Recycled Paper Sludge (RPS)-Derived Nanocellulose: Production, Detection and Water Treatment Application
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
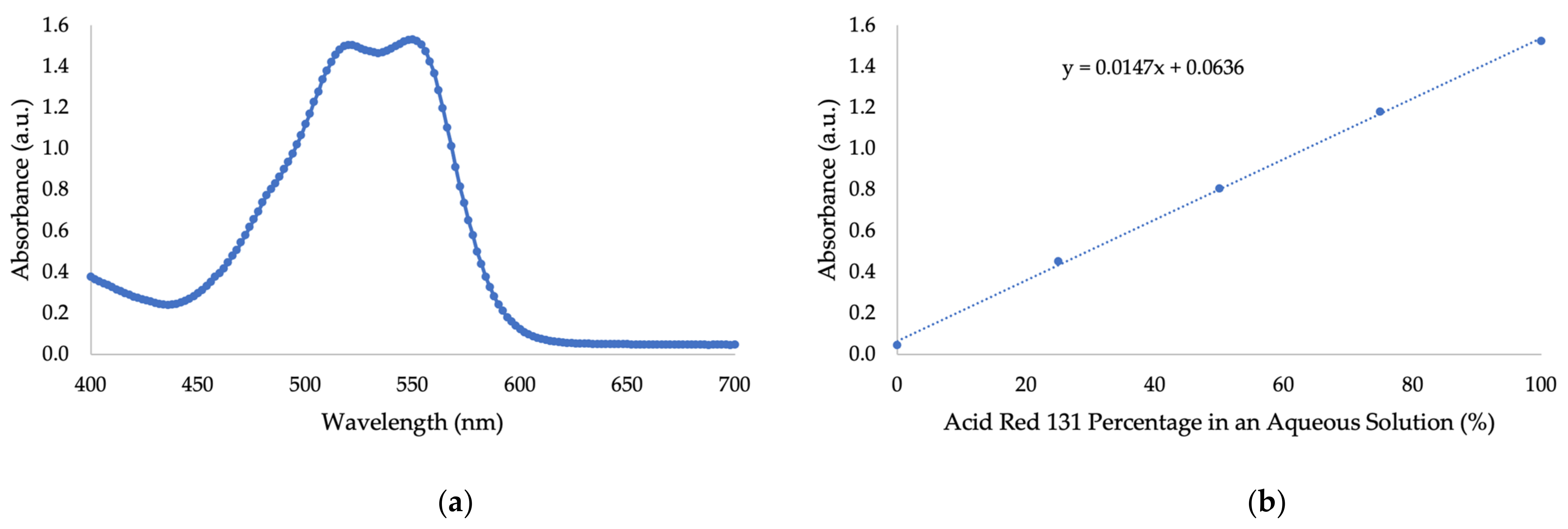
2.2. UV/Vis Absorbance of Nanocellulose and Sulfuric Acid
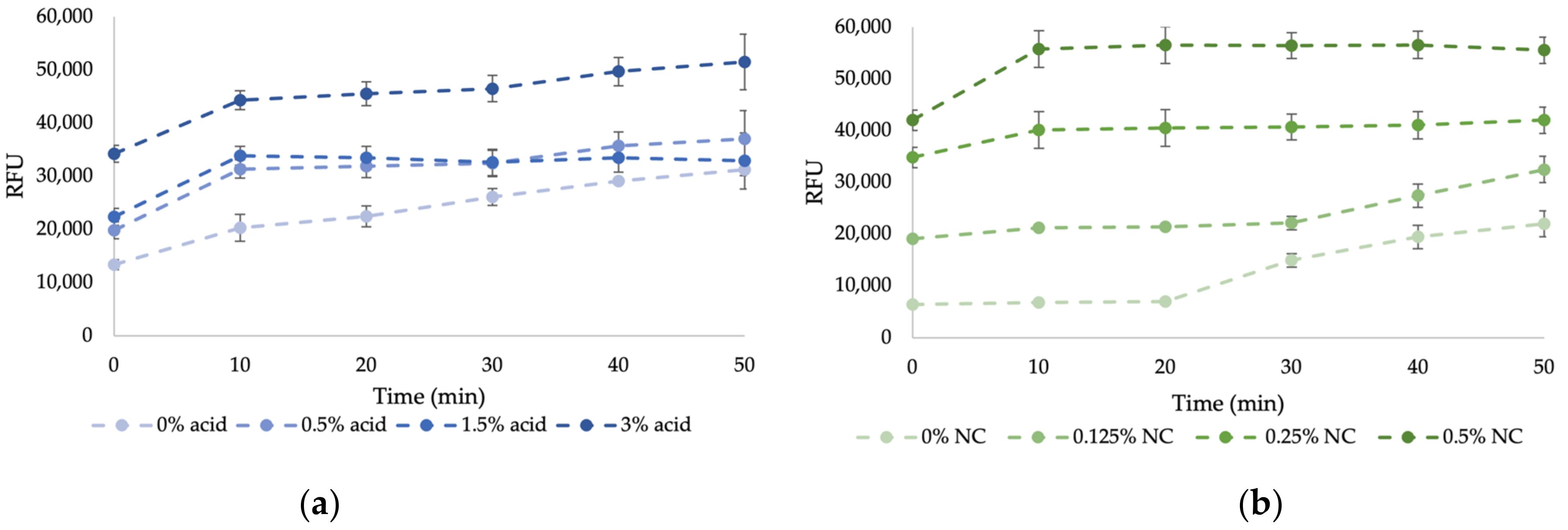
2.3. In-Situ Detection of Nanocellulose by Calcofluor White Fluorescence Staining
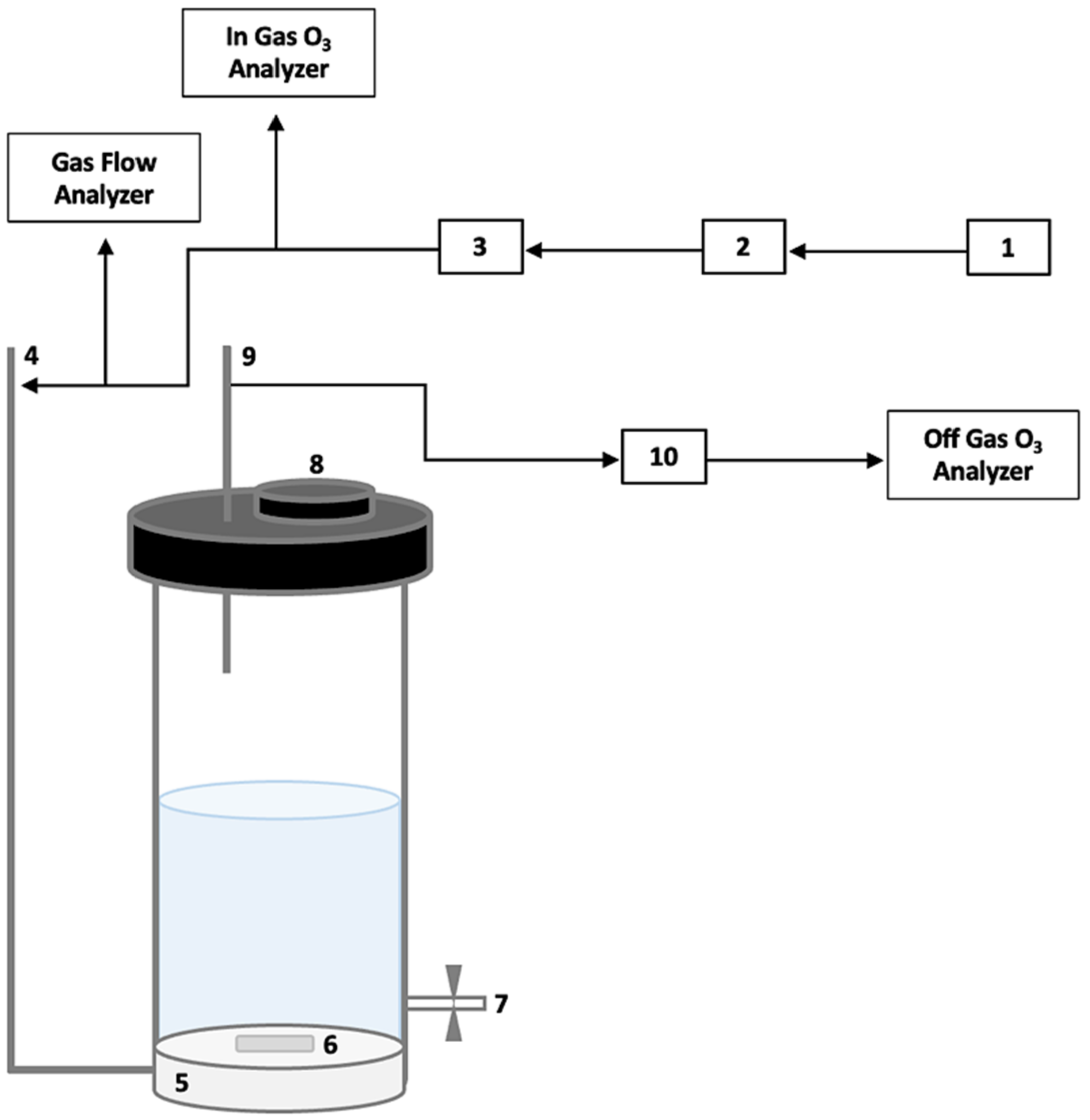
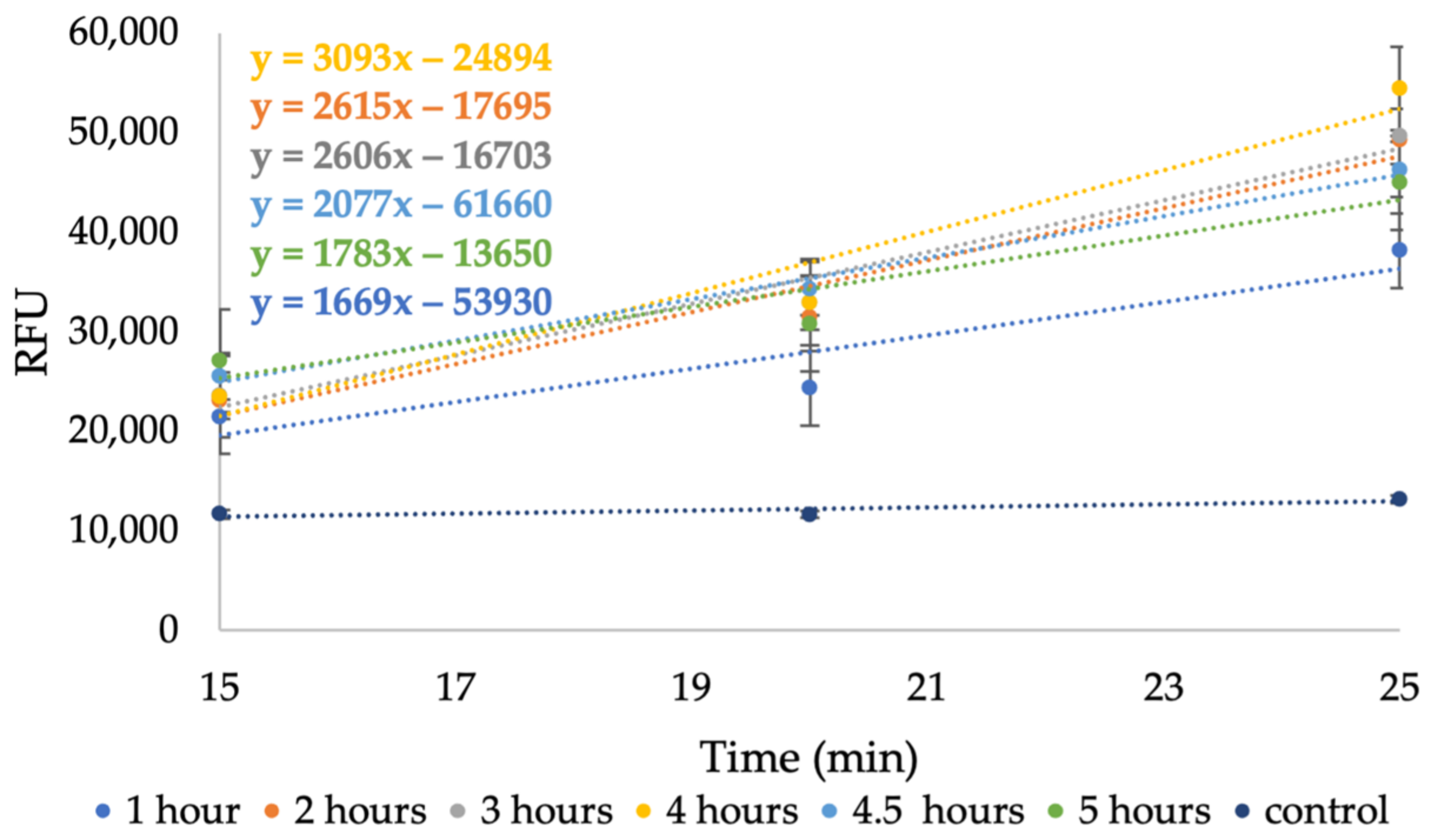
2.4. Ozonation Pretreatment
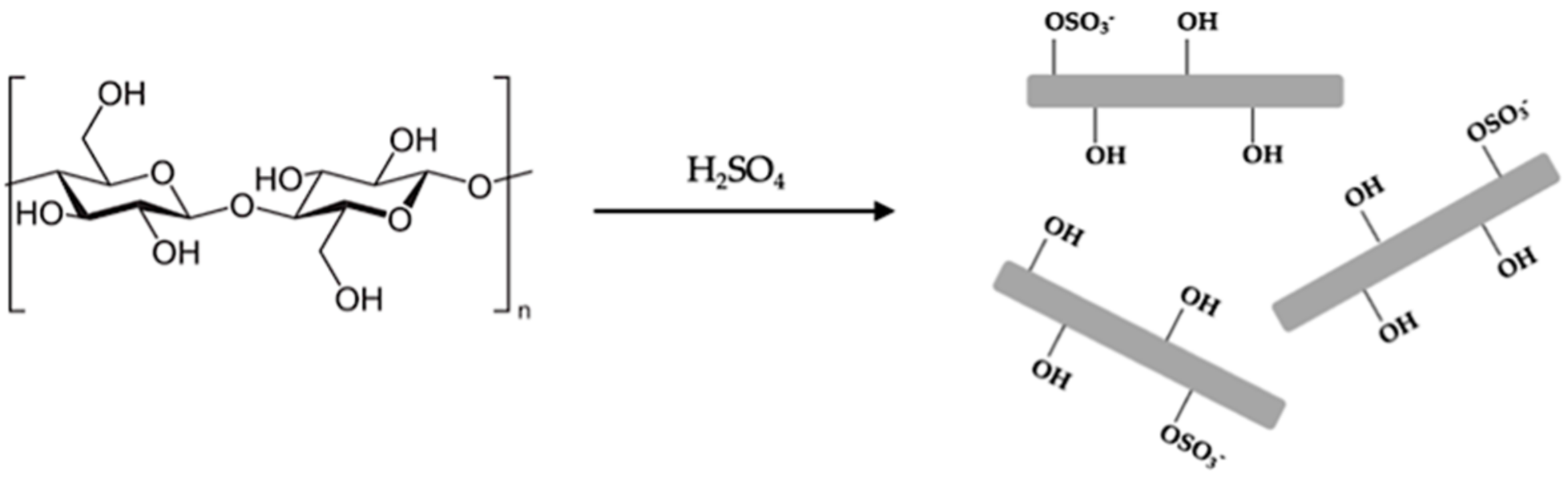
