An Insight into Neuropeptides Inhibitors in the Biology of Colorectal Cancer: Opportunity and Translational Perspectives
Abstract
:1. Introduction
2. Most Significant Neurotransmitters in Cancer Development and Progression
3. Neuropeptides: Peptidergic Neurotransmitters
4. Promising Neuropeptides Inhibitors for the Development of Effective Cancer Therapy
4.1. Emerging Neuropeptides Inhibitors in Colorectal Cancer
4.1.1. Bombesin
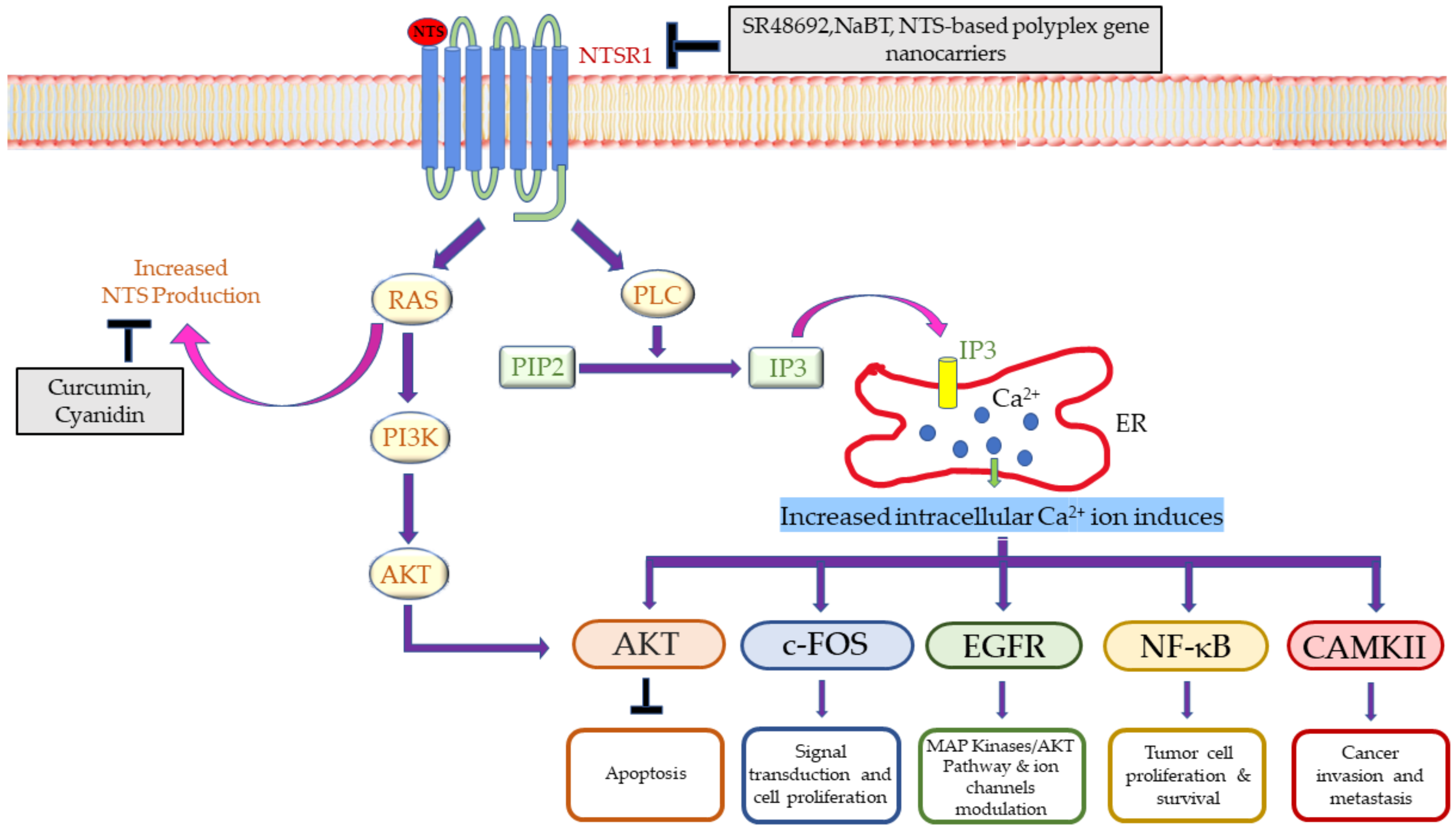
4.1.2. Neurotensin
4.1.3. Vasoactive Intestinal Peptide
4.1.4. Substance P
4.1.5. Neuropeptide Y
4.1.6. Orexins
4.2. Neuropeptides Inhibitors: A Promising Approach also for Other Cancers
4.2.1. Breast Cancer
4.2.2. Prostate Cancer
4.2.3. Glioblastoma
4.2.4. Lung Cancer
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mains, R.E.; Eipper, B.A. Peptides. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Siegel, G.J., Agranoff, B.W., Albers, R.W., Fisher, S.K., Uhler, M.D., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Nässel, D.R.; Zandawala, M. Recent advances in neuropeptide signaling in Drosophila, from genes to physiology and behavior. Prog. Neurobiol. 2019, 179, 101607. [Google Scholar] [CrossRef]
- Carraway, R.; Leeman, S.E. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J. Biol. Chem. 1973, 248, 6854–6861. [Google Scholar] [CrossRef]
- Carraway, R.; Leeman, S.E. The amino acid sequence of a hypothalamic peptide, neurotensin. J. Biol. Chem. 1975, 250, 1907–1911. [Google Scholar] [CrossRef]
- Juhász, T.; Helgadottir, S.L.; Tamás, A.; Reglődi, D.; Zákány, R. PACAP and VIP signaling in chondrogenesis and osteogenesis. Peptides 2015, 66, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Ganea, D. Vasoactive intestinal peptide: A neuropeptide with pleiotropic immune functions. Amino Acids 2013, 45, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Datar, P.; Srivastava, S.; Coutinho, E.; Govil, G. Substance P: Structure, function, and therapeutics. Curr. Top. Med. Chem. 2004, 4, 75–103. [Google Scholar] [CrossRef]
- Harrison, S.; Geppetti, P. Substance p. Int. J. Biochem. Cell Biol. 2001, 33, 555–576. [Google Scholar] [CrossRef]
- Gonzalez, N.; Moody, T.W.; Igarashi, H.; Ito, T.; Jensen, R.T. Bombesin-related peptides and their receptors: Recent advances in their role in physiology and disease states. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 58. [Google Scholar] [CrossRef]
- Weber, H.C. Regulation and signaling of human bombesin receptors and their biological effects. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Heilig, M.; Widerlöv, E. Neurobiology and clinical aspects of neuropeptide Y. Crit. Rev. Neurobiol. 1995, 9, 115–136. [Google Scholar]
- Decressac, M.; Barker, R. Neuropeptide Y and its role in CNS disease and repair. Exp. Neurol. 2012, 238, 265–272. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.-J.; Kodji, X.; Brain, S. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef] [PubMed]
- Rezaeian, A.H.; Isokane, T.; Nishibori, M.; Chiba, M.; Hiraiwa, N.; Yoshizawa, M.; Yasue, H. αCGRP and βCGRP transcript amount in mouse tissues of various developmental stages and their tissue expression sites. Brain Dev. 2009, 31, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology 2007, 191, 391–431. [Google Scholar] [CrossRef]
- Noori, S.; Friedlich, P.; Seri, I. Developmentally regulated cardiovascular, renal, and neuroendocrine effects of dopamine. NeoReviews 2003, 4, 283–288. [Google Scholar] [CrossRef]
- Peaston, R.T.; Weinkove, C. Measurement of catecholamines and their metabolites. Ann. Clin. Biochem. 2004, 41, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.M. Structural studies of metabolic products of dopamine. IV. Crystal and molecular structure of (-)-noradrenaline. Acta Chem. Scandinavica. Ser. B Org. Chem. Biochem. 1975, 29, 871–876. [Google Scholar] [CrossRef]
- Schinka, J.A.; Busch, R.M.; Robichaux-Keene, N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol. Psychiatry 2004, 9, 197–202. [Google Scholar] [CrossRef]
- Young, S.N. How to increase serotonin in the human brain without drugs. J. Psychiatry Neurosci. JPN 2007, 32, 394–399. [Google Scholar]
- Kellogg, D.L., Jr.; Zhao, J.L.; Coey, U.; Green, J.V. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J. Appl. Physiol. 2005, 98, 629–632. [Google Scholar] [CrossRef]
- Tiwari, P.; Dwivedi, S.; Singh, M.P.; Mishra, R.; Chandy, A. Basic and modern concepts on cholinergic receptor: A review. Asian Pac. J. Trop. Dis. 2013, 3, 413–420. [Google Scholar] [CrossRef]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Xu, E. The role and the mechanism of gamma-aminobutyric acid during central nervous system development. Neurosci. Bull. 2008, 24, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ntountoumi, C.; Vlastaridis, P.; Mossialos, D.; Stathopoulos, C.; Iliopoulos, I.; Promponas, V.; Oliver, S.G.; Amoutzias, G.D. Low complexity regions in the proteins of prokaryotes perform important functional roles and are highly conserved. Nucleic Acids Res. 2019, 47, 9998–10009. [Google Scholar] [CrossRef]
- Nieto-Alamilla, G.; Márquez-Gómez, R.; García-Gálvez, A.M.; Morales-Figueroa, G.E.; Arias-Montaño, J.A. The Histamine H3 Receptor: Structure, Pharmacology, and Function. Mol. Pharmacol. 2016, 90, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Hanoun, M.; Maryanovich, M.; Arnal-Estapé, A.; Frenette, P.S. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron 2015, 86, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Boilly, B.; Faulkner, S.; Jobling, P.; Hondermarck, H. Nerve dependence: From regeneration to cancer. Cancer Cell 2017, 31, 342–354. [Google Scholar] [CrossRef]
- Körner, M.; Reubi, J.C. NPY receptors in human cancer: A review of current knowledge. Peptides 2007, 28, 419–425. [Google Scholar] [CrossRef]
- Gao, Z.; Lei, W.I.; Lee, L.T.O. The Role of Neuropeptide-Stimulated cAMP-EPACs Signalling in Cancer Cells. Molecules 2022, 27, 311. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Bhuyan, P.P.; Dash, S.R.; Ki, J.-S.; Adhikary, S.P.; Ragusa, A.; Jena, M. Cyanobacteria and Algae-Derived Bioactive Metabolites as Antiviral Agents: Evidence, Mode of Action, and Scope for Further Expansion; A Comprehensive Review in Light of the SARS-CoV-2 Outbreak. Antioxidants 2022, 11, 354. [Google Scholar] [CrossRef]
