Abstract
PACAP is widely expressed throughout the body. It exerts a beneficial role in the eye, including the cornea. The corneal epithelium is regularly exposed to diverse types of insults, including ultraviolet B (UV-B) radiation. Previously, we showed the protective role played by PACAP in counteracting UV-B ray insults in human corneal endothelial cells; however, its involvement in corneal epithelium protection against ROS induced by UV-B radiation, and the underlying mechanisms, remain to be determined. Here, we demonstrated that the peptide treatment reduced UV-B-induced ROS generation by playing an anti-apoptotic role via JNK-signaling pathway inhibition. Overall, our results can provide guidance in the therapeutic use of PACAP for the treatment of epithelial corneal damage.
1. Introduction
Sunlight covers a wide range of the electromagnetic spectrum. Visible light, ultraviolet (UV) rays, and infrared rays reach the Earth’s surface to form terrestrial sunlight. There are three types of UV radiation: UV-A, UV-B, and UV-C. Since UV-C is completely absorbed by the ozone layer, the UV radiation component of terrestrial light consists of ~4% UV-B and ~96% UV-A. The depletion of the ozone layer causes increased human exposure to UV rays. The overexposure of eyes to UV radiation causes harmful effects in the cornea, lens, and retina [1,2,3]. In particular, the cornea, a natural UV-B filter, represents the most exposed structure of the eye to oxidative stress since it absorbs the major part of UV-B at 300 nm [4,5]. The corneal epithelial layer is the most anterior region of the eye, characterized by about six cell layers. It exhibits a smooth, regular surface and consists of nonkeratinized stratified squamous cells. Beneath the epithelium is a fibrous meshwork called Bowman’s layer, which is a condensation of collagen and proteoglycans. The stroma, representing 90% of the corneal thickness, is formed by a collagen-rich central layer in contact with the underlying Descemet’s membrane, which sustains the endothelial cells’ monolayer of posterior cornea [6].
UV-B rays’ dangerous mechanism of action is composed of the aberrant increase in reactive oxygen species (ROS) [7]. The UV-B-induced apoptotic process is regulated by some intracellular signaling, including the JNK pathway mediation of UV-B/ROS-induced apoptosis [8,9,10].
The healthy cornea contains antioxidant molecules involved in the scavenging of ROS [11]. However, when UV-B-induced ROS overwhelmed the corneal antioxidant mechanisms, a reduction in its epithelium thickness was observed [12].
Corneal epithelial damage affects millions of people around the world. Therefore, there is increased interest in discovering new strategies to repair corneal damage and promote proper corneal re-epithelialization.
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a neuropeptide belonging to the secretin and histidine-isoleucine (PHI) superfamily peptides. It exerts multiple activities in a tissue-specific manner by binding to G protein-coupled receptors comprising PAC1, VPAC-1, and VPAC-2 receptors [13,14,15]. Moreover, PACAP promotes the activity-dependent neuroprotective protein (ADNP) [16,17]. Accordingly, NAP, the smallest active element of ADNP, plays a beneficial role, acting in a synergic way with PACAP [18,19,20]. PACAP is widely expressed in the nervous system and shows a neuroprotective role in several neurodegenerative diseases [21,22,23,24,25,26,27,28]. The peptide and its receptors are distributed in different regions of the eye [29,30,31,32]. The effects of the peptide in the visual system were repeatedly described, especially in the retina, where PACAP showed a neuroprotective role against different types of insult [33,34,35,36,37]. In the cornea, PACAP displayed different pleiotropic effects. It decreased corneal endothelial cell loss after transplantation, protected the corneal surface by stimulating tear secretion, and defended the endothelium against UV damage [38,39,40,41,42]. The involvement of PACAP in corneal epithelium protection against UV-B-radiation-induced ROS formation, and the underlying mechanisms therein, remains to be determined. Here, we assessed the expression of PACAP and its receptors in rabbit corneal epithelium. Then, we investigated its role in UV-B-irradiation-induced damage in rabbit corneal epithelial cells. Our results demonstrated that the peptide was strongly expressed in the corneal epithelial layer. Moreover, it affected UV-B-induced ROS generation and JNK pathway activation, counteracting apoptotic cell death induced by UV-B irradiation.
2. Materials and Methods
2.1. Animals
The experiments were performed on male New Zealand White rabbits (n = 12), in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2. Immunohistochemistry (IHC) Analysis
PACAP, PAC1R, VPAC1R, and VPAC2R expression in rabbit cornea was analyzed through immunohistochemical analysis [43] using the following primary antibodies: rabbit anti-PACAP (H-76, sc-25439, 1:200), rabbit anti-PAC1 receptor (H-55, sc-30018, 1:300), rabbit anti-VPAC1 (H-130, sc-30019, 1:200), and rabbit anti-VPAC2 (H-50, sc-30020, 1:200) diluted in PBS and 1%BSA. The immunoreaction was observed using 3,3′-diaminobenzidine solution. The samples were lightly counterstained with hematoxylin.
2.3. Cell Cultures
Experiments were performed on rabbit corneal cell lines (Statens Seruminstitut Rabbit Cornea (SIRC) treated with vehicle (CTRL), or in the presence of PACAP (100 nM)) and subjected to 30 s UV-B rays at a dose of 20 mJ/cm2 through UVllink (UVItec, Cambridge, UK).
2.4. Cell Viability Assay
Cell viability was investigated through an MTT assay (11465007001, Roche Diagnostics, Indianapolis, IN, USA) as previously demonstrated [43]. The absorbance was measured with the a Varioskan reader (Thermo Fisher Scientific, Waltham, MA, USA).
2.5. Assessment of Cell Death Using Fluorescence Microscope
The SIRC cells were cultured at a density of 4 × 103 cells/well in 500 μL of the culture medium. The day after, cells were incubated in the control medium (CTRL) in the presence of 100 nM PACAP, or exposed to 30s UV-B-irradiation (20 mJ/cm2) in the presence or absence of 100 nM PACAP. After 24 h, cells were incubated with 0.4 μg/mL Hoechst 33342 dye as previously described [43]. Two investigators carried out the counting of apoptotic nuclei independently.
2.6. Western Blot Analysis
Western blot analysis was performed as described by D’Amico et al. [18]. Membranes were incubated with the following primary antibodies: rabbit anti-PACAP (1:500); rabbit anti-PAC1 receptor (1:1000); rabbit anti-VPAC1 (1:500); rabbit anti-VPAC2 (1:500); mouse anti-Bcl2 (sc-509, 1:200); mouse anti-Bax (sc-20067, 1:200); rabbit anti-JNK (sc-571; 1:200); mouse anti-p-JNK (sc-6254, 1:200), and rabbit anti-β-tubulin, which was used as a loading control (sc-9104, 1:500). Densitometric analyses were performed using ImageJ software (last access 22 March 2022).
2.7. Detection of ROS
To quantitatively assess the ROS production, the DCFDA/H2DCFDA assay (ab113851, Abcam, Cambridge, UK) was performed on SIRC cells cultured at a density of 1 × 104 cells/well for 24 h. ROS concentration was measured with the Varioskan reader.
Mitochondrial-derived ROS (mtROS) detection was assessed using the MitoSOX™ Red Mitochondrial Superoxide Indicator. The MACSQuant Analyzer 10 was used to measure mtROS.
2.8. Statistical Analysis
Data reported mean ± SEM. One-way analysis of variance was performed, and statistical significance was evaluated by the Tukey–Kramer post hoc test (p ≤ 0.05).