2.5. Production of Nanocellulose from Whatman Filter Paper
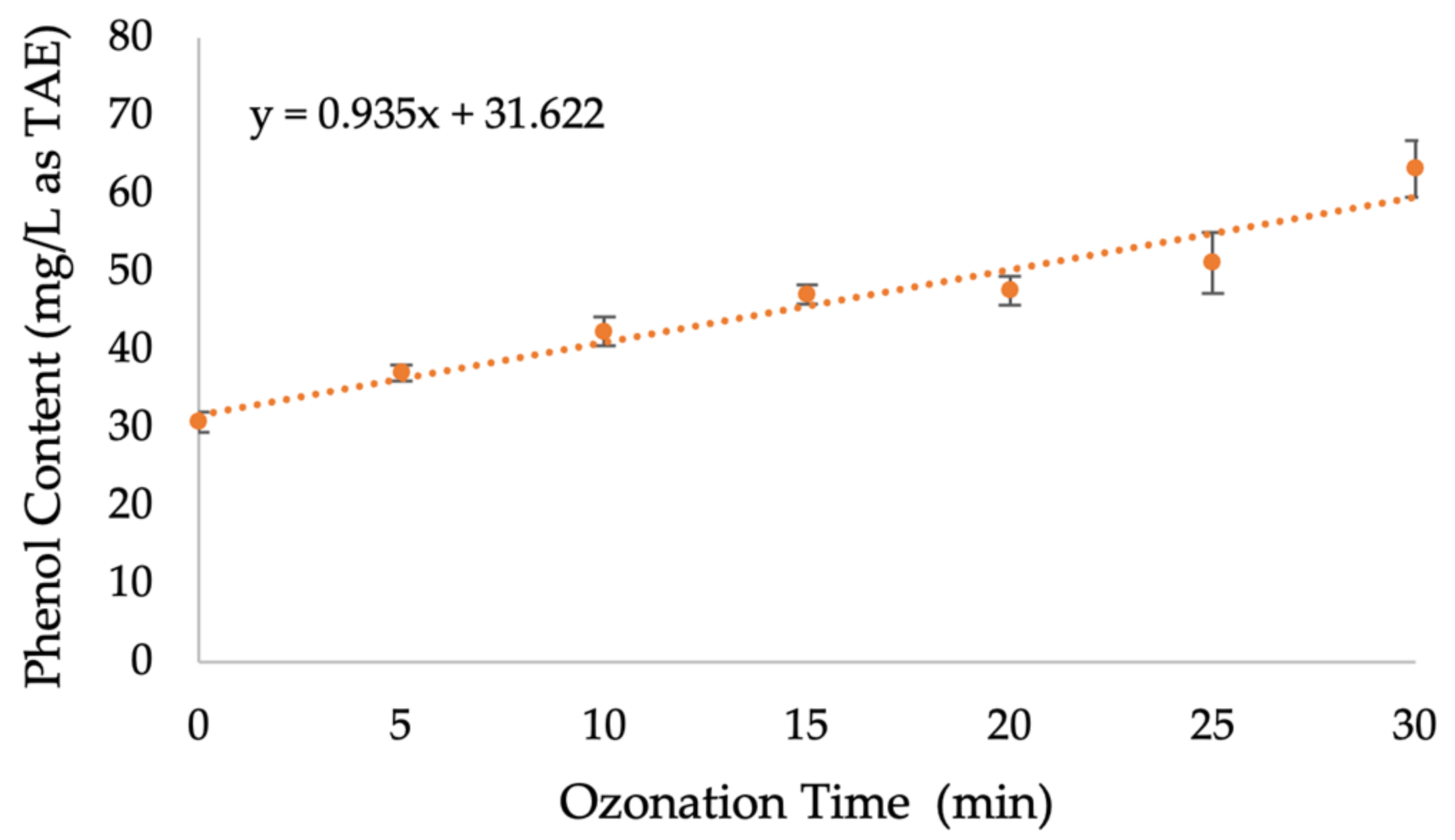
2.6. Determination of Phenolic Content Released from RPS during Ozonation Pretreatment
2.7. Production of Nanocellulose from Recycled Paper Sludge (RPS)
2.8. Zeta Potential Measurements
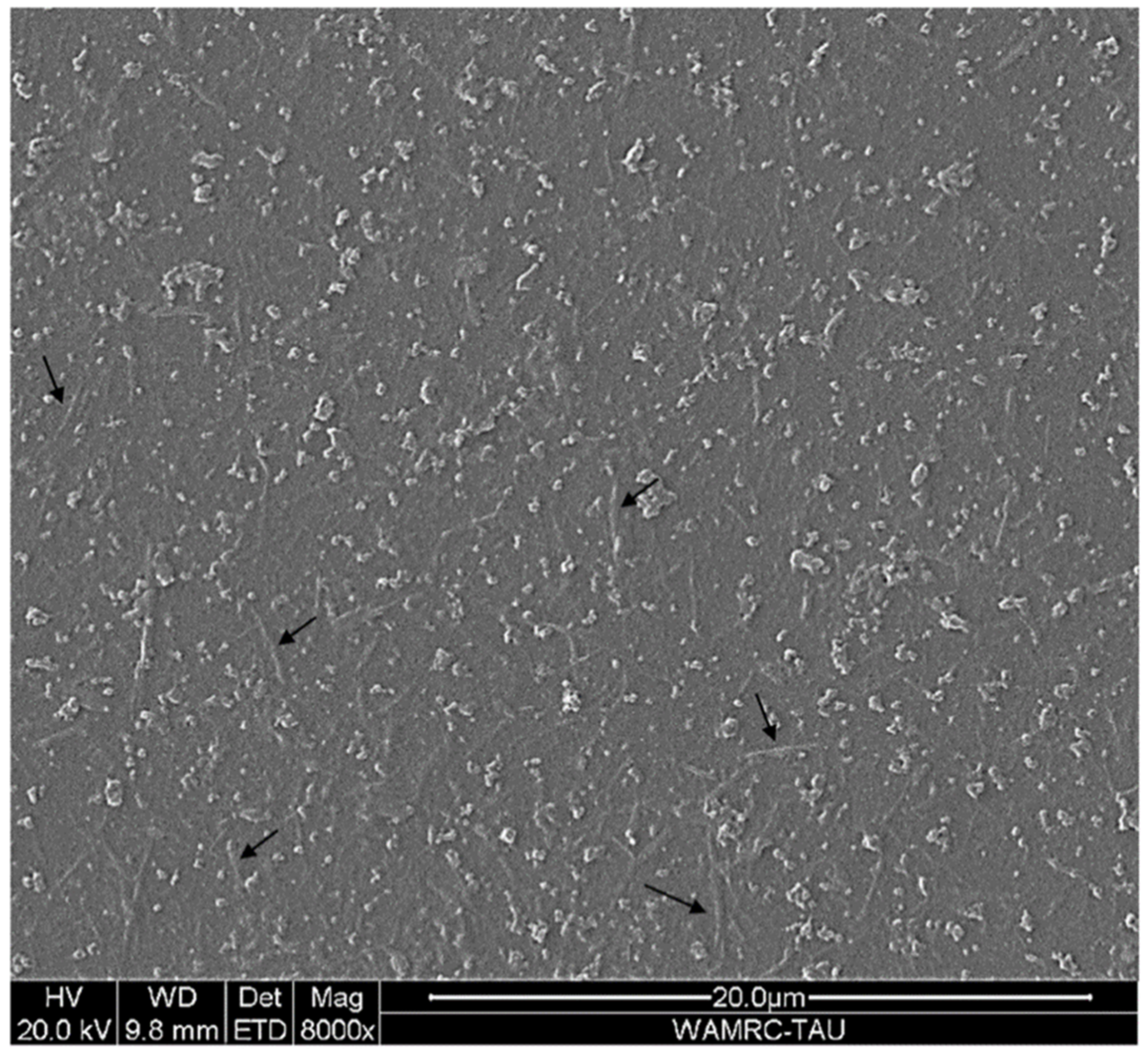
2.9. Scanning Electron Microscopy (SEM) Imaging
2.10. Adsorption of Acid Red 131 Dye by Crude NC
3. Results
3.1. UV/Vis Absorbance of Nanocellulose and Sulfuric Acid
