Horizontal Histopathology Correlation with In Vivo Reflectance Confocal Microscopy in Inflammatory Skin Diseases: A Review
Abstract
:1. Introduction
2. Reflectance Confocal Microscopy and Horizontal Histopathology
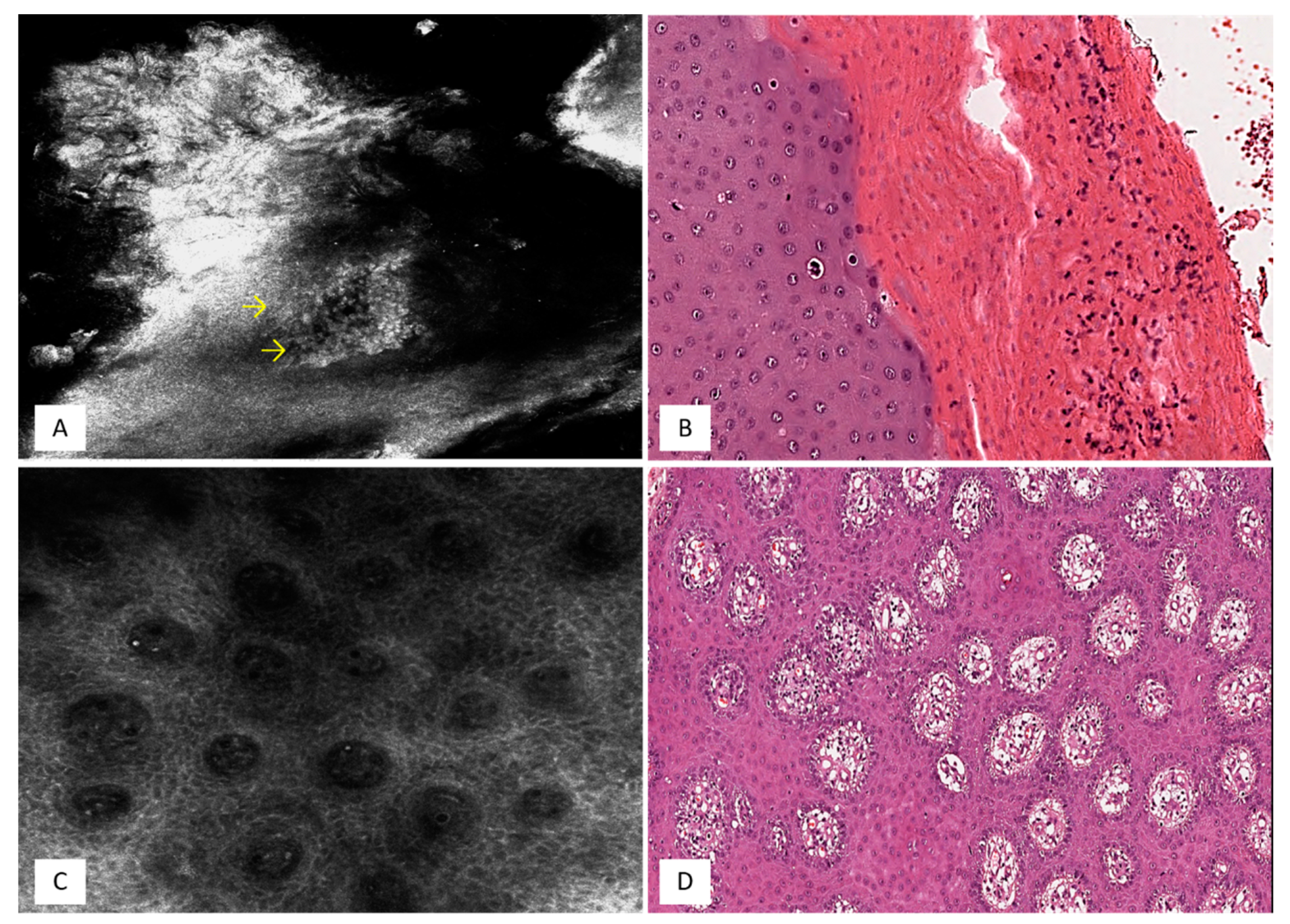
3. Psoriasis
4. Discoid Lupus Erythematosus
5. Eczema
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Broggi, G.; Verzì, A.E.; Caltabiano, R.; Micali, G.; Lacarrubba, F. Correlation between in vivo Reflectance Confocal Microscopy and Horizontal Histopathology in skin cancer: A review. Front. Oncol. 2021, 11, 653140. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.A.; Caruntu, C.; Lixandru, D.; Tampa, M.; Georgescu, S.R.; Constantin, M.M.; Constantin, C.; Neagu, M.; Zurac, S.A.; Boda, D. In vivo confocal laser scanning microscopy imaging of skin inflammation: Clinical applications and research directions. Exp. Ther. Med. 2019, 17, 1004–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahriari, N.; Rabinovitz, H.; Oliviero, M.; Grant-Kels, J.M. Reflectance confocal microscopy: Melanocytic and nonmelanocytic. Clin. Dermatol. 2021, 39, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Ko, R.F.; Smidt, A.C.; Durkin, J.R. Reflectance confocal microscopy in pediatric dermatology: A state-of-the-art review. Pediatr. Dermatol. 2021, 38, 1488–1499. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, Z.; Xu, W.; Zhou, X.; Tang, S.; Song, C.; Fan, W. Diagnostic value of horizontal versus vertical sections for scarring and non-scarring alopecia: A systematic review and meta-analysis. Eur. J. Dermatol. 2016, 26, 361–369. [Google Scholar] [CrossRef]
- Olsen, E.A.; Bergfeld, W.F.; Cotsarelis, G.; Price, V.H.; Shapiro, J.; Sinclair, R.; Solomon, A.; Sperling, L.; Stenn, K.; Whiting, D.A.; et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J. Am. Acad. Dermatol. 2003, 48, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Palo, S.; Biligi, D.S. Utility of horizontal and vertical sections of scalp biopsies in various forms of primary alopecias. J. Lab. Physicians 2018, 10, 95–100. [Google Scholar] [CrossRef]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Prim. 2016, 2, 16082. [Google Scholar] [CrossRef]
- Langley, R.G.; Krueger, G.G.; Griffiths, C.E. Psoriasis: Epidemiology, clinical features, and quality of life. Ann. Rheum. Dis. 2005, 64 (Suppl. 2), ii18–ii23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Lacarrubba, F.; Pellacani, G.; Gurgone, S.; Verzì, A.E.; Micali, G. Advances in non-invasive techniques as aids to the diagnosis and monitoring of therapeutic response in plaque psoriasis: A review. Int. J. Dermatol. 2015, 54, 626–634. [Google Scholar] [CrossRef]