- Mallamaci, R.; Budriesi, R.; Clodoveo, M.L.; Biotti, G.; Micucci, M.; Ragusa, A.; Curci, F.; Muraglia, M.; Corbo, F.; Franchini, C. Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation. Molecules 2021, 26, 1072. [Google Scholar] [CrossRef] [PubMed]
- Quarta, A.; Gaballo, A.; Pradhan, B.; Patra, S.; Jena, M.; Ragusa, A. Beneficial Oxidative Stress-Related trans-Resveratrol Effects in the Treatment and Prevention of Breast Cancer. Appl. Sci. 2021, 11, 11041. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Jit, B.P.; Ragusa, A.; Jena, M. Preliminary Investigation of the Antioxidant, Anti-Diabetic, and Anti-Inflammatory Activity of Enteromorpha intestinalis Extracts. Molecules 2021, 26, 1171. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Nayak, R.; Patra, S.; Jit, B.P.; Ragusa, A.; Jena, M. Bioactive Metabolites from Marine Algae as Potent Pharmacophores against Oxidative Stress-Associated Human Diseases: A Comprehensive Review. Molecules 2021, 26, 37. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Bhuyan, P.P.; Behera, P.K.; Mandal, A.K.; Behera, C.; Ki, J.-S.; Adhikary, S.P.; MubarakAli, D.; et al. A state-of-the-art review on fucoidan as an antiviral agent to combat viral infections. Carbohydr. Polym. 2022, 291, 119551. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Patil, S.; Bhutia, S.K.; Jena, M. Enteromorpha compressa extract induces anticancer activity through apoptosis and autophagy in Oral cancer. Mol. Biol. Rep. 2020, 47, 9567–9578. [Google Scholar] [CrossRef]
- Pradhan, B.; Bhuyan, P.P.; Patra, S.; Nayak, R.; Behera, P.K.; Behera, C.; Behera, A.K.; Ki, J.-S.; Jena, M. Beneficial effects of seaweeds and seaweed-derived bioactive compounds: Current evidence and future prospective. Biocatal. Agric. Biotechnol. 2022, 39, 102242. [Google Scholar] [CrossRef]
- Pradhan, B.; Ki, J.-S. Phytoplankton Toxins and Their Potential Therapeutic Applications: A Journey toward the Quest for Potent Pharmaceuticals. Mar. Drugs 2022, 20, 271. [Google Scholar] [CrossRef]
- Pradhan, B.; Kim, H.; Abassi, S.; Ki, J.-S. Toxic Effects and Tumor Promotion Activity of Marine Phytoplankton Toxins: A Review. Toxins 2022, 14, 397. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Bhuyan, P.P.; Patra, S.; Behera, C.; Sahoo, S.; Ki, J.-S.; Quarta, A.; Ragusa, A.; Jena, M. Algal Phlorotannins as Novel Antibacterial Agents with Reference to the Antioxidant Modulation: Current Advances and Future Directions. Mar. Drugs 2022, 20, 403. [Google Scholar] [CrossRef]
- Quarta, A.; Amorín, M.; Aldegunde, M.J.; Blasi, L.; Ragusa, A.; Nitti, S.; Pugliese, G.; Gigli, G.; Granja, J.R.; Pellegrino, T. Novel synthesis of platinum complexes and their intracellular delivery to tumor cells by means of magnetic nanoparticles. Nanoscale 2019, 11, 23482–23497. [Google Scholar] [CrossRef]
- Zafar, M.S.; Quarta, A.; Marradi, M.; Ragusa, A. Recent Developments in the Reduction of Oxidative Stress through Antioxidant Polymeric Formulations. Pharmaceutics 2019, 11, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragusa, A.; Priore, P.; Giudetti, A.M.; Ciccarella, G.; Gaballo, A. Neuroprotective Investigation of Chitosan Nanoparticles for Dopamine Delivery. Appl. Sci. 2018, 8, 474. [Google Scholar] [CrossRef]
- Zacheo, A.; Bizzarro, L.; Blasi, L.; Piccirillo, C.; Cardone, A.; Gigli, G.; Ragusa, A.; Quarta, A. Lipid-Based Nanovesicles for Simultaneous Intracellular Delivery of Hydrophobic, Hydrophilic, and Amphiphilic Species. Front. Bioeng. Biotechnol. 2020, 8, 690. [Google Scholar] [CrossRef]
- Li, J.; Tian, Y.; Wu, A. Neuropeptide Y receptors: A promising target for cancer imaging and therapy. Regen. Biomater. 2015, 2, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Basu, B.; Shome, S.; Jadhav, T.; Roy, S.; Majumdar, J.; Dasgupta, P.S.; Basu, S. Dopamine, by acting through its D2 receptor, inhibits insulin-like growth factor-I (IGF-I)-induced gastric cancer cell proliferation via up-regulation of Krüppel-like factor 4 through down-regulation of IGF-IR and AKT phosphorylation. Am. J. Pathol. 2010, 177, 2701–2707. [Google Scholar] [CrossRef]
- Chakroborty, D.; Chowdhury, U.R.; Sarkar, C.; Baral, R.; Dasgupta, P.S.; Basu, S. Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J. Clin. Investig. 2008, 118, 1380–1389. [Google Scholar] [CrossRef]
- Sarkar, C.; Chakroborty, D.; Chowdhury, U.R.; Dasgupta, P.S.; Basu, S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin. Cancer Res. 2008, 14, 2502–2510. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Kim, H.; Toyofuku, Y.; Lynn, F.C.; Chak, E.; Uchida, T.; Mizukami, H.; Fujitani, Y.; Kawamori, R.; Miyatsuka, T.; Kosaka, Y. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 2010, 16, 804–808. [Google Scholar] [CrossRef]
- Sarrouilhe, D.; Mesnil, M. Serotonin and human cancer: A critical view. Biochimie 2019, 161, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.P.; Marshall, A.M.; Hernandez, L.L.; Buckley, A.R.; Horseman, N.D. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res. 2009, 11, R81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soll, C.; Jang, J.H.; Riener, M.O.; Moritz, W.; Wild, P.J.; Graf, R.; Clavien, P.A. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology 2010, 51, 1244–1254. [Google Scholar] [CrossRef]
- Jiang, S.-H.; Li, J.; Dong, F.-Y.; Yang, J.-Y.; Liu, D.-J.; Yang, X.-M.; Wang, Y.-H.; Yang, M.-W.; Fu, X.-L.; Zhang, X.-X.; et al. Increased Serotonin Signaling Contributes to the Warburg Effect in Pancreatic Tumor Cells Under Metabolic Stress and Promotes Growth of Pancreatic Tumors in Mice. Gastroenterology 2017, 153, 277–291.e219. [Google Scholar] [CrossRef]
- Krizanova, O.; Babula, P.; Pacak, K. Stress, catecholaminergic system and cancer. Stress 2016, 19, 419–428. [Google Scholar] [CrossRef]
- Badino, G.R.; Novelli, A.; Girardi, C.; Di Carlo, F. Evidence for functional beta-adrenoceptor subtypes in CG-5 breast cancer cell. Pharmacol. Res. 1996, 33, 255–260. [Google Scholar] [CrossRef]
- Sarkar, C.; Chakroborty, D.; Basu, S. Neurotransmitters as regulators of tumor angiogenesis and immunity: The role of catecholamines. J. Neuroimmune Pharmacol. 2013, 8, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorf, S.K.; Cole, S.; Costanzo, E.; Bradley, S.; Coffin, J.; Jabbari, S.; Rainwater, K.; Ritchie, J.M.; Yang, M.; Sood, A.K. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin. Cancer Res. 2003, 9, 4514–4521. [Google Scholar]