3.2. In-Situ Detection of Nanocellulose by Calcofluor White Fluorescence Staining
3.3. Production of Nanocellulose from Whatman Filter Paper
3.4. Determination of Phenolic Content Released from RPS during Ozonation Pretreatment
3.5. Production of Nanocellulose from Recycled Paper Sludge (RPS)
3.6. Zeta Potential Measurements
3.7. Adsorption of Acid Red 131 Dye by Crude NC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Additional Figures


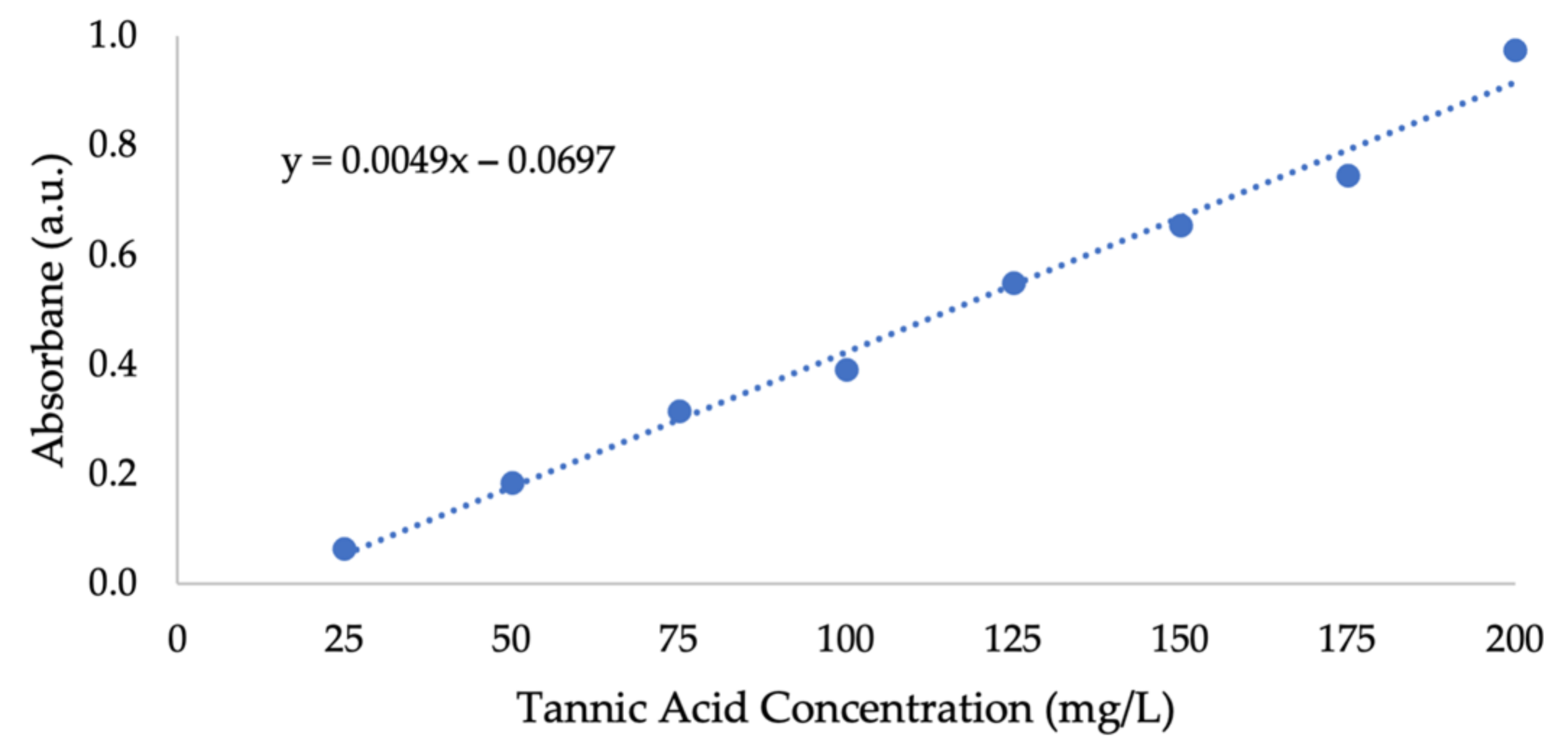
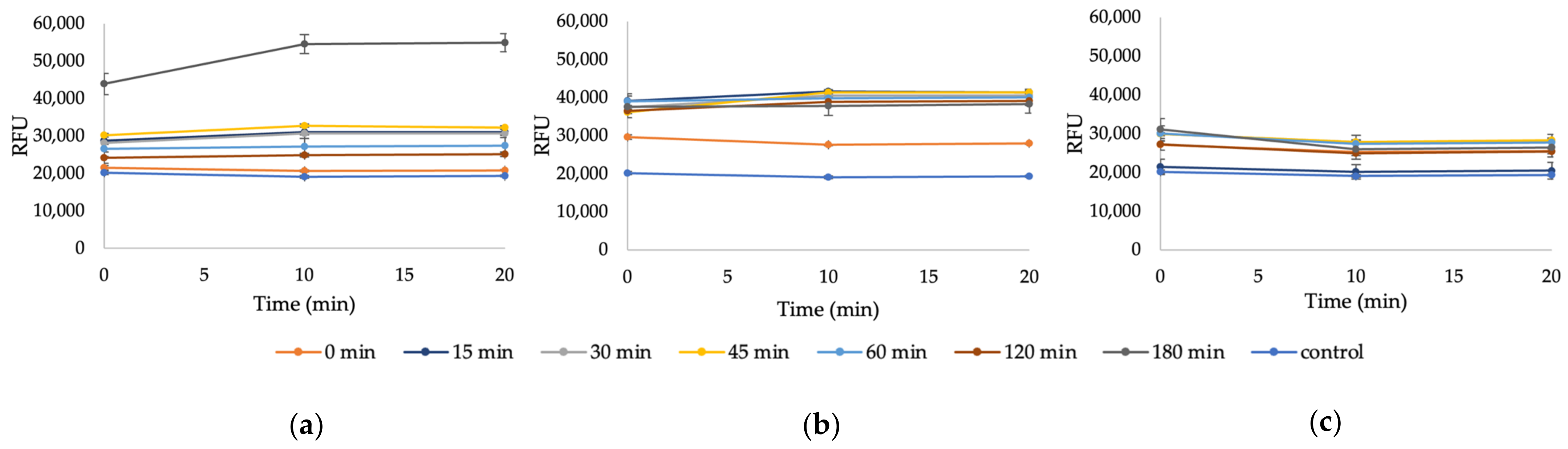
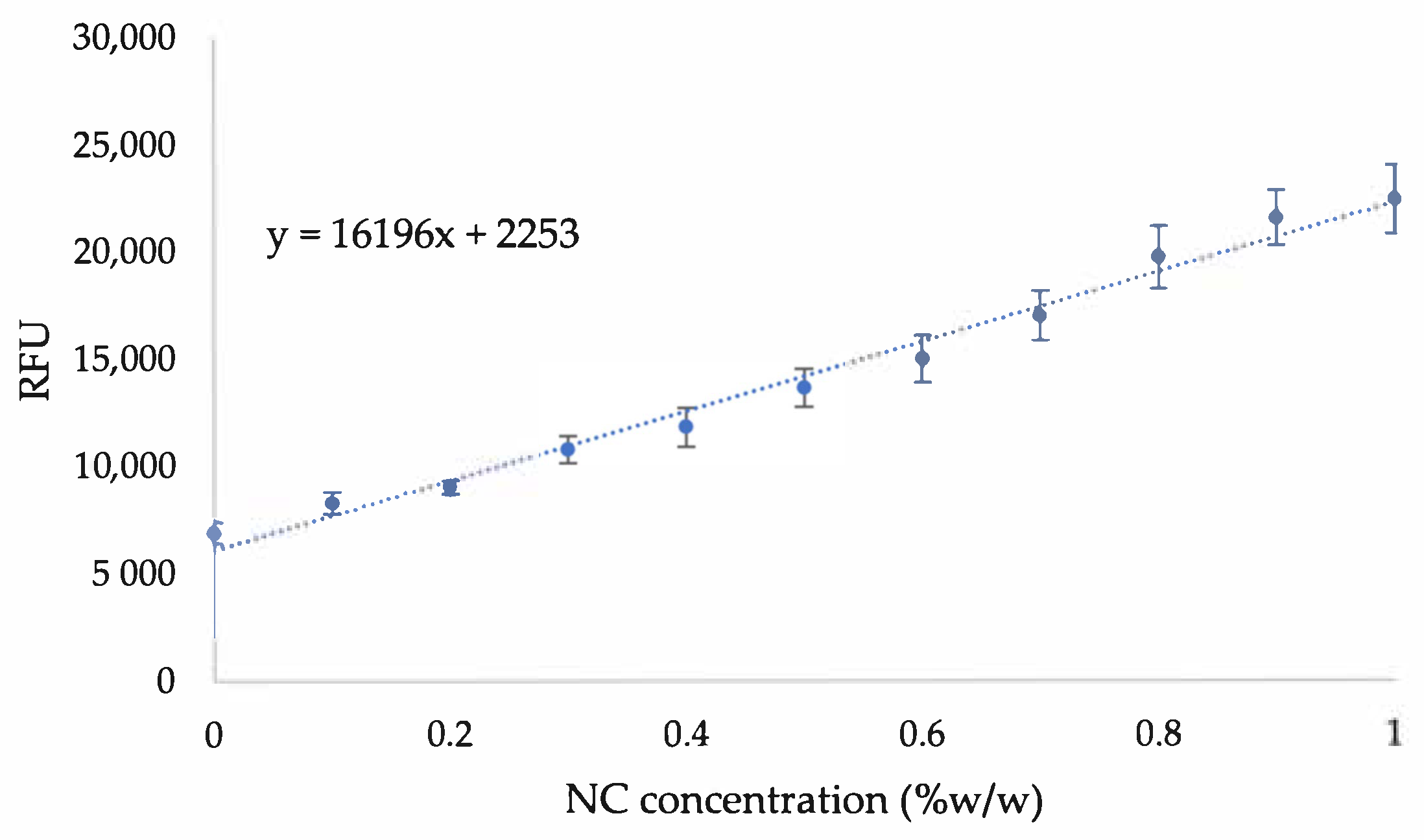