- Lacarrubba, F.; Musumeci, M.L.; Ferraro, S.; Stinco, G.; Verzì, A.E.; Micali, G. A three-cohort comparison with videodermatoscopic evidence of the distinct homogeneous bushy capillary microvascular pattern in psoriasis vs atopic dermatitis and contact dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 701–703. [Google Scholar] [CrossRef]
- Verzì, A.E.; Lacarrubba, F.; Caltabiano, R.; Broggi, G.; Musumeci, M.L.; Micali, G. Reflectance Confocal Microscopy Features of Plaque Psoriasis Overlap With Horizontal Histopathological Sections: A Case Series. Am. J. Dermatopathol. 2019, 41, 355–357. [Google Scholar] [CrossRef]
- Nasca, M.R.; Lacarrubba, F.; Caltabiano, R.; Micali, G. Image Gallery: Reproduction of the Auspitz sign by videodermatoscopy, confocal microscopy and horizontal histopathology. Br. J. Dermatol. 2019, 180, e178. [Google Scholar] [CrossRef] [Green Version]
- Walling, H.W.; Sontheimer, R.D. Cutaneous lupus erythematosus: Issues in diagnosis and treatment. Am. J. Clin. Dermatol. 2009, 10, 365–381. [Google Scholar] [CrossRef]
- Michel, M.; Johanet, C.; Meyer, O.; Francès, C.; Wittke, F.; Michel, C.; Arfi, S.; Tournier-Lasserve, E.; Piette, J.C.; Group for Research on Auto-Immune Disorders (GRAID). Familial lupus erythematosus. Clinical and immunologic features of 125 multiplex families. Medicine 2001, 80, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Żychowska, M.; Żychowska, M. Dermoscopy of discoid lupus erythematosus—a systematic review of the literature. Int. J. Dermatol. 2021, 60, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, A.M.; Halbert, A.R.; Rohr, J.B. Discoid lupus erythematosus. Australas. J. Dermatol. 1995, 36, 3–10, quiz 11-2. [Google Scholar] [CrossRef] [PubMed]
- Lacarrubba, F.; Verzì, A.E.; Caltabiano, R.; Broggi, G.; Di Natale, A.; Micali, G. Discoid lupus erythematosus: Reflectance confocal microscopy features correlate with horizontal histopathological sections. Ski. Res. Technol. 2019, 25, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Rożalski, M.; Rudnicka, L.; Samochocki, Z. Atopic and Non-atopic Eczema. Acta Dermatovenerol. Croat. 2016, 24, 110–115. [Google Scholar] [PubMed]
- Sohn, A.; Frankel, A.; Patel, R.V.; Goldenberg, G. Eczema. Mt. Sinai J. Med. 2011, 78, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Broggi, G.; Lacarrubba, F.; Verzì, A.E.; Micali, G.; Caltabiano, R. Confocal microscopy features of patch-stage mycosis fungoides and their correlation with horizontal histopathological sections. A case series. J. Cutan. Pathol. 2019, 46, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Broggi, G.; Verzì, A.E.; Lacarrubba, F.; Caltabiano, R.; Di Natale, A.; Micali, G. Correlation between reflectance confocal microscopy features and horizontal histopathology in cutaneous squamous cell carcinoma in situ: A case series. J. Cutan. Pathol. 2020, 47, 777–780. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Ma, S.J.; Mo, Y.; Huo, S.T.; Wen, Y.Q.; Chen, Q. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: A meta-analysis. J. Cancer Res. Clin. Oncol. 2017, 143, 1627–1635. [Google Scholar] [CrossRef]
