Pituitary Adenylate Cyclase-Activating Polypeptide Protects Corneal Epithelial Cells against UV-B-Induced Apoptosis via ROS/JNK Pathway Inhibition
Abstract
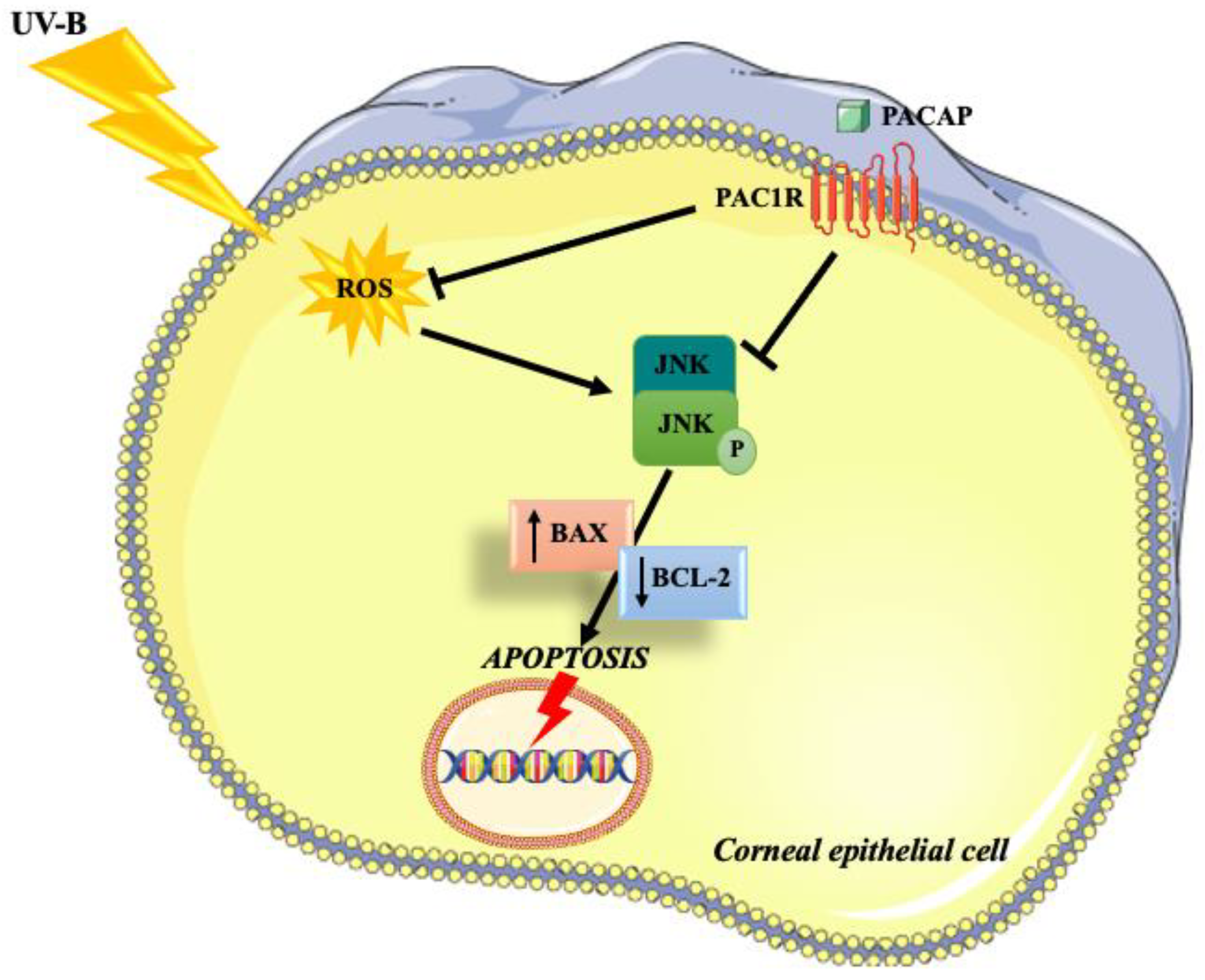
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Immunohistochemistry (IHC) Analysis
2.3. Cell Cultures
2.4. Cell Viability Assay
2.5. Assessment of Cell Death Using Fluorescence Microscope
2.6. Western Blot Analysis
2.7. Detection of ROS
2.8. Statistical Analysis
3. Results
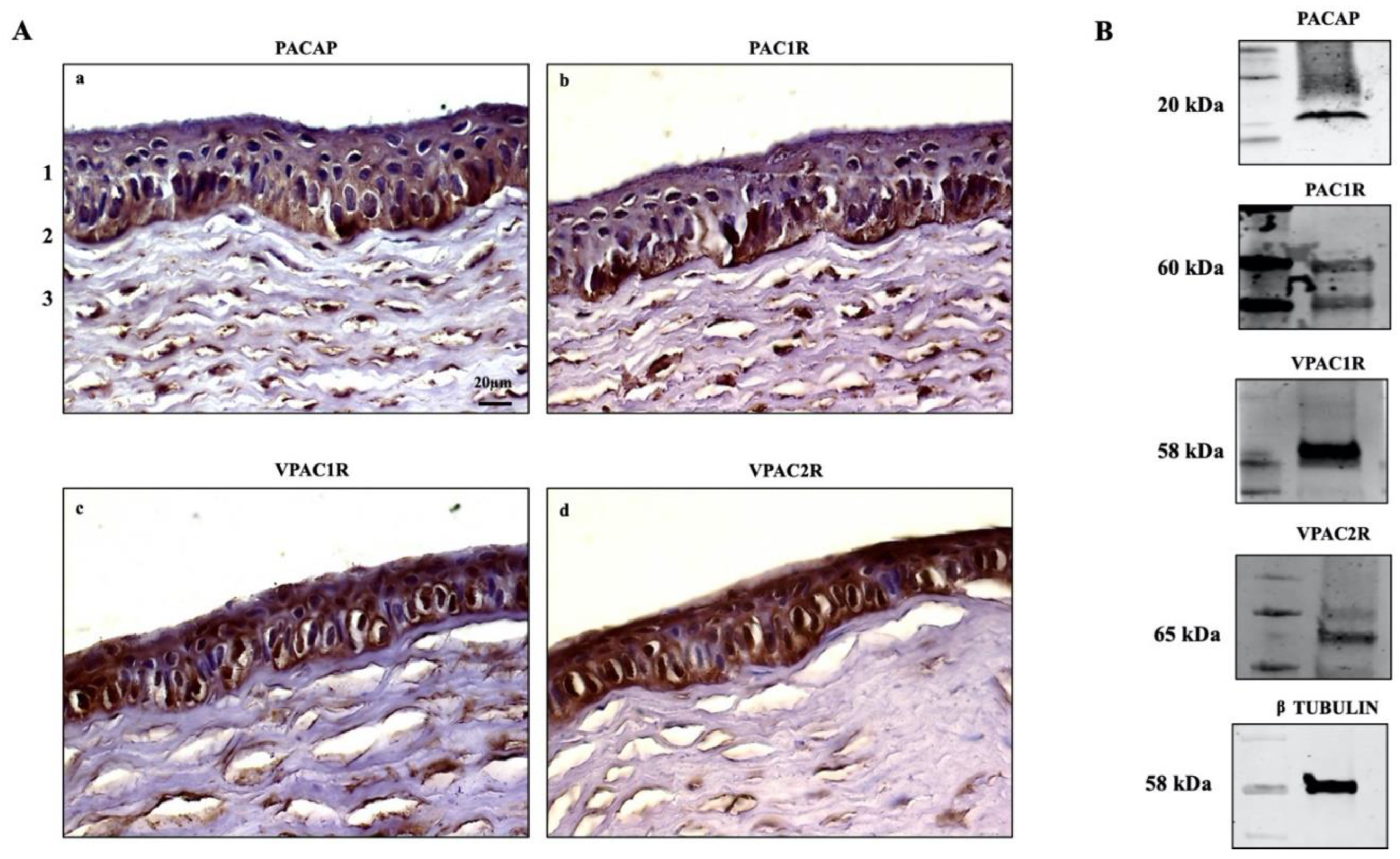
3.1. Detection of PACAP, PAC1R and VPACRs in Corneal Epithelium
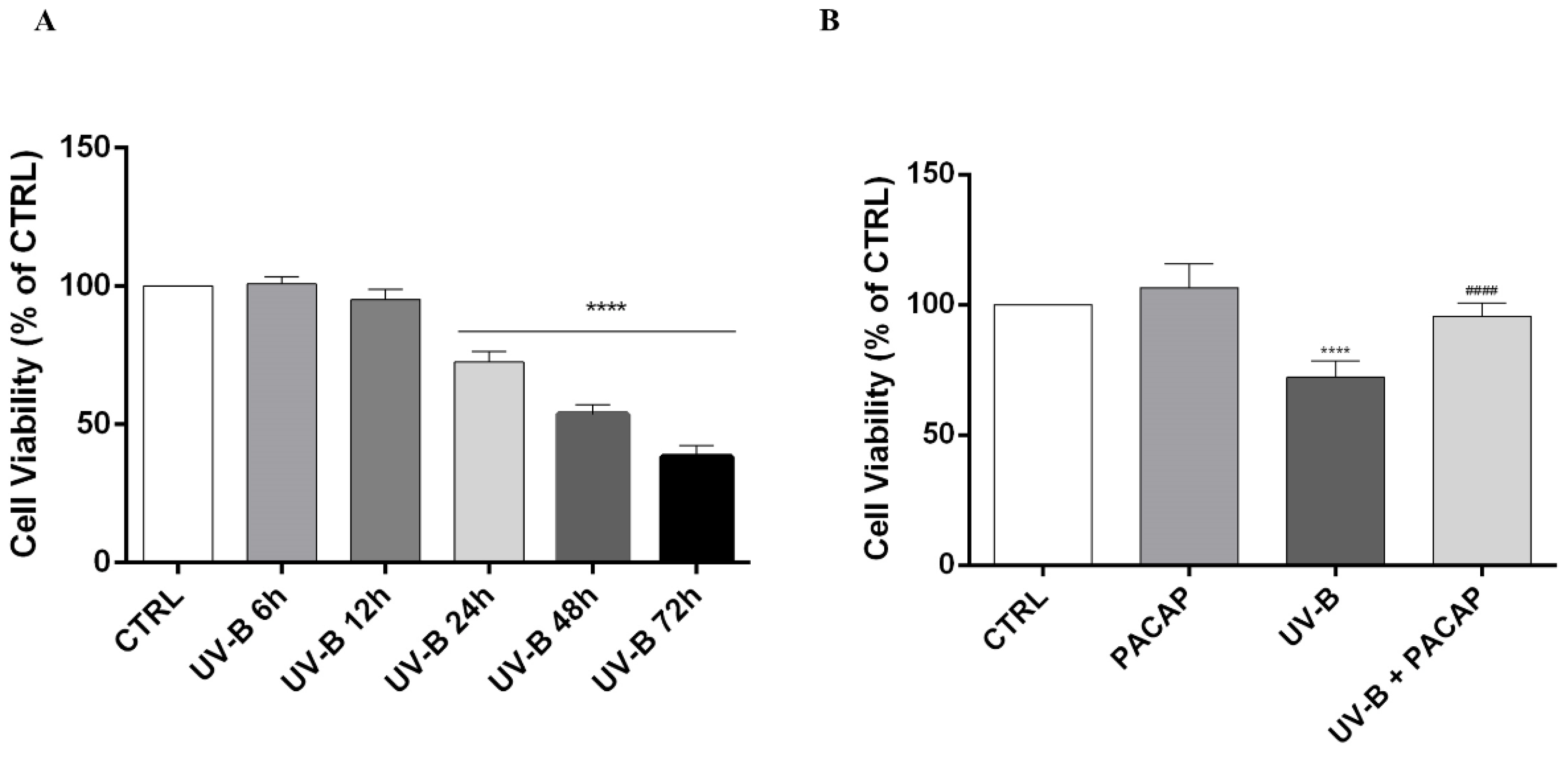
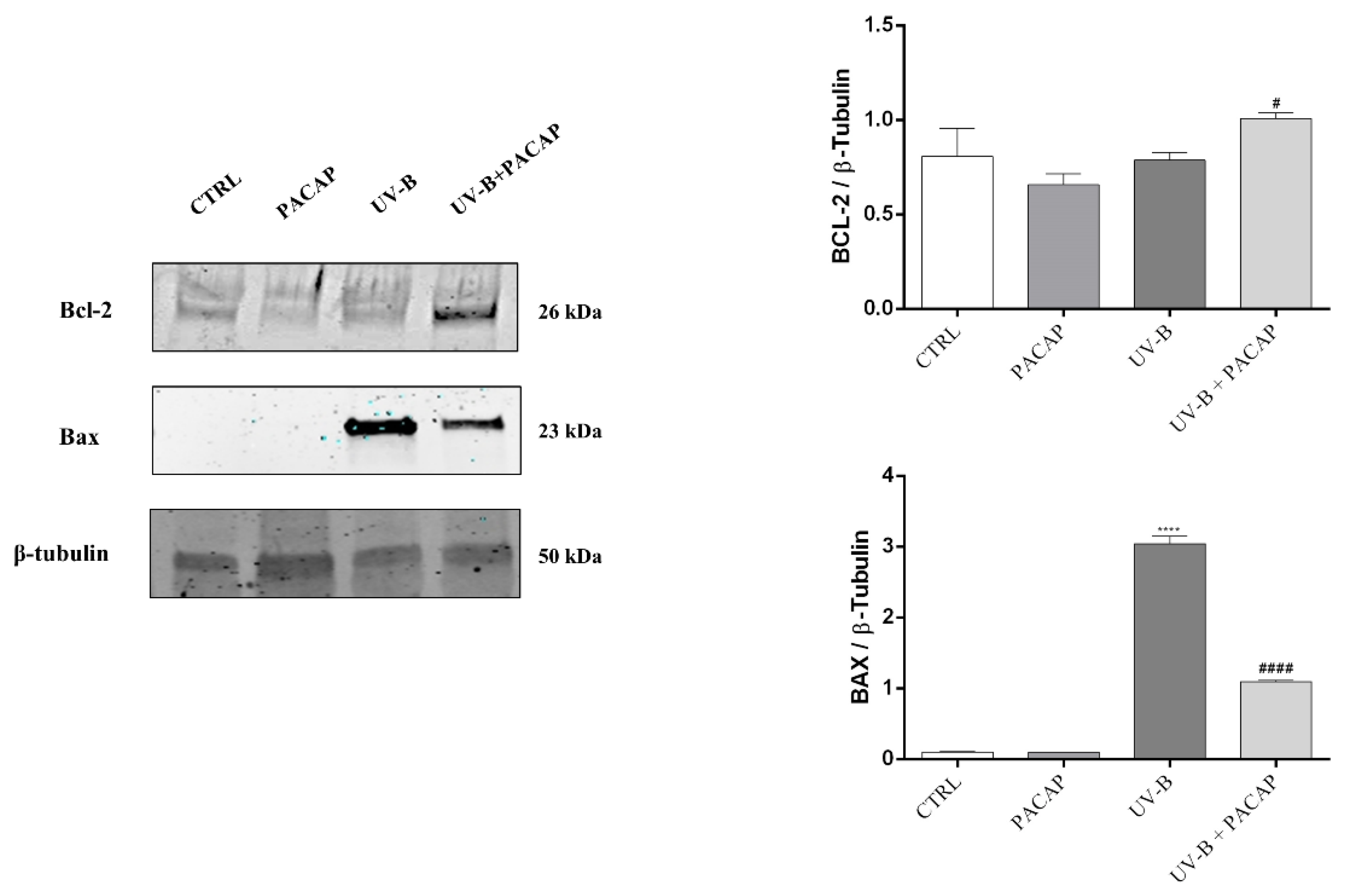
3.2. Effect of PACAP Treatment on UV-B-Induced Apoptotic Cell Death
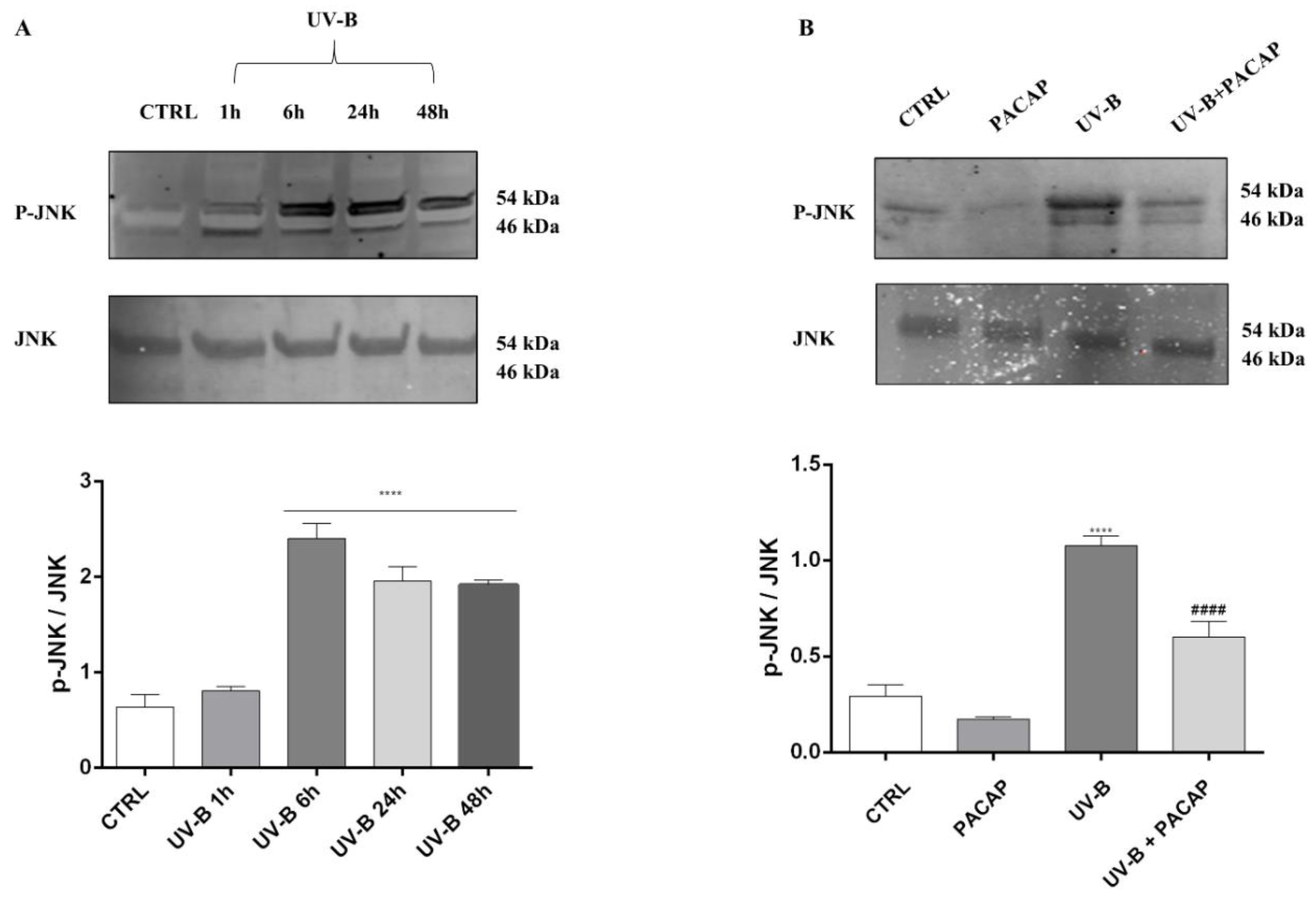
3.3. PACAP Decreased the Production of ROS Induced by UV-B Rays on Corneal Epithelial Cells via the JNK Pathway Inhibition
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sliney, D.H. How light reaches the eye and its components. Int. J. Toxicol. 2002, 21, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Heck, D.E.; Gerecke, D.R.; Vetrano, A.M.; Laskin, J.D. Solar ultraviolet radiation as a trigger of cell signal transduction. Toxicol. Appl. Pharmacol. 2004, 195, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Azzam, N.; Levanon, D.; Dovrat, A. Effects of UV-A irradiation on lens morphology and optics. Exp. Gerontol. 2004, 39, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Bashir, H.; Seykora, J.T.; Lee, V. Invisible Shield: Review of the Corneal Epithelium as a Barrier to UV Radiation, Pathogens, and Other Environmental Stimuli. J. Ophthalmic Vis. Res. 2017, 12, 305–311. [Google Scholar] [CrossRef]
- Mesa, R.; Bassnett, S. UV-B-induced DNA damage and repair in the mouse lens. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6789–6797. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian. J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef]
