privacy in the internet of things. IEEE Cloud Comput. 2016, 3, 36–45. [Google Scholar] [CrossRef]
- Solanas, A.; Patsakis, C.; Conti, M.; Vlachos, I.S.; Ramos, V.; Falcone, F.; Postolache, O.; Pérez-Martínez, P.A.; Di Pietro, R.; Perrea, D.N.; et al. Smart health: A context-aware health paradigm within smart cities. IEEE Commun. Mag. 2014, 52, 74–81. [Google Scholar] [CrossRef]
- Martinz-Ballest, A.; Pérez-Martínez, P.A.; Solanas, A. The pursuit of citizens’ privacy: A privacy-aware smart city is possible. IEEE Commun. Mag. 2013, 51, 136–141. [Google Scholar] [CrossRef]
- Eckhoff, D.; Wagner, I. Privacy in the smart city- applications, technologies, challenges, and solutions. IEEE Commun. Surv. Tutor. 2017, 20, 489–516. [Google Scholar] [CrossRef] [Green Version]
- Alghanim, A.A.; Rahman, S.M.M.; Hossain, M.A. Privacy analysis of smart city healthcare services. In Proceedings of the 2017 IEEE International Symposium on Multimedia (ISM), Taichung, Taiwan, 11–13 December 2017; pp. 394–398. [Google Scholar]
- Storm, D. MEDJACK: Hackers Hijacking Medical Devices to Create Backdoors in Hospital Networks Computer World. 2015. Available online: https://www.computerworld.com/article/2932371/medjack-hackers-hijacking-medical-devices-to-create-backdoors-in-hospital-networks.html (accessed on 1 October 2021).
- McMahon, E.; Williams, R.; El, M.; Samtani, S.; Patton, M.; Chen, H. Assessing medical device vulnerabilities on the Internet of Things. In Proceedings of the IEEE International Conference on Intelligence and Security Informatics (ISI), Beijing, China, 22–24 July 2017; pp. 176–178. [Google Scholar]
- Wang, L.; Ali, Y.; Nazir, S.; Niazi, M. ISA evaluation framework for security of internet of health things system using AHP-TOPSIS methods. IEEE Access 2020, 8, 152316–152332. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hossain, M.S.; Showail, A.J.; Alrajeh, N.A.; Alhamid, M.F. A secure, private, and explainable IoHT framework to support sustainable health monitoring in a smart city. Sustain. Cities Soc. 2021, 72, 103083. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hossain, M.S.; Islam, M.S.; Alrajeh, N.A.; Muhammad, G. Secure and provenance enhanced Internet of health things framework: A blockchain managed federated learning approach. IEEE Access 2020, 8, 205071–205087. [Google Scholar] [CrossRef]
- Ketu, S.; Mishra, P.K. Mishra Internet of Healthcare Things: A contemporary survey. J. Netw. Comput. Appl. 2021, 192, 103179. [Google Scholar] [CrossRef]
- Mamdouh, M.; Awad, A.I.; Khalaf, A.A.; Hamed, H.F. Authentication and Identity Management of IoHT Devices: Achievements, Challenges, and Future Directions. Comput. Secur. 2021, 111, 102491. [Google Scholar] [CrossRef]
- Somasundaram, R.; Thirugnanam, M. Review of security challenges in healthcare internet of things. Wirel. Netw. 2021, 27, 5503–5509. [Google Scholar] [CrossRef]
- Sivan, R.; Zukarnain, Z.A. Security and Privacy in Cloud-Based E-Health System. Symmetry 2021, 13, 742. [Google Scholar] [CrossRef]
- Parashar, A.; Rishishwar, S. Security challenges in IoT. In Proceedings of the Third International Conference on Advances in Electrical, Electronics, Information, Communication and Bio-Informatics (AEEICB), Chennai, India, 27–28 February 2017; pp. 446–449. [Google Scholar]
- Morghan, H.; Hashmi, U.S.; Imran, A. Edge computing in smart health care systems: Review, challenges, and research directions. Trans. Emerg. Telecommun. Technol. 2019, e3710. [Google Scholar] [CrossRef]