3. Results
3.1. Detection of PACAP, PAC1R and VPACRs in Corneal Epithelium
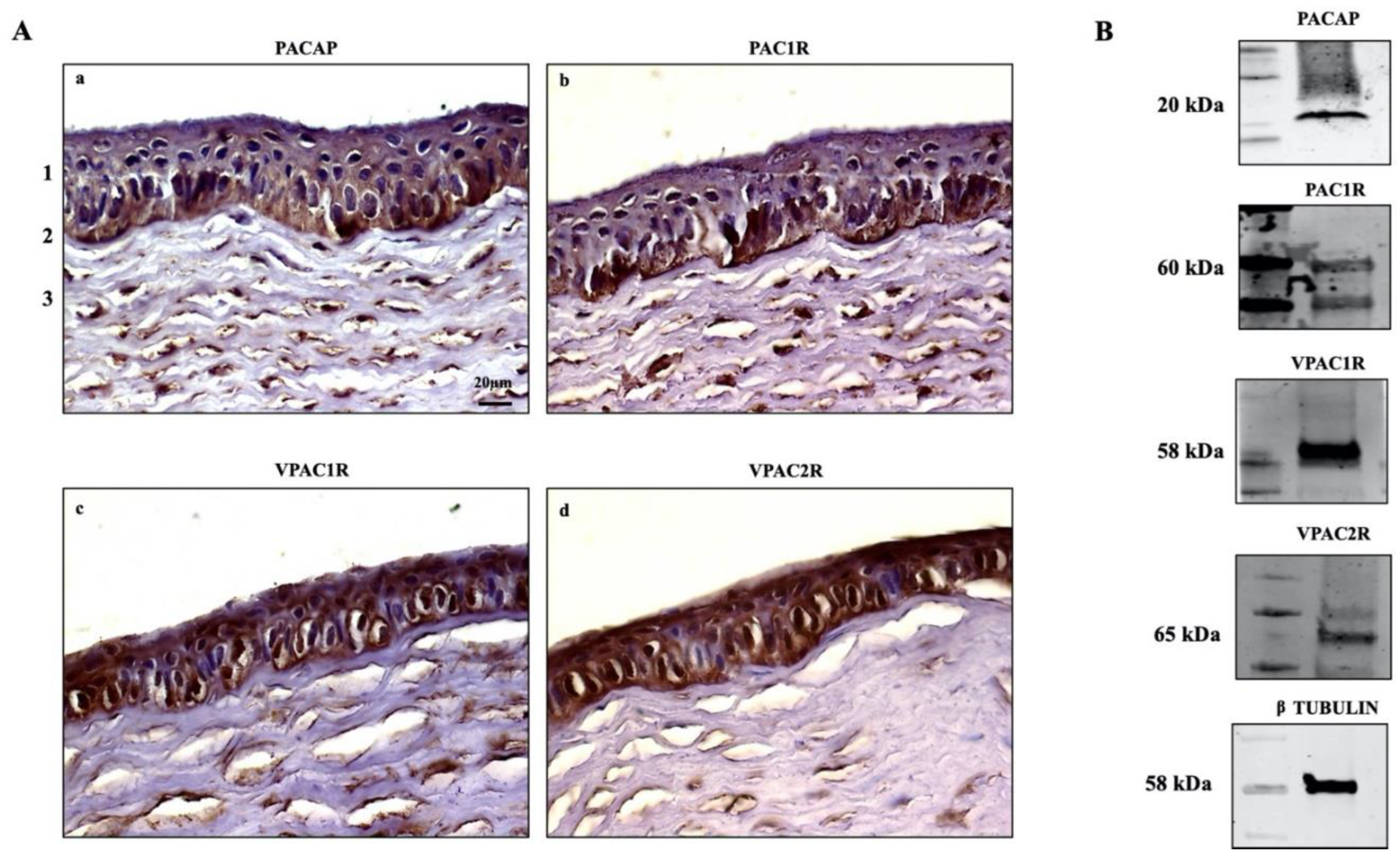
The expression of PACAP and its receptors in the eye has been widely reported [43,44,45]. PACAP-positive cells have been detected in the corneal endothelial layer [39,42], and PACAP-IR has been found in nerve terminals running through the stroma [29]. Here, we investigated the endogenous expression of PACAP and its receptors in the cornea of rabbits (n = 12) through IHC analysis. We found the positive expression of PACAP and its receptors in all examined sections. In particular, PACAP was expressed through the entire height of the corneal epithelium in all examined zones but showed higher signal intensity in the cells laying on the basement membrane. Weak expression of the peptide was detected in the stroma (Figure 1Aa). The labeling of PAC1R resembled the localization of PACAP, as the receptor was clearly expressed in both the basal epithelial layer as well as in the stroma (Figure 1Ab). The VPAC1 and VPAC2 receptors were found in all epithelial cell layers. The signal intensity of VPACRs differed in the stroma, where VPAC1R was highly expressed in comparison to VPAC2R (Figure 1Ac–d). The endogenous expression of PACAP, PAC1R, VPAC1R, and VPAC2R was also investigated in rabbit corneal epithelial cells. As shown in Figure 1B, the peptide and its receptors are expressed in SIRC cells.
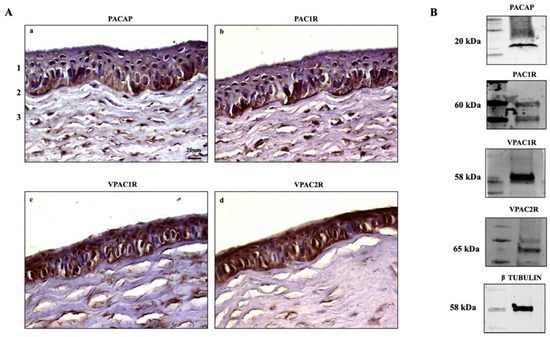
Figure 1.
(A) Immunodetection of PACAP (a), PAC1R (b), VPAC1R (c), and VPAC2R (d) in rabbit cornea: (1) epithelium, (2) Bowman’s membrane, (3) stroma. Original magnification ×200, scale bar: 20 μm. (B) Representative immunoblot of PACAP and its receptors in SIRC cells.
3.2. Effect of PACAP Treatment on UV-B-Induced Apoptotic Cell Death
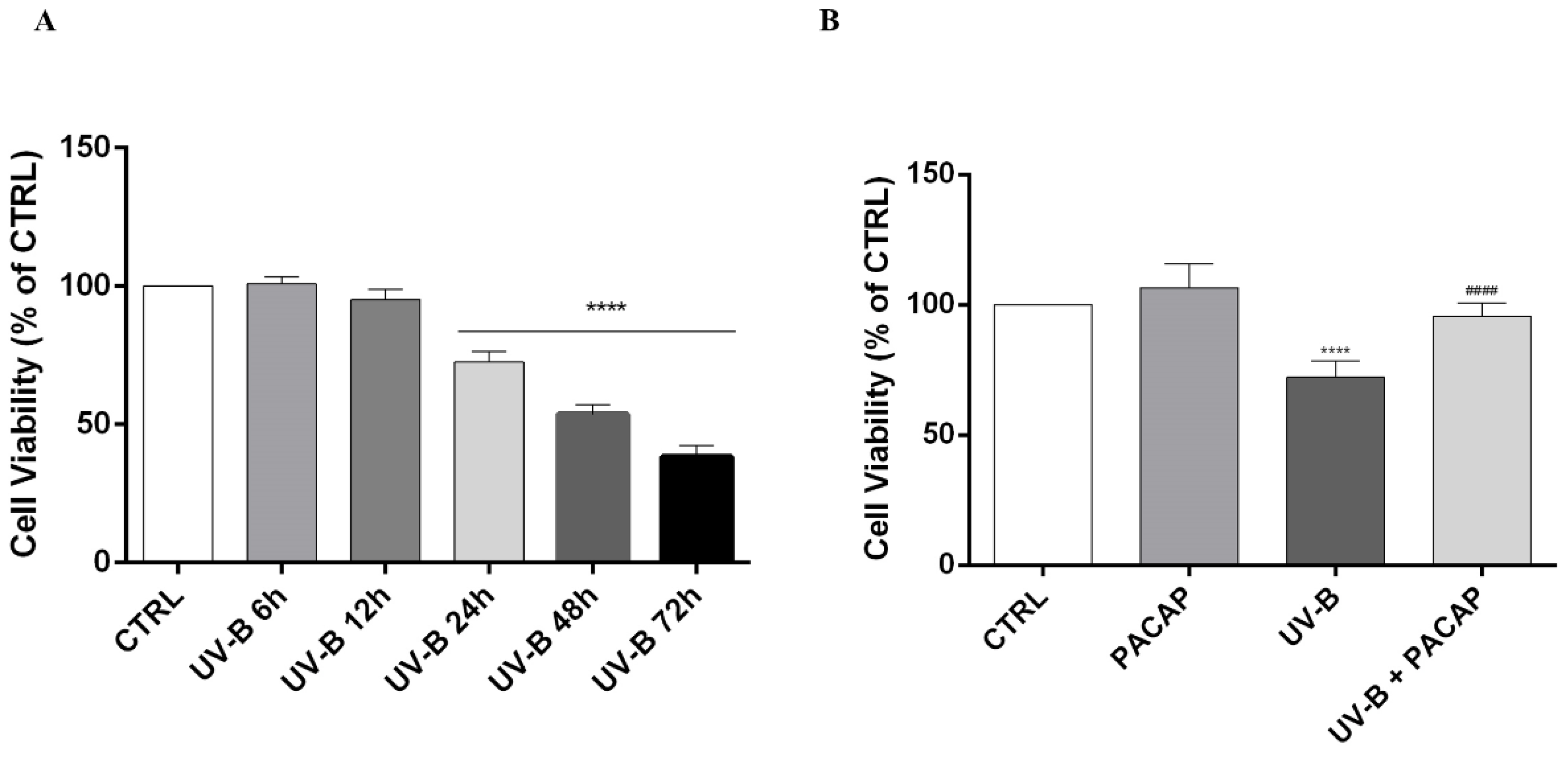
Studies showed that UV-B insult injures the cornea by causing apoptotic cell death [46,47,48,49]. Here, it was found that UV-B rays (20 mJ/cm2) significantly decreased cell viability in a time-dependent manner starting from 24 h following UV-B insult (**** p < 0.0001 vs. CTRL) (Figure 2A). Therefore, we chose this time point to investigate the effect of PACAP treatment on UV-B-exposed cells’ viability. As shown in Figure 2B, the peptide increased cell viability when compared with the UV-B group, confirming its protective role (#### p < 0.0001 vs. UV-B).