- Ben-Eliyahu, S.; Yirmiya, R.; Liebeskind, J.C.; Taylor, A.N.; Gale, R.P. Stress increases metastatic spread of a mammary tumor in rats: Evidence for mediation by the immune system. Brain Behav. Immun. 1991, 5, 193–205. [Google Scholar] [CrossRef]
- Masur, K.; Niggemann, B.; Zanker, K.S.; Entschladen, F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by β-blockers. Cancer Res. 2001, 61, 2866–2869. [Google Scholar]
- Wessler, I.K.; Kirkpatrick, C.J. Non-neuronal acetylcholine involved in reproduction in mammals and honeybees. J. Neurochem. 2017, 142, 144–150. [Google Scholar] [CrossRef]
- Nimmakayala, R.K.; Seshacharyulu, P.; Lakshmanan, I.; Rachagani, S.; Chugh, S.; Karmakar, S.; Rauth, S.; Vengoji, R.; Atri, P.; Talmon, G.A. Cigarette smoke induces stem cell features of pancreatic cancer cells via PAF1. Gastroenterology 2018, 155, 892–908.e896. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Sekhon, H.S.; Fu, X.W.; Maier, M.; Jia, Y.; Duan, J.; Proskosil, B.J.; Gravett, C.; Lindstrom, J.; Mark, G.P. Activated cholinergic signaling provides a target in squamous cell lung carcinoma. Cancer Res. 2008, 68, 4693–4700. [Google Scholar] [CrossRef]
- Bierhaus, A.; Wolf, J.; Andrassy, M.; Rohleder, N.; Humpert, P.M.; Petrov, D.; Ferstl, R.; von Eynatten, M.; Wendt, T.; Rudofsky, G.; et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. USA 2003, 100, 1920–1925. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.D.; Campisi, J.; Sharkey, C.M.; Kennedy, S.L.; Nickerson, M.; Greenwood, B.N.; Fleshner, M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 2005, 135, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Paleari, L.; Cesario, A.; Fini, M.; Russo, P. α7-Nicotinic receptor antagonists at the beginning of a clinical era for NSCLC and Mesothelioma? Drug Discov. Today 2009, 14, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Horiguchi, K.; Sunaga, H.; Moriwaki, Y.; Misawa, H.; Kasahara, T.; Tsuji, S.; Kawashima, K. SLURP-1, an endogenous α7 nicotinic acetylcholine receptor allosteric ligand, is expressed in CD205(+) dendritic cells in human tonsils and potentiates lymphocytic cholinergic activity. J. Neuroimmunol. 2014, 267, 43–49. [Google Scholar] [CrossRef]
- Tata, A.M. Muscarinic acetylcholine receptors: New potential therapeutic targets in antinociception and in cancer therapy. Recent Pat. CNS Drug Discov. 2008, 3, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Del Bufalo, A.; Milic, M.; Salinaro, G.; Fini, M.; Cesario, A. Cholinergic receptors as target for cancer therapy in a systems medicine perspective. Curr. Mol. Med. 2014, 14, 1126–1138. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Sekiya, H.; Namiki, S.; Sakamoto, H.; Iinuma, S.; Yamasaki, M.; Watanabe, M.; Hirose, K.; Iino, M. Imaging extrasynaptic glutamate dynamics in the brain. Proc. Natl. Acad. Sci. USA 2010, 107, 6526–6531. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, E.A.; Olney, J.W. Glutamate antagonists: Deadly liaisons with cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 5947–5948. [Google Scholar] [CrossRef]
- Kalariti, N.; Pissimissis, N.; Koutsilieris, M. The glutamatergic system outside the CNS and in cancer biology. Expert Opin. Investig. Drugs 2005, 14, 1487–1496. [Google Scholar] [CrossRef]
- Yoo, B.C.; Jeon, E.; Hong, S.-H.; Shin, Y.-K.; Chang, H.J.; Park, J.-G. Metabotropic glutamate receptor 4-mediated 5-Fluorouracil resistance in a human colon cancer cell line. Clin. Cancer Res. 2004, 10, 4176–4184. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.J.; Yoo, B.C.; Lim, S.-B.; Jeong, S.-Y.; Kim, W.H.; Park, J.-G. Metabotropic glutamate receptor 4 expression in colorectal carcinoma and its prognostic significance. Clin. Cancer Res. 2005, 11, 3288–3295. [Google Scholar] [CrossRef]
- Zhang, X.; Du, Z.; Liu, J.; He, J. Γ-aminobutyric acid receptors affect the progression and migration of tumor cells. J. Recept. Signal Transduct. Res. 2014, 34, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.Y.; Yang, S.-D.; Ju, W.; Ahn, J.-H. Aberrant epigenetic regulation of GABRP associates with aggressive phenotype of ovarian cancer. Exp. Mol. Med. 2017, 49, e335. [Google Scholar] [CrossRef]
- Wang, T.; Huang, W.; Chen, F. Baclofen, a GABAB receptor agonist, inhibits human hepatocellular carcinoma cell growth in vitro and in vivo. Life Sci. 2008, 82, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M. Neurotransmission and cancer: Implications for prevention and therapy. Anti-Cancer Drugs 2008, 19, 655–671. [Google Scholar] [CrossRef]
- Jiang, S.-H.; Zhu, L.-L.; Zhang, M.; Li, R.-K.; Yang, Q.; Yan, J.-Y.; Zhang, C.; Yang, J.-Y.; Dong, F.-Y.; Dai, M.; et al. GABRP regulates chemokine signalling, macrophage recruitment and tumour progression in pancreatic cancer through tuning KCNN4-mediated Ca2+ signalling in a GABA-independent manner. Gut 2019, 68, 1994–2006. [Google Scholar] [CrossRef]
- Johnson, S.K.; Haun, R.S. The gamma-aminobutyric acid A receptor pi subunit is overexpressed in pancreatic adenocarcinomas. JOP 2005, 6, 136–142. [Google Scholar] [PubMed]
- Takehara, A.; Hosokawa, M.; Eguchi, H.; Ohigashi, H.; Ishikawa, O.; Nakamura, Y.; Nakagawa, H. γ-Aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor π subunit. Cancer Res. 2007, 67, 9704–9712. [Google Scholar] [CrossRef]
- Xia, S.; He, C.; Zhu, Y.; Wang, S.; Li, H.; Zhang, Z.; Jiang, X.; Liu, J. GABABR-Induced EGFR Transactivation Promotes Migration of Human Prostate Cancer Cells. Mol. Pharmacol. 2017, 92, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Semba, S.; Ito, M.; Takeda, H.; Kawata, S.; Yamakawa, M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer 2002, 94, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Dupouy, S.; Mourra, N.; Gompel, A.; Alifano, M.; Forgez, P. The potential use of the neurotensin high affinity receptor 1 as a biomarker for cancer progression and as a component of personalized medicine in selective cancers. Biochimie 2011, 93, 1369–1378. [Google Scholar] [CrossRef]
- Qiu, S.; Pellino, G.; Fiorentino, F.; Rasheed, S.; Darzi, A.; Tekkis, P.; Kontovounisios, C. A Review of the Role of Neurotensin and Its Receptors in Colorectal Cancer. Gastroenterol. Res. Pract. 2017, 2017, 6456257. [Google Scholar] [CrossRef]