- Cao, Y.; Hou, P.; Brown, D.; Wang, J.; Chen, S. Distributed analytics and edge intelligence: Pervasive health monitoring at the era of fog computing. In Proceedings of the 2015 Workshop on Mobile Big Data (Mobidata), Hangzhou, China, 21 June 2015. [Google Scholar]
- Hu, R.; Pham, H.; Buluschek, P.; Gatica-Perez, D. Elderly people living alone: Detecting home visits with ambient and wearable sensing. In Proceedings of the 2nd International Workshop on Multimedia for Personal Health and Health Care (MMHealth), Mountain View, CA, USA, 23 October 2017. [Google Scholar]
- Baktir, A.C.; Tunca, C.; Ozgovde, A.; Salur, G.; Ersoy, C. SDN-based multi-tier computing and communication architecture for pervasive healthcare. IEEE Access 2018, 6, 56765–56781. [Google Scholar] [CrossRef]
- Brito, C.; Pinto, L.; Marinho, V.; Paiva, S.; Pinto, P. A Review on Recent Advances in Implanted Medical Devices Security. In Proceedings of the 2021 16th Iberian Conference on Information Systems and Technologies (CISTI), 2021, Chaves, Portugal, 23–26 June 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Thakar, A.T.; Pandya, S. Survey of IoT enables healthcare devices. In Proceedings of the 2017 International Conference on Computing Methodologies and Communication (ICCMC), Erode, India, 18–19 July 2017. [Google Scholar]
- Li, X.; Huang, X.; Li, C.; Yu, R.; Shu, L. EdgeCare: Leveraging edge computing for collaborative data management in mobile healthcare systems. IEEE Access 2019, 7, 22011–22025. [Google Scholar] [CrossRef]
- Perez, A.J.; Zeadally, S. Recent Advances in Wearable Sensing Technologies. Sensors 2021, 21, 6828. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zheng, G.; Ma, H.; Wang, X.; Ji, B.; Wu, H. A Survey of Routing Protocols in WBAN for Healthcare Applications. Sensors 2019, 19, 1638. [Google Scholar] [CrossRef] [Green Version]
- Baker, S.B.; Xiang, W.; Atkinson, I. Internet of things for smart healthcare: Technologies, challenges, and opportunities. IEEE Access 2017, 5, 26521–26544. [Google Scholar] [CrossRef]
- Saboor, A.; Mustafa, A.; Ahmad, R.; Khan, M.A.; Haris, M.; Hameed, R. Evolution of Wireless Standards for Health Monitoring. In Proceedings of the 2019 9th Annual Information Technology, Electromechanical Engineering and Microelectronics Conference (IEMECON), Jaipur, India, 13–15 March 2019; pp. 268–272. [Google Scholar] [CrossRef]
- Saboor, A.; Ahmad, R.; Ahmed, W.; Kiani, A.K.; Moullec, Y.L.; Alam, M.M. On Research Challenges in Hybrid Medium-Access Control Protocols for IEEE 802.15.6 WBANs. IEEE Sens. J. 2019, 19, 8543–8555. [Google Scholar] [CrossRef]
- Taleb, H.; Nasser, A.; Andrieux, G. Wireless technologies, medical applications and future challenges in WBAN: A survey. Wirel. Netw. 2021, 27, 5271–5295. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Paso, T.; Mucchi, L. ETSI SmartBAN in Medical IoT. In Proceedings of the 2021 XXXIVth General Assembly and Scientific Symposium of the International Union of Radio Science (URSI GASS), Rome, Italy, 28 August–4 September 2021. [Google Scholar] [CrossRef]
- Negra, R.; Jemili, I.; Belghith, A. Wireless body area networks: Applications and technologies. Procedia Comput. Sci. 2016, 83, 1274–1281. [Google Scholar] [CrossRef] [Green Version]
- Khajenasiri, I.; Zhu, P.; Verhelst, M.; Gielen, G. A low-energy ultra-wideband internet-of-things radio system for multi-standard smart-home energy management. IEIE Trans. Smart Process. Comput. 2015, 4, 354–365. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Ghosh, S.; Behere, A.; Ghosh, S.K.; Buyya, R. Internet of Health Things (IoHT) for personalized health care using integrated edge-fog-cloud network. J. Ambient. Intell. Hum. Comput. 2021, 12, 943–959. [Google Scholar] [CrossRef]