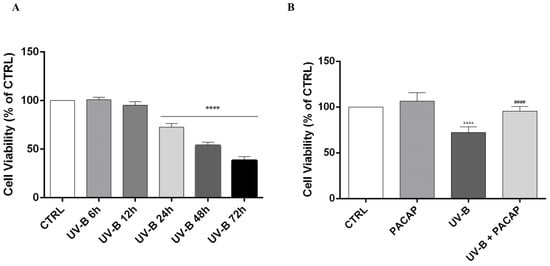
Figure 2.
(A) MTT assay performed on SIRC cells at different time points. (B) Cell viability evaluated at 24 h on different experimental groups. **** p < 0.0001 vs. CTRL; #### p < 0.0001 vs. UV-B.
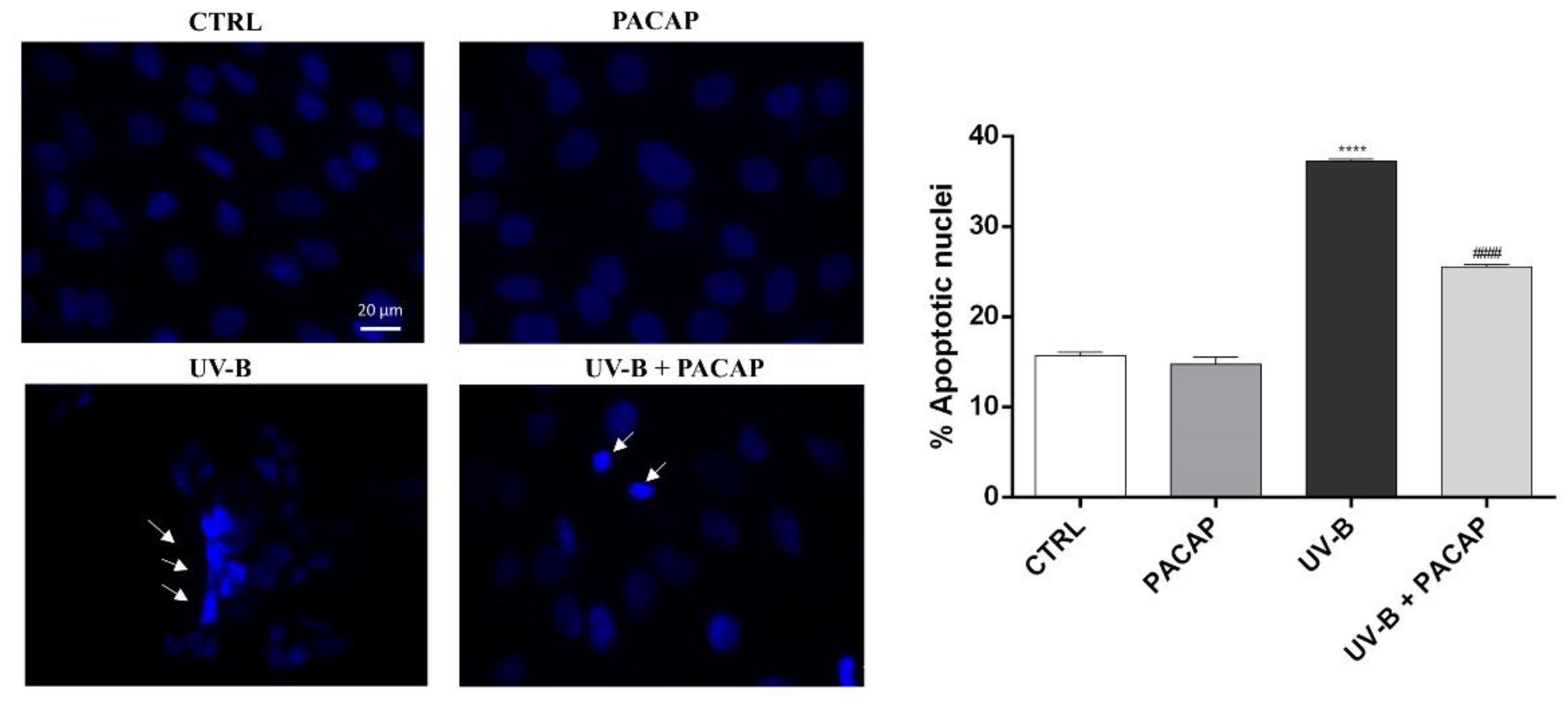
To further analyze the protective effect of PACAP on UV-B-mediated cell damage, the nuclei of SIRC cells exposed to UV-B were stained with Hoechst 33342. As shown in Figure 3, significant fragmentation and nuclear condensation, characteristic of apoptotic death, was observed in the UV-B-exposed group, in comparison with the control (**** p <0.0001 vs. CTRL). When the cells were treated with PACAP, the number of apoptotic nuclei in the UV-B-exposed cells was markedly reduced (#### p < 0.0001 vs. UV-B).
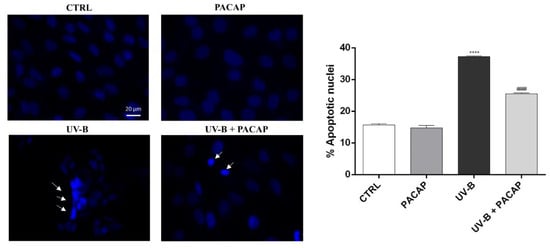
Figure 3.
Assessment of cell death using Hoechst 33342 staining. Scale bar: 20 μm. **** p < 0.0001 vs. CTRL; #### p < 0.0001 vs. UV-B.