- Black, A.T.; Gordon, M.K.; Heck, D.E.; Gallo, M.A.; Laskin, D.L.; Laskin, J.D. UVB light regulates expression of antioxidants and inflammatory mediators in human corneal epithelial cells. Biochem. Pharmacol. 2011, 81, 873–880. [Google Scholar] [CrossRef] [Green Version]
- McCubrey, J.A.; Lahair, M.M.; Franklin, R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 2006, 8, 1775–1789. [Google Scholar] [CrossRef]
- Liu, J.; Lin, A. Role of JNK activation in apoptosis: A double-edged sword. Cell Res. 2005, 15, 36–42. [Google Scholar] [CrossRef]
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant defenses in the ocular surface. Ocul. Surf. 2009, 7, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Kolozsvári, L.; Nógrádi, A.; Hopp, B.; Bor, Z. UV absorbance of the human cornea in the 240- to 400-nm range. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2165–2168. [Google Scholar]
- Harmar, A.J.; Arimura, A.; Gozes, I.; Journot, L.; Laburthe, M.; Pisegna, J.R.; Rawlings, S.R.; Robberecht, P.; Said, S.I.; Sreedharan, S.P.; et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 1998, 50, 265–270. [Google Scholar] [PubMed]
- Arimura, A.; Shioda, S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: Neuroendocrine and endocrine interaction. Front. Neuroendocrinol. 1995, 16, 53–88. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Bassan, M.; Zamostiano, R.; Davidson, A.; Pinhasov, A.; Giladi, E.; Perl, O.; Bassan, H.; Blat, C.; Gibney, G.; Glazner, G.; et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J. Neurochem. 1999, 72, 1283–1293. [Google Scholar] [CrossRef] [Green Version]
- Zamostiano, R.; Pinhasov, A.; Gelber, E.; Steingart, R.A.; Seroussi, E.; Giladi, E.; Bassan, M.; Wollman, Y.; Eyre, H.J.; Mulley, J.C.; et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J. Biol. Chem. 2001, 276, 708–714. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, A.G.; Scuderi, S.; Maugeri, G.; Cavallaro, S.; Drago, F.; D’Agata, V. NAP reduces murine microvascular endothelial cells proliferation induced by hyperglycemia. J. Mol. Neurosci. 2014, 54, 405–413. [Google Scholar] [CrossRef]