- Mazzilli, S.; Vollono, L.; Diluvio, L.; Botti, E.; Costanza, G.; Campione, E.; Donati, M.; Prete, M.D.; Orlandi, A.; Bianchi, L.; et al. The combined role of clinical, reflectance confocal microscopy and dermoscopy applied to chronic discoid cutaneous lupus and subacutus lupus erythematosus: A case series and literature review. Lupus 2021, 30, 125–133. [Google Scholar] [CrossRef]
- Li, J.; Garfinkel, J.; Zhang, X.; Wu, D.; Zhang, Y.; de Haan, K.; Wang, H.; Liu, T.; Bai, B.; Rivenson, Y.; et al. Biopsy-free in vivo virtual histology of skin using deep learning. Light Sci. Appl. 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]


| Skin Level | RCM | HHSs | |
|---|---|---|---|
| Psoriasis [8] | -S corneum -S granulosum/spinosum -DEJ |
-Bright structures with detached keratinocytes -Hyperrefractile nuclei surrounded by dark cytoplasm -Dark, roundish areas containing bright particles -Islands of keratinocytes with broadened white or grayish outlines -Dark circles within the dermal papillae | -Hyperkeratosis -Parakeratosis -Munro microabscesses -Acanthosis -Papillomatosis |
| DLE [14] | S corneum DEJ |
-Roundish areas filled with highly refractive material -Disarrangement -Small polygonal/round bright cells | -Dilated follicles with infundibular hyperkeratosis -Vacuolar degeneration -Inflammatory infiltrate |
| Eczema [17] | S granulosum/spinosum | -Expanded intercellular spaces -Round-shaped hyporefractive spaces -Scattered roundish, mildly refractive cells | -Spongiosis -Vesicles -Lymphocytic exocytosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broggi, G.; Verzì, A.E.; Lacarrubba, F.; Micali, G.; Caltabiano, R. Horizontal Histopathology Correlation with In Vivo Reflectance Confocal Microscopy in Inflammatory Skin Diseases: A Review. Appl. Sci. 2022, 12, 1930. https://doi.org/10.3390/app12041930
Broggi G, Verzì AE, Lacarrubba F, Micali G, Caltabiano R. Horizontal Histopathology Correlation with In Vivo Reflectance Confocal Microscopy in Inflammatory Skin Diseases: A Review. Applied Sciences. 2022; 12(4):1930. https://doi.org/10.3390/app12041930
Chicago/Turabian StyleBroggi, Giuseppe, Anna Elisa Verzì, Francesco Lacarrubba, Giuseppe Micali, and Rosario Caltabiano. 2022. "Horizontal Histopathology Correlation with In Vivo Reflectance Confocal Microscopy in Inflammatory Skin Diseases: A Review" Applied Sciences 12, no. 4: 1930. https://doi.org/10.3390/app12041930