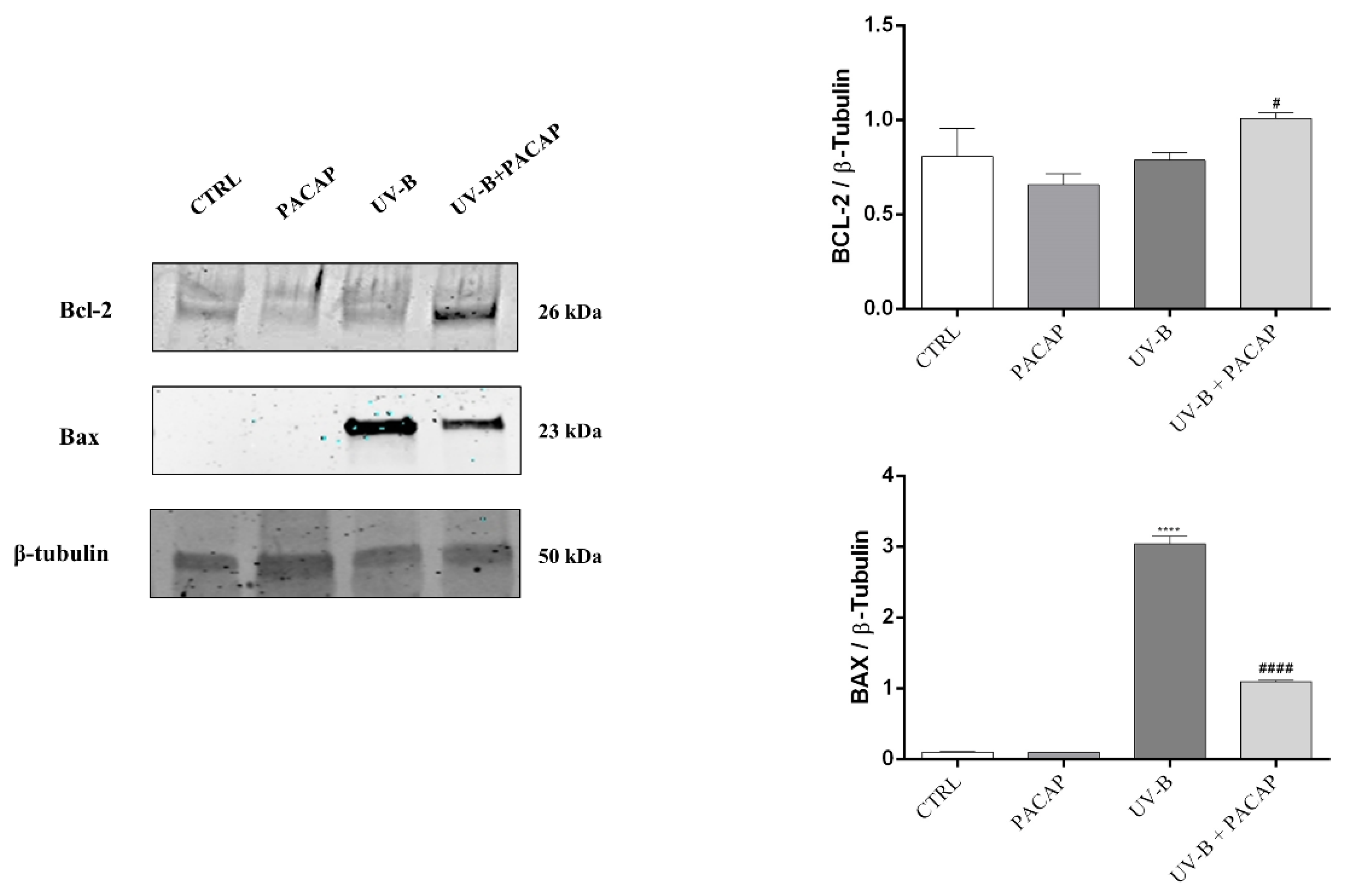
Considering that apoptotic cell death is regulated by Bcl-2 family proteins, key mediators, we investigated the role of PACAP to modulate the expression of the anti-apoptotic protein, Bcl-2, and pro-apoptotic protein, Bax. As shown in Figure 4, PACAP markedly increased the expression of Bcl-2 protein in UV-B-exposed cells (# p < 0.05 vs. UV-B). In contrast, the expression levels of the pro-apoptotic protein Bax, which drastically increased following UV-B exposure (**** p < 0.0001 vs. CTRL), were significantly downregulated by treatment with the peptide (#### p < 0.0001 vs. UV-B). These results confirmed that PACAP inhibits corneal epithelial cells’ apoptotic death activated by UV-B ray exposure.
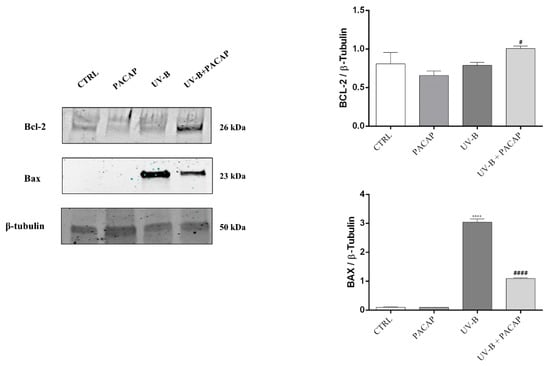
Figure 4.
Expression of Bcl-2 and Bax proteins at 24 h in different experimental groups. **** p < 0.0001 vs. CTRL; # p < 0.05, and #### p < 0.0001 vs. UV-B.
3.3. PACAP Decreased the Production of ROS Induced by UV-B Rays on Corneal Epithelial Cells via the JNK Pathway Inhibition
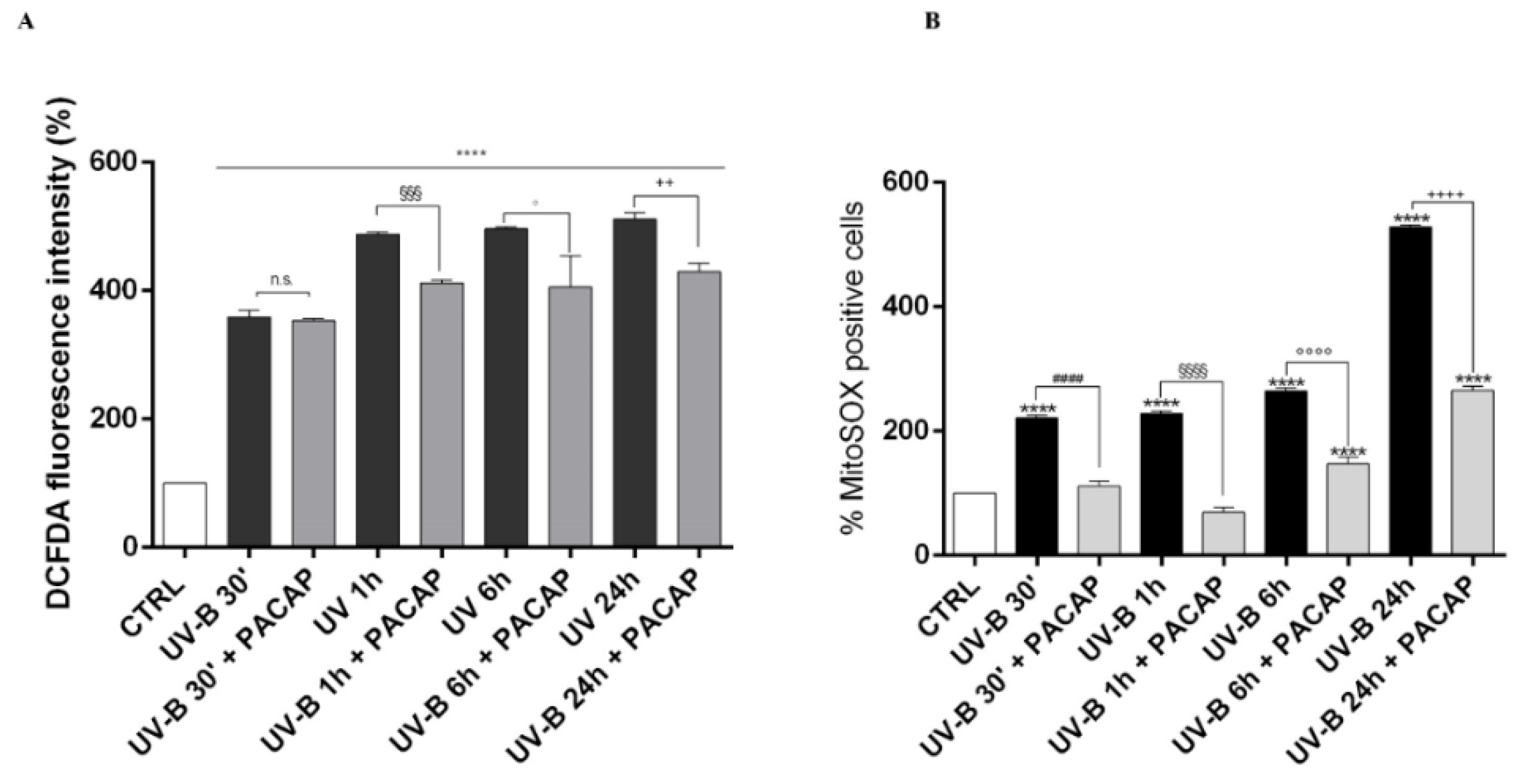
Considering that corneal injuries induced by UV-B are predominantly triggered by oxidative stress [50,51], we analyzed the time-course of ROS generation in corneal epithelial cells after UV-B radiation exposure (20 mJ/cm2) by using the DCFDA assay and the Mito-SOX™ Red fluorescence indicator (Figure 5A,B). The cellular ROS production significantly increased in a time-dependent manner starting from 30 s after UV-B irradiation (**** p < 0.0001 vs. CTRL). Treatment with the peptide significantly counteracted ROS formation starting from one hour after UV-B irradiation (20 mJ/cm2 for 30 s) (Figure 5A). Similarly, a time-dependent increase in mitochondrial ROS formation was observed in cells exposed to UV-B irradiation (**** p < 0.0001 vs. CTRL) (Figure 5B). PACAP treatment strongly reduced UV-B-induced mitochondrial ROS formation at all time points considered.