- Tang, B.; Yong, X.; Xie, R.; Li, Q.-W.; Yang, S.-M. Vasoactive intestinal peptide receptor-based imaging and treatment of tumors (Review). Int. J. Oncol. 2014, 44, 1023–1031. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Niewiadomski, P.; Nowak, J.Z. Receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in the goose cerebral cortex. Pol. J. Pharmacol. 2004, 56, 203–211. [Google Scholar]
- Garnier, A.; Vykoukal, J.; Hubertus, J.; Alt, E.; Von Schweinitz, D.; Kappler, R.; Berger, M.; Ilmer, M. Targeting the neurokinin-1 receptor inhibits growth of human colon cancer cells. Int. J. Oncol. 2015, 47, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Mehboob, R.; Gilani, S.A.; Hassan, A.; Tanvir, I.; Javaid, S.; Khalid, S.; Hasan, S.; Waseem, H.; Alwazzan, A.; Munoz, M. Prognostic Significance of Substance P/Neurokinin 1 Receptor and Its Association with Hormonal Receptors in Breast Carcinoma. Biomed. Res. Int. 2020, 2021, 5577820. [Google Scholar] [CrossRef]
- Bajo, A.M.; Schally, A.V.; Groot, K.; Szepeshazi, K. Bombesin antagonists inhibit proangiogenic factors in human experimental breast cancers. Br. J. Cancer 2004, 90, 245–252. [Google Scholar] [CrossRef]
- Chanda, N.; Kattumuri, V.; Shukla, R.; Zambre, A.; Katti, K.; Upendran, A.; Kulkarni, R.R.; Kan, P.; Fent, G.M.; Casteel, S.W.; et al. Bombesin functionalized gold nanoparticles show in vitro and in vivo cancer receptor specificity. Proc. Natl. Acad. Sci. USA 2010, 107, 8760–8765. [Google Scholar] [CrossRef]
- Moreno, P.; Ramos-Álvarez, I.; Moody, T.W.; Jensen, R.T. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin. Ther. Targets 2016, 20, 1055–1073. [Google Scholar] [CrossRef]
- Moody, T.W.; Lee, L.; Ramos-Alvarez, I.; Iordanskaia, T.; Mantey, S.A.; Jensen, R.T. Bombesin Receptor Family Activation and CNS/Neural Tumors: Review of Evidence Supporting Possible Role for Novel Targeted Therapy. Front. Endocrinol. 2021, 12, 728088. [Google Scholar] [CrossRef]
- Tilan, J.; Kitlinska, J. Neuropeptide Y (NPY) in tumor growth and progression: Lessons learned from pediatric oncology. Neuropeptides 2016, 55, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.; Naranjo, A.; Van Ryn, C.; Tilan, J.U.; Trinh, E.; Yang, C.; Tsuei, J.; Hong, S.-H.; Wang, H.; Izycka-Swieszewska, E.; et al. Neuropeptide Y as a Biomarker and Therapeutic Target for Neuroblastoma. Am. J. Pathol. 2016, 186, 3040–3053. [Google Scholar] [CrossRef]
- Martinez, A.; Miller, M.J.; Unsworth, E.J.; Siegfried, J.M.; Cuttitta, F. Expression of adrenomedullin in normal human lung and in pulmonary tumors. Endocrinology 1995, 136, 4099–4105. [Google Scholar] [CrossRef]
- Rocchi, P.; Boudouresque, F.; Zamora, A.J.; Muracciole, X.; Lechevallier, E.; Martin, P.-M.; Ouafik, L.H. Expression of adrenomedullin and peptide amidation activity in human prostate cancer and in human prostate cancer cell lines. Cancer Res. 2001, 61, 1196–1206. [Google Scholar]
- Michelsen, J.; Thiesson, H.; Walter, S.; Ottosen, P.D.; Skøtt, O.; Jensen, B.L. Tissue expression and plasma levels of adrenomedullin in renal cancer patients. Clin. Sci. 2006, 111, 61–70. [Google Scholar] [CrossRef]
- Nakata, T.; Seki, N.; Miwa, S.; Kobayashi, A.; Soeda, J.; Nimura, Y.; Kawasaki, S.; Miyagawa, S. Identification of genes associated with multiple nodules in hepatocellular carcinoma using cDNA microarray: Multicentric occurrence or intrahepatic metastasis? Hepatogastroenterology 2008, 55, 865–872. [Google Scholar]
- Tsang, W.Y.; Chan, J.K. Neural invasion in intraductal carcinoma of the breast. Hum. Pathol. 1992, 23, 202–204. [Google Scholar] [CrossRef]
- Wang, J.; He, Y. Diagnostic role of NPY methylation in patients with colorectal cancer. JUSTC 2022, 52, 2. [Google Scholar] [CrossRef]
- Souazé, F.; Viardot-Foucault, V.; Roullet, N.; Toy-Miou-Leong, M.; Gompel, A.; Bruyneel, E.; Comperat, E.; Faux, M.C.; Mareel, M.; Rostène, W.; et al. Neurotensin receptor 1 gene activation by the Tcf/β-catenin pathway is an early event in human colonic adenomas. Carcinogenesis 2006, 27, 708–716. [Google Scholar] [CrossRef]
- Dupouy, S.; Viardot-Foucault, V.; Alifano, M.; Souazé, F.; Plu-Bureau, G.; Chaouat, M.; Lavaur, A.; Hugol, D.; Gespach, C.; Gompel, A.; et al. The neurotensin receptor-1 pathway contributes to human ductal breast cancer progression. PLoS ONE 2009, 4, e4223. [Google Scholar] [CrossRef]
- Munoz, M.; Covenas, R. Involvement of substance P and the NK-1 receptor in cancer progression. Peptides 2013, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids 2014, 46, 1727–1750. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Coveñas, R. Neurokinin-1 receptor antagonists as antitumor drugs in gastrointestinal cancer: A new approach. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2016, 22, 260. [Google Scholar] [CrossRef]
- Maoret, J.J.; Anini, Y.; Rouyer-Fessard, C.; Gully, D.; Laburthe, M. Neurotensin and a non-peptide neurotensin receptor antagonist control human colon cancer cell growth in cell culture and in cells xenografted into nude mice. Int. J. Cancer 1999, 80, 448–454. [Google Scholar] [CrossRef]
- Brzozowska, M.; Całka, J. Occurrence and distribution of galanin in the physiological and inflammatory states in the mammalian gastrointestinal tract. Front. Immunol. 2021, 11, 602070. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, P.; Chai, C.; Shao, L.; Mao, H.; Qiao, D.; Kong, G.; Dong, X.; Shi, M.; Zhang, Z. Galanin expression is down-regulated in patients with gastric cancer. J. Int. Med. Res. 2019, 47, 1241–1249. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Godlewski, J.; Kieżun, J.; Kraziński, B.E.; Kmieć, Z. Colorectal cancer patients exhibit increased levels of galanin in serum and colon tissues. Oncol. Lett. 2016, 12, 3323–3329. [Google Scholar] [CrossRef]
- Kiezun, J.; Godlewski, J.; Krazinski, B.E.; Kozielec, Z.; Kmiec, Z. Galanin Receptors (GalR1, GalR2, and GalR3) Expression in Colorectal Cancer Tissue and Correlations to the Overall Survival and Poor Prognosis of CRC Patients. Int. J. Mol. Sci. 2022, 23, 3735. [Google Scholar] [CrossRef]