- D’Amico, A.G.; Maugeri, G.; Rasà, D.; Federico, C.; Saccone, S.; Lazzara, F.; Fidilio, A.; Drago, F.; Bucolo, C.; D’Agata, V. NAP modulates hyperglycemic-inflammatory event of diabetic retina by counteracting outer blood retinal barrier damage. J. Cell. Physiol. 2019, 234, 5230–5240. [Google Scholar] [CrossRef]
- D’Amico, A.G.; Maugeri, G.; Musumeci, G.; Reglodi, D.; D’Agata, V. PACAP and NAP: Effect of Two Functionally Related Peptides in Diabetic Retinopathy. J. Mol. Neurosci. 2021, 71, 1525–1535. [Google Scholar] [CrossRef]
- Solés-Tarrés, I.; Cabezas-Llobet, N.; Vaudry, D.; Xifró, X. Protective Effects of Pituitary Adenylate Cyclase-Activating Polypeptide and Vasoactive Intestinal Peptide Against Cognitive Decline in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 221. [Google Scholar] [CrossRef] [PubMed]
- Dejda, A.; Jolivel, V.; Bourgault, S.; Seaborn, T.; Fournier, A.; Vaudry, H.; Vaudry, D. Inhibitory effect of PACAP on caspase activity in neuronal apoptosis: A better understanding towards therapeutic applications in neurodegenerative diseases. J. Mol. Neurosci. 2008, 36, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Kiss, P.; Lubics, A.; Tamas, A. Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr. Pharm. Des. 2011, 17, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J.A. VIP and PACAP: Neuropeptide modulators of CNS inflammation, injury, and repair. Br. J. Pharmacol. 2013, 169, 512–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maugeri, G.; D’Amico, A.G.; Morello, G.; Reglodi, D.; Cavallaro, S.; D’Agata, V. Differential Vulnerability of Oculomotor Versus Hypoglossal Nucleus During ALS: Involvement of PACAP. Front. Neurosci. 2020, 14, 805. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Musumeci, G.; Reglodi, D.; D’Agata, V. Effects of Pacap on Schwann Cells: Focus on Nerve Injury. Int. J. Mol. Sci. 2020, 21, 8233. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; Federico, C.; Saccone, S.; Morello, G.; La Cognata, V.; Cavallaro, S.; D’Agata, V. Molecular mechanisms involved in the protective effect of pituitary adenylate cyclase-activating polypeptide in an in vitro model of amyotrophic lateral sclerosis. J. Cell. Physiol. 2019, 234, 5203–5214. [Google Scholar] [CrossRef]
- D’Amico, A.G.; Maugeri, G.; Saccone, S.; Federico, C.; Cavallaro, S.; Reglodi, D.; D’Agata, V. PACAP Modulates the Autophagy Process in an In Vitro Model of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 21, 2943. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Alm, P.; Håkanson, R. Distribution and effects of pituitary adenylate cyclase-activating peptide in the rabbit eye. Neuroscience 1995, 69, 297–308. [Google Scholar] [CrossRef]
- Nilsson, S.F.; De Neef, P.; Robberecht, P.; Christophe, J. Characterization of ocular receptors for pituitary adenylate cyclase activating polypeptide (PACAP) and their coupling to adenylate cyclase. Exp. Eye Res. 1994, 58, 459–467. [Google Scholar] [CrossRef]
- Nakajima, E.; Walkup, R.D.; Fujii, A.; Shearer, T.R.; Azuma, M. Pituitary adenylate cyclase-activating peptide induces neurite outgrowth in cultured monkey trigeminal ganglion cells: Involvement of receptor PAC1. Mol. Vis. 2013, 19, 174–183. [Google Scholar] [PubMed]
- Toth, D.; Szabo, E.; Tamas, A.; Juhasz, T.; Horvath, G.; Fabian, E.; Opper, B.; Szabo, D.; Maugeri, G.; D’Amico, A.G.; et al. Protective Effects of PACAP in Peripheral Organs. Front. Endocrinol. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Szabadfi, K.; Reglodi, D.; Szabo, A.; Szalontai, B.; Valasek, A.; Setalo, G., Jr.; Kiss, P.; Tamas, A.; Wilhelm, M.; Gabriel, R. Pituitary Adenylate Cyclase Activating Polypeptide, A Potential Therapeutic Agent for Diabetic Retinopathy in Rats: Focus on the Vertical Information Processing Pathway. Neurotox. Res. 2016, 29, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Atlasz, T.; Szabadfi, K.; Kiss, P.; Tamas, A.; Toth, G.; Reglodi, D.; Gabriel, R. Evaluation of the protective effects of PACAP with cell-specific markers in ischemia-induced retinal degeneration. Brain Res. Bull. 2010, 81, 497–504. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Bucolo, C.; D’Agata, V. Protective effect of PACAP-38 on retinal pigmented epithelium in an in vitro and in vivo model of diabetic retinopathy through EGFR-dependent mechanism. Peptides 2019, 119, 170108. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Gagliano, C.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. VIP Family Members Prevent Outer Blood Retinal Barrier Damage in a Model of Diabetic Macular Edema. J. Cell. Physiol. 2017, 232, 1079–1085. [Google Scholar] [CrossRef]
- Atlasz, T.; Szabadfi, K.; Kiss, P.; Marton, Z.; Griecs, M.; Hamza, L.; Gaal, V.; Biro, Z.; Tamas, A.; Hild, G.; et al. Effects of PACAP in UV-A radiation-induced retinal degeneration models in rats. J. Mol. Neurosci. 2011, 43, 51–57. [Google Scholar] [CrossRef]
- Fukiage, C.; Nakajima, T.; Takayama, Y.; Minagawa, Y.; Shearer, T.R.; Azuma, M. PACAP induces neurite outgrowth in cultured trigeminal ganglion cells and recovery of corneal sensitivity after flap surgery in rabbits. Am. J. Ophthalmol. 2007, 143, 255–262. [Google Scholar] [CrossRef]
- Shioda, S.; Takenoya, F.; Hirabayashi, T.; Wada, N.; Seki, T.; Nonaka, N.; Nakamachi, T. Effects of PACAP on Dry Eye Symptoms, and Possible Use for Therapeutic Application. J. Mol. Neurosci. 2019, 68, 420–426. [Google Scholar] [CrossRef]
- Wang, Z.; Shan, W.; Li, H.; Feng, J.; Lu, S.; Ou, B.; Ma, M.; Ma, Y. The PACAP-derived peptide MPAPO facilitates corneal wound healing by promoting corneal epithelial cell proliferation and trigeminal ganglion cell axon regeneration. Int. J. Biol. Sci. 2019, 15, 2676–2691. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Castrogiovanni, P.; Saccone, S.; Federico, C.; Reibaldi, M.; Russo, A.; Bonfiglio, V.; Avitabile, T.; Longo, A.; et al. PACAP through EGFR transactivation preserves human corneal endothelial integrity. J. Cell. Biochem. 2019, 120, 10097–10105. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; Longo, A.; D’Amico, A.G.; Rasà, D.M.; Reibaldi, M.; Russo, A.; Bonfiglio, V.; Avitabile, T.; D’Agata, V. Trophic effect of PACAP on human corneal endothelium. Peptides 2018, 99, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Nakamachi, T.; Ohtaki, H.; Seki, T.; Yofu, S.; Kagami, N.; Hashimoto, H.; Shintani, N.; Baba, A.; Mark, L.; Lanekoff, I.; et al. PACAP suppresses dry eye signs by stimulating tear secretion. Nat. Commun. 2016, 7, 12034. [Google Scholar] [CrossRef] [PubMed]
- Shioda, S.; Takenoya, F.; Wada, N.; Hirabayashi, T.; Seki, T.; Nakamachi, T. Pleiotropic and retinoprotective functions of PACAP. Anat. Sci. Int. 2016, 91, 313–324. [Google Scholar] [CrossRef]
- D’Agata, V.; Cavallaro, S. Functional and molecular expression of PACAP/VIP receptors in the rat retina. Brain Res. Mol. Brain Res. 1998, 54, 161–164. [Google Scholar] [CrossRef]
- Rogers, C.S.; Chan, L.M.; Sims, Y.S.; Byrd, K.D.; Hinton, D.L.; Twining, S.S. The effects of sub-solar levels of UV-A and UV-B on rabbit corneal and lens epithelial cells. Exp. Eye Res. 2004, 78, 1007–1014. [Google Scholar] [CrossRef]
- Kulms, D.; Zeise, E.; Pöppelmann, B.; Schwarz, T. DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way. Oncogene 2002, 21, 5844–5851. [Google Scholar] [CrossRef] [Green Version]
- Kronschläger, M.; Talebizadeh, N.; Yu, Z.; Meyer, L.M.; Löfgren, S. Apoptosis in Rat Cornea After In Vivo Exposure to Ultraviolet Radiation at 300 nm. Cornea 2015, 34, 945–949. [Google Scholar] [CrossRef]
- Young, A.R. Acute effects of UVR on human eyes and skin. Prog. Biophys. Mol. Biol. 2006, 92, 80–85. [Google Scholar] [CrossRef]