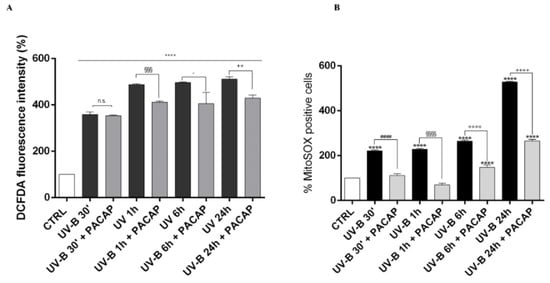
Figure 5.
Detection of intracellular ROS production in SIRC cells after UV-B exposure through DCFDA (A), or MitoSOX™ Red (B). **** p < 0.0001 vs. CTRL; #### p < 0.0001 vs. UV-B 30′; §§§ p < 0.001 and §§§§ p < 0.0001 vs. UV-B 1 h; ° p < 0.05 and °°°° p < 0.0001 vs. UV-B 6 h; ++ p < 0.01 and ++++ p < 0.0001 vs. UV-B 24 h.
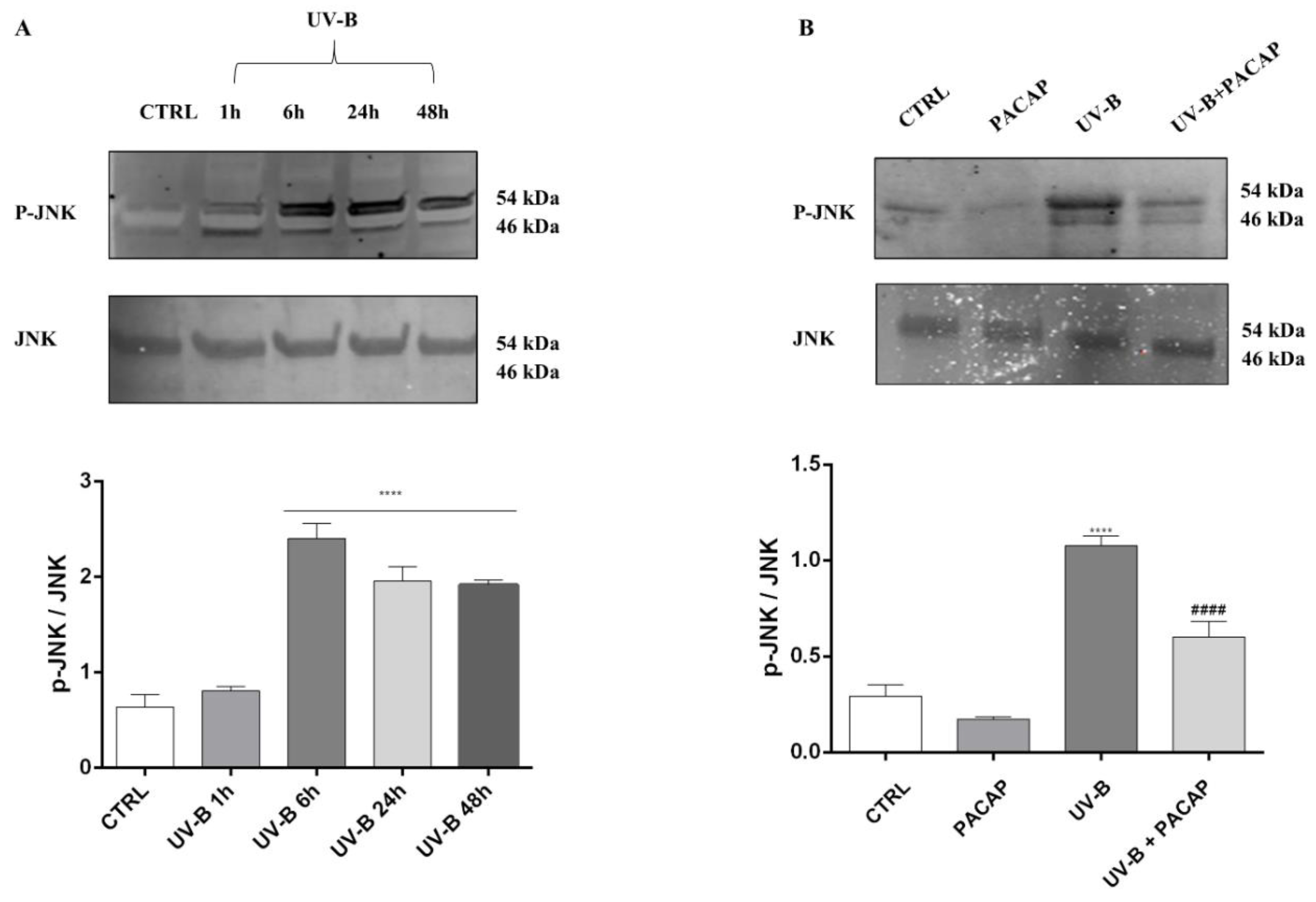
The literature shows that JNK activation is crucial as it represents the signaling pathway downstream of ROS, leading to apoptotic cell death [50,51]. Therefore, to investigate whether this pathway was linked to UV-B ROS-generation-induced cell death, we first measured the phosphorylated JNK (p-JNK) and total JNK at different time points in cells exposed to UV-B rays, through Western blot analysis. The significant activation of JNK was observed starting from 6 h after UV-B irradiation (**** p < 0.0001 vs. CTRL) (Figure 6A), before assessing the PACAP effect on JNK phosphorylation after 6 h from UV-B insult. As observed in Figure 6B, PACAP treatment strongly reduced p-JNK (#### p < 0.0001 vs. UV-B).
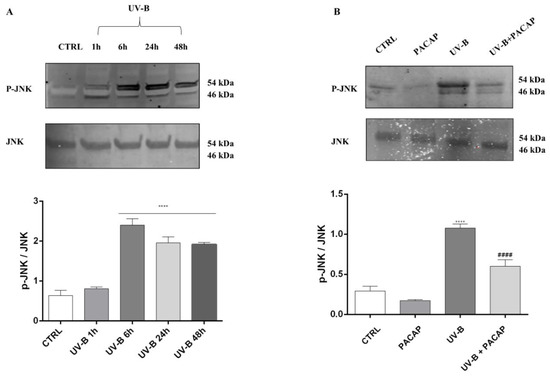
Figure 6.
(A) JNK activation induced by UV-B at different time points. (B) JNK phosphorylation expression at 6 h. **** p < 0.0001 vs. CTRL; #### p < 0.0001 vs. UV-B.
4. Discussion
Ozone layer depletion causes a continuous and more pronounced exposure of the cornea to UV-B rays, which provokes the formation of ROS, including superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl (OH·) radicals. ROS overproduction is associated with different ocular disorders, e.g., cataracts, age-related macular degeneration, glaucoma, inflammation, and corneal alteration [52]. The human eye has several defense mechanisms, including enzymes and antioxidants (e.g., glutathione peroxidase and ascorbic acid) which protect ocular tissues against oxidative injury [53]. However, these are gradually reduced during UV-B irradiation, as opposed to the pro-oxidant enzymes such as xanthine oxidoreductase/xanthine oxidase, which remain at normal values or may even increase.