- Talaat, I.; Yakout, N.; Soliman, A.; Venkatakhalam, T.; Vinod, A.; Eldohaji, L.; Nair, V.; Hareedy, A.; Kandil, A.; Abdel-Rahman, W. Evaluation of Galanin Expression in Colorectal Cancer: An Immunohistochemical and Transcriptomic Study. Front. Oncol. 2022, 12, 877147. [Google Scholar] [CrossRef] [PubMed]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Rai, S.; Singh, M.P.; Srivastava, S. Computational Intelligence-Based Gene Expression Analysis in Colorectal Cancer: A Review. In Computational Intelligence in Oncology; Studies in Computational, Intelligence; Raza, K., Ed.; Springer: Singapore, 2022; Volume 1016, pp. 387–410. [Google Scholar]
- Singh, M.P.; Rai, S.; Singh, N.K.; Srivastava, S. Transcriptomic landscape of early age onset of colorectal cancer identifies novel genes and pathways in Indian CRC patients. Sci. Rep. 2021, 11, 11765. [Google Scholar] [CrossRef]
- Kasprzak, A.; Adamek, A. The Neuropeptide System and Colorectal Cancer Liver Metastases: Mechanisms and Management. Int. J. Mol. Sci. 2020, 21, 3494. [Google Scholar] [CrossRef]
- Reubi, J.C.; Wenger, S.; Schmuckli-Maurer, J.; Schaer, J.-C.; Gugger, M. Bombesin receptor subtypes in human cancers: Detection with the universal radioligand 125I-[D-Tyr6, β-Ala11, Phe13, Nle14]-bombesin(6–14). Clin. Cancer Res. 2002, 8, 1139–1146. [Google Scholar] [PubMed]
- Srivastava, A.; Rai, S.; Bisht, D.; Sachan, M.; Jit, B.P.; Srivastava, S. Targeting the altered tyrosine kinases in colorectal cancer: From inhibitors to drugs. In Protein Kinase Inhibitors: From Discovery to Therapeutics; Hassan, I., Noor, S., Eds.; Academic Press: London, UK, 2022; pp. 361–391. [Google Scholar]
- Sancho, V.; Di Florio, A.; Moody, T.W.; Jensen, R.T. Bombesin receptor-mediated imaging and cytotoxicity: Review and current status. Curr. Drug Deliv. 2011, 8, 79–134. [Google Scholar] [CrossRef] [PubMed]
- Maoret, J.-J.; Pospaï, D.; Rouyer-Fessard, C.; Couvineau, A.; Laboisse, C.; Voisin, T.; Laburthe, M. Neurotensin receptor and its mRNA are expressed in many human colon cancer cell lines but not in normal colonic epithelium: Binding studies and RT-PCR experiments. Biochem. Biophys. Res. Commun. 1994, 203, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jackson, L.N.; Johnson, S.M.; Wang, Q.; Evers, B.M. Suppression of Neurotensin Receptor Type 1 Expression and Function by Histone Deacetylase Inhibitors in Human Colorectal Cancers. Mol. Cancer Ther. 2010, 9, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Iwase, K.; Evers, B.M.; Hellmich, M.R.; Kim, H.J.; Higashide, S.; Gully, D.; Townsend, C.M., Jr. Indirect inhibitory effect of a neurotensin receptor antagonist on human colon cancer (LoVo) growth. Surg. Oncol. 1996, 5, 245–251. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Ives, K.L.; Evers, B.M. Curcumin inhibits neurotensin-mediated interleukin-8 production and migration of HCT116 human colon cancer cells. Clin. Cancer Res. 2006, 12, 5346–5355. [Google Scholar] [CrossRef]
- Briviba, K.; Abrahamse, S.L.; Pool-Zobel, B.L.; Rechkemmer, G. Neurotensin-and EGF-induced metabolic activation of colon carcinoma cells is diminished by dietary flavonoid cyanidin but not by its glycosides. Nutr. Cancer 2001, 41, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.E.; Rembao, J.D.; Hernandez-Baltazar, D.; Castillo-Rodriguez, R.A.; Tellez-Lopez, V.M.; Flores-Martinez, Y.M.; Orozco-Barrios, C.E.; Rubio, H.A.; Sánchez-García, A.; Ayala-Davila, J.; et al. Safety of the intravenous administration of neurotensin-polyplex nanoparticles in BALB/c mice. Nanomedicine 2014, 10, 745–754. [Google Scholar] [CrossRef]
- Levy, A.; Gal, R.; Granoth, R.; Dreznik, Z.; Fridkin, M.; Gozes, I. In vitro and in vivo treatment of colon cancer by VIP antagonists. Regul. Pept. 2002, 109, 127–133. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Ru, G.-Q.; Ma, Y.-Y.; Xie, J.; Chen, W.-Y.; Wang, H.-J.; Wang, S.-B.; Li, L.; Jin, K.-T.; He, X.-L. High expression of substance P and its receptor neurokinin-1 receptor in colorectal cancer is associated with tumor progression and prognosis. OncoTargets Ther. 2016, 9, 3595. [Google Scholar]
- Xiang, H.; Toyoshima, Y.; Shen, W.; Wang, X.; Okada, N.; Kii, S.; Sugiyama, K.; Nagato, T.; Kobayashi, H.; Ikeo, K. IFN-α/β-mediated NK2R expression is related to the malignancy of colon cancer cells. Cancer Sci. 2022, 113, 2513. [Google Scholar] [CrossRef]
- Medeiros, P.J.; Al-Khazraji, B.K.; Novielli, N.M.; Postovit, L.M.; Chambers, A.F.; Jackson, D.N. Neuropeptide Y stimulates proliferation and migration in the 4T1 breast cancer cell line. Int. J. Cancer 2012, 131, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Chakroborty, D.; Goswami, S.; Fan, H.; Frankel, W.L.; Basu, S.; Sarkar, C. Neuropeptide Y, a paracrine factor secreted by cancer cells, is an independent regulator of angiogenesis in colon cancer. Br. J. Cancer 2022, 1–10. [Google Scholar] [CrossRef]
- Couvineau, A.; Nicole, P.; Gratio, V.; Voisin, T. The Orexin receptors: Structural and anti-tumoral properties. Front. Endocrinol. 2022, 13, 931970. [Google Scholar] [CrossRef]
- Messal, N.; Fernandez, N.; Dayot, S.; Gratio, V.; Nicole, P.; Prochasson, C.; Chantret, I.; LeGuilloux, G.; Jarry, A.; Couvelard, A. Ectopic expression of OX1R in ulcerative colitis mediates anti-inflammatory effect of orexin-A. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 3618–3628. [Google Scholar] [CrossRef]
- Alain, C.; Pascal, N.; Valérie, G.; Thierry, V. Orexins/Hypocretins and Cancer: A Neuropeptide as Emerging Target. Molecules 2021, 26, 4849. [Google Scholar] [CrossRef]
- Rouet-Benzineb, P.; Rouyer-Fessard, C.; Jarry, A.; Avondo, V.; Pouzet, C.; Yanagisawa, M.; Laboisse, C.; Laburthe, M.; Voisin, T. Orexins acting at native OX1 receptor in colon cancer and neuroblastoma cells or at recombinant OX1 receptor suppress cell growth by inducing apoptosis. J. Biol. Chem. 2004, 279, 45875–45886. [Google Scholar] [CrossRef] [PubMed]
- Voisin, T.; El Firar, A.; Fasseu, M.; Rouyer-Fessard, C.; Descatoire, V.; Walker, F.; Paradis, V.; Bedossa, P.; Henin, D.; Lehy, T. Aberrant Expression of OX1 Receptors for Orexins in Colon Cancers and Liver Metastases: An Openable Gate to ApoptosisOrexin Receptor in Colon Cancers and Liver Metastases. Cancer Res. 2011, 71, 3341–3351. [Google Scholar] [CrossRef]
- Srivastava, A.; Jit, B.P.; Dash, R.; Panda, M.K. Bioactive lipid: A novel diagnostic approach for retinoblastoma in clinical management. Int. J. Mol. Immun. Oncol. 2021, 6, 136–139. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A.; et al. Green Synthesis of Gold Nanoparticles Using Plant Extracts as Beneficial Prospect for Cancer Theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef]