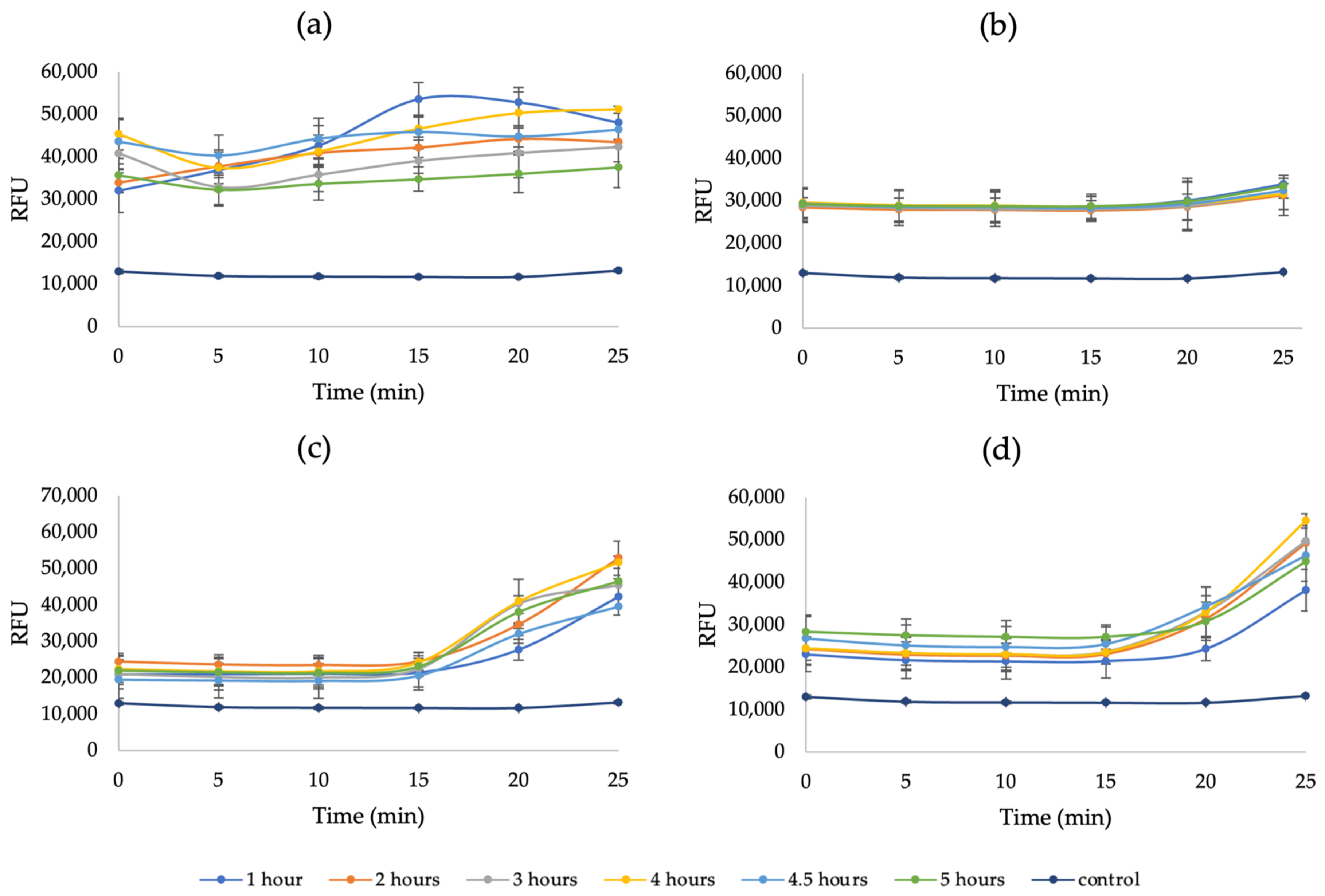
References
- Bajpai, P. Generation of waste in pulp and paper mills. In Management of Pulp and Paper Mill Waste; Springer: Cham, Switzerland, 2015; pp. 9–17. [Google Scholar]
- Lekha, P.; Andrew, J.E.; Gibril, M.; Sithole, B. Pulp and Paper mill sludge: A potential resource for producing high-value products. J. Tech. Assoc. Pulp Pap. Ind. S. Afr. 2017, 1, 16. [Google Scholar]
- Peretz, R.; Mamane, H.; Wissotzky, E.; Sterenzon, E.; Gerchman, Y. Making cardboard and paper recycling more sustainable: Recycled paper sludge for energy production and water-treatment applications. Waste Biomass Valoriz. 2021, 12, 1599–1608. [Google Scholar] [CrossRef]
- Nair, A.S.; Al-Battashi, H.; Al-Akzawi, A.; Annamalai, N.; Gujarathi, A.; Al-Bahry, S.; Dhillon, G.S.; Sivakumar, N. Waste office paper: A potential feedstock for cellulase production by a novel strain Bacillus velezensis ASN1. Waste Manag. 2018, 79, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Peretz, R.; Sterenzon, E.; Gerchman, Y.; Vadivel, V.K.; Luxbacher, T.; Mamane, H. Nanocellulose production from recycled paper mill sludge using ozonation pretreatment followed by recyclable maleic acid hydrolysis. Carbohydr. Polym. 2019, 216, 343–351. [Google Scholar] [CrossRef]
- KSH Solutions Inc. Pulp and Paper Mill Sludge Disposal Methods; KSH Solutions Inc.: North Charleston, SC, USA, 2013; Available online: https://www.ksh.ca/wp-content/uploads/2015/10/EN_Pulp-and-Paper-Mill-Sludge-Disposal-Methods_20jan2015.pdf (accessed on 19 December 2021).