- Wada, T.; Penninger, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.M.; Liu, Z.G. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2006, 40, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Cejka, C.; Cejkova, J. Oxidative stress to the cornea, changes in corneal optical properties, and advances in treatment of corneal oxidative injuries. Oxid. Med. Cell. Longev. 2015, 2015, 591530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Takagi-Morishita, Y.; Yamada, N.; Sugihara, A.; Iwasaki, T.; Tsujimura, T.; Terada, N. Mouse uterine epithelial apoptosis is associated with expression of mitochondrial voltage-dependent anion channels, release of cytochrome C from mitochondria, and the ratio of Bax to Bcl-2 or Bcl-X. Biol. Reprod. 2003, 68, 1178–1184. [Google Scholar] [CrossRef] [Green Version]
- Balaiya, S.; Murthy, R.K.; Brar, V.S.; Chalam, K.V. Evaluation of ultraviolet light toxicity on cultured retinal pigment epithelial and retinal ganglion cells. Clin. Ophthalmol. 2010, 4, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzawa, A.; Ichijo, H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid. Redox Signal. 2005, 7, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yu, S.J.; Oh, H.J.; Lee, J.Y.; Kim, Y.; Sohn, J. Panaxydol induces apoptosis through an increased intracellular calcium level, activation of JNK and p38 MAPK and NADPH oxidase-dependent generation of reactive oxygen species. Apoptosis 2011, 16, 347–358. [Google Scholar] [CrossRef]
- Ramiro-Cortés, Y.; Guemez-Gamboa, A.; Morán, J. Reactive oxygen species participate in the p38-mediated apoptosis induced by potassium deprivation and staurosporine in cerebellar granule neurons. Int. J. Biochem. Cell Biol. 2011, 43, 1373–1382. [Google Scholar] [CrossRef]
- Szabo, A.; Danyadi, B.; Bognar, E.; Szabadfi, K.; Fabian, E.; Kiss, P.; Mester, L.; Manavalan, S.; Atlasz, T.; Gabriel, R.; et al. Effect of PACAP on MAP kinases, Akt and cytokine expressions in rat retinal hypoperfusion. Neurosci. Lett. 2012, 523, 93–98. [Google Scholar] [CrossRef]