- Sarma, M.K.; Ningthoujam, R.; Panda, M.K.; Babu, P.J.; Srivastava, A.; Das, M.; Singh, Y.D. Translational healthcare system through bioinformatics. In Translational Bioinformatics Applications in Healthcare; CRC Press: Boca Raton, FL, USA, 2021; pp. 3–21. [Google Scholar]
- Quartara, L.; Altamura, M. Tachykinin receptors antagonists: From research to clinic. Curr. Drug Targets 2006, 7, 975–992. [Google Scholar] [CrossRef] [PubMed]
- Langford, P.; Chrisp, P. Fosaprepitant and aprepitant: An update of the evidence for their place in the prevention of chemotherapy-induced nausea and vomiting. Core Evid. 2010, 5, 77–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigioni, M.; Benzo, A.; Irrissuto, C.; Maggi, C.A.; Goso, C. Role of NK-1 and NK-2 tachykinin receptor antagonism on the growth of human breast carcinoma cell line MDA-MB-231. Anticancer Drugs 2005, 16, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Pei, G.; Zhao, Z.; Kim, S.T.; German, A.; Robinson, P. Substance P antagonism as a novel therapeutic option to enhance efficacy of cisplatin in triple negative breast cancer and protect PC12 cells against cisplatin-induced oxidative stress and apoptosis. Cancers 2021, 13, 3871. [Google Scholar] [CrossRef]
- Buchegger, F.; Bonvin, F.; Kosinski, M.; Schaffland, A.O.; Prior, J.; Reubi, J.C.; Bläuenstein, P.; Tourwé, D.; Garayoa, E.G.; Delaloye, A.B. Radiolabeled Neurotensin Analog, 99mTc-NT-XI, Evaluated in Ductal Pancreatic Adenocarcinoma Patients. J. Nucl. Med. 2003, 44, 1649–1654. [Google Scholar]
- García-Garayoa, E.; Bläuenstein, P.; Blanc, A.; Maes, V.; Tourwé, D.; Schubiger, P.A. A stable neurotensin-based radiopharmaceutical for targeted imaging and therapy of neurotensin receptor-positive tumours. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 37–47. [Google Scholar] [CrossRef]
- Khan, I.U.; Zwanziger, D.; Böhme, I.; Javed, M.; Naseer, H.; Hyder, S.W.; Beck-Sickinger, A.G. Breast-cancer diagnosis by neuropeptide Y analogues: From synthesis to clinical application. Angew. Chem. Int. Ed. Engl. 2010, 49, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Morgat, C.; Mishra, A.K.; Varshney, R.; Allard, M.; Fernandez, P.; Hindié, E. Targeting neuropeptide receptors for cancer imaging and therapy: Perspectives with bombesin, neurotensin, and neuropeptide-Y receptors. J. Nucl. Med. 2014, 55, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Dozio, E.; Boghossian, S.; Bovo, G.; Martos Riaño, V.; Motta, M.; Magni, P. Activation of the Y1 receptor by neuropeptide Y regulates the growth of prostate cancer cells. Endocrinology 2006, 147, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Dufes, C.; Alleaume, C.; Montoni, A.; Olivier, J.-C.; Muller, J.-M. Effects of the vasoactive intestinal peptide (VIP) and related peptides on glioblastoma cell growth in vitro. J. Mol. Neurosci. 2003, 21, 91–102. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Jensen, R.T.; Battey, J.F.; Spindel, E.R.; Benya, R.V. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 2008, 60, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Hohla, F.; Schally, A.V. Targeting gastrin releasing peptide receptors: New options for the therapy and diagnosis of cancer. Cell Cycle 2010, 9, 1738–1741. [Google Scholar] [CrossRef]
- Flores, D.G.; Meurer, L.; Uberti, A.F.; Macedo, B.R.; Lenz, G.; Brunetto, A.L.; Schwartsmann, G.; Roesler, R. Gastrin-releasing peptide receptor content in human glioma and normal brain. Brain Res. Bull. 2010, 82, 95–98. [Google Scholar] [CrossRef]
- Roesler, R.; Brunetto, A.T.; Abujamra, A.L.; de Farias, C.B.; Brunetto, A.L.; Schwartsmann, G. Current and emerging molecular targets in glioma. Expert Rev. Anticancer Ther. 2010, 10, 1735–1751. [Google Scholar] [CrossRef] [PubMed]
- Roesler, R.; Schwartsmann, G. Gastrin-releasing peptide receptors in the central nervous system: Role in brain function and as a drug target. Front. Endocrinol. 2012, 3, 159. [Google Scholar] [CrossRef]
- Cochaud, S.; Meunier, A.-C.; Monvoisin, A.; Bensalma, S.; Muller, J.-M.; Chadéneau, C. Neuropeptides of the VIP family inhibit glioblastoma cell invasion. J. Neurooncol. 2015, 122, 63–73. [Google Scholar] [CrossRef]
- Moody, T.W.; Nuche-Berenguer, B.; Jensen, R.T. Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Ramos-Alvarez, I.; Moody, T.W.; Mantey, S.A.; Jensen, R.T. Neuropeptide bombesin receptor activation stimulates growth of lung cancer cells through HER3 with a MAPK-dependent mechanism. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118625. [Google Scholar] [CrossRef]
- Reubi, J.C.; Läderach, U.; Waser, B.; Gebbers, J.O.; Robberecht, P.; Laissue, J.A. Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res. 2000, 60, 3105–3112. [Google Scholar]
- Moody, T.W.; Moreno, P.; Jensen, R.T. Neuropeptides as lung cancer growth factors. Peptides 2015, 72, 106–111. [Google Scholar] [CrossRef]
- Moody, T.W.; Pert, C.B.; Gazdar, A.F.; Carney, D.N.; Minna, J.D. High levels of intracellular bombesin characterize human small-cell lung carcinoma. Science 1981, 214, 1246–1248. [Google Scholar] [CrossRef]
- Korman, L.Y.; Carney, D.N.; Citron, M.L.; Moody, T.W. Secretin/vasoactive intestinal peptide-stimulated secretion of bombesin/gastrin releasing peptide from human small cell carcinoma of the lung. Cancer Res. 1986, 46, 1214–1218. [Google Scholar] [PubMed]
- Moody, T.W.; Carney, D.N.; Korman, L.Y.; Gazdar, A.F.; Minna, J.D. Neurotensin is produced by and secreted from classic small cell lung cancer cells. Life Sci. 1985, 36, 1727–1732. [Google Scholar] [CrossRef]
- Hassan, S.; Dobner, P.R.; Carraway, R.E. Involvement of MAP-kinase, PI3-kinase and EGF-receptor in the stimulatory effect of Neurotensin on DNA synthesis in PC3 cells. Regul. Pept. 2004, 120, 155–166. [Google Scholar] [CrossRef]
- Younes, M.; Wu, Z.; Dupouy, S.; Lupo, A.M.; Mourra, N.; Takahashi, T.; Fléjou, J.F.; Trédaniel, J.; Régnard, J.F.; Damotte, D.; et al. Neurotensin (NTS) and its receptor (NTSR1) causes EGFR, HER2 and HER3 over-expression and their autocrine/paracrine activation in lung tumors, confirming responsiveness to erlotinib. Oncotarget 2014, 5, 8252–8269. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Leyton, J.; Garcia-Marin, L.; Jensen, R.T. Nonpeptide gastrin releasing peptide receptor antagonists inhibit the proliferation of lung cancer cells. Eur. J. Pharmacol. 2003, 474, 21–29. [Google Scholar] [CrossRef]