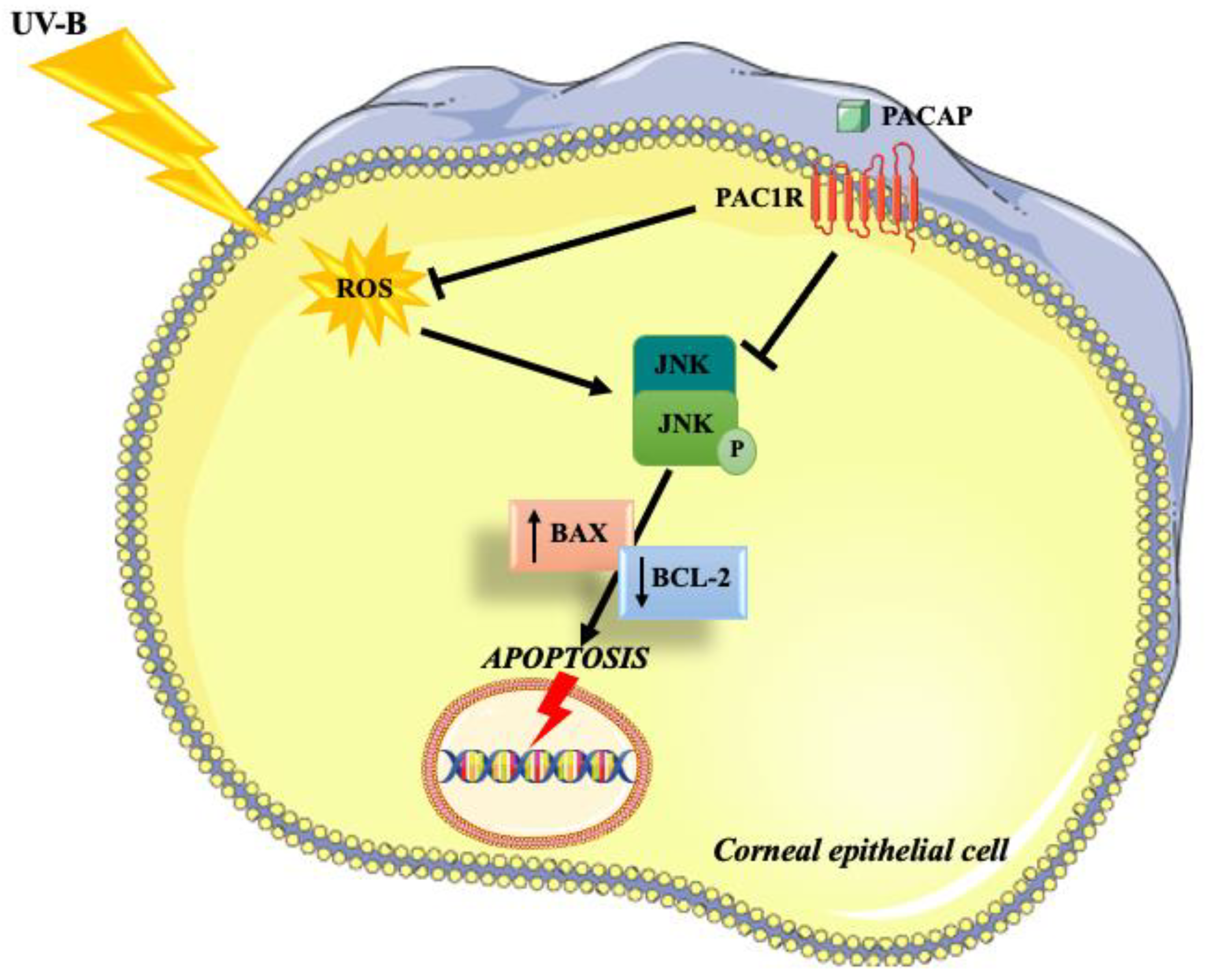
Many works demonstrated that PACAP exerts an important antioxidant and anti-apoptotic role in different cell types throughout the body [54,55,56]. The present study aimed to investigate the role of the peptide in corneal epithelium protection against UV-B radiation and elucidate the associated molecular mechanisms (Figure 7).
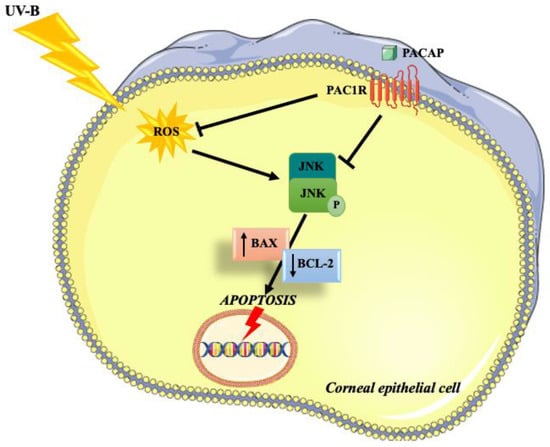
Figure 7.
Schematic representation of the protective effects exerted by PACAP on corneal epithelium against UV-B-induced apoptosis via ROS/JNK pathway inhibition.
We first analyzed the endogenous expression of PACAP and its receptors in the corneal epithelium. PACAP and PAC1R are found in the rabbit corneal epithelium, specifically in basal cells. VPAC1R and VPAC2R were observed in the entire length of the epithelial layers as well as in the stroma (Figure 1).
To explore the role of PACAP in corneal epithelium, we exposed SIRC cells to UV-B insult. It is widely recognized that UV-B radiation leads to corneal cell apoptotic death due to DNA damage and the production of ROS [47,48,49]. Here, we confirmed this evidentially, as well as demonstrating that treatment with PACAP significantly increased cell viability (Figure 2B) by preventing UV-B-induced apoptotic death (Figure 3). These data were also confirmed by the analysis of proteins Bcl-2/Bax. High levels of Bax protein were detected in the UV-B group (Figure 4), consistent with previous findings showing that UV-B irradiation increased pro-apoptotic Bax gene expression [54,55]. PACAP treatment significantly increased Bcl-2 protein expression and reduced the pro-apoptotic protein Bax in SIRC cells exposed to UV-B insult, confirming the anti-apoptotic mechanism involved in the protective effect of PACAP against UV-B-induced corneal epithelial damage.
As previously shown, UV-B irradiation induced ROS formation on the ocular surface [56], leading to DNA and mitochondrial damage, mitochondrial membrane depolarization, lipid peroxidation, and protein denaturation [56]. Here, we showed that UV-B insult induced a time-dependent increase in cellular ROS formation, as well as mitochondrial superoxide anion generation, whereas treatment with PACAP significantly reversed these changes (Figure 5A,B). Therefore, PACAP reduced oxidative stress directly through ROS scavenging and protected cells against UV-B radiation through the modulation of the JNK pathway. ROS induces different pathways, including JNK [9], which serves a central role in the apoptotic event [57,58]. Accordingly, we showed that UV-B insult triggers the activation of the JNK pathway, whereas PACAP treatment counteracts its phosphorylation (Figure 6). Correspondingly, previous findings show that PACAP exerts important cytoprotective effects by suppressing the JNK pathway [59,60].
The therapeutic use of PACAP has some limitations due to its poor in vivo stability. In fact, the peptide is degraded by different peptidases in the blood, including the dipeptidyl peptidase IV, which binds PACAP 1-38 to the PAC1 receptor antagonist, and other enzymes such as carboxypeptidase, endopeptidase, and prohormone convertase [61,62]. However, a recent paper showed that PACAP in a water solution can be preserved for relatively long periods of time [62]. Consequently, the present findings, along with the aforementioned studies, demonstrate that PACAP protects the cornea from UV-B damage, encouraging new investigations. In fact, the main limitation of the present work is that it was executed on immortalized corneal epithelial cells that fail to perfectly replicate the complex architecture and organization of the epithelial corneal layer. Therefore, further in vivo studies will be needed to confirm the corneal protective effects displayed by PACAP.