- Mamdouh, M.; Awad, A.I.; Hamed, H.F.A.; Khalaf, A.A.M. Outlook on Security and Privacy. In IoHT: Key Challenges and Future Vision, Proceedings of the International Conference on Artificial Intelligence and Computer Vision (AICV 2020). Advances in Intelligent Systems and Computing, Cairo, Egypt, 8–10 April, 2020; Hassanien, A.E., Azar, A., Gaber, T., Oliva, D., Tolba, F., Eds.; Springer: Cham, Switzerland, 2020; p. 1153. [Google Scholar] [CrossRef]
- Meyer, J.; Kazakova, A.; Büsing, M.; Boll, S. Visualization of complex health data on mobile devices. In Proceedings of the 2016 ACM Workshop on Multimedia for Personal Health and Health Care (MMHealth), Amsterdam, The Netherlands, 16 October 2016. [Google Scholar]
- Rolim, C.O.; Koch, F.L.; Westphall, C.B.; Werner, J.; Fracalossi, A.; Salvador, G.S. A cloud computing solution for patient’s data collection in health care institutions. In Proceedings of the 2010 Second International Conference on eHealth, Telemedicine, and Social Medicine, St. Maarten, The Netherlands, 2–16 October 2010. [Google Scholar]
- Perera, C.; McCormick, C.; Bandara, A.K.; Price, B.A.; Nuseibeh, B. Privacy-by-design framework for assessing internet of things applications and platforms. In Proceedings of the 6th International Conference on the Internet of Things, Stuttgart, Germany, 7–9 November 2016; pp. 83–92. [Google Scholar]
- Stork, M.; Vancura, V. Hidden pacemaker pulses detection based on wavelet and Hilbert-Huang transform. In Proceedings of the IEEE International Conference on Applied Electronics, Pilsen, Czech Republic, 9–10 September 2014; pp. 285–288. [Google Scholar]
- Samani, M.M.; Mahnam, A. Design and implementation of an ultra low power wireless neuro-stimulator system. In Proceedings of the 17th Iranian Conference of Biomedical Engineering (ICBME), Isfahan, Iran, 3–4 November 2010; pp. 1–4. [Google Scholar]
- Lucisano, J.Y.; Routh, T.L.; Lin, J.T.; Gough, D.A. Glucose monitoring in individuals with diabetes using a long-term implanted sensor/telemetry system and mode. IEEE Trans. Biomed. Eng. 2016, 64, 198–1993. [Google Scholar]
- Hiremath, S.; Yang, G.; Mankodiya, K. Wearable internet of things: Concept, architectural components and promises for person-centered healthcare. In Proceedings of the International Conference on Wireless Mobile Communication and Healthcare-Transforming Healthcare Through Innovations in Mobile and Wireless Technologies (MOBIHEALTH), Athens, Greece, 3–5 November 2014; pp. 304–307. [Google Scholar]
- Birgit, L.; Andrei, P. ActiGait®: A Partly Implantable Drop-Foot Stimulator System. In Introduction to Neural Engineering for Motor Rehabilitation; Farina, D., Jensen, W., Akay, M., Eds.; IEEE: Piscataway, NJ, USA, 2013; pp. 421–423. [Google Scholar]
- Hansen, J.H.; Ali, H.; Saba, J.N.; Charan, M.R.; Mamun, N.; Ghosh, R.; Brueggeman, A. Cci-mobile: Design and evaluation of a cochlear implant and hearing aid research platform for speech scientists and engineers. In Proceedings of the 2019 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Chicago, IL, USA, 19–22 May 2019; pp. 1–4. [Google Scholar]
- Caffey, S.; Po-Ying, L.; Jeffrey, B. Remote-Controlled Drug Pump Devices. U.S. Patent 8,285,328, 9 October 2012. [Google Scholar]
- Zhao, Y.; Wang, J.; Zhang, Y.; Liu, H.; Chen, Z.A.; Lu, Y.; Dai, Y.; Xu, L.; Gao, S. Flexible and Wearable EMG and PSD Sensors Enabled Locomotion Mode Recognition for IoHT Based In-home Rehabilitation. IEEE Sens. J. 2021, 21, 26311–26319. [Google Scholar] [CrossRef]