- Moreno, P.; Mantey, S.A.; Lee, S.H.; Ramos-Álvarez, I.; Moody, T.W.; Jensen, R.T. A possible new target in lung-cancer cells: The orphan receptor, bombesin receptor subtype-3. Peptides 2018, 101, 213–226. [Google Scholar] [CrossRef]
- Bajo, A.M.; Schally, A.V.; Krupa, M.; Hebert, F.; Groot, K.; Szepeshazi, K. Bombesin antagonists inhibit growth of MDA-MB-435 estrogen-independent breast cancers and decrease the expression of the ErbB-2/HER-2 oncoprotein and c-jun and c-fos oncogenes. Proc. Natl. Acad. Sci. USA 2002, 99, 3836–3841. [Google Scholar] [CrossRef]
- Schwartsmann, G.; DiLeone, L.; Horowitz, M.; Schunemann, D.; Cancella, A.; Pereira, A.; Richter, M.; Souza, F.; Da Rocha, A.B.; Souza, F. A phase I trial of the bombesin/gastrin-releasing peptide (BN/GRP) antagonist RC3095 in patients with advanced solid malignancies. Investig. New Drugs 2006, 24, 403–412. [Google Scholar] [CrossRef]
- Sotomayor, S.; Muñoz-Moreno, L.; Carmena, M.J.; Schally, A.V.; Sánchez-Chapado, M.; Prieto, J.C.; Bajo, A.M. Regulation of HER expression and transactivation in human prostate cancer cells by a targeted cytotoxic bombesin analog (AN-215) and a bombesin antagonist (RC-3095). Int. J. Cancer 2010, 127, 1813–1822. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.I.; Jutila, M.A.; Quinn, M.T. Gastrin-releasing peptide/neuromedin B receptor antagonists PD176252, PD168368, and related analogs are potent agonists of human formyl-peptide receptors. Mol. Pharmacol. 2011, 79, 77–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moody, T.W.; Jensen, R.T.; Garcia, L.; Leyton, J. Nonpeptide neuromedin B receptor antagonists inhibit the proliferation of C6 cells. Eur. J. Pharmacol. 2000, 409, 133–142. [Google Scholar] [CrossRef]
- Cai, R.; Qin, Y.; Ertl, T.; Schally, A.V. New pseudononapeptide bombesin antagonists with C-terminal leu-psi (ch2n) tac-nh2 show high binding-affinity to bombesin/grp receptors on cfpac-1 human pancreatic-cancer cells. Int. J. Oncol. 1995, 6, 1165–1172. [Google Scholar] [CrossRef]
- Baum, R.P.; Singh, A.; Schuchardt, C.; Kulkarni, H.R.; Klette, I.; Wiessalla, S.; Osterkamp, F.; Reineke, U.; Smerling, C. 177Lu-3BP-227 for neurotensin receptor 1–targeted therapy of metastatic pancreatic adenocarcinoma: First clinical results. J. Nucl. Med. 2018, 59, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Chiles, J.; Casibang, M.; Moody, E.; Chan, D.; Davis, T.P. SR48692 is a neurotensin receptor antagonist which inhibits the growth of small cell lung cancer cells. Peptides 2001, 22, 109–115. [Google Scholar] [CrossRef]
- Moody, T.W.; Leyton, J.; Chan, D.; Brenneman, D.C.; Fridkin, M.; Gelber, E.; Levy, A.; Gozes, I. VIP receptor antagonists and chemotherapeutic drugs inhibit the growth of breast cancer cells. Breast Cancer Res. Treat. 2001, 68, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Wu, W.K.K.; Ng, S.S.M.; Yu, L.; Li, H.T.; Wong, C.C.M.; Wu, Y.C.; Zhang, L.; Ren, S.X.; Sun, X.G. A novel peptide specifically targeting the vasculature of orthotopic colorectal cancer for imaging detection and drug delivery. J. Control. Release 2010, 148, 292–302. [Google Scholar] [CrossRef]
- Li, Z.J.; Cho, C.H. Neurotransmitters, more than meets the eye—Neurotransmitters and their perspectives in cancer development and therapy. Eur. J. Pharmacol. 2011, 667, 17–22. [Google Scholar] [CrossRef] [PubMed]

| Neurotransmitters | Sources | Expressed | Description | Refs. |
|---|---|---|---|---|
| Peptidergic neuropeptides | ||||
| Neurotensin (C78H121N21O20) | Hypothalamus and intestine | Hypothalamus, bronchial epithelial cell, lymph node, anterior pituitary, jejunal mucosa, superficial temporal artery, hippocampus proper, duodenum, appendix and gallbladder. | Neurotensin is thought to regulate the luteinizing hormone, prolactin release and brain dopamine system. Involved in a wide variety of biological effects, such as histamine release, vasodilation, gastrointestinal (GI) muscle modulation and motility, and stimulation of intestinal secretion. | [3,4] |
| Vasoactive intestinal polypeptide | Gut, pancreas, and hypothalamus | Appendix, rectum, gastric mucosa, endothelial cell, transverse colon, cingulate gyrus and prefrontal cortex | It performs several functions in the body, such as relaxation of muscle in digestive tract and heart, control of fluid secretion, increase in glycogenolysis and reduction of the blood pressure. | [5,6] |
| Substance P (C63H98N18O13S) | Brain, spinal cord and intestine | Hypothalamus, amygdala and periaqueductal gray | Substance P is associated with inflammation, pain, anxiety, mood, cell migration and angiogenesis. | [7,8] |
| Bombesin (C71H110N24O18S) | Brain | - | It mediates gastrin release and was also termed gastrin-releasing peptide. It participates in various processes, such as glucose homeostasis, circadian rhythm, thermoregulation, and many GI processes. | [9,10] |
| Neuropeptide Y (C190H287N55O57) | Brain and circulating platelets | Cerebral cortex, thalamus, brainstem, hypothalamus, amygdala, prostate, and hippocampus | Neuropeptide Y plays a crucial role in food intake, reducing stress and pain, lowering blood pressure, and storing of energy. | [11,12] |
| Calcitonin gene-related peptide (CGRP) | Peripheral and central neuron | placental syncytiotrophoblast, villous vascular endothelial cells, and decidua | Calcitonin gene-related peptide is implicated in vasodilation, appetite suppression, stem cell mobilization, and homeostasis. | [13,14] |
| Biogenic neurotransmitter | ||||
| Dopamine (C8H11NO2) | Substantia nigra ventral brain | Brain, blood vessels, kidneys, pancreas, and gastrointestinal tract | Dopamine regulates norepinephrine inhibition, vasodilation, increases sodium excretion, reduces insulin production and gastrointestinal motility. | [15,16] |
| Epinephrine and Norepinephrine (C9H13NO3) | Adrenal gland and medulla oblongata | Heart, liver, lungs, muscles, and brain | Epinephrine has effects on increasing blood sugar, heart rate, smooth muscle contraction, and pupil dilation | [17,18] |