- Faubert, P.; Barnabé, S.; Bouchard, S.; Côté, R.; Villeneuve, C. Pulp and paper mill sludge management practices: What are the challenges to assess the impacts on greenhouse gas emissions? Resour. Conserv. Recycl. 2016, 108, 107–133. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Xu, T.; Feng, H.; Chen, S. Greenhouse gas emissions from landfills: A review and bibliometric analysis. Sustainability 2019, 11, 2282. [Google Scholar] [CrossRef] [Green Version]
- Trache, D.; Hussin, M.H.; Haafiz, M.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V.K. Nanocellulose Polymer Nanocomposites: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Kalia, S.; Kaith, B.S.; Kaur, I. Cellulose Fibers: Bio-and Nano-Polymer Composites: Green Chemistry and Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials; Walter de Gruyter GmbH & Co. KG: Berlin, Germany, 2012. [Google Scholar]
- Eichhorn, S.J. Cellulose nanowhiskers: Promising materials for advanced applications. Soft Matter 2011, 7, 303–315. [Google Scholar] [CrossRef]
- Kumari, A.; Singla, R.; Guliani, A.; Walia, S.; Acharya, A.; Yadav, S.K. Nanoscale materials in targeted drug delivery. In Nanoscale Materials in Targeted Drug Delivery, Theragnosis and Tissue Regeneration; Springer: Singapore, 2016; pp. 1–19. [Google Scholar]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties, and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Dias, O.A.T.; Konar, S.; Leão, A.L.; Yang, W.; Tjong, J.; Sain, M. Current state of applications of nanocellulose in flexible energy and electronic devices. Front. Chem. 2020, 8, 420. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties, and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef]
- Vartiainen, J.; Pöhler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Tapper, U.; Lahtinen, P.; Kapanen, A.; Putkisto, K.; et al. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Sacui, I.A.; Nieuwendaal, R.C.; Burnett, D.J.; Stranick, S.J.; Jorfi, M.; Weder, C.; Foster, E.J.; Olsson, R.T.; Gilman, J.W. Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods. ACS Appl. Mater. Interfaces 2014, 6, 6127–6138. [Google Scholar] [CrossRef]
- Fang, W.; Arola, S.; Malho, J.M.; Kontturi, E.; Linder, M.B. Laaksonen, P. Noncovalent dispersion and functionalization of cellulose nanocrystals with proteins and polysaccharides. Biomacromolecules 2016, 17, 1458–1465. [Google Scholar] [CrossRef]
- Gaspar, D.; Fernandes, S.N.; De Oliveira, A.G.; Fernandes, J.G.; Grey, P.; Pontes, R.V.; Pereira, L.; Martins, R.; Godinho, M.H.; Fortunato, E. Nanocrystalline cellulose applied simultaneously as the gate dielectric and the substrate in flexible field effect transistors. Nanotechnology 2014, 25, 094008. [Google Scholar] [CrossRef] [Green Version]
- Hoeng, F.; Denneulin, A.; Bras, J. Use of nanocellulose in printed electronics: A review. Nanoscale 2016, 8, 13131–13154. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Glasser, W.G.; Atalla, R.H.; Blackwell, J.; Malcolm Brown, R.; Burchard, W.; French, A.D.; Klemm, D.O.; Nishiyama, Y. About the structure of cellulose: Debating the Lindman hypothesis. Cellulose 2012, 19, 589–598. [Google Scholar] [CrossRef]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Medronho, B.; Romano, A.; Miguel, M.G.; Stigsson, L.; Lindman, B. Rationalizing cellulose (in) solubility: Reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose 2012, 19, 581–587. [Google Scholar] [CrossRef]
- Petrucci, R.H. General Chemistry: Principles and Modern Applications, 6th ed.; Pearson: Prentice Hall, NJ, USA, 1993; pp. 785–808. [Google Scholar]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Rosen, Y.; Mamane, H.; Gerchman, Y. Short ozonation of lignocellulosic waste as energetically favorable pretreatment. Bioenergy Res. 2019, 12, 292–301. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Travaini, R.; Martín-Juárez, J.; Lorenzo-Hernando, A.; Bolado-Rodríguez, S. Ozonolysis: An advantageous pretreatment for lignocellulosic biomass revisited. Bioresour. Technol. 2016, 199, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Contreras Iglesias, S. Degradation and Biodegradability Enhancement of Nitrobenzene and 2,4-Dichlorophenol by Means of Advanced Oxidation Processes based on Ozone. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, November 2002. Available online: https://www.tesisenred.net/bitstream/handle/10803/1520/TOL167A.pdf?sequence=1 (accessed on 6 February 2022).
- Tian, D.; Chandra, R.P.; Lee, J.S.; Lu, C.; Saddler, J.N. A comparison of various lignin-extraction methods to enhance the accessibility and ease of enzymatic hydrolysis of the cellulosic component of steam-pretreated poplar. Biotechnol. Biofuels 2017, 10, 157. [Google Scholar] [CrossRef]
- Hemmati, F.; Jafari, S.M.; Taheri, R.A. Optimization of homogenization-sonication technique for the production of cellulose nanocrystals from cotton linter. Int. J. Biol. Macromol. 2019, 137, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Hsieh, Y.L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, L.; Zhu, J.Y.; Yang, R. Tailored and integrated production of carboxylated cellulose nanocrystals (CNC) with nanofibrils (CNF) through maleic acid hydrolysis. ChemNanoMat 2017, 3, 328–335. [Google Scholar] [CrossRef]
- Peretz, R.; Gerchman, Y.; Mamane, H. Ozonation of tannic acid to model biomass pretreatment for bioethanol production. Bioresour. Technol. 2017, 241, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.M.; Sin, L.T.; Tee, T.T.; Bee, S.T.; Hui, D.; Low, C.Y.; Rahmat, A.R. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [Green Version]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Liu, C.; Li, B.; Du, H.; Lv, D.; Zhang, Y.; Yu, G.; Mu, X.; Peng, H. Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohydr. Polym. 2016, 151, 716–724. [Google Scholar] [CrossRef]
- Abitbol, T.; Kloser, E.; Gray, D.G. Estimation of the surface sulfur content of cellulose nanocrystals prepared by sulfuric acid hydrolysis. Cellulose 2013, 20, 785–794. [Google Scholar] [CrossRef]
- Bruice, P.Y. Organic Chemistry, 8th ed.; Pearson: London, UK, 2016; pp. 259–260, 340, 702–704. [Google Scholar]
- Wang, Q.Q.; Zhu, J.Y.; Reiner, R.S.; Verrill, S.P.; Baxa, U.; McNeil, S.E. Approaching zero cellulose loss in cellulose nanocrystal (CNC) production: Recovery and characterization of cellulosic solid residues (CSR) and CNC. Cellulose 2012, 19, 2033–2047. [Google Scholar] [CrossRef]