Author Contributions
Conceptualization, G.M. (Grazia Maugeri) and V.D.; Funding Acquisition, G.M. (Grazia Maugeri) and S.G.; Investigation, G.M. (Grazia Maugeri), A.G.D., B.M., E.P. and C.G.; Resources, S.G., C.B. and G.M. (Giuseppe Musumeci); Visualization, C.B.; Writing, Grazia Maugeri, A.G.D. and V.D.; Supervision, V.D. All authors have read and agreed to the published version of the manuscript.
Funding
PIAno di inCEntivi per la RIcerca di Ateneo 2020/2022 Linea di Intervento 3 Starting. Grant “REPAIR” and Linea Intervento 2 “NanoRET”, University of Catania, Italy; Italian Ministry of University and Research (MUR) (grant number PRIN 2017TSHBXZ_003).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institution of Animal Care and Use Committee of Catania University (#303).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sliney, D.H. How light reaches the eye and its components. Int. J. Toxicol. 2002, 21, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Heck, D.E.; Gerecke, D.R.; Vetrano, A.M.; Laskin, J.D. Solar ultraviolet radiation as a trigger of cell signal transduction. Toxicol. Appl. Pharmacol. 2004, 195, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Azzam, N.; Levanon, D.; Dovrat, A. Effects of UV-A irradiation on lens morphology and optics. Exp. Gerontol. 2004, 39, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Bashir, H.; Seykora, J.T.; Lee, V. Invisible Shield: Review of the Corneal Epithelium as a Barrier to UV Radiation, Pathogens, and Other Environmental Stimuli. J. Ophthalmic Vis. Res. 2017, 12, 305–311. [Google Scholar] [CrossRef]
- Mesa, R.; Bassnett, S. UV-B-induced DNA damage and repair in the mouse lens. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6789–6797. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian. J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef]
- Black, A.T.; Gordon, M.K.; Heck, D.E.; Gallo, M.A.; Laskin, D.L.; Laskin, J.D. UVB light regulates expression of antioxidants and inflammatory mediators in human corneal epithelial cells. Biochem. Pharmacol. 2011, 81, 873–880. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Lahair, M.M.; Franklin, R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 2006, 8, 1775–1789. [Google Scholar] [CrossRef]
- Liu, J.; Lin, A. Role of JNK activation in apoptosis: A double-edged sword. Cell Res. 2005, 15, 36–42. [Google Scholar] [CrossRef]
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant defenses in the ocular surface. Ocul. Surf. 2009, 7, 176–185. [Google Scholar] [CrossRef]
- Kolozsvári, L.; Nógrádi, A.; Hopp, B.; Bor, Z. UV absorbance of the human cornea in the 240- to 400-nm range. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2165–2168. [Google Scholar]
- Harmar, A.J.; Arimura, A.; Gozes, I.; Journot, L.; Laburthe, M.; Pisegna, J.R.; Rawlings, S.R.; Robberecht, P.; Said, S.I.; Sreedharan, S.P.; et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 1998, 50, 265–270. [Google Scholar] [PubMed]
- Arimura, A.; Shioda, S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: Neuroendocrine and endocrine interaction. Front. Neuroendocrinol. 1995, 16, 53–88. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Bassan, M.; Zamostiano, R.; Davidson, A.; Pinhasov, A.; Giladi, E.; Perl, O.; Bassan, H.; Blat, C.; Gibney, G.; Glazner, G.; et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J. Neurochem. 1999, 72, 1283–1293. [Google Scholar] [CrossRef]
- Zamostiano, R.; Pinhasov, A.; Gelber, E.; Steingart, R.A.; Seroussi, E.; Giladi, E.; Bassan, M.; Wollman, Y.; Eyre, H.J.; Mulley, J.C.; et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J. Biol. Chem. 2001, 276, 708–714. [Google Scholar] [CrossRef]
- D’Amico, A.G.; Scuderi, S.; Maugeri, G.; Cavallaro, S.; Drago, F.; D’Agata, V. NAP reduces murine microvascular endothelial cells proliferation induced by hyperglycemia. J. Mol. Neurosci. 2014, 54, 405–413. [Google Scholar] [CrossRef]
- D’Amico, A.G.; Maugeri, G.; Rasà, D.; Federico, C.; Saccone, S.; Lazzara, F.; Fidilio, A.; Drago, F.; Bucolo, C.; D’Agata, V. NAP modulates hyperglycemic-inflammatory event of diabetic retina by counteracting outer blood retinal barrier damage. J. Cell. Physiol. 2019, 234, 5230–5240. [Google Scholar] [CrossRef]
- D’Amico, A.G.; Maugeri, G.; Musumeci, G.; Reglodi, D.; D’Agata, V. PACAP and NAP: Effect of Two Functionally Related Peptides in Diabetic Retinopathy. J. Mol. Neurosci. 2021, 71, 1525–1535. [Google Scholar] [CrossRef]
- Solés-Tarrés, I.; Cabezas-Llobet, N.; Vaudry, D.; Xifró, X. Protective Effects of Pituitary Adenylate Cyclase-Activating Polypeptide and Vasoactive Intestinal Peptide Against Cognitive Decline in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 221. [Google Scholar] [CrossRef] [PubMed]
- Dejda, A.; Jolivel, V.; Bourgault, S.; Seaborn, T.; Fournier, A.; Vaudry, H.; Vaudry, D. Inhibitory effect of PACAP on caspase activity in neuronal apoptosis: A better understanding towards therapeutic applications in neurodegenerative diseases. J. Mol. Neurosci. 2008, 36, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Kiss, P.; Lubics, A.; Tamas, A. Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr. Pharm. Des. 2011, 17, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J.A. VIP and PACAP: Neuropeptide modulators of CNS inflammation, injury, and repair. Br. J. Pharmacol. 2013, 169, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Morello, G.; Reglodi, D.; Cavallaro, S.; D’Agata, V. Differential Vulnerability of Oculomotor Versus Hypoglossal Nucleus During ALS: Involvement of PACAP. Front. Neurosci. 2020, 14, 805. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Musumeci, G.; Reglodi, D.; D’Agata, V. Effects of Pacap on Schwann Cells: Focus on Nerve Injury. Int. J. Mol. Sci. 2020, 21, 8233. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; Federico, C.; Saccone, S.; Morello, G.; La Cognata, V.; Cavallaro, S.; D’Agata, V. Molecular mechanisms involved in the protective effect of pituitary adenylate cyclase-activating polypeptide in an in vitro model of amyotrophic lateral sclerosis. J. Cell. Physiol. 2019, 234, 5203–5214. [Google Scholar] [CrossRef]
- D’Amico, A.G.; Maugeri, G.; Saccone, S.; Federico, C.; Cavallaro, S.; Reglodi, D.; D’Agata, V. PACAP Modulates the Autophagy Process in an In Vitro Model of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 21, 2943. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Alm, P.; Håkanson, R. Distribution and effects of pituitary adenylate cyclase-activating peptide in the rabbit eye. Neuroscience 1995, 69, 297–308. [Google Scholar] [CrossRef]
- Nilsson, S.F.; De Neef, P.; Robberecht, P.; Christophe, J. Characterization of ocular receptors for pituitary adenylate cyclase activating polypeptide (PACAP) and their coupling to adenylate cyclase. Exp. Eye Res. 1994, 58, 459–467. [Google Scholar] [CrossRef]
- Nakajima, E.; Walkup, R.D.; Fujii, A.; Shearer, T.R.; Azuma, M. Pituitary adenylate cyclase-activating peptide induces neurite outgrowth in cultured monkey trigeminal ganglion cells: Involvement of receptor PAC1. Mol. Vis. 2013, 19, 174–183. [Google Scholar] [PubMed]