| Serotonin (C₁₀H₁₂N₂O) | Enteric nervous system located in the GI tract and brain | - | Sleep, emotion, mood, wound healing, immune regulation, and insulin secretion are some of the important cognitive and peripheral functions modulated by serotonin. | [19,20] |
| Acetylcholine (Ach, C7NH16O2+) | Motor neurons, parasympathetic nervous system and brain | Skeleton muscle, brain, and other organs | Ach is a well-known neurotransmitter of the neuronal system, it is also synthesized in non-neuronal cells including mesothelial, adipocytes, fibroblast, epithelial, endothelial, and cancer cells. | [21,22] |
| Amino acids | ||||
| Gamma-aminobutyric acid-GABA, (C4H9NO2) | Brain | - | GABA is an inhibitory neurotransmitter that blocks messages or nerve signals between nerves and CNS, though its function is well defined in reducing the feeling of stress, anxiety, and fear. | [23,24] |
| Glycine (C₂H₅NO₂) | Kidneys and liver | - | Glycine is an inhibitory neurotransmitter of the central nervous system CNS, produced naturally in the body and important for the healthy development of bones, muscles, and tissues. | [25] |
| Histamine (C5H9N3) | Basophils and mast cells | - | It plays a central role in inflammatory response and as itching mediator. | [26] |
| Neuropeptides | Number of Amino Acids | Discovery | Related Cancers | Tumorigenic Properties | Refs. |
|---|---|---|---|---|---|
| Neurotensin | 13 | Carraway and Leeman in 1973 | Pancreatic, lung, breast, prostate, and colorectal cancer | Increased cell proliferation and migration | [86,87] |
| Vasoactive intestinal polypeptide (VIP) | 28 | Said and Mutt in 1970 | Neuroblastoma, pituitary adenomas, colorectal cancer, endometrial, and lung cancer | Increased cell proliferation, metastasis, invasion, and angiogenesis | [88,89] |
| Substance P | 11 | Von Euler and Gaddum in 1931 | Glioblastoma, breast, acute lymphoblastic Leukemia, colorectal cancer, melanoma, and gastric cancer | Increased cell proliferation, migration, invasion, and angiogenesis; pro-inflammatory effect | [90,91] |
| Bombesin | 14 | Battey and Wada in 1991 | Prostate, gastric, lung, breast, colorectal cancer, renal cell carcinoma, small cell lung carcinoma, neuroendocrine, squamous, colon, and pituitary cancer | Promoted vascularization, tumor growth, and differentiation | [92,93,94,95] |
| Neuropeptide Y | 36 | Tatemoto and Mutt in 1982 | Neuroblastoma, colorectal cancer, breast, Ewing sarcoma, and prostate cancer | Induced cell growth, vascularization, and angiogenesis; pro-inflammatory effect | [46,96,97] |
| Calcitonin gene-related peptide (CGRP) | 37 | Amara and colleagues in 1982 | Prostate, lung, colorectal cancer, pancreatic, ovarian, endometrial, pituitary, renal, and hepatic cancer | Promoted angiogenesis, lymphangiogenesis, cell growth, neovascularization, proliferation, and migration | [98,99,100,101] |
| Neuropeptide | Cancer | Drugs/Antagonists | Targeted Receptors | Refs. |
|---|---|---|---|---|
| Bombesin (neuromedin B/gastrin-releasing peptide) | Small cell lung carcinoma | PD176252, PD168368 | Gastrin-releasing peptide receptors-GRPR, neuromedin B receptor-NMBR | [169,170] |
| Bantag-1 | Bombesin-receptor subtype-3 | |||
| Breast | RC-3095, RC-3940-II | Gastrin-releasing peptide receptors-GRPR | [92,171,172] | |
| CRC | RC-3095, AN-215 | Gastrin-releasing peptide receptors-GRPR | [121,154] | |
| Prostate | BAY 86-7548, 64Cu-CB-TE2A-AR-06, RC-3095 | Gastrin-releasing peptide receptors-GRPR | [149,173] | |
| Ovary | PD176252 | Gastrin-releasing peptide receptors-GRPR, neuromedin B receptor-NMBR | [174] | |
| Glioma | PD176252, PD168368 | Neuromedin B receptor-NMBR | [175] | |
| Pancreatic | RC-3095, RC-3925-II, RC-3940-II and RC-3950-II | Gastrin-releasing peptide receptors-GRPR | [173,176] | |
| Neurotensin | Breast | 99mTc-NT-XIX, 188Re-NT-XIX, 18F-DEG-VS-NT | Neurotensin receptor-NTSR | [145,146] |
| Pancreatic adenocarcinoma | 177 Lu-3BP-227 | Neurotensin receptor-1 NTSR-1 | [177] | |
| CRC | Sodium butyrate, SR48692, Curcumin, Cyanidin, SR 48692, 177 Lu-3BP-227 | Neurotensin receptor-NTSR | [109,124] | |
| Small cell lung carcinoma | SR48692 | Neurotensin receptor-1 NTSR-1 | [178] | |
| Vasoactive intestinal peptide-VIP | CRC | NTS6-11VIP7-28 | Vasoactive intestinal peptide receptor-VPAC1/2 | [128] |
| Breast | VIP hybrid | Vasoactive intestinal peptide receptor VIP receptor | [179] | |
| Substance P | Breast | Aprepitant, L-732,138, L-733,060, CP-96345, C-9994, MEN 11467, SR14033, Spantide III | Neurokinin 1 receptor-NK1R | [91,142,143,144] |
| CRC | Spantide 1, Aprepitant, Fosaprepitant, GR 159897 | Neurokinin 1 receptor-NK1R | [90,129,130] | |
| Neuropeptide Y | Breast | BIBP3226 | Y1R | [131] |
| BIIE0246 | Y2R | |||
| L-152,804 | Y5R | |||
| Colon adenocarcinoma | - | Y2R | [132] | |
| Prostate | BIBP32226 | Y1R | [150] | |
| Orexin | Gastrointestinal tumors and CRC | NSC-87877, PD169316 | Orexin receptor -OX1R | [136,137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, A.; Rikhari, D.; Pradhan, B.; Bharadwaj, K.K.; Gaballo, A.; Quarta, A.; Jena, M.; Srivastava, S.; Ragusa, A. An Insight into Neuropeptides Inhibitors in the Biology of Colorectal Cancer: Opportunity and Translational Perspectives. Appl. Sci. 2022, 12, 8990. https://doi.org/10.3390/app12188990
Srivastava A, Rikhari D, Pradhan B, Bharadwaj KK, Gaballo A, Quarta A, Jena M, Srivastava S, Ragusa A. An Insight into Neuropeptides Inhibitors in the Biology of Colorectal Cancer: Opportunity and Translational Perspectives. Applied Sciences. 2022; 12(18):8990. https://doi.org/10.3390/app12188990
Chicago/Turabian StyleSrivastava, Ankit, Deeksha Rikhari, Biswajita Pradhan, Kaushik Kumar Bharadwaj, Antonio Gaballo, Alessandra Quarta, Mrutyunjay Jena, Sameer Srivastava, and Andrea Ragusa. 2022. "An Insight into Neuropeptides Inhibitors in the Biology of Colorectal Cancer: Opportunity and Translational Perspectives" Applied Sciences 12, no. 18: 8990. https://doi.org/10.3390/app12188990
APA StyleSrivastava, A., Rikhari, D., Pradhan, B., Bharadwaj, K. K., Gaballo, A., Quarta, A., Jena, M., Srivastava, S., & Ragusa, A. (2022). An Insight into Neuropeptides Inhibitors in the Biology of Colorectal Cancer: Opportunity and Translational Perspectives. Applied Sciences, 12(18), 8990. https://doi.org/10.3390/app12188990