- Rao, S.; Dubey, S.; Deb, S.; Hughes, Z.; Seo, Y.S.; Nguyen, M.Q.; Tang, S.J.; Abell, T.; Lahr, C.; Chiao, J.C. Wireless gastric stimulators. In Proceedings of the Texas Symposium on Wireless and Microwave Circuits and Systems, Waco, TX, USA, 3–4 April 2014; pp. 1–4. [Google Scholar]
- Zareei, M.; Zarei, A.; Budiarto, R.; Omar, M.A. A comparative study of short range wireless sensor network on high density networks. In Proceedings of the 17th Asia-Pacific Conference on Communications, APCC, Sabah, Malaysia, 2–5 October 2011; pp. 247–252. [Google Scholar]
- Fouladi, B.; Ghanoun, S. Security Evaluation of the Z-Wave Wireless Protocol; Black Hat: Las Vegas, NV, USA, 2013; Volume 24, pp. 1–2. [Google Scholar]
- Fatima, I.; Ahmad, A.; Ali, S.; Ali, M.; Baig, M. ITriple-Band circular polarized antenna for WLAN/WiFi/Bluetooth/WiMAX applications. Prog. Electromagn. Res. C 2021, 109, 65–75. [Google Scholar] [CrossRef]
- Varshney, G.; Gupta, H. A security framework for IOT devices against wireless threats. In Proceedings of the 2nd International Conference on Telecommunication and Networks (TEL-NET), Noida, India, 10–11 August 2017; pp. 1–6. [Google Scholar]
- Xie, L.; Yang, G.; Mantysalo, M.; Xu, L.L.; Jonsson, F.; Zheng, L.R. Heterogeneous integration of bio-sensing system-on-chip and printed electronics. IEEE J. Emerg. Sel. Top. Circuits Syst. 2012, 4, 672–682. [Google Scholar] [CrossRef]
- Lindquist, A.; Johansson, P.; Petersson, G.; Saveman, B.I.; Nilsson, G. The use of the personal digital assistant (PDA) among personnel and students in health care: A review. J. Med. Internet Res. 2008, 10, e1038. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, J. Automatic discovery and installation of wearable bio signal devices in ubiquitous healthcare system. In Proceedings of the 9th International Conference on Advanced Communication Technology, Gangwon, Korea, 12–14 February 2007; pp. 412–414. [Google Scholar]
- da Costa, C.A.; Pasluosta, C.F.; Eskofier, B.; da Silva, D.B.; Righi, R.d. Internet of health things: Toward intelligent vital signs monitoring in hospital wards. Artif. Intell. Med. 2018, 89, 61–69. [Google Scholar] [CrossRef]
- Rajit, N.; Thanachayanont, A. A 1-V CMOS low-power resistor-based temperature sensor for human body temperature monitoring. In Proceedings of the 34th International Technical Conference on Circuits/Systems, Computers and Communications (ITC-CSCC), JeJu, Korea, 23–26 June 2019; pp. 1–4. [Google Scholar]
- Yousefzadeh, B.; Shalmany, S.H.; Makinwa, K.A. A BJT-based temperature-to-digital converter with inaccuracy from −55 °C to +125 °C in 0.16. IEEE J. Solid State Circuits 2017, 52, 1044–1052. [Google Scholar] [CrossRef]
- Bai, B.; Nazir, S.; Bai, Y.; Anees, A. Security and provenance for Internet of Health Things: A systematic literature review. J. Softw. Evol. Process. 2021, 33, e2335. [Google Scholar] [CrossRef]
- Esha, N.H.; Tasmim, M.R.; Huq, S.; Mahmud, M.; Kaiser, M.S. Trust IoHT: A Trust Management Model for Internet of Healthcare Things. In Proceedings of the International Conference on Data Science and Applications, Kolkata, India, 10–11 April 2021; 2021; pp. 47–57. [Google Scholar]
- MacDermott, A.; Kendrick, P.; Idowu, I.; Ashall, M.; Shi, Q. Securing things in the healthcare internet of things. In Proceedings of the Global IoT Summit (GIoTS), New York, NY, USA, 7–21 June 2019; pp. 1–6. [Google Scholar]