- Toth, D.; Szabo, E.; Tamas, A.; Juhasz, T.; Horvath, G.; Fabian, E.; Opper, B.; Szabo, D.; Maugeri, G.; D’Amico, A.G.; et al. Protective Effects of PACAP in Peripheral Organs. Front. Endocrinol. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Szabadfi, K.; Reglodi, D.; Szabo, A.; Szalontai, B.; Valasek, A.; Setalo, G., Jr.; Kiss, P.; Tamas, A.; Wilhelm, M.; Gabriel, R. Pituitary Adenylate Cyclase Activating Polypeptide, A Potential Therapeutic Agent for Diabetic Retinopathy in Rats: Focus on the Vertical Information Processing Pathway. Neurotox. Res. 2016, 29, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Atlasz, T.; Szabadfi, K.; Kiss, P.; Tamas, A.; Toth, G.; Reglodi, D.; Gabriel, R. Evaluation of the protective effects of PACAP with cell-specific markers in ischemia-induced retinal degeneration. Brain Res. Bull. 2010, 81, 497–504. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Bucolo, C.; D’Agata, V. Protective effect of PACAP-38 on retinal pigmented epithelium in an in vitro and in vivo model of diabetic retinopathy through EGFR-dependent mechanism. Peptides 2019, 119, 170108. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Gagliano, C.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. VIP Family Members Prevent Outer Blood Retinal Barrier Damage in a Model of Diabetic Macular Edema. J. Cell. Physiol. 2017, 232, 1079–1085. [Google Scholar] [CrossRef]
- Atlasz, T.; Szabadfi, K.; Kiss, P.; Marton, Z.; Griecs, M.; Hamza, L.; Gaal, V.; Biro, Z.; Tamas, A.; Hild, G.; et al. Effects of PACAP in UV-A radiation-induced retinal degeneration models in rats. J. Mol. Neurosci. 2011, 43, 51–57. [Google Scholar] [CrossRef]
- Fukiage, C.; Nakajima, T.; Takayama, Y.; Minagawa, Y.; Shearer, T.R.; Azuma, M. PACAP induces neurite outgrowth in cultured trigeminal ganglion cells and recovery of corneal sensitivity after flap surgery in rabbits. Am. J. Ophthalmol. 2007, 143, 255–262. [Google Scholar] [CrossRef]
- Shioda, S.; Takenoya, F.; Hirabayashi, T.; Wada, N.; Seki, T.; Nonaka, N.; Nakamachi, T. Effects of PACAP on Dry Eye Symptoms, and Possible Use for Therapeutic Application. J. Mol. Neurosci. 2019, 68, 420–426. [Google Scholar] [CrossRef]
- Wang, Z.; Shan, W.; Li, H.; Feng, J.; Lu, S.; Ou, B.; Ma, M.; Ma, Y. The PACAP-derived peptide MPAPO facilitates corneal wound healing by promoting corneal epithelial cell proliferation and trigeminal ganglion cell axon regeneration. Int. J. Biol. Sci. 2019, 15, 2676–2691. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Castrogiovanni, P.; Saccone, S.; Federico, C.; Reibaldi, M.; Russo, A.; Bonfiglio, V.; Avitabile, T.; Longo, A.; et al. PACAP through EGFR transactivation preserves human corneal endothelial integrity. J. Cell. Biochem. 2019, 120, 10097–10105. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; Longo, A.; D’Amico, A.G.; Rasà, D.M.; Reibaldi, M.; Russo, A.; Bonfiglio, V.; Avitabile, T.; D’Agata, V. Trophic effect of PACAP on human corneal endothelium. Peptides 2018, 99, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Nakamachi, T.; Ohtaki, H.; Seki, T.; Yofu, S.; Kagami, N.; Hashimoto, H.; Shintani, N.; Baba, A.; Mark, L.; Lanekoff, I.; et al. PACAP suppresses dry eye signs by stimulating tear secretion. Nat. Commun. 2016, 7, 12034. [Google Scholar] [CrossRef] [PubMed]
- Shioda, S.; Takenoya, F.; Wada, N.; Hirabayashi, T.; Seki, T.; Nakamachi, T. Pleiotropic and retinoprotective functions of PACAP. Anat. Sci. Int. 2016, 91, 313–324. [Google Scholar] [CrossRef]
- D’Agata, V.; Cavallaro, S. Functional and molecular expression of PACAP/VIP receptors in the rat retina. Brain Res. Mol. Brain Res. 1998, 54, 161–164. [Google Scholar] [CrossRef]
- Rogers, C.S.; Chan, L.M.; Sims, Y.S.; Byrd, K.D.; Hinton, D.L.; Twining, S.S. The effects of sub-solar levels of UV-A and UV-B on rabbit corneal and lens epithelial cells. Exp. Eye Res. 2004, 78, 1007–1014. [Google Scholar] [CrossRef]
- Kulms, D.; Zeise, E.; Pöppelmann, B.; Schwarz, T. DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way. Oncogene 2002, 21, 5844–5851. [Google Scholar] [CrossRef]
- Kronschläger, M.; Talebizadeh, N.; Yu, Z.; Meyer, L.M.; Löfgren, S. Apoptosis in Rat Cornea After In Vivo Exposure to Ultraviolet Radiation at 300 nm. Cornea 2015, 34, 945–949. [Google Scholar] [CrossRef]
- Young, A.R. Acute effects of UVR on human eyes and skin. Prog. Biophys. Mol. Biol. 2006, 92, 80–85. [Google Scholar] [CrossRef]
- Wada, T.; Penninger, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef]
- Shen, H.M.; Liu, Z.G. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2006, 40, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Cejka, C.; Cejkova, J. Oxidative stress to the cornea, changes in corneal optical properties, and advances in treatment of corneal oxidative injuries. Oxid. Med. Cell. Longev. 2015, 2015, 591530. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Takagi-Morishita, Y.; Yamada, N.; Sugihara, A.; Iwasaki, T.; Tsujimura, T.; Terada, N. Mouse uterine epithelial apoptosis is associated with expression of mitochondrial voltage-dependent anion channels, release of cytochrome C from mitochondria, and the ratio of Bax to Bcl-2 or Bcl-X. Biol. Reprod. 2003, 68, 1178–1184. [Google Scholar] [CrossRef][Green Version]
- Balaiya, S.; Murthy, R.K.; Brar, V.S.; Chalam, K.V. Evaluation of ultraviolet light toxicity on cultured retinal pigment epithelial and retinal ganglion cells. Clin. Ophthalmol. 2010, 4, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, A.; Ichijo, H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid. Redox Signal. 2005, 7, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yu, S.J.; Oh, H.J.; Lee, J.Y.; Kim, Y.; Sohn, J. Panaxydol induces apoptosis through an increased intracellular calcium level, activation of JNK and p38 MAPK and NADPH oxidase-dependent generation of reactive oxygen species. Apoptosis 2011, 16, 347–358. [Google Scholar] [CrossRef]
- Ramiro-Cortés, Y.; Guemez-Gamboa, A.; Morán, J. Reactive oxygen species participate in the p38-mediated apoptosis induced by potassium deprivation and staurosporine in cerebellar granule neurons. Int. J. Biochem. Cell Biol. 2011, 43, 1373–1382. [Google Scholar] [CrossRef]
- Szabo, A.; Danyadi, B.; Bognar, E.; Szabadfi, K.; Fabian, E.; Kiss, P.; Mester, L.; Manavalan, S.; Atlasz, T.; Gabriel, R.; et al. Effect of PACAP on MAP kinases, Akt and cytokine expressions in rat retinal hypoperfusion. Neurosci. Lett. 2012, 523, 93–98. [Google Scholar] [CrossRef]
- Rácz, B.; Gallyas, F., Jr.; Kiss, P.; Tóth, G.; Hegyi, O.; Gasz, B.; Borsiczky, B.; Ferencz, A.; Roth, E.; Tamás, A.; et al. The neuroprotective effects of PACAP in monosodium glutamate-induced retinal lesion involve inhibition of proapoptotic signaling pathways. Regul. Pept. 2006, 137, 20–26. [Google Scholar] [CrossRef]
- Bourgault, S.; Vaudry, D.; Dejda, A.; Doan, N.D.; Vaudry, H.; Fournier, A. Pituitary adenylate cyclase-activating polypeptide: Focus on structure-activity relationships of a neuroprotective Peptide. Curr. Med. Chem. 2009, 16, 4462–4480. [Google Scholar] [CrossRef] [PubMed]
- Gourlet, P.; Vandermeers, A.; Robberecht, P.; Deschodt-Lanckman, M. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP-27, but not PACAP-38) degradation by the neutral endopeptidase EC 3.4.24.11. Biochem. Pharmacol. 1997, 54, 509–515. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).