- Rácz, B.; Gallyas, F., Jr.; Kiss, P.; Tóth, G.; Hegyi, O.; Gasz, B.; Borsiczky, B.; Ferencz, A.; Roth, E.; Tamás, A.; et al. The neuroprotective effects of PACAP in monosodium glutamate-induced retinal lesion involve inhibition of proapoptotic signaling pathways. Regul. Pept. 2006, 137, 20–26. [Google Scholar] [CrossRef]
- Bourgault, S.; Vaudry, D.; Dejda, A.; Doan, N.D.; Vaudry, H.; Fournier, A. Pituitary adenylate cyclase-activating polypeptide: Focus on structure-activity relationships of a neuroprotective Peptide. Curr. Med. Chem. 2009, 16, 4462–4480. [Google Scholar] [CrossRef] [PubMed]
- Gourlet, P.; Vandermeers, A.; Robberecht, P.; Deschodt-Lanckman, M. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP-27, but not PACAP-38) degradation by the neutral endopeptidase EC 3.4.24.11. Biochem. Pharmacol. 1997, 54, 509–515. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maugeri, G.; D’Amico, A.G.; Magrì, B.; Pricoco, E.; Giallongo, C.; Musumeci, G.; Bucolo, C.; Giunta, S.; D’Agata, V. Pituitary Adenylate Cyclase-Activating Polypeptide Protects Corneal Epithelial Cells against UV-B-Induced Apoptosis via ROS/JNK Pathway Inhibition. Appl. Sci. 2022, 12, 3435. https://doi.org/10.3390/app12073435
Maugeri G, D’Amico AG, Magrì B, Pricoco E, Giallongo C, Musumeci G, Bucolo C, Giunta S, D’Agata V. Pituitary Adenylate Cyclase-Activating Polypeptide Protects Corneal Epithelial Cells against UV-B-Induced Apoptosis via ROS/JNK Pathway Inhibition. Applied Sciences. 2022; 12(7):3435. https://doi.org/10.3390/app12073435
Chicago/Turabian StyleMaugeri, Grazia, Agata Grazia D’Amico, Benedetta Magrì, Elisabetta Pricoco, Cesarina Giallongo, Giuseppe Musumeci, Claudio Bucolo, Salvatore Giunta, and Velia D’Agata. 2022. "Pituitary Adenylate Cyclase-Activating Polypeptide Protects Corneal Epithelial Cells against UV-B-Induced Apoptosis via ROS/JNK Pathway Inhibition" Applied Sciences 12, no. 7: 3435. https://doi.org/10.3390/app12073435