- Baccelli, E.; Hahm, O.; Günes, M.; Wählisch, M.; Schmidt, T. OS for the IoT-goals, challenges, and solutions. In Proceedings of the Interdisciplinaire sur la Sécurité Globale (WISG2013) Workshop, Troyes, France, 22 January 2013; pp. 1–6. [Google Scholar]
- Chung, B.; Kim, J.; Jeon, Y. On-demand security configuration for IoT devices. In Proceedings of the International Conference on Information and Communication Technology Convergence (ICTC), Jeju, Korea, 19–21 October 2016; pp. 1082–1084. [Google Scholar]
- Zhou, W.; Jia, Y.; Peng, A.; Zhang, Y.; Liu, P. The effect of IoT new features on security and privacy: New threats, existing solutions, and challenges yet to be solved. IEEE Internet Things J. 2018, 6, 1606–1616. [Google Scholar] [CrossRef] [Green Version]
- Foukia, N.; Billard, D.; Solana, E. PISCES: A framework for privacy by design in IoT. In Proceedings of the 14th Annual Conference on Privacy, Security and Trust (PST), Auckland, New Zealand, 12–14 December 2016; pp. 706–713. [Google Scholar]
- van Rest, J.; Boonstra, D.; Everts, M.; van Rijn, M.; van Paassen, R. Designing privacy-by-design. In Proceedings of the 1st Annual Privacy Forum, Lecture Notes in Computer Science, Limassol, Cyprus, 10–11 October 2012; Springer: Berlin/Heidelberg, Germany, 2012; Volume 8319, pp. 55–72. [Google Scholar]
- Weber, R.H. Internet of things-new security and privacy challenges. Comput. Law Secur. 2010, 26, 23–30. [Google Scholar] [CrossRef]
- Neuhaus, C.; Polze, A.; Chowdhury, M.M. Survey on Healthcare IT Systems: Standards, Regulations and Security; University Potsdam: Potsdam, Germany, 2011. [Google Scholar]
- Swartz, N. Canada reviews PIPEDA. Inform. Manag. 2007, 41(2), 8. [Google Scholar]
- Danzon, M.; Litvinov, S.K. EUROHEALTH Programme. World Health Stat. Q. Rapp. Trimest. De Stat. Sanit. Mond. 1993, 46, 153–157. [Google Scholar]
- de Hert, P.; Papakonstantinou, V. The proposed data protection regulation replacing directive 95/46/EC: A sound system for the protection of individuals. Comput. Law Secur. Rev. 2012, 28, 130–142. [Google Scholar] [CrossRef]
- Georgiou, D.; Lambrinoudakis, C. Compatibility of a security policy for a cloud-based healthcare system with the EU general data protection regulation (GDPR). Information 2020, 11, 586. [Google Scholar] [CrossRef]
- Maeve, M. E-government in Australia: The challenge to privacy of personal information. Int. J. Inf. Technol. 2002, 10, 327. [Google Scholar]
- Balkhair, A. Kingdom of Saudi Arabia The National eHealth Program. 2014. Available online: https://www.itu.int/ITU-D/cyb/events/2012/e-health/Nat_eH_Dev/Session%204/KSA-MOH-Presentation-SaudiArabia%20FINAL.pdf (accessed on 1 October 2021).
- Zheng, M. Surveillance and disease control in COVID-19: Big data application in public health. In Proceeding of the International Conference on Applications and Techniques in Cyber Security and Intelligence, Fuyang, China, 19–21 June 2021; Springer: Berlin/Heidelberg, Germany, 2021; pp. 565–570. [Google Scholar]
- Overhage, J.M.; Ryan, P.B.; Reich, C.G.; Hartzema, A.G.; Stang, P.E. Validation of a common data model for active safety surveillance research. J. Am. Med. Inform. Assoc. 2012, 19, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Mattoo, A.; Meltzer, J.P. International data flows and privacy: The conflict and its resolution. J. Int. Econ. Law 2018, 21, 769–789. [Google Scholar] [CrossRef] [Green Version]
- Hemalatha, P. Monitoring and securing the healthcare data harnessing IOT and blockchain technology. Turk. J. Comput. Math. Educ. 2021, 12, 2554–2561. [Google Scholar]