- Bian, H.; Chen, L.; Dai, H.; Zhu, J.Y. Integrated production of lignin containing cellulose nanocrystals (LCNC) and nanofibrils (LCNF) using an easily recyclable di-carboxylic acid. Carbohydr. Polym. 2017, 167, 167–176. [Google Scholar] [CrossRef]
- Herth, W. Calcofluor white and Congo red inhibit chitin microfibril assembly of Poterioochromonas: Evidence for a gap between polymerization and microfibril formation. J. Cell Biol. 1980, 87, 442–450. [Google Scholar] [CrossRef]
- Maddar, F.M.; Perry, D.; Brooks, R.; Page, A.; Unwin, P.R. Nanoscale surface charge visualization of human hair. Anal. Chem. 2019, 91, 4632–4639. [Google Scholar] [CrossRef]
- Harrington, B.J.; Raper, K.B. Use of a fluorescent brightener to demonstrate cellulose in the cellular slime molds. Appl. Microbiol. 1968, 16, 106–113. [Google Scholar] [CrossRef]
- Herth, W.; Schnepf, E. The fluorochrome, calcofluor white, binds oriented to structural polysaccharide fibrils. Protoplasma 1980, 105, 129–133. [Google Scholar] [CrossRef]
- Harrington, B.J.; Hageage, G.J., Jr. Calcofluor white: A review of its uses and applications in clinical mycology and parasitology. Lab. Med. 2003, 34, 361–367. [Google Scholar] [CrossRef]
- Harrington, B.J.; Hageage, G.J., Jr. Calcofluor white: Tips for improving its use. Clin. Microbiol. Newsl. 1991, 13, 3–5. [Google Scholar] [CrossRef]
- Peretz, R.; Mamane, H.; Sterenzon, E.; Gerchman, Y. Rapid quantification of cellulose nanocrystals by Calcofluor White fluorescence staining. Cellulose 2019, 26, 971–977. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Antunes, F.E.; Fernández-García, M.P.; Ventura, J.; Araújo, J.P.; Romano, A.; Lindman, B. Unusual extraction and characterization of nanocrystalline cellulose from cellulose derivatives. J. Mol. Liq. 2015, 210, 106–112. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Margraf, T.; Karnopp, A.R.; Rosso, N.D.; Granato, D. Comparison between Folin-Ciocalteu and Prussian Blue assays to estimate the total phenolic content of juices and teas using 96-well microplates. J. Food Sci. 2015, 80, C2397–C2403. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Kamboj, A.; Gupta, R.; Rana, A.; Kaur, R. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from extracts of Terminalia bellerica. Eur. J. Biomed. Pharm. Sci. 2015, 2, 201–215. [Google Scholar]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Box, J.D. Investigation of the Folin-Ciocalteu phenol reagent for the determination of polyphenolic substances in natural waters. Water Res. 1983, 17, 511–525. [Google Scholar] [CrossRef]
- Spectranomics Protocols: Total Phenol and Tannin Determination, Carnegie Institution for Science 2011; Modified from Ainsworth and Gillespie (2007) for Phenols, Toth and Pavia (2001) and Makaar (2007) for Tannins. Zenodo.org. Available online: https://zenodo.org/record/3247631/files/CSP_protocol_Phenols_Tannins_Analysis.pdf?download=1 (accessed on 19 December 2021).
- Stana-Kleinschek, K.; Ribitsch, V. Electrokinetic properties of processed cellulose fibers. Colloids Surf. A Physicochem. Eng. Asp. 1998, 140, 127–138. [Google Scholar] [CrossRef]
- Grancaric, A.M.; Tarbuk, A.; Pusic, T. Electrokinetic properties of textile fabrics. Coloration Technol. 2005, 121, 221–227. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Nattich, M.; Zaucha, M. Electrokinetics of particle covered surfaces. Curr. Opin. Colloid Interface Sci. 2010, 15, 175–183. [Google Scholar] [CrossRef]
- Bukšek, H.; Luxbacher, T.; Petrinić, I. Zeta potential determination of polymeric materials using two differently designed measuring cells of an electrokinetic analyzer. Acta Chim. Slov. 2010, 57, 700–706. [Google Scholar]
- Daskal, S.; Ayalon, O.; Shechter, M. The state of municipal solid waste management in Israel. Waste Manag. Res. 2018, 36, 527–534. [Google Scholar] [CrossRef]
- Oz, Y.B.; Mamane, H.; Menashe, O.; Cohen-Yaniv, V.; Kumar, R.; Kruh, L.I.; Kurzbaum, E. Treatment of olive mill wastewater using ozonation followed by an encapsulated acclimated biomass. J. Environ. Chem. Eng. 2018, 6, 5014–5023. [Google Scholar]
- Horwath, W. Carbon cycling and formation of soil organic matter. In Soil Microbiology, Ecology, and Biochemistry; Academic Press: Cambridge, MA, USA, 2007; pp. 303–339. [Google Scholar]
- Zhang, Y.; Naebe, M. Lignin: A review on structure, properties, and applications as a light-colored UV absorber. ACS Sustain. Chem. Eng. 2021, 9, 1427–1444. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Vocciante, M.; Bruzzone, A.G.; Fabiano, B. A Critical Analysis on Green and Low-Temperature Methods for the Production of Carbon Nanoparticles. Chem. Eng. Trans. 2021, 86, 805–810. [Google Scholar]
- Bharimalla, A.K.; Deshmukh, S.P.; Patil, P.G.; Vigneshwaran, N. Energy efficient manufacturing of nanocellulose by chemo-and bio-mechanical processes: A review. World J. Nano Sci. Eng. 2015, 5, 204. [Google Scholar] [CrossRef] [Green Version]
- Luxbacher, T. Electrokinetic properties of natural fibres. In Handbook of Natural Fibres; Woodhead Publishing: Sawston, UK, 2020; pp. 323–353. [Google Scholar]




| Hydrolysis (min) | COD (mg/L) | NC (mg/L) | Yield |
|---|---|---|---|
| 30 | 455 | 382 | 0.76% |
| 120 | 494 | 417 | 0.83% |
| 180 | 578 | 488 | 0.98% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslennikov, A.; Peretz, R.; Vadivel, V.K.; Mamane, H. Recycled Paper Sludge (RPS)-Derived Nanocellulose: Production, Detection and Water Treatment Application. Appl. Sci. 2022, 12, 3077. https://doi.org/10.3390/app12063077
Maslennikov A, Peretz R, Vadivel VK, Mamane H. Recycled Paper Sludge (RPS)-Derived Nanocellulose: Production, Detection and Water Treatment Application. Applied Sciences. 2022; 12(6):3077. https://doi.org/10.3390/app12063077
Chicago/Turabian StyleMaslennikov, Alona, Roi Peretz, Vinod Kumar Vadivel, and Hadas Mamane. 2022. "Recycled Paper Sludge (RPS)-Derived Nanocellulose: Production, Detection and Water Treatment Application" Applied Sciences 12, no. 6: 3077. https://doi.org/10.3390/app12063077
APA StyleMaslennikov, A., Peretz, R., Vadivel, V. K., & Mamane, H. (2022). Recycled Paper Sludge (RPS)-Derived Nanocellulose: Production, Detection and Water Treatment Application. Applied Sciences, 12(6), 3077. https://doi.org/10.3390/app12063077