- Lydahl, D. Standard tools for non-standard care: The values and scripts of a person-centred assessment protocol. Health 2021, 25, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, J.; Yu, B.; Shull, P.B. Validity of FitBit, Jawbone UP, Nike+ and other wearable devices for level and stair walking. Gait Posture 2016, 48, 36–41. [Google Scholar] [CrossRef] [PubMed]





| IoHT Devices | Protocol | Range | Frequency Spectrum | Data Transmission Rate | Security Protocols |
|---|---|---|---|---|---|
| Pacemaker | BLE/WiFi/Cellular | 400–500 m | 2.4-5 GHz, ISM Band, 700–2100 MHz | 1–3 Mbit/s | Secure SDN, NIST Standard |
| Hear Rate Monitor | ANT +/BLE | 400 m | 2.5 GHz | 60 Kbps–3 Mbit/s | 8-Byte Network Key, 128-bit AES |
| Temperature Sensor | IEEE 802.15.4/Zigbee | 10 m | 2.4-2.48 GHz | 250 Kbps | Symmetric Cryptography |
| ECG Sensor | WiFi | 50 m | 2.4–2.5 GHz, ISM Band | 1–3 Mbit/s | WPA-2 |
| Blood Pressure Monitor | Bluetooth 3.0 + EDR Technology | 10 m | 2.45 GHz | 3 Mbit/s | AES-CMAC Encryption |
| EMG Sensors | BLE | 400 m | 2.45 GHz | 1 Mbit/s | Link Layer Encryption |
| PPG Sensors | BLE | 400 m | 2.45 GHz | 1 Mbit/s | Link Layer Encryption |
| Position Sensors | BLE | 400 m | 2.45 GHz | 1 Mbit/s | Link Layer Encryption |
| Cuffless B.P. Sensors | BLE | 400 m | 2.45 GHz | 1 Mbit/s | Link Layer Encryption |
| Motion Sensors | Radio Frequency | 150 m | 433.92 MHz | 10 Kbps | SPECK/SIMON |
| Air Flow Sensors | Bluetooth 3.0 | 100–150 m | 2.45 GHz | 1–3 Mbit/s | Symmetric Cryptography |
| Protocol | Frequency | Range | Data Transfer Rate | Energy Consumption | Security |
|---|---|---|---|---|---|
| Bluetooth | 2.40–2.48 GHz | 10–50 m | 1–3 Mbps | 0.01–50 W | SAFER Block Cipher |
| BLE | 2.40 GHz | 400–1000 m | 125 Kbps–2 Mbps | 50–100 micro W | AES-CCM Cipher |
| ZigBee | 860 MHz–2.40 GHz | 10–100 m | 20–250 Kbps | 10–100 micro W | AES-CCM/CBC |
| LoRaWAN | 433–923 MHz | 2–7 km | 27 Kbps | 50–80 micro W | AEA-CMAC |
| ANT | 2.40 GHz | 30 m | 60 Kbps | 42–72 micro W | AES-CBC |
| UWB | 4.3 GHz | 10 m | 1 Mbps | 5.31 micro W | CRC |
| RuBee | 131 kHz | 15 m | 9.6 Kbps | 40 nano W | AES |
| Regulation | Country/ Region | Device Layer | Protocol Layer | Intermediate Layer | Management Layer | Feedback Layer |
|---|---|---|---|---|---|---|
| HIPAA | America | Yes | Yes | No | Yes | No |
| PIPEDA | Canada | Yes | No | No | No | No |
| EURO HEALTH | EU | Yes | No | No | No | No |
| GDPR | EU | Yes | Yes | No | No | No |
| The Privacy Act | Australia | Yes | Yes | No | No | No |
| SHIEP | KSA | Yes | Yes | No | Yes | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, J.; Ahmad, R.; Kiani, A.K.; Ahmad, T.; Saeed, S.; Almuhaideb, A.M. Data Protection and Privacy of the Internet of Healthcare Things (IoHTs). Appl. Sci. 2022, 12, 1927. https://doi.org/10.3390/app12041927
Shahid J, Ahmad R, Kiani AK, Ahmad T, Saeed S, Almuhaideb AM. Data Protection and Privacy of the Internet of Healthcare Things (IoHTs). Applied Sciences. 2022; 12(4):1927. https://doi.org/10.3390/app12041927
Chicago/Turabian StyleShahid, Jahanzeb, Rizwan Ahmad, Adnan K. Kiani, Tahir Ahmad, Saqib Saeed, and Abdullah M. Almuhaideb. 2022. "Data Protection and Privacy of the Internet of Healthcare Things (IoHTs)" Applied Sciences 12, no. 4: 1927. https://doi.org/10.3390/app12041927
APA StyleShahid, J., Ahmad, R., Kiani, A. K., Ahmad, T., Saeed, S., & Almuhaideb, A. M. (2022). Data Protection and Privacy of the Internet of Healthcare Things (IoHTs). Applied Sciences, 12(4), 1927. https://doi.org/10.3390/app12041927